Abstract
Context:
Placental angiogenesis contributes to the pathogenesis of preeclampsia (PE) that affects 5–8% of all human pregnancies. MicroRNA (miRNA) are a class of noncoding 21- to 25-nucleotide RNA that negatively regulate gene expression posttranscriptionly.
Objective:
The aim of this study was to test the hypothesis that miRNA are differentially expressed in healthy term and PE placentas and a subclass of angiogenesis-associated miRNA are increased by PE.
Design:
Total miRNA were extracted from villous placental tissues from healthy term and severe preeclamptic pregnancies. Differential miRNA expression was analyzed by microarray and real-time quantitative PCR. Angiogenesis-associated miRNA were analyzed by target prediction databases. In situ hybridization was used to localize miRNA. Target verification was performed by transfection of miRNA precursors or antagomirs into endothelial and BeWo cells and luciferase reporter assays.
Results:
Three highly expressed miRNA (miR-17, -20a, and -20b) were found significantly increased in PE compared with healthy term placentas (n = 10 per group). They target on the same group of genes important for angiogenesis. miR-20b was expressed primarily in villous syncytiotrophoblasts in term placenta. Overexpression or inhibition of miR-20b differentially regulated mRNA expression of those genes in endothelial vs. trophoblast cells. Luciferase reporter assay showed that miR-20b targets EPHB4 and ephrin-B2 that have been shown to be critical for early human placental development. Placental ephrin-B2 mRNA was significantly down-regulated in PE compared with normotensive pregnancies.
Conclusion:
miR-17, miR-20a, and miR-20b are differentially regulated in human placentas by PE. They regulate EPHB4 and ephrin-B2 expression in trophoblast and endothelial cells via the same “seed” sequence, suggesting their roles in early placental development.
Preeclampsia (PE) is a human pregnancy-specific disorder characterized by new onset of hypertension and proteinuria. PE is a multiple organ syndrome affecting 5–8% of all pregnancies worldwide and remains a leading cause of maternal and neonatal mortality and morbidity (1, 2). Although its etiology remains elusive, the placenta is definitely involved in the pathogenesis of PE because removal of the placenta but not the fetus eradicates the clinical manifestations (3). Both conventional and microarray approaches have identified a substantial number of differentially expressed genes in the transcriptome of human placentas from PE compared with normotensive healthy term deliveries (4–6). These studies clearly suggest that differential gene expression is associated with the pathogenesis of PE. Genetic and epigenetic pathways have been suspected to cause the alterations of placental transcriptome by PE (7, 8). However, how placental gene expression is differentially regulated is largely unknown.
The microRNA (miRNA) represent a class of noncoding, 21- to 25-nucleotide small RNA that play a key regulatory role in gene expression via posttranscriptional repression. Derived from a stem-loop precursor, miRNA regulate gene expression by binding primarily to the 3′-untranslated region (UTR) of the target mRNA, resulting in translational repression and mRNA degradation based on the complementary pairing between the miRNA “seed” sequence centered on the nucleotide 2–7 of the miRNA and the 3′-UTR of the target mRNA (9). Because the pairing can be either a perfect or an imperfect complement, one miRNA is able to regulate the expression of multiple genes, and one gene can be regulated by more than one miRNA. Since initially being discovered in Caenorhabditis elegans, a large number of miRNA have been identified in many species. In humans, more than 1000 miRNA have been detected to date, and this number is still increasing (10). It is estimated that miRNA regulate the expression of approximately 30% of all human genes (11), thereby participating in nearly all fundamental cellular processes, including cell differentiation, proliferation, migration, apoptosis, etc. (12).
In mammals, a placental vasculature must form during embryogenesis; it further expands during placentation and grows exponentially in parallel with the extremely fast-growing fetus during the third trimester of pregnancy (2, 13). New vessel formation from preexisting capillaries (angiogenesis) is a vital mechanism for the expansion and extensive vascular growth at the maternal, fetal, and placental interface. Derangement of placental vasculature compromises fetal growth, which is inevitably seen in various complicated pregnancies including PE, gestational diabetes, intrauterine growth restriction, and low birth weight (1, 2). Because compelling evidence has shown that miRNA play a critical role in angiogenesis and endothelial cell function (14, 15) and PE has been postulated as an endothelial disease (16, 17), we hypothesize that miRNA are differentially expressed in human placentas from PE vs. normotensive term deliveries, and a subclass of angiogenesis-associated miRNA are increased by PE. By using miRNA microarray and real-time reverse transcriptase PCR [quantitative PCR (qPCR)] analyses, we identified several PE-up-regulated miRNA of the miR-17-92 cluster that regulates angiogenesis (14), including miR-17, miR-20a, and miR-20b. Target analysis suggests that they regulate the expression of Eph receptor B4 (EPHB4) and ephrin-B2 that are critical for vascular patterning and trophoblast invasion during placentation (18). Thus, our data may have suggested a potential miRNA pathway for regulating early placental development.
Materials and Methods
Placental collection
Collection of placentas from severe PE and normotensive term deliveries (n = 10 per group) was approved by the Institutional Review Board, Meriter Hospital, and the Health Sciences Institutional Review Boards, the University of Wisconsin-Madison, and followed the recommended guidelines for using human subjects. All the normal and severe PE placentas were obtained immediately after cesarean section delivery. The diagnostic criteria for normotensive and preeclamptic pregnancies were based on American College of Obstetricians and Gynecologists guidelines (19). Severe PE was defined as maternal blood pressures of at least 160/110 mm Hg in two separate readings at least 6 h apart and significant quantitative proteinuria (≥2+ on urine dipstick or >2 × g in a 24-h urine), oliguria, cerebral or visual disturbances, pulmonary edema or cyanosis, epigastric or right upper-quarter pain, impaired liver function, thrombocytopenia, or fetal growth restriction. None of the study subjects had signs of infection. Smokers were excluded. Patients' ages were similar (23 ± 1.2 yr) between normotensive and PE pregnancies. All the PE pregnancies did not have any other maternal complications. Gestational ages for normotensive pregnancies (39 ± 0.2 wk) were significantly (P < 0.05) longer than PE (33 ± 1.2 wk). Fetal weights for normotensive pregnancies (3433 ± 96.2 g) were higher (P < 0.05) than PE pregnancies (1811 ± 309.1 g). Placental villous tissues were randomly sampled free of the decidua, snap-frozen in liquid nitrogen, and kept in −80 C until used. Placental tissues were also fixed in 4% paraformaldehyde overnight at 4 C for in situ hybridization analysis.
