Abstract
Context:
During conditions of ovarian suppression, women with premenstrual dysphoria (PMD) experience abnormal behavioral responses to physiological levels of ovarian steroids. Although hypothalamic-pituitary-adrenal (HPA) axis dysregulation frequently accompanies depression, and ovarian steroids regulate HPA axis responsivity, the role of HPA axis dysregulation in PMD is not known. We hypothesized that women with PMD would show abnormalities of HPA axis function analogous to those reported in depressive illness, and that ovarian steroids would differentially regulate HPA axis function in women with PMD compared with asymptomatic controls (AC).
Objective:
Our objective was to characterize the HPA axis response to physiological levels of estradiol and progesterone in women with PMD and AC.
Design and Setting:
We conducted an open-label trial of the GnRH agonist depot Lupron with ovarian steroid replacement administered in a double-blind crossover design in an outpatient clinic.
Participants:
Forty-three women (18 with prospectively confirmed PMD and 25 AC) participated.
Interventions:
Women received Lupron for 6 months. After 3 months of hypogonadism, women received 5 wk each of estradiol (100-μg patch daily) or progesterone (suppositories 200 mg twice daily). During each condition, combined dexamethasone-suppression/CRH-stimulation tests and 24-h urinary free cortisol levels were performed.
Main Outcome Measures:
Plasma cortisol and ACTH levels were evaluated.
Results:
HPA axis function was similar in PMD compared with AC. In all, progesterone significantly increased the secretion of cortisol compared with estradiol [area under the curve (t74 = 3.1; P < 0.01)] and urinary free cortisol (t74 = 3.2; P < 0.01) and ACTH compared with hypogonadism [area under the curve (t74 = 2.4; P < 0.05)].
Conclusions:
HPA axis regulation is normal in PMD, suggesting that the pathophysiology of PMD differs from major depression. As observed previously, progesterone but not estradiol up-regulates HPA axis function in women.
Women with premenstrual dysphoria (PMD) have affective and behavioral disturbances linked to the luteal phase of their menstrual cycles. Abnormalities of the hypothalamic-pituitary-adrenal (HPA) axis have been postulated to contribute to these disturbances in behavior, because HPA axis dysregulation is associated with depression, and ovarian steroids can regulate HPA axis function (1–3). In healthy women, HPA axis function increases during the luteal phase compared with the follicular phase of the menstrual cycle, and after progesterone (but not estradiol) replacement during GnRH agonist-induced ovarian suppression (1, 4). By contrast, women with PMD failed to show this luteal-phase increase (1).
We previously demonstrated that women with PMD have abnormal mood and behavioral responses to physiological changes in ovarian steroids (5). Specifically, ovarian suppression with a GnRH agonist, depot Lupron, caused a remission of symptoms in women with PMD, and replacement of ovarian steroids with either estradiol or progesterone (but not placebo) was associated with typical PMD symptoms in women with PMD but not in asymptomatic controls (AC). Thus, the HPA axis in women with PMD might respond abnormally to progesterone and contribute to the luteal phase-related affective abnormalities.
Reports of HPA axis function in PMD are inconsistent (6–28). Compared with controls, blunted HPA axis responses are frequently observed, but both enhanced and normal HPA axis function is reported.
In this study, we performed dexamethasone (Dex)/CRH challenge studies during GnRH agonist-induced hypogonadism with and without physiological levels of estradiol and progesterone replacement in women with prospectively confirmed PMD and in AC.
Subjects and Methods
Participant selection
Participants were women between the ages of 18 and 48 yr who were medication free, not medically ill, and not pregnant. All women received remuneration according to guidelines from the National Institutes of Health (NIH) Healthy Volunteer Office. The study protocol was reviewed and approved by the National Institute of Mental Health Institutional Review Board, and all women gave written consent to study participation.
Women with PMD were self-referred in response to newspaper advertisements or were referred by their physician. The diagnosis of PMD was confirmed prospectively before entry into this study by self-administered symptom ratings (a 100-mm visual analog scale) completed daily over three consecutive menstrual cycles. The diagnosis of PMD was confirmed when participants' ratings showed at least a 30% increase in mean negative mood (i.e. sadness, anxiety, or irritability) in the week before menses compared with the week after menses, adjusted for the range of the scale used by each participant.
Asymptomatic controls were recruited through local advertisements. Daily self-ratings over 2 months before entering the study confirmed a historical lack of menstrual-related mood or behavior disturbances.
The women with PMD had no current Axis I psychiatric diagnosis or any diagnoses within the past 2 yr per Structured Clinical Interview for DSM-IV (29), whereas controls had neither current nor past Axis I diagnoses.
Study design
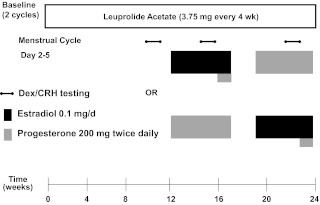
For the entire 6 months of the study, all participants received monthly injections of the GnRH agonist leuprolide acetate, Lupron (3.75 mg). Plasma FSH, LH, estradiol, and progesterone levels were checked at each study visit to confirm adequate gonadal suppression. After 3 months of Lupron alone, all participants received estradiol (100 μg daily by skin patch) and progesterone (200 mg vaginal suppository twice daily) replacement lasting for 5 wk each while continuing to receive monthly Lupron injections (Fig. 1).
Fig. 1.
Study schematic and Dex/CRH testing. All participants received injections of the GnRH agonist leuprolide acetate (Lupron) at a dose of 3.75 mg every 4 wk. Plasma FSH, LH, estradiol, and progesterone levels were checked at each study visit to confirm adequate gonadal suppression. After 3 months of unopposed leuprolide acetate, all participants entered the hormonal addback phase while continuing to receive monthly leuprolide injections. The women were randomly assigned to receive 5 wk each of transdermal 17β-estradiol (E) (Watson Pharmaceuticals, Inc., Corona, CA; Novum Pharmaceutical Research Services, Pittsburgh, PA; Novartis Pharmaceuticals Corp., East Hanover, NJ) at a dose of 0.1 mg/d and progesterone (P) vaginal suppositories (Upsher-Smith Laboratories, Minneapolis, MN; NIH Pharmacy, Bethesda, MD) at a dose of 200 mg twice daily in a double-blind, placebo-controlled, crossover fashion with a 2-wk washout period between the periods of hormone administration. In addition, during the fifth week of estradiol, all women received both estradiol and progesterone to induce menses. During the hormonal addback phases, all women received both active or placebo patches and suppositories each day to blind both researchers and participants to the hormonal addback. Dex/CRH testing was developed for affective disorders by Holsboer and colleagues (37). Participants take 1.5 mg Dex orally at 2300 h the night before the test. The next day, while fasting, participants present to the outpatient clinic to have blood sampling while resting in a supine position. Baseline samples are drawn at 1445 and 1455 h. At 1500 h, participants are given ovine CRH 100 μg iv, and blood is drawn at 15, 30, 45, 60, and 75 min after the CRH infusion.