RNA isolation
Enriched small RNA with size less than 200 nucleotides were isolated from 50–100 mg of the frozen placenta tissue samples using mirVana RNA Isolation Kit (Ambion, Austin, TX) according to the manufacturer's protocol. For the transfected cells, total RNA were extracted using Trizol reagent (Invitrogen, Carlsbad, CA) following the manufacturer's protocol.
miRNA microarray analysis
The differential miRNA expression profile was analyzed by a miRNA microarray service provider (LC Sciences, Houston, TX) on three pairs of control and PE samples. The array included 894 unique probes to cover all miRNA available in the version 14.0 of the miRBase database. Briefly, paired Cy3 (control) and Cy5 (PE)-labeled small RNA samples were hybridized to a dual-color microarray platform using uParaflo microfluidics chips. The raw data of hybridization signals were analyzed, including background subtraction and normalization using the locally weighted scatterplot smoothing method. Only transcripts with a signal intensity higher than 3 × (background sd) and spot coefficient of variation (CV) < 0.5 [CV = (sd)/(signal intensity)] were listed as detectable. In addition, when there were repeating probes on the array, transcripts were listed as detectable if at least 50% of the repeating probes were above detection level. Transcripts with signal intensity less than 500 were considered as low expression. Differential analysis was performed using a Student's t test to analyze the statistical significance of the signal differences between the two groups.
Quantitative real-time RT-PCR
Real-time RT-PCR was performed on all the control and PE tissue samples to confirm the miRNA microarray results. The miRNA from each sample were reverse-transcribed into cDNA using RT2 miRNA First Strand Kit (SABiosciences, Frederick, MD) according to the manufacturer's protocol. The real-time PCR was performed using RT2 miRNA qPCR Assay (SABiosciences) for each miRNA. The comparative CT method (ΔΔCT method) was used to calculate the miRNA expression, with the universally expressed small nuclear RNA U6 used as the endogenous control.
For mRNA gene expression analysis, cDNA was synthesized using random primers in reverse transcription, and real-time qPCR was performed with gene-specific primers listed in Supplemental Table 1 (published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). Ribosomal protein L19 was amplified as the endogenous control.
miRNA target predictions
Predictions of the target genes of miRNA were performed by combinatorial utilization of four different online databases, including TargetScan 5.1 (www.targetscan.org), PicTar (http://pictar.mdc-berlin.de), RegRNA (http://regrna.mbc.nctu.edu.tw/php/browse.php), and miRNA target detection software Miranda (www.microrna.org). Additionally, PubMed searches were performed to identify experimentally validated target genes of miRNA.
miRNA in situ hybridization
miRNA in situ hybridizations were performed on formalin-fixed and paraffin-embedded (FFPE) placenta tissue samples using miRCURY LNA MicroRNA ISH Optimization Kit (Exiqon, Vedbaek, Denmark) and double digoxigenin-labeled LNA probes for miR-20b (50 nm; Exiqon), human U6 (5 nm; Exiqon), or scrambled miRNA control probes (50 nm; Exiqon) according to the manufacturer's protocol. The hybridization was performed at 55 C for 1 h. All the sections were counter-stained with Nuclear Fast Red (Vector Laboratories, Burlingame, CA). U6 small nuclear RNA probe was used as the positive control, and scrambled miRNA probe was used as the negative control.
Cell culture and transfection
Human umbilical vein endothelial cells (HUVEC) were isolated from human umbilical cords collected from the University of California Irvine Medical Center, with approval of the Institutional Review Board (20). The cells were grown in endothelial cell medium with 10% fetal bovine serum (ScienCell Research Laboratories, Carlsbad, CA) and used within three to five passages. BeWo trophoblast cells (American Type Culture Collection, Manassas, VA) were cultured in DMEM/F12 medium with 10% fetal bovine serum. miR-20b precursor, miR-20b antagomir, and pre-miR negative control no. 1 (Ambion) were transfected into HUVEC or BeWo cells using siPORT NeoFX transfection agent (Ambion). After transfection, the cells were allowed to incubate for 48 h at 37 C, followed by RNA extraction.
Generation of luciferase reporter constructs and luciferase assay
The 3′-UTR fragments of ephrin-B2 (1003 bp) and EPHB4 (223 bp) containing the predicted conserved binding sites of miR-20b were PCR amplified and inserted into pMIR-REPORT vector (Ambion) downstream of the luciferase open reading frame. The constructs were termed pMIR-EFNB2 and pMIR-EPHB4, respectively. HEK293 cells were transfected with 5 pmol of pre-miR-20b or pre-miR-scramble control in a 96-well plate. Cells were cotransfected with 25 ng of pMIR-EFNB2, pMIR-EPHB4, or pMIR-REPORT and 5 ng of reference pRL-TK Renilla luciferase reporter plasmid (Promega, Madison, WI). Transfections were performed using Lipofectamine 2000 (Invitrogen), according to the manufacturer's protocol. Cells were lysed 24 h after transfection, and both firefly and Renilla luciferase activities were measured using Dual Luciferase Reporter Assay (Promega). Firefly luciferase activity was normalized to Renilla luciferase activity.