Procedures
The combined Dex (1.5 mg)/CRH (100 μg iv)-stimulation test was conducted in all participants after 6 wk of hypogonadism and again after at least 2 wk of estradiol addback and progesterone addback (Fig. 1). Additionally, urinary free cortisol (UFC) collections were completed before Dex administration before each Dex/CRH test.
Assay methodology
Plasma hormone levels of estradiol and progesterone were assayed by ELISA (ALPCO Diagnostics kit, Core Laboratory, Johns Hopkins, Baltimore, MD). Plasma cortisol was analyzed by RIA (Siemens Healthcare, Core Laboratory, Johns Hopkins, Baltimore, MD), and plasma ACTH was analyzed by ELISA immunoassay (ALPCO Diagnostics, Salem, NH). Additionally, plasma was assayed for both cortisol-binding globulin (CBG) (ALPCO Diagnostics kit, Core Laboratory, Johns Hopkins, Baltimore, MD) and Dex [HPLC tandem mass spectrometry (MS/MS); Esoterix, Calabasas Hills, CA]. Urine cortisol levels were included if either the 24-h urine volume or the urine creatinine were within the NIH Clinical Pathology Department's normal range. In 34 women, UFC was analyzed by on-line turbulent flow HPLC and detection by LC-MS/MS (Mayo Medical Labs, Rochester, MN); however, for seven women, UFC was analyzed at another laboratory also by LC-MS/MS (Department of Laboratory Medicine, National Institutes of Health, Bethesda, MD). Each participant's three UFC were analyzed by the same assay. Thus, UFC were analyzed as the percentage of the upper limit of the normal range for the particular assay.
On the day of CRH administration, the rating for premenstrual tension observer scale (PMTS-R) (30) was completed to assess the presence (or absence) of PMD symptoms. The PMTS-R is an observer-completed rating that measures mood, behavior, and physical symptoms on a 36-point scale, with scores of 10 or lower consistent with PMD symptoms and scores of 5 or lower consistent with the absence of significant PMD symptoms (31).
To document a history of childhood trauma, which can impact HPA activity (32), the Childhood Trauma Questionnaire (33) was completed once by each woman at baseline before Lupron administration.
Statistical analysis
Analyses were done with Systat version 11 statistical software (Systat Software, Chicago, IL). Age and body mass index were compared between the diagnostic groups with a Student's t test.
Plasma ACTH and cortisol levels were analyzed by ANOVA with repeated measures (ANOVA-R), with hormone conditions and time points of the procedure (baseline, 15–75 min) as the within-subjects factors and diagnostic group as the between-subjects factor. Areas under the curves (AUC) for ACTH and cortisol, 24-h UFC, baseline levels of estradiol, progesterone, Dex, and CBG, and PMTS-R scores were analyzed by ANOVA-R with hormone condition as the within-subjects factor and diagnostic group as the between-subjects factor. To avoid violations of sphericity assumptions, we employed Greenhouse-Geisser corrections of the F ratios. Post hoc Bonferroni t tests evaluated significant differences observed in each ANOVA-R; P values < 0.05 were considered significant. The analyses of ACTH and cortisol response were repeated with the diagnostic group and trauma group (moderate-severe exposure vs. no exposure) (see Table 3) as the between-subjects factors and, in women with PMD only, past history of depression as the between-subjects factor. Finally, the AUC of ACTH and cortisol and 24-h UFC values were compared in PMD women who were symptomatic (PMTS-R scores ≥ 10) or not during progesterone addback. In women with PMD, the relationship between symptom ratings (PMTS-R) and HPA axis measures were analyzed by Spearman rank correlation.
Table 3.
Plasma levels of cortisol, ACTH, CBG, and Dex in women with PMD and controls across the three hormone conditions
| Hypogonadism |
Estradiol addback |
Progesterone addback |
||||
|---|---|---|---|---|---|---|
| Controls | PMD | Controls | PMD | Controls | PMD | |
| UFC (μ g/24 h) | 20.8 ± 11.5 | 16.3 ± 7.9 | 19.6 ± 12.8 | 15.5 ± 9.6 | 31.0 ± 30.2 | 55.3 ± 74.6 |
| Cortisol AUC (μg/dl·min) | 216.8 ± 219.0 | 192.6 ± 195.9 | 176.3 ± 190.5 | 148.7 ± 177.2 | 237.4 ± 187.7 | 266.5 ± 263.9 |
| ACTH AUC (pg/ml·min) | 609.6 ± 399.7 | 631.7 ± 657.8 | 720.0 ± 552.8 | 613.6 ± 622.2 | 740.7 ± 523.9 | 979.6 ± 1062.0 |
| CBG (μg/ml) | 65.4 ± 21.4 | 59.5 ± 9.4 | 59.0 ± 15.4 | 58.9 ± 10.6 | 62.3 ± 15.3 | 62.4 ± 11.0 |
| Dex (ng/dl) | 123.0 ± 72.8 | 145.1 ± 100.0 | 120.2 ± 65.1 | 165.0 ± 146.6 | 127.8 ± 84.2 | 146.2 ± 114.6 |
Results are shown as mean ± sd. AUC for ACTH and cortisol were determined by a baseline-corrected trapezoidal integration method. We observed no significant differences between women with PMD and asymptomatic controls in any measure of HPA axis function. There were no significant main or interactive effects of diagnosis on the individual plasma cortisol levels from baseline to + 75 min (P value is NS). Nor were there significant main or interactive effects of diagnosis on the cortisol AUC (P value is NS). Similarly, there were no significant main or interactive effects of diagnosis in the plasma ACTH levels from baseline to +75 min (P value is NS). Nor was there a significant main effect of diagnosis in the AUC for plasma ACTH (P value is NS). There was a nonsignificant trend in the ACTH AUC reflecting an increased AUC ACTH in women with PMD compared with controls during progesterone [F(2,74) (diagnosis × hormone condition) = 3.0; P = 0.06]. Post hoc analysis showed no significant differences between the AUC ACTH during progesterone when the values of all 18 women with PMD (three of whom were excluded from the ANOVA-R due to missing data during one hormone condition (Bonferroni t111 = 1.2; P value is NS). Finally, there were no significant differences between women with PMD and controls in the adjusted UFC values (P value is NS). There also were no significant main or interactive effects of diagnosis or hormone condition on measures of CBG (P value is NS). Similarly, there were no significant effects of diagnosis or hormone condition on plasma Dex levels (P value is NS). In women with PMD, the presence of a past history of major depression had no significant effects on the AUC of either cortisol or ACTH across hormone conditions (P value is NS). Finally, no significant effects of trauma exposure were observed in AUC of cortisol or ACTH during any hormone condition. Plasma cortisol was analyzed by RIA (Siemens Healthcare, Core Laboratory, Johns Hopkins, Baltimore, MD), with intraassay and interassay coefficients of variation of 4.5–7.4 and 8.6–9.6%, respectively. Plasma ACTH was analyzed by ELISA (ALPCO Diagnostics, Salem, NH; Core Laboratory, Johns Hopkins, Baltimore, MD), with intraassay and interassay coefficients of variation of 7.8 and 9.7–9.9%, respectively. Plasma Dex was analyzed by LC-MS/MS (Esoterix Laboratories, Calabasas Hills, California), with intraassay and interassay CV of 8.2 and 6.6%, respectively. CBG was analyzed by RIA (ALPCO Diagnostics), with intraassay and interassay coefficients of variation of 4.9 and 7.9%, respectively. Samples from individual women across each hormone condition were run within the same assay, and both women with PMD and controls were paired to be run within the same assay.