Statistics
Results are presented as mean ± sd. Statistical analyses were performed using SigmaStat 3.5 (Systat Software Inc., San Jose, CA). Student's t test was used for comparison between controls and preeclamptics, and ANOVA was used for comparison among the three trimesters. Significance was defined as P < 0.05.
Results
Up-regulation of miR-17, -20a, and -20b in severe PE placentas
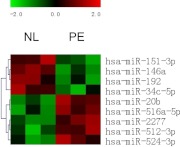
miRNA microarray analysis from three pairs of normal and PE placenta samples detected a total of 615 miRNA expressed in human placenta, with 69 highly expressed miRNA (signal > 500) and 546 low abundant miRNA (signal < 500) (Supplemental Table 2). Differential analysis identified nine miRNA with differential expression (P < 0.05) between PE and normal controls, including five up-regulated miRNA (miR-20b, miR-516a-5p, miR-512–3p, miR-2277, and miR-524-3p) and four down-regulated miRNA (miR-151-3p, miR-146a, miR-192, and miR-34c-5p) by PE (Fig. 1). Among them, three miRNA (miR-20b, miR-516a-5p, and miR-512-3p) were abundantly expressed with signal intensity more than 500. By looking for their potential target genes, four of the nine miRNA, namely miR-20b, miR-151-3p, miR-524-3p, and miR-34c-5p, were identified to be important for angiogenesis (Table 1). Real-time qPCR was then performed to verify the differential expression of these four miRNA identified by differential microarray analysis. Two abundant miRNA, miR-20a and miR-17, up-regulated in PE with P more than 0.05, but less than 0.1 by miRNA microarray analysis, were identified to have the same seed sequence as miR-20b and target on the same set of angiogenesis-related genes as miR-20b (see Fig. 3). Thus, their expression was also verified by real-time qPCR. Another miRNA, miR-1975, which has the highest expression signal among all the miRNA identified with P value less than 0.1, was also included in the qPCR verification.
Fig. 1.
The heat map of differentially expressed miRNA in normal and preeclamptic placentas with P < 0.05 determined by microarray analysis. Green indicates down-regulation, and red indicates up-regulation. Each column represents a single microarray analysis. Columns 1–3 and columns 4–6 display the results from three normal placenta and three preeclamptic placenta samples, respectively. Similarities or differences in the miRNA expression patterns are represented in the dendrogram.
Table 1.
Comparison of the microarray and real-time RT-PCR results for the miRNA of interest
| MicroRNA name | Microarray results (3 pairs NL vs. PE) |
Real-time PCR results (10 pairs) |
Predicted targets | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NL mean | PE mean | Change | P value | NL mean | PE mean | Change | P value | ||
| hsa-miR-17 | 770 | 971 | Up | 0.096 | 1.37 ± 0.60 | 2.76 ± 1.42 | Up | 0.011 | HIF1A VEGFA |
| hsa-miR-20a | 800 | 1019 | Up | 0.069 | 1.39 ± 0.59 | 2.78 ± 1.17 | Up | 0.004 | EFNB2 |
| hsa-miR-20b | 538 | 695 | Up | 0.010 | 1.41 ± 0.69 | 2.39 ± 1.27 | Up | 0.045 | EPHB4 |
| hsa-miR-34c | 30 | 19 | Down | 0.046 | 1.36 ± 0.79 | 1.77 ± 1.49 | Unchanged | 0.450 | NOS3 |
| has-miR-151-3p | 104 | 86 | Down | 0.023 | 1.21 ± 0.95 | 1.18 ± 0.61 | Unchanged | 0.950 | HIF1A |
| has-miR-524-3p | 77 | 95 | Up | 0.039 | 1.02 ± 0.39 | 1.44 ± 0.61 | Up | 0.082 | NOS3 |
| hsa-miR-1975 | 9200 | 3865 | Down | 0.088 | 0.77 ± 0.21 | 0.76 ± 0.29 | Unchanged | 0.930 | Unknown |
NL, Normal.
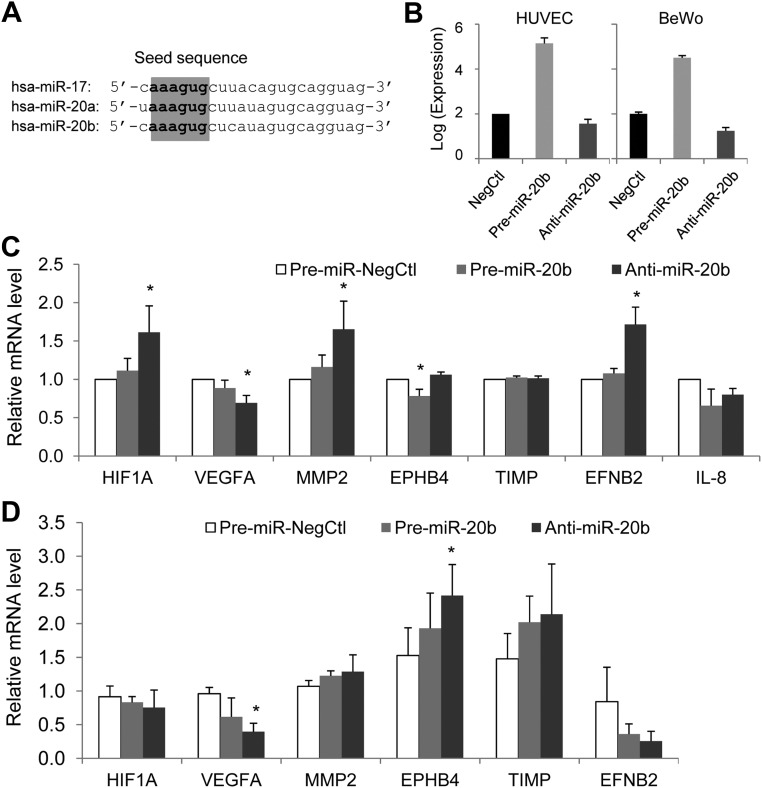
Fig. 3.
The sequence of hsa-miR-17, -20a, and -20b and overexpression and down-regulation of miR-20b in HUVEC and BeWo cells. A, Sequence alignment of hsa-miR-17, -20a, and -20b shows that the three miRNA share the same seed sequence. B, Overexpression and down-regulation of miR-20b was achieved by transfection of miR-20b precursor or antagomir in HUVEC and BeWo cells (n = 3). C and D, The relative expression of the predicted target genes of miR-20b in the HUVEC (C) or BeWo cells (D) transfected with pre-miR negative control, pre-miR-20b or miR-20b antagomir. Data were summed as mean ± sd from four independent experiments. *, P < 0.05 vs. control.
With 10 pairs of normal and PE placental samples, real-time qPCR analysis revealed that the levels of miR-17, miR-20a, and miR-20b in the severe PE placentas were approximately 1-fold greater than that in the normal controls (P < 0.05) (Table 1). However, the levels of miR-1975, miR-34c, and miR-151-3p in PE placentas did not differ from that of normal controls. The level of miR-524-3p was approximately 40% greater in PE placentas than that of normal controls, but it did not reach statistical significance (P = 0.08).
Localization of miR-20b in human placentas
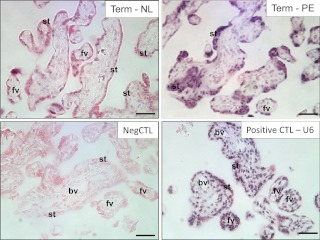
Because miR-20b was the only one identified with significant differential expression between PE and normal placenta (P < 0.05) by microarray analysis, we then took miR-20b as an example to study its localization in placenta by in situ hybridization (Fig. 2). In the healthy term placenta, miR-20b expression was detected in the syncytium and some of the villous mesoblasts, but not in the capillary endothelial cells. The hybridization signal of miR-20b seemed to be more intensive in the PE placentas, which was mainly localized in the syncytium.
Fig. 2.
In situ hybridization of miRNA-20b in paraffin-embedded placental tissue sections from the healthy term and PE placentas. st, Syncytiotrophoblast; bv, blood vessel; fv, floating villi; NL, normal; CTL, control; Neg, negative. Bar, 50 μm.
Predicted target genes of miR-17, -20a, and -20b
In silico analysis using miRNA target prediction databases identified hundreds to thousands of potential target genes of miR-17, -20a, and -20b. Because the three miRNA share the same “seed” sequence (Fig. 3A), they target on similar predicted genes, including many angiogenesis-related factors listed in Table 1. All three computational algorithms, i.e. TargetScan, PicTar, and Miranda, identified several angiogenesis-related genes, including hypoxia-induced factor 1, α-subunit (HIF1A), IL-8, Eph receptor B4 (EPHB4) and tissue inhibitor of metalloproteinase 2 (TIMP2) as the targets of miR-17, 20a and 20b. Vascular endothelial growth factor A (VEGFA), ephrin-B2 (EFNB2) and matrix metallopeptidase 2 (MMP2) were predicted as the targets by TargetScan and Miranda, but not PicTar. TargetScan search also showed that the putative binding sequences of miR-17, -20a, and -20b in the 3′-UTR of the predicted targets were conserved among species.
Using both HUVEC and trophoblast BeWo cells as the cell models, we studied the effect of miR-20b on the mRNA expression of the predicted gene targets (Fig. 3B). Overexpression of miR-20b significantly reduced EPHB4 expression by about 20% in HUVEC (Fig. 3C); however, it did not significantly affect EPHB4 expression in BeWo cells (Fig. 3D). Transfection of miR-20b antagomir to inhibit miR-20b expression significantly increased the expression of HIF1A, MMP2, and EFNB2 by about 60–70% in HUVEC and EPHB4 expression in BeWo cells. Inhibition of miR-20b also significantly reduced VEGFA expression in both HUVEC and BeWo cells. IL-8 mRNA was not detected in BeWo cells.
Ephrin-B2 and EPHB4 are the direct targets of miR-20b
To determine whether ephin-B2 and EPHB4 are the direct targets of miR-20b, miRNA reporter vectors were constructed with ephrin-B2 3′-UTR fragment (pMIR-EFNB2) or EPHB4 3′-UTR fragment (pMIR-EPHB4) containing the miR-20b binding region. When pre-miR-20b was cotransfected with either pMIR-EFNB2 or pMIR-EPHB4 in HEK293 cells, it significantly decreased the luciferase reporter gene expression in comparison with the negative control precursor miRNA (P = 0.002 and 0.003, respectively). As a control, when pre-miR-20b was cotransfected with the pMIR-REPORT plasmid without the 3′-UTR, it did not affect the luciferase gene expression as compared with the control precursor miRNA cotransfected cells (Fig. 4).
Fig. 4.