Results
Participants
Eighteen women with PMD and 25 AC completed the study (Table 1). Women with PMD were significantly older (Table 1) but did not differ by body mass index. Two of five women with PMD and a history of major depression reported a postpartum onset.
Table 1.
Demographics of participants
| Controls | PMD | |
|---|---|---|
| n | 25 | 18 |
| Age (yr),a mean ± sd | 33.3 ± 8.2 | 40.2 ± 6.2 |
| Body mass index (kg/m2), mean ± sd | 25.0 ± 6.0 | 27.7 ± 5.9 |
| Psychiatric history (number of women) | ||
| Major depression episodes | 0 | 5 |
| CTQ scores | ||
| Moderate-severe exposure | 5 | 5 |
| No exposure | 13 | 10 |
All women were in good medical health confirmed by medical history, physical exam, neurological exam, gynecological exam, PAP smear within the last year, laboratory tests, and electrocardiogram. The Childhood Trauma Questionnaire (CTQ), assesses five types of maltreatment, each out of a 25-point scale: emotional abuse, physical abuse, sexual abuse, emotional neglect, and physical neglect (33). Using cutoffs specified by Heim and colleagues (59), participants were grouped by moderate-severe exposure to childhood trauma (consistent with scores of emotional abuse > 12, physical abuse > 9, sexual abuse > 7, emotional neglect > 14, and physical neglect > 9) or no such exposure. Completed CTQ scales were available in only 33 participants (15 PMD, 18 controls). Based on the scores, the participants were split into two groups: one with moderate-severe exposure to trauma (n = 10) and one without exposure to trauma (n = 23). Five of 15 women with PMD (33%) reported moderate-severe exposure to trauma.
P < 0.01 by two-sample t test with Bonferroni adjusted P value.
Plasma estradiol and progesterone levels
Plasma estradiol and progesterone levels differed across hormone conditions (Table 2); however, there were no significant main or interactive effects of diagnosis.
Table 2.
Symptom ratings, plasma estradiol and progesterone levels across the three hormone conditions
| Hypogonadism |
Estradiol addback |
Progesterone addback |
||||
|---|---|---|---|---|---|---|
| Controls | PMD | Controls | PMD | Controls | PMD | |
| PMTS-R | 2.1 ± 3.6 | 1.9 ± 2.6 | 1.2 ± 2.3 | 4.2 ± 4.4 | 0.8 ± 1.3 | 7.1 ± 7.1 |
| Estradiol (pg/ml) | 10.6 ± 4.3 | 10.5 ± 4.7 | 82.1 ± 74.3 | 50.6 ± 31.6 | 10.1 ± 6.7 | 10.2 ± 3.8 |
| Progesterone (ng/ml) | 0.4 ± 0.1 | 0.7 ± 0.4 | 0.4 ± 0.2 | 0.6 ± 0.3 | 18.7 ± 7.6 | 20.4 ± 9.7 |
Results are shown as mean ± sd. PMTS-R scores showed a significant effect of diagnosis [F(1,41) = 15.5; P < 0.001] and diagnosis by hormone condition [F(2,82 = 9.0; P < 0.001] that on post hoc testing reflected a significant difference between the PMTS-R scores in women with PMD compared with controls during both estradiol and progesterone [Bonferroni t123 = 5.3, P < 0.01 (progesterone); Bonferroni t123 = 2.5, P < 0.05 (estradiol)], whereas there was no significant difference between women with PMD and controls during hypogonadism (Bonferroni t123 = 0.2; P value is NS). ANOVA-R of the plasma estradiol and progesterone levels during each of the three hormonal conditions did not show any significant main or interactive effects of diagnosis with hormone condition (P value is NS for all comparisons). However, as expected by the design, significant differences were observed between the estradiol levels during estradiol compared with either hypogonadism or progesterone (Bonferroni t82 = 7.7, P < 0.01; Bonferroni t82 = 7.8, P < 0.01) and significant increases in plasma progesterone levels during progesterone compared with both hypogonadism and estradiol (Bonferroni t82 = 17.8, P < 0.01; Bonferroni t82 = 17.9, P < 0.01). Plasma levels of estradiol during the estradiol addback were similar to those during the early to midfollicular phase, and plasma levels of progesterone during the progesterone addback were similar to those during the luteal phase of an ovulatory menstrual cycle. Plasma progesterone and estradiol were analyzed by ELISA (ALPCO Diagnostics kit, Core Laboratory, Johns Hopkins, Baltimore, MD), with intraassay and interassay coefficients of variation, respectively, as follows: progesterone, 7.0–7.3 and 8.0–9.2%; and estradiol, 7.0 and 4.4–5.5%.
Symptom ratings
As expected from our previous study (5), symptoms remitted during Lupron alone and recurred during the ovarian steroid addback phase in the women with PMD but not in controls. These changes were reflected in significant diagnosis by hormone condition effects in the PMTS-R scores, with higher scores in women with PMD during both progesterone and estradiol addbacks (Table 2).
HPA axis function
Three women (two PMD, one control) did not suppress plasma cortisol levels during one of their three Dex/CRH studies, based on plasma cortisol levels higher than 4 μg/dl at 1500 h before CRH administration (34). This occurred during hypogonadism (i.e. Lupron alone) in women with PMD and during progesterone in a control woman. Another woman with PMD had incomplete Dex/CRH data during estradiol due to inadequate patency of her iv line.
PMD compared with controls
We observed no significant differences between women with PMD and AC in any measure of HPA axis function (Table 3). There were no significant effects of diagnosis in the individual plasma ACTH or cortisol levels (Fig. 2), the plasma ACTH or cortisol AUC, 24-h UFC, plasma CBG, or plasma Dex levels. A post hoc analysis of the AUC ACTH during progesterone, which included the data from the three women with PMD whose data were excluded from the ANOVA-R because of missing values during either hypogonadal or estradiol conditions showed no significant differences between the AUC ACTH during progesterone in these 18 women with PMD and the 24 controls [Bonferroni t111 = 1.2; P value is not significant (NS)].
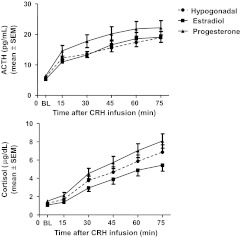
Fig. 2.
Stimulated plasma ACTH and cortisol levels in women with PMD and controls after CRH administration across three hormone conditions (mean ± sem). There were no significant main or interactive effects of diagnosis in the pattern of plasma ACTH and cortisol levels at baseline or after CRH administration. ANOVA-R showed significant effects of hormone condition and hormone condition by time with plasma ACTH levels. Post hoc analysis of the individual time points showed the highest ACTH levels during progesterone compared with estradiol and hypogonadism. Plasma ACTH levels during progesterone were significantly higher than those during hypogonadism at times +45 and +60 min [Bonferroni (range) t444 = 2.7–2.9; P < 0.05] and higher at a trend level of significance at +30 min. Plasma ACTH levels during progesterone also were significantly higher than those during estradiol at time +30 min (Bonferroni t444 = 2.9; P < 0.05). Plasma ACTH levels were not significantly different during estradiol compared with hypogonadism (Bonferroni t444 < 1.0; P value is NS for all comparisons). Individual plasma cortisol values also showed significant effects of hormone condition and hormone condition by time. Overall, plasma cortisol levels were lowest during estradiol and highest during progesterone with plasma levels during hypogonadism between those of estradiol and progesterone. Post hoc analysis of the individual time points showed plasma cortisol levels during progesterone were higher than those during hypogonadism at a trend level of significance at times +60 and +75 (Bonferroni t444 = 2.3 and 2.5; P < 0.1 with 18 comparisons). Significantly higher plasma cortisol levels during progesterone also were observed compared with estradiol at times +30, +45, +60, and +75 (P < 0.05 at +30 and P < 0.01 at +45, +60, and +75; Bonferroni t444 = 3.2, 3.8, 4.4, and 5.5, respectively). Finally, plasma cortisol levels were higher (at a trend level of significance) during hypogonadism compared with estradiol at time +75 (Bonferroni t444 = 2.9; P < 0.1).
There were no significant differences in measures of either plasma cortisol or ACTH between the six symptomatic (i.e. PMTS-R scores ≥ 10) and 12 nonsymptomatic women with PMD. Nor were there significant correlations observed between PMTS-R scores and the AUC of either ACTH or cortisol. Finally, in women with PMD, AUC of both cortisol and ACTH did not differ in those with and those without a past history of depression.
Effects of hormone conditions in PMD and controls
There was a significant effect of hormone condition [F(2,74) = 5.7; P < 0.01] on ACTH AUC with the highest value during progesterone and the lowest during hypogonadism (P < 0.05). The ACTH AUC during progesterone also was greater compared with estradiol (but not significantly), and although lower, the ACTH AUC during hypogonadism was not significantly different from that during estradiol (Fig. 3).
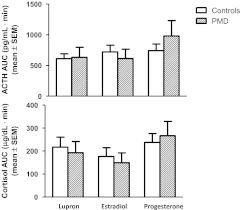
Fig. 3.
AUC of plasma ACTH and cortisol in women with PMD and controls after the administration of CRH (100 μg) across three hormone conditions (mean ± sem). Women with PMD did not differ from controls in their AUC of ACTH and cortisol. ANOVA-R of the ACTH AUC showed a significant effect of hormone condition [F(2,74) = 5.7; P < 0.01] with the highest ACTH AUC during progesterone and the lowest during hypogonadism (Bonferroni t74 = 2.3; P < 0.05). The ACTH AUC during progesterone also was greater compared with estradiol (but not significantly), and although lower, the ACTH AUC during hypogonadism was not significantly different from that during estradiol. The cortisol AUC was significantly higher during progesterone compared with estradiol (Bonferroni t74 = 3.4; P < 0.01), whereas the AUC cortisol was higher, but not significantly so, during progesterone compared with hypogonadism and during hypogonadism compared with estradiol (Bonferroni t74 = 1.7; P value is NS for both comparisons).
There was a significant effect of hormone condition and hormone condition by time on individual plasma ACTH levels from baseline to +75 min [F(2,74) (hormone condition) = 5.8, P < 0.01; F(10,370) (hormone condition by time) = 2.9, P < 0.05]. Post hoc analyses of the individual time points (after adjustment of the P value for 18 comparisons) showed the highest ACTH levels during progesterone compared with either estradiol or hypogonadism. Plasma ACTH levels during progesterone were significantly higher than those during hypogonadism at time +45 and +60 min (P < 0.05 for both comparisons) and higher at a trend level of significance at time +30 min. Plasma ACTH levels during progesterone also were significantly higher than those during estradiol at time +30 min (P < 0.05) and higher but at a trend level of significance at times +15 and +45 min (P < 0.1 for both comparisons). Plasma ACTH levels were not significantly different at any time point during estradiol compared with hypogonadism (P value is NS for all comparisons).
The cortisol AUC was significantly higher during progesterone compared with estradiol (P < 0.01), whereas the AUC cortisol was higher, but not significantly so, during progesterone compared with hypogonadism and during hypogonadism compared with estradiol (P value is NS for both comparisons).
Analysis of the individual plasma cortisol values from baseline to time +75 min across the three hormone conditions showed significant effects of hormone condition and hormone condition by time [F(2,74) (hormone condition) = 9.2, P = 0.001; F(10,370) (hormone condition by time) = 4.7, P = 0.002]. Overall, plasma cortisol levels were lowest during estradiol and highest during progesterone addback with intermediate levels during hypogonadism. Post hoc analyses of the individual time points after adjustment of the P value for 18 comparisons showed significantly higher plasma cortisol levels during progesterone compared with estradiol at times +30, +45, +60, and +75 min (P < 0.05 at +30 min and P < 0.01 at +45, +60, and +75 min). Baseline plasma cortisol was not significantly different on progesterone compared with estradiol. Plasma cortisol levels were higher during progesterone than during hypogonadism, but only at a trend level of significance, at times +60 and +75 min (P < 0.1 with adjustment for 18 comparisons). Plasma cortisol levels were higher (at a trend level) during hypogonadism compared with estradiol at time +75 min.
UFC (Table 3) were available in 41 women (18 PMD, 23 controls). A significantly higher percent maximal UFC was observed on progesterone compared with hypogonadism and estradiol [ANOVA-R (hormone condition) F(2,74) = 7.8, P = 0.001; Bonferroni t74 = 3.2, P < 0.01 for both comparisons].