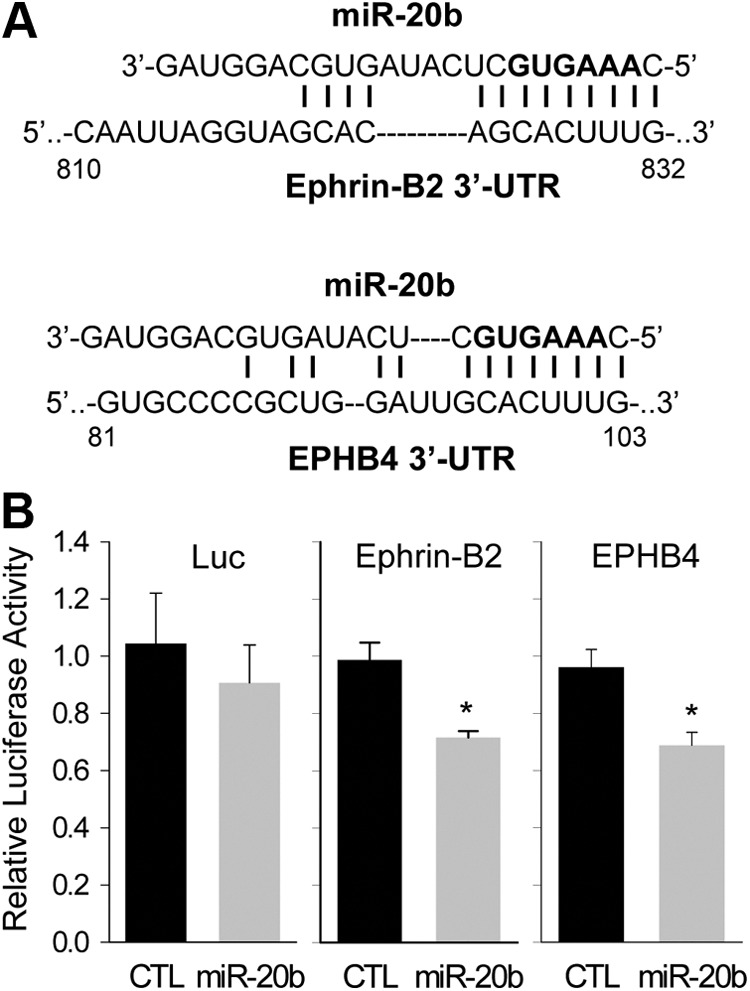
miR-20b targets 3′-UTR of ephrin B2 and EPHB4. A, The complementary between miR-20b and its predicted binding sites in the Ephrin-B2 and EPHB4 3′-UTR. The bold nucleotides in the miR-20b sequence indicate the seed sequence. B, The Ephrin-B2 3′-UTR or EPHB4 3′-UTR fragment containing the predicted miR-20b binding site was inserted downstream of the luciferase reporter gene in the pMIR-Report plasmid. The pMIR-Report plasmid with or without 3′-UTR (Luc) was transfected into HEK293 cells with pre-miR-negative control or pre-miR-20b. Luciferase reporter gene expression was measured. *, P < 0.05 vs. control. CTL, Control.
Ephrin-B2 and EPHB4 expression in normal term and PE placentas
Because ephrin-B2 and EPHB4 were shown as the direct targets of miR-20b and miR-20b was up-regulated in PE placenta, we then analyzed the expression of ephrin-B2 and EPHB4 expression in the placenta using real-time qPCR. Ephrin-B2 was significantly down-regulated in PE placentas compared with normal term placenta. However, levels of EPHB4 mRNA did not differ between PE and normal term placentas (Fig. 5).
Fig. 5.
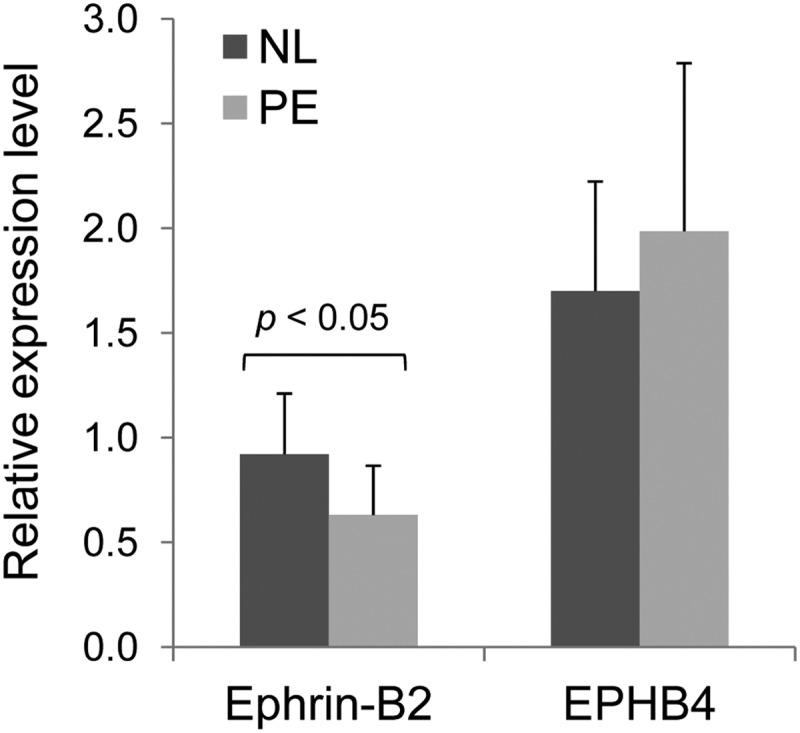
Ephrin B2 and EPHB4 mRNA expressions in preeclamptic and normal (NL) term human placentas (n = 10 per group) as determined by real-time qPCR.
Discussion
miRNA are important regulators for angiogenesis (14). To date, many miRNA have been identified to regulate angiogenesis, including the miR-17-92 cluster (14, 15). This polycistronic cluster contains six miRNA that are processed from a common precursor transcript, i.e. miR-17, miR-18, miR-19a, miR-19b-1, miR-20a, and miR-92a-1. Based on their seed sequences, the six miRNA can be grouped into four families: the miR-17 family (miR-17 and miR-20a), the miR-18 family, the miR-19 family, and the miR-92 family (21). The miR-17-92 cluster has two paralogs in mammals, miR-106a-363 and miR-106b-25, generated by ancient gene duplications. They contain homologous miRNA to those encoded by miR-17-92 (21). miR-20b is located in the miR-106a-363 cluster and belongs to the miR-17 family. It is noteworthy that miR-17, -20a, and -20b share identical seed sequence, thus possibly possessing overlapping functions by targeting on a similar set of genes.