The 10 women (five with PMD, five controls) with moderate-severe childhood trauma exposure had similar HPA axis function compared with the women without such a history.
Discussion
This study, the first to examine HPA axis function in women with PMD under experimentally controlled hormone conditions, yielded two main findings. First, no differences in Dex-suppressed/CRH-stimulated HPA axis activity were observed between women with PMD and controls. Thus, in contrast to depression, PMD is characterized by neither abnormal glucocorticoid feedback regulation nor increased central hypothalamic drive. Second, progesterone increased measures of HPA axis function in all women, regardless of diagnosis. Progesterone's effects are evident in multiple measures including UFC and CRH-stimulated plasma cortisol and ACTH levels. In contrast to progesterone, estradiol had no significant effects on measures of HPA axis function.
Not all previous reports find abnormalities of HPA axis function in PMD. Compared with controls, both blunted and enhanced HPA axis responses are reported, although a normal HPA axis function is most frequently reported (6–28). These inconsistencies may reflect differences in the menstrual cycle phase when studies are performed, the challenge paradigms used to examine the HPA axis, or the presence or absence of PMD symptoms. Our findings are consistent with most reports, which do not identify differences in HPA function between women with PMD and controls. Specifically, women with PMD and controls do not differ in 24-h UFC secretion (6, 7), basal plasma cortisol or ACTH secretion (including single morning or evening levels and overnight and 24-h serial every 20–30 min sampling, as well as single daily basal levels obtained repeatedly across the menstrual cycle) (1, 6, 8–19), and plasma cortisol response to either Dex suppression (7, 11, 20) or ACTH stimulation (11).
Nonetheless, a few studies identified abnormalities in basal hormone secretion, reporting both lower (6, 21–23) and higher (24) basal plasma cortisol levels, lower salivary cortisol levels (25), and lower plasma ACTH levels (14) in women with PMD compared with controls. Both Rabin et al. (6) and Facchinetti et al. (26) reported higher AUC cortisol responses to CRH stimulation in women with PMD (albeit in the former study, the higher AUC occurred in the context of lower baseline evening cortisol levels in the women with PMD). Finally, no significant diagnostic differences in exercise-stimulated ACTH and cortisol were observed in the follicular or luteal phase (1); however, women with PMD failed to show the luteal-phase increase (compared with the follicular phase) in stimulated cortisol and ACTH secretion observed in AC. Overall, most studies employing either an endocrine challenge or serial plasma sampling suggest that the regulation of plasma cortisol and ACTH secretion at the levels of the hypothalamus, pituitary, and adrenal is normal in women with PMD.
In contrast to studies of values at baseline and with HPA stimulation, reports of pharmacological agents that stimulate serotonergic (5HT) system function or induce emotional stress more frequently demonstrate abnormalities of HPA axis response in PMD. Blunted HPA axis response to 5HT stimulation was observed in three studies (15, 18, 19) but not in three others (16, 23, 24). One additional study reported an increased ACTH/cortisol response to the selective serotonin reuptake inhibitor paroxetine (17). Similarly, studies in which psychological or emotional stress was induced to challenge the HPA axis report blunted (22, 27) or increased (28) response or no differences in the responses (21) of women with PMD and controls. The apparent discrepancies between the 5HT- and stress-challenge studies and those examining glucocorticoid feedback and CRH activation of the HPA axis could reflect differences in the regulatory circuitry being stimulated under the different challenge conditions. For example, hypothalamic nuclei and nonlimbic or cortical systems involved in glucocorticoid feedback inhibition may respond normally in women with PMD, whereas abnormalities in limbic and cortical areas could be more evident when 5HT or emotional stress challenges are employed.
We used the Dex/CRH stimulation test to evaluate the integrity of glucocorticoid receptor (GR) feedback in PMD and to evaluate the hypothalamic activation of the HPA axis (i.e. CRH and vasopressinergic activity) compared with controls. Additionally, the Dex/CRH test is emerging as an important probe of HPA axis dysfunction in depression (with better diagnostic utility than the Dex suppression test) and is now used in studies of depression more often than the CRH stimulation test. The HPA axis response to the combined Dex/CRH test is increased in depression compared with healthy controls, and responses often normalize after successful antidepressant therapy (35–38). Thus, the extensive experience with the Dex/CRH test in depression facilitates comparisons of HPA axis function between PMD and other forms of affective disorder. The findings of normal HPA axis regulation in women with PMD under conditions of Dex/CRH challenge stand in marked contrast to the abnormal Dex/CRH response in patients with depression. Thus, despite the overlapping characteristics of family history, treatment response characteristics, and symptom complaints (39), PMD and major depression do not appear to share the same pathophysiology with respect to either GR feedback regulation or central vasopressinergic or CRH stimulation of the axis.
Our observation that progesterone increased HPA axis response is consistent with previous reports documenting luteal-phase enhancement of cortisol and ACTH secretion compared with the follicular phase. Studies evaluating salivary cortisol levels (25) and plasma cortisol levels after Dex (40) as well as after exercise challenge (1, 4, 41) reveal higher levels in the luteal phase. Luteal-phase enhancement of HPA axis function also has been observed in response to the 5HT agent m-chlorophenylpiperazine (m-CPP) in regularly cycling premenopausal women (15). In the study by Roca et al. (1), the effects of estradiol and progesterone on exercise-stimulated HPA axis function were isolated and examined separately under conditions of GnRH agonist-induced ovarian suppression. Consistent with findings in the present study, Roca et al. (1) observed HPA axis secretion to be significantly increased during progesterone but not estradiol. Thus, several different challenge paradigms and both naturalistic and experimental study designs demonstrate that progesterone is an important regulator of HPA axis function that enhances stimulated HPA axis function in women.
Progesterone's diverse actions provide multiple sites at which it could regulate the HPA axis. Progesterone can modulate CRH promoter activity (in placental tissue) through either of its receptor isoforms acting on cAMP response elements (42). It can interfere with glucocorticoid negative feedback by directly binding type I and type II GR (43, 44). Progesterone also can increase chaperone proteins that would serve to increase the HPA axis response to stimulation (45). Finally, the effects of progesterone could be mediated by its neurosteroid metabolites (e.g. allopregnanolone), which bind γ-aminobutyric acid (GABA)-A receptors and modulate GABAergic inhibition of the HPA axis. In rodents, GABAergic inhibition directly inhibits ACTH and corticosterone secretion (46), and allopregnanolone blunts stress-stimulated cortisol responses (47). These data suggest that neurosteroid metabolites of progesterone do not mediate the effects of progesterone observed in this study.
In contrast to the effects of progesterone, we observed that estradiol did not increase HPA axis responsivity compared with hypogonadism. Despite the considerable literature in animals documenting the effects of estradiol to increase HPA axis function (2, 3), few studies have replicated these findings in humans. In an identical hormone manipulation protocol, Roca et al. (1) observed no differences in basal or exercise-stimulated levels of cortisol and ACTH during estradiol compared with the hypogonadal phase in young women. Indeed, two stress paradigm studies in postmenopausal women found that estradiol decreased stimulated cortisol and ACTH responses compared with placebo (48, 49). Additionally, healthy postmenopausal women, who are estrogen deficient, have enhanced Dex/CRH cortisol responses compared with younger, premenopausal women (50–52). Nonetheless, had we employed a challenge that involved more stressful conditions similar to those employed in animal studies, it is possible that we would have observed a pattern of HPA axis response to estradiol more consistent with that observed in animal studies.
Ovarian steroids may regulate several confounders that influence HPA axis function, including CBG and Dex levels. We observed no effect of estradiol or progesterone on CBG levels, similar to the study by Roca et al. (1). Therefore, our findings of enhanced HPA axis function on progesterone and unchanged HPA axis function on estradiol were not due to correspondingly different levels in free cortisol. Although progesterone binds to CBG, its affinity is much less than that of cortisol, and at the physiological levels in this study, it would not be expected to have significant effects (53). Moreover, progesterone binding to CBG would increase negative feedback by increasing free cortisol. Previous studies have found oral estrogen treatment to significantly increase serum CBG and total serum cortisol levels compared with controls (54). However, unlike oral estrogens, transdermal estradiol, the form of estradiol employed in both this study and that by Roca et al. (1), did not increase CBG levels (55). Second, sex steroids can potentially alter the absorption or clearance of Dex (56, 57). In this study, plasma levels of Dex at the time of testing did not differ across hormonal states or across diagnostic groups. Thus, the findings in the study were likely independent of any hormone-mediated change in Dex metabolism.
The hormone manipulation paradigm used here provided a standardized exposure to estradiol and progesterone in both PMD and controls and separate evaluation of the effects of estradiol and progesterone, neither of which is possible in naturalistic studies performed across the menstrual cycle. Nonetheless, several possible limitations of this study deserve mention. First, the small size of our samples decreases our power to detect type II errors; consequently, it is possible that differences in HPA axis response between women with PMD and controls could have been detected with a larger sample size. Second, an age-related increase in the cortisol and ACTH responses to Dex/CRH testing has been reported (58). Although there was an average difference of only 5 yr between the women with PMD and the controls, it is possible that the older age of the women with PMD contributed to the nonsignificantly elevated ACTH and cortisol secretion observed compared with the younger controls. The clinical implications of progesterone's enhancement of HPA axis function remain to be clarified. These effects of progesterone are observed in both patients and controls and are reported in several different studies, suggesting, therefore that progesterone's actions to increase HPA axis function is a common phenomenon that could serve an adaptive purpose. Regardless, it does not appear that an abnormal HPA axis response to gonadal steroids contributes to the symptomatology seen in PMD.
Acknowledgments
We are indebted to Ms. Karla Thompson for her outstanding clinical support of this study.
This publication was made possible by Grant Number UL1 RR 025005 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
The funding for E.E.L. was made possible through the Clinical Research Training Program, a public-private partnership supported jointly by the NIH and Pfizer Inc. (via a grant to the Foundation for NIH from Pfizer Inc.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AC
- Asymptomatic controls
- ANOVA-R
- ANOVA with repeated measures
- AUC
- area under the curve
- CBG
- cortisol-binding globulin
- Dex
- dexamethasone
- GABA
- γ-aminobutyric acid
- GR
- glucocorticoid receptor
- HPA
- hypothalamic-pituitary-adrenal
- 5HT
- serotonergic
- MS/MS
- tandem mass spectrometry
- NS
- not significant
- PMD
- premenstrual dysphoria
- PMTS-R
- premenstrual tension observer scale
- UFC
- urinary free cortisol.
References
- 1. Roca CA, Schmidt PJ, Altemus M, Deuster P, Danaceau MA, Putnam K, Rubinow DR. 2003. Differential menstrual cycle regulation of hypothalamic-pituitary-adrenal axis in women with premenstrual syndrome and controls. J Clin Endocrinol Metab 88:3057–3063 [DOI] [PubMed] [Google Scholar]
- 2. Viau V. 2002. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. J Neuroendocrinol 14:506–513 [DOI] [PubMed] [Google Scholar]
- 3. Handa RJ, Weiser MJ, Zuloaga DG. 2009. A role for the androgen metabolite, 5α-androstane-3β,17β-diol, in modulating oestrogen receptor β-mediated regulation of hormonal stress reactivity. J Neuroendocrinol 21:351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Altemus M, Roca C, Galliven E, Romanos C, Deuster P. 2001. Increased vasopressin and adrenocorticotropin responses to stress in the midluteal phase of the menstrual cycle. J Clin Endocrinol Metab 86:2525–2530 [DOI] [PubMed] [Google Scholar]
- 5. Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. 1998. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med 338:209–216 [DOI] [PubMed] [Google Scholar]
- 6. Rabin DS, Schmidt PJ, Campbell G, Gold PW, Jensvold M, Rubinow DR, Chrousos GP. 1990. Hypothalamic-pituitary-adrenal function in patients with the premenstrual syndrome. J Clin Endocrinol Metab 71:1158–1162 [DOI] [PubMed] [Google Scholar]