We have shown herein that human placental expressions of miR-17, -20a, and -20b are significantly up-regulated by PE. Several recent differential miRNA expression studies also revealed higher expression of other angiogenesis-associated miRNA in PE placenta, including miR-210, miR-16, and miR-222 (22–24). Of note, the significantly PE-up-regulated angiogenesis-associated miRNA identified in our current study were not reported in any of the previous studies that had also used microarray profiling (22–24). Surprisingly, there was minimum overlapping among the PE-regulated miRNA identified by these previous studies, and some even reported opposite findings. For example, Enquobahrie et al. (25) found that miRNA-584 was down-regulated in PE (25), opposite to the findings by Zhu et al. (24). Although the cause of these discrepancies is currently unknown, potential factors may be related to the differences in sample collection (delivery methods and sample size), RNA extraction methods, the miRNA array methods used, etc. Noack et al. (26) found up-regulation of several miRNA in severe PE placentas using FFPE placental tissues by qPCR, including angiogenesis-associated miRNA-182 (26); however, differences in RNA quality have been found to present between snap-frozen fresh tissues and FFPE tissue samples (27). In addition, different control groups were used in different studies. Pineles et al. (28) used patients with spontaneous preterm labor and delivery as their controls. Regardless, identifying the PE-up-regulated angiogenesis-associated miRNA, including miR-17, -20a, and -20b (current study) and miR-210, miR-16, and miR-222 (22–24), signifies an important step for investigating a potentially important role of angiogenesis-associated miRNA in placental physiology and pathophysiology. Because proangiogenic and antiangiogenic imbalance contributes to the pathogenesis of many pregnancy complications, including PE (29), these findings may have suggested a miRNA pathway for regulating placental angiogenesis during PE.
In addition, our other findings may have suggested that PE-up-regulated miR-17, -20a, and -20b are possibly important for regulating early human placental development for the following reasons. First, target prediction analysis indicated that these miRNA share the same seed sequence, thus targeting the same set of genes, including HIF1A, MMP2, VEGFA, TIMP2, IL-8, ephrin-B2, EPHB4, etc. Second, using miR-20b as an example and HUVEC and BeWo as the cell models, we have verified that overexpression or down-regulation of miR-20b indeed regulates differential mRNA expression of these genes in HUVEC and BeWo cells. Third, we have shown that ephrin-B2 and EPHB4 are the two primary targets of miR-20b, which is verified by using reporter gene expression assays. These results are intriguing because of the critical roles that ephrin-B2 and EPHB4 play in vascular patterning and trophoblast invasion during early human placental development (18).
EPHB4 and ephrin-B2 belong to the large families of Eph receptor tyrosine kinases and their ephrin ligands, respectively. Interaction of Eph receptors and cell surface-bound ephrin ligands mediates various cellular responses such as cell adhesion, repulsion, and migration (30, 31). The Eph-ephrin signaling system is essential for angiogenesis, embryonic, and neural development (31). Ephrin-B2 and EPHB4 are highly expressed in the cardiovascular system, and they represent the respective markers for arterial and venous identity. Ephrin-B2 is predominantly expressed in arterial endothelial cells, whereas EPHB4 is mostly expressed in the venous endothelium (32, 33). Recent studies revealed a proangiogenic function of ephrin-B2 by regulating internalization and signaling activities of vascular endothelial growth factor receptors VEGFR2 and VEGFR3 in endothelial cells (34, 35). The spatiotemporal expression patterns of EPHB4 and ephrin-B2 observed in human cytotrophoblasts suggest their important roles in human placentation (36, 37). It has been shown that the Eph-ephrin interactions pattern cytotrophoblast invasion during human placentation by generating repulsive signals to direct cytotrophoblast invasion toward the uterine wall and allowing invasive cytotrophoblast migration only toward ephrin-B2 expressing arterioles, but not EPHB4-expressing veins (18). Our findings that EPHB4 and ephrin-B2 are the direct targets of miR-20b suggest that miR-20b, and possibly miR-17 and miR-20a as well, may have important roles in placental development, especially vascular remodeling and cytotrophoblast invasion via regulating EPHB4 and ephrin-B2 expression. Moreover, we have found that ephrin-B2 is significantly down-regulated in PE placentas compared with healthy term placentas. Together with the finding that miR-20b is up-regulated in PE placentas, these data further suggest that up-regulation of miR-20b impairs vascular remodeling during placentation in PE placenta by down-regulating ephrin-B2 expression. It is also possible that altering the expression levels of these miRNA may provide a potential route for correcting impaired vascular remodeling and inadequate trophoblast invasion in complicated pregnancies.
Apart from targeting EPHB4 and ephrin-B2 expression, the angiogenesis-associated miRNA (i.e. miR-17, -20a, and -20b) also target several other genes important for placental angiogenesis, including HIF1A, MMP2, VEGFA, TIMP2, and IL-8. HIF1A is a transcription factor highly sensitive to oxygen tension critical for placental development and function through regulating the expression of many hypoxia-responsive genes, including VEGFA (38). MMP2 and TIMP2 are critical for regulating extracellular matrix degradation that is the initial angiogenic response (39). Accordingly, these miRNA may regulate multiple steps of the angiogenesis process, including increased expression of angiogenic factors, matrix breakdown, endothelial cell proliferation, migration, and tube formation (40).