- 7. Haskett RF, Steiner M, Carroll BJ. 1984. A psychoendocrine study of premenstrual tension syndrome: a model for endogenous depression? J Affect Disord 6:191–199 [DOI] [PubMed] [Google Scholar]
- 8. Rubinow DR, Hoban MC, Grover GN, Galloway DS, Roy-Byrne PP, Andersen R, Merriam GR. 1988. Changes in plasma hormones across the menstrual cycle in patients with menstrually related mood disorder and in control subjects. Am J Obstet Gynecol 158:5–11 [DOI] [PubMed] [Google Scholar]
- 9. Parry BL, Javeed S, Laughlin GA, Hauger R, Clopton P. 2000. Cortisol circadian rhythms during the menstrual cycle and with sleep deprivation in premenstrual dysphoric disorder and normal control subjects. Biol Psychiatry 48:920–931 [DOI] [PubMed] [Google Scholar]
- 10. Mortola JF, Girton L, Yen SSC. 1989. Depressive episodes in premenstrual syndrome. Am J Obstet Gynecol 161:1682–1687 [DOI] [PubMed] [Google Scholar]
- 11. Lombardi I, Luisi S, Quirici B, Monteleone P, Bernardi F, Liut M, Casarosa E, Palumbo M, Petraglia F, Genazzani AR. 2004. Adrenal response to adrenocorticotropic hormone stimulation in patients with premenstrual syndrome. Gynecol Endocrinol 18:79–87 [DOI] [PubMed] [Google Scholar]
- 12. Rosenstein DL, Kalogeras KT, Kalafut M, Malley J, Rubinow DR. 1996. Peripheral measures of arginine vasopressin, atrial natriuretic peptide and adrenocorticotropic hormone in premenstrual syndrome. Psychoneuroendocrinology 21:347–359 [DOI] [PubMed] [Google Scholar]
- 13. Bloch M, Schmidt PJ, Su TP, Tobin MB, Rubinow DR. 1998. Pituitary-adrenal hormones and testosterone across the menstrual cycle in women with premenstrual syndrome and controls. Biol Psychiatry 43:897–903 [DOI] [PubMed] [Google Scholar]
- 14. Redei E, Freeman EW. 1993. Preliminary evidence for plasma adrenocorticotropin levels as biological correlates of premenstrual symptoms. Acta Endocrinol 128:536–542 [DOI] [PubMed] [Google Scholar]
- 15. Su TP, Schmidt PJ, Danaceau M, Murphy DL, Rubinow DR. 1997. Effect of menstrual cycle phase on neuroendocrine and behavioral responses to the serotonin agonist m-chlorophenylpiperazine in women with premenstrual syndrome and controls. J Clin Endocrinol Metab 82:1220–1228 [DOI] [PubMed] [Google Scholar]
- 16. Bancroft J, Cook A. 1995. The neuroendocrine reponse to d-fenfluramine in women with premenstrual depression. J Affect Disord 36:57–64 [DOI] [PubMed] [Google Scholar]
- 17. Inoue Y, Terao T, Iwata N, Okamoto K, Kojima H, Okamoto T, Yoshimura R, Nakamura J. 2007. Fluctuating serotonergic function in premenstrual dysphoric disorder and premenstrual syndrome: findings from neuroendocrine challenge tests. Psychopharmacology (Berl) 190:213–219 [DOI] [PubMed] [Google Scholar]
- 18. Bancroft J, Cook A, Davidson D, Bennie J, Goodwin G. 1991. Blunting of neuroendocrine responses to infusion of l-tryptophan in women with perimenstrual mood change. Psychol Med 21:305–312 [DOI] [PubMed] [Google Scholar]
- 19. Steiner M, Yatham LN, Coote M, Wilkins A, Lepage P. 1999. Serotonergic dysfunction in women with pure premenstrual dysphoric disorder: is the fenfluramine challenge test still relevant? Psychiatry Res 87:107–115 [DOI] [PubMed] [Google Scholar]
- 20. Roy-Byrne PP, Rubinow DR, Gwirtsman H, Hoban MC, Grover GN. 1986. Cortisol response to dexamethasone in women with premenstrual syndrome. Neuropsychobiology 16:61–63 [DOI] [PubMed] [Google Scholar]
- 21. Girdler SS, Sherwood A, Hinderliter AL, Leserman J, Costello NL, Straneva PA, Pedersen CA, Light KC. 2003. Biological correlates of abuse in women with premenstrual dysphoric disorder and healthy controls. Psychosom Med 65:849–856 [DOI] [PubMed] [Google Scholar]
- 22. Girdler SS, Pedersen CA, Straneva PA, Leserman J, Stanwyck CL, Benjamin S, Light KC. 1998. Dysregulation of cardiovascular and neuroendocrine responses to stress in premenstrual dysphoric disorder. Psychiatry Res 81:163–178 [DOI] [PubMed] [Google Scholar]
- 23. Veeninga AT, Westenberg HGM. 1992. Serotonergic function and late luteal phase dysphoric disorder. Psychopharmacology 108:153–158 [DOI] [PubMed] [Google Scholar]
- 24. Rasgon N, McGuire M, Tanavoli S, Fairbanks L, Rapkin A. 2000. Neuroendocrine response to an intravenous l-tryptophan challenge in women with premenstrual syndrome. Fertil Steril 73:144–149 [DOI] [PubMed] [Google Scholar]
- 25. Odber J, Cawood EH, Bancroft J. 1998. Salivary cortisol in women with and without premenstrual mood changes. J Psychosom Res 45:557–568 [DOI] [PubMed] [Google Scholar]
- 26. Facchinetti F, Fioroni L, Martignoni E, Sances G, Costa A, Genazzani AR. 1994. Changes of opioid modulation of the hypothalamo-pituitary-adrenal axis in patients with severe premenstrual syndrome. Psychosom Med 56:418–422 [DOI] [PubMed] [Google Scholar]
- 27. Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. 2001. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry 49:788–797 [DOI] [PubMed] [Google Scholar]
- 28. Van Goozen SH, Frijda NH, Wiegant VM, Endert E, Van de Poll NE. 1996. The premenstrual phase and reactions to aversive events: a study of hormonal influences on emotionality. Psychoneuroendocrinology 21:479–497 [DOI] [PubMed] [Google Scholar]
- 29. American Psychiatric Association 1994. Diagnostic and statistical manual of mental disorders fourth edition. Washington, DC: American Psychiatric Association [Google Scholar]
- 30. Steiner M, Haskett RF, Carroll BJ. 1980. Premenstrual tension syndrome: the development of research diagnostic criteria and new rating scales. Acta Psychiatr Scand 62:177–190 [DOI] [PubMed] [Google Scholar]
- 31. Steiner M, Haskett RF, Osmun JN, Carroll BJ. 1980. Treatment of premenstrual tension with lithium carbonate. Acta Psychiatr Scand 61:96–102 [DOI] [PubMed] [Google Scholar]
- 32. Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. 2000. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA 284:592–597 [DOI] [PubMed] [Google Scholar]
- 33. Scher CD, Stein MB, Asmundson GJ, McCreary DR, Forde DR. 2001. The Childhood Trauma Questionnaire in a community sample: psychometric properties and normative data. J Trauma Stress 14:843–857 [DOI] [PubMed] [Google Scholar]
- 34. Rush AJ, Giles DE, Schlesser MA, Orsulak PJ, Parker CR, Jr, Weissenburger JE, Crowley GT, Khatami M, Vasavada N. 1996. The dexamethasone suppression test in patients with mood disorders. J Clin Psychiatry 57:470–484 [DOI] [PubMed] [Google Scholar]
- 35. Ising M, Künzel HE, Binder EB, Nickel T, Modell S, Holsboer F. 2005. The combined dexamethasone/CRH test as a potential surrogate marker in depression. Prog Neuropsychopharmacol Biol Psychiatry 29:1085–1093 [DOI] [PubMed] [Google Scholar]
- 36. Watson S, Gallagher P, Smith MS, Ferrier IN, Young AH. 2006. The dex/CRH test: is it better than the DST? Psychoneuroendocrinology 31:889–894 [DOI] [PubMed] [Google Scholar]
- 37. Holsboer F, von Bardeleben U, Wiedemann K, Müller OA, Stalla GK. 1987. Serial assessment of corticotropin-releasing hormone response after dexamethasone in depression. Implications for pathophysiology of DST nonsuppression. Biol Psychiatry 22:228–234 [DOI] [PubMed] [Google Scholar]
- 38. Heuser I, Yassouridis A, Holsboer F. 1994. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. J Psychiatr Res 28:341–356 [DOI] [PubMed] [Google Scholar]
- 39. Endicott J, Amsterdam J, Eriksson E, Frank E, Freeman E, Hirschfeld R, Ling F, Parry B, Pearlstein T, Rosenbaum J, Rubinow D, Schmidt P, Severino S, Steiner M, Stewart DE, Thys-Jacobs S. 1999. Is premenstrual dysphoric disorder a distinct clinical entity? J Women's Health Gend Based Med 8:663–679 [DOI] [PubMed] [Google Scholar]
- 40. Altemus M, Redwine L, Leong YM, Yoshikawa T, Yehuda R, Detera-Wadleigh S, Murphy DL. 1997. Reduced sensitivity to glucocorticoid feedback and reduced glucocorticoid receptor mRNA expression in the luteal phase of the menstrual cycle. Neuropsychopharmacology 17:100–109 [DOI] [PubMed] [Google Scholar]
- 41. Lavoie JM, Dionne N, Helie R, Brisson GR. 1987. Menstrual cycle phase dissociation of blood glucose homeostasis during exercise. J Appl Physiol 62:1084–1089 [DOI] [PubMed] [Google Scholar]
- 42. Ni X, Hou Y, Yang R, Tang X, Smith R, Nicholson RC. 2004. Progesterone receptors A and B differentially modulate corticotropin-releasing hormone gene expression through a cAMP regulatory element. Cell Mol Life Sci 61:1114–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Turner BB. 1997. Influence of gonadal steroids on brain corticosteriod receptors: a minireview. Neurochem Res 22:1375–1385 [DOI] [PubMed] [Google Scholar]
- 44. Xu X, Hoebeke J, Björntorp P. 1990. Progestin binds to the glucocorticoid receptor and mediates antiglucocorticoid effect in rat adipose precursor cells. J Steroid Biochem 36:465–471 [DOI] [PubMed] [Google Scholar]
- 45. Wochnik GM, Rüegg J, Abel GA, Schmidt U, Holsboer F, Rein T. 2005. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J Biol Chem 280:4609–4616 [DOI] [PubMed] [Google Scholar]
- 46. Jones MT, Gillham B, Altaher AR, Nicholson SA, Campbell EA, Watts SM, Thody A. 1984. Clinical and experimental studies on the role of GABA in the regulation of ACTH secretion: a review. Psychoneuroendocrinology 9:107–123 [DOI] [PubMed] [Google Scholar]
- 47. Patchev VK, Hassan AH, Holsboer DF, Almeida OF. 1996. The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology 15:533–540 [DOI] [PubMed] [Google Scholar]
- 48. Komesaroff PA, Esler MD, Sudhir K. 1999. Estrogen supplementation attenuates glucocorticoid and catecholamine responses to mental stress in perimenopausal women. J Clin Endocrinol Metab 84:606–610 [DOI] [PubMed] [Google Scholar]
- 49. Lindheim SR, Legro RS, Bernstein L, Stanczyk FZ, Vijod MA, Presser SC, Lobo RA. 1992. Behavioral stress responses in premenopausal and postmenopausal women and the effects of estrogen. Am J Obstet Gynecol 167:1831–1836 [DOI] [PubMed] [Google Scholar]
- 50. Seeman TE, Robbins RJ. 1994. Aging and hypothalamic-pituitary-adrenal response to challenge in humans. Endocr Rev 15:233–260 [DOI] [PubMed] [Google Scholar]
- 51. Born J, Ditschuneit I, Schreiber M, Dodt C, Fehm HL. 1995. Effects of age and gender on pituitary-adrenocortical responsiveness in humans. Eur J Endocrinol 132:705–711 [DOI] [PubMed] [Google Scholar]
- 52. Kudielka BM, Schmidt-Reinwald AK, Hellhammer DH, Kirschbaum C. 1999. Psychological and endocrine responses to psychosocial stress and dexamethasone/corticotropin-releasing hormone in healthy postmenopausal women and young controls: the impact of age and a two-week estradiol treatment. Neuroendocrinology 70:422–430 [DOI] [PubMed] [Google Scholar]
- 53. Cameron A, Henley D, Carrell R, Zhou A, Clarke A, Lightman S. 2010. Temperature-responsive release of cortisol from its binding globulin: a protein thermocouple. J Clin Endocrinol Metab 95:4689–4695 [DOI] [PubMed] [Google Scholar]
- 54. Wiegratz I, Jung-Hoffmann C, Kuhl H. 1995. Effect of two oral contraceptives containing ethinylestradiol and gestodene or norgestimate upon androgen parameters and serum binding proteins. Contraception 51:341–346 [DOI] [PubMed] [Google Scholar]
- 55. Qureshi AC, Bahri A, Breen LA, Barnes SC, Powrie JK, Thomas SM, Carroll PV. 2007. The influence of the route of oestrogen administration on serum levels of cortisol-binding globulin and total cortisol. Clin Endocrinol (Oxf) 66:632–635 [DOI] [PubMed] [Google Scholar]
- 56. Low SC, Chapman KE, Edwards CR, Seckl JR. 1994. ‘Liver-type’ 11 beta-hydroxysteroid dehydrogenase cDNA encodes reductase but not dehydrogenase activity in intact mammalian COS-7 cells. J Mol Endocrinol 13:167–174 [DOI] [PubMed] [Google Scholar]
- 57. Finken MJ, Andrews RC, Andrew R, Walker BR. 1999. Cortisol metabolism in healthy young adults: sexual dimorphism in activities of A-ring reductases, but not 11β-hydroxysteroid dehydrogenases. J Clin Endocrinol Metab 84:3316–3321 [DOI] [PubMed] [Google Scholar]
- 58. Heuser IJ, Gotthardt U, Schweiger U, Schmider J, Lammers CH, Dettling M, Holsboer F. 1994. Age-associated changes of pituitary-adrenocortical hormone regulation in humans: importance of gender. Neurobiol Aging 15:227–231 [DOI] [PubMed] [Google Scholar]
- 59. Heim C, Young LJ, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. 2009. Lower CSF oxytocin concentrations in women with a history of childhood abuse. Mol Psychiatry 14:954–958 [DOI] [PubMed] [Google Scholar]