It is noteworthy that miR-20b is likely differentially expressed in the trophoblast cells and capillary endothelial cells in human term placenta, indicating that the angiogenesis-associated miRNA may have different functions in trophoblast and endothelial cells. This idea is supported by our in vitro“gain and loss of function” studies using HUVEC and BeWo cell models transfected with miR-20b or its antagomirs. Inhibition of miR-20b significantly increased HIF1A in HUVEC but does not affect HIF1A expression in BeWo cells, indicating that an angiogenic miRNA pathway may preferentially regulate hypoxia-responsive gene expression in endothelial cells. Interestingly, suppression of miR-20b with its antagomirs unexpectedly decreased VEGF mRNA expression in both HUVEC and BeWo cells. This is opposite to the inhibitory effect of miR-20b on VEGF expression observed in tumor cells (41), indicating cell-specific effects of miR-20b. Moreover, miR-20b regulates IL-8 expression in HUVEC but not in BeWo cells. IL-8 is one of the most potent chemotactic and activating factors for neutrophils, potentiating host defense mechanism against inflammation (42). This finding suggests that these “so-called” angiogenesis-associated miRNA may also regulate cell-cell interactions among endothelial cells, immune cells, and trophoblasts in the placenta.
In summary, differential microarray profiling and real-time qPCR analyses of miRNA expression in human placentas have identified up-regulation of human placental angiogenesis-associated miRNA (miR-17, miR-20a, and miR-20b) in PE compared with normal pregnancies. Target identification of miR-20b has revealed that they may play important roles in regulating placental angiogenesis, trophoblast differentiation, host defense mechanism, and cell-cell communications via regulating cell-specific expression of target genes.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health Grants RO1 HL74947, RO1 HL70562, and R21 HL98746 (to D.-b.C.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- EFNB2
- Ephrin-B2
- EPHB4
- Eph receptor B4
- FFPE
- formalin-fixed and paraffin-embedded
- HIF1A
- hypoxia-induced factor 1, α-subunit
- HUVEC
- human umbilical vein endothelial cell
- miRNA
- microRNA
- MMP2
- matrix metallopeptidase 2
- PE
- preeclampsia
- qPCR
- quantitative PCR
- TIMP2
- tissue inhibitor of metalloproteinase 2
- UTR
- untranslated region
- VEGFA
- vascular endothelial growth factor A.
References
- 1. Redman CW, Sargent IL. 2005. Latest advances in understanding preeclampsia. Science 308:1592–1594 [DOI] [PubMed] [Google Scholar]
- 2. Kanasaki K, Kalluri R. 2009. The biology of preeclampsia. Kidney Int 76:831–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roberts JM, Cooper DW. 2001. Pathogenesis and genetics of pre-eclampsia. Lancet 357:53–56 [DOI] [PubMed] [Google Scholar]
- 4. Enquobahrie DA, Meller M, Rice K, Psaty BM, Siscovick DS, Williams MA. 2008. Differential placental gene expression in preeclampsia. Am J Obstet Gynecol 199:566.e1–566.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sitras V, Paulssen RH, Grønaas H, Leirvik J, Hanssen TA, Vårtun A, Acharya G. 2009. Differential placental gene expression in severe preeclampsia. Placenta 30:424–433 [DOI] [PubMed] [Google Scholar]
- 6. Mouillet JF, Chu T, Sadovsky Y. 2011. Expression patterns of placental microRNAs. Birth Defects Res A Clin Mol Teratol 91:737–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chelbi ST, Vaiman D. 2008. Genetic and epigenetic factors contribute to the onset of preeclampsia. Mol Cell Endocrinol 282:120–129 [DOI] [PubMed] [Google Scholar]
- 8. Kulkarni A, Chavan-Gautam P, Mehendale S, Yadav H, Joshi S. 2011. Global DNA methylation patterns in placenta and its association with maternal hypertension in pre-eclampsia. DNA Cell Biol 30:79–84 [DOI] [PubMed] [Google Scholar]
- 9. Bartel DP. 2009. MicroRNAs: target recognition and regulatory functions. Cell 136:215–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, Barad O, Barzilai A, Einat P, Einav U, Meiri E, Sharon E, Spector Y, Bentwich Z. 2005. Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet 37:766–770 [DOI] [PubMed] [Google Scholar]
- 11. Baek D, Villén J, Shin C, Camargo FD, Gygi SP, Bartel DP. 2008. The impact of microRNAs on protein output. Nature 455:64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang C. 2008. MicroRNomics: a newly emerging approach for disease biology. Physiol Genomics 33:139–147 [DOI] [PubMed] [Google Scholar]
- 13. Cudihy D, Lee RV. 2009. The pathophysiology of pre-eclampsia: current clinical concepts. J Obstet Gynaecol 29:576–582 [DOI] [PubMed] [Google Scholar]
- 14. Wang S, Olson EN. 2009. AngiomiRs—key regulators of angiogenesis. Curr Opin Genet Dev 19:205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu F, Yang Z, Li G. 2009. Role of specific microRNAs for endothelial function and angiogenesis. Biochem Biophys Res Commun 386:549–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. George EM, Granger JP. 2011. Endothelin: key mediator of hypertension in preeclampsia. Am J Hypertens 24:964–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Powe CE, Levine RJ, Karumanchi SA. 2011. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation 123:2856–2869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Red-Horse K, Kapidzic M, Zhou Y, Feng KT, Singh H, Fisher SJ. 2005. EPHB4 regulates chemokine-evoked trophoblast responses: a mechanism for incorporating the human placenta into the maternal circulation. Development 132:4097–4106 [DOI] [PubMed] [Google Scholar]
- 19. 2007. Clinical management guidelines for obstetrician-gynecologists. ACOG Practice Bulletin. Vol. II, no. 23, January 2002. Washington, DC: American College of Obstetricians and Gynecologists; 640–648 [Google Scholar]
- 20. Zhang HH, Feng L, Livnat I, Hoh JK, Shim JY, Liao WX, Chen DB. 2010. Estradiol-17β stimulates specific receptor and endogenous nitric oxide-dependent dynamic endothelial protein S-nitrosylation: analysis of endothelial nitrosyl-proteome. Endocrinology 151:3874–3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, Newman J, Bronson RT, Crowley D, Stone JR, Jaenisch R, Sharp PA, Jacks T. 2008. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell 132:875–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mayor-Lynn K, Toloubeydokhti T, Cruz AC, Chegini N. 2011. Expression profile of microRNAs and mRNAs in human placentas from pregnancies complicated by preeclampsia and preterm labor. Reprod Sci 18:46–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu Y, Li P, Hao S, Liu L, Zhao J, Hou Y. 2009. Differential expression of microRNAs in the placentae of Chinese patients with severe pre-eclampsia. Clin Chem Lab Med 47:923–929 [DOI] [PubMed] [Google Scholar]
- 24. Zhu XM, Han T, Sargent IL, Yin GW, Yao YQ. 2009. Differential expression profile of microRNAs in human placentas from preeclamptic pregnancies vs normal pregnancies. Am J Obstet Gynecol 200:661.e1–661.e7 [DOI] [PubMed] [Google Scholar]
- 25. Enquobahrie DA, Abetew DF, Sorensen TK, Willoughby D, Chidambaram K, Williams MA. 2011. Placental microRNA expression in pregnancies complicated by preeclampsia. Am J Obstet Gynecol 204:178.e12–178.e21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Noack F, Ribbat-Idel J, Thorns C, Chiriac A, Axt-Fliedner R, Diedrich K, Feller AC. 2011. miRNA expression profiling in formalin-fixed and paraffin-embedded placental tissue samples from pregnancies with severe preeclampsia. J Perinat Med 39:267–271 [DOI] [PubMed] [Google Scholar]
- 27. Scicchitano MS, Dalmas DA, Bertiaux MA, Anderson SM, Turner LR, Thomas RA, Mirable R, Boyce RW. 2006. Preliminary comparison of quantity, quality, and microarray performance of RNA extracted from formalin-fixed, paraffin-embedded, and unfixed frozen tissue samples. J Histochem Cytochem 54:1229–1237 [DOI] [PubMed] [Google Scholar]
- 28. Pineles BL, Romero R, Montenegro D, Tarca AL, Han YM, Kim YM, Draghici S, Espinoza J, Kusanovic JP, Mittal P, Hassan SS, Kim CJ. 2007. Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. Am J Obstet Gynecol 196:261.e1–261.e6 [DOI] [PubMed] [Google Scholar]
- 29. Espinoza J, Uckele JE, Starr RA, Seubert DE, Espinoza AF, Berry SM. 2010. Angiogenic imbalances: the obstetric perspective. Am J Obstet Gynecol 203:17.e1–17.e8 [DOI] [PubMed] [Google Scholar]
- 30. Kuijper S, Turner CJ, Adams RH. 2007. Regulation of angiogenesis by Eph-ephrin interactions. Trends Cardiovasc Med 17:145–151 [DOI] [PubMed] [Google Scholar]
- 31. Mosch B, Reissenweber B, Neuber C, Pietzsch J. 2010. Eph receptors and ephrin ligands: important players in angiogenesis and tumor angiogenesis. J Oncol 2010:135285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim YH, Hu H, Guevara-Gallardo S, Lam MT, Fong SY, Wang RA. 2008. Artery and vein size is balanced by Notch and ephrin B2/EphB4 during angiogenesis. Development 135:3755–3764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang HU, Chen ZF, Anderson DJ. 1998. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 93:741–753 [DOI] [PubMed] [Google Scholar]
- 34. Sawamiphak S, Seidel S, Essmann CL, Wilkinson GA, Pitulescu ME, Acker T, Acker-Palmer A. 2010. Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature 465:487–491 [DOI] [PubMed] [Google Scholar]
- 35. Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Lüthi U, Barberis A, Benjamin LE, Mäkinen T, Nobes CD, Adams RH. 2010. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 465:483–486 [DOI] [PubMed] [Google Scholar]
- 36. Chennakesava CS, Di Santo S, Ziemiecki A, Schneider H, Andres AC. 2006. Differential expression of the receptor tyrosine kinase EphB4 and its ligand Ephrin-B2 during human placental development. Placenta 27:959–967 [DOI] [PubMed] [Google Scholar]
- 37. Goldman-Wohl D, Greenfield C, Haimov-Kochman R, Ariel I, Anteby EY, Hochner-Celnikier D, Farhat M, Yagel S. 2004. Eph and ephrin expression in normal placental development and preeclampsia. Placenta 25:623–630 [DOI] [PubMed] [Google Scholar]
- 38. Pringle KG, Kind KL, Sferruzzi-Perri AN, Thompson JG, Roberts CT. 2010. Beyond oxygen: complex regulation and activity of hypoxia inducible factors in pregnancy. Hum Reprod Update 16:415–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sounni NE, Janssen M, Foidart JM, Noel A. 2003. Membrane type-1 matrix metalloproteinase and TIMP-2 in tumor angiogenesis. Matrix Biol 22:55–61 [DOI] [PubMed] [Google Scholar]
- 40. Kuhnert F, Kuo CJ. 2010. miR-17-92 angiogenesis micromanagement. Blood 115:4631–4633 [DOI] [PubMed] [Google Scholar]
- 41. Lei Z, Li B, Yang Z, Fang H, Zhang GM, Feng ZH, Huang B. 2009. Regulation of HIF-1α and VEGF by miR-20b tunes tumor cells to adapt to the alteration of oxygen concentration. PLoS One 4:e7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Baggiolini M, Walz A, Kunkel SL. 1989. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest 84:1045–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.