Abstract
Context:
Thyroid cancer predominately affects women, carries a worse prognosis in older age, and may have higher mortality in men. Superimposed on these observations is the fact that most women have attained menopause by age 55 yr.
Objective:
The objective of the study was to determine whether men contribute disproportionately to papillary thyroid cancer (PTC) mortality or whether menopause affects PTC prognosis.
Design:
Gender-specific mortality was normalized using age-matched subjects from the U.S. population. Multivariate Cox proportional hazard regression models incorporating gender, age, and National Thyroid Cancer Treatment Cooperative Study Group stage were used to model disease-specific survival (DSS).
Participants and Setting:
Patients were followed in a prospective registry.
Main Outcome Measure:
The relationships between gender, age, and PTC outcomes were analyzed.
Results:
The unadjusted hazard ratio (HR) for DSS for women was 0.40 [confidence interval (CI) 0.24–0.65]. This female advantage diminished when DSS was adjusted for age at diagnosis and stage with a HR encompassing unity (HR 0.72, CI 0.44–1.19). Additional multivariate models of DSS considering gender, disease stage, and various age groupings showed that the DSS for women diagnosed at under 55 yr was improved over men (HR 0.33, CI 0.13–0.81). However, the HR for DSS increased to become similar to men for women diagnosed at 55–69 yr (HR 1.01, CI 0.42–2.37) and at 70 yr or greater (HR 1.17, CI 0.48–2.85).
Conclusions:
Although the overall outcome of women with PTC is similar to men, subgroup analysis showed that this composite outcome is composed of two periods with different outcomes. The first period is a period with better outcomes for women than men when the diagnosis occurs at younger than 55 yr; the second is a period with similar outcomes for both women and men diagnosed at ages greater than 55 yr. These data raise the question of whether an older age cutoff would improve current staging systems. We hypothesize that older age modifies the effect of gender on outcomes due to menopause-associated hormonal alterations.
Despite a higher incidence of differentiated thyroid cancer (DTC), women may enjoy more favorable DTC outcomes than men. Overall survival (OS) was reported to be better in women in four DTC cohorts, both by univariate and multivariate analyses (1–4). One of these cohorts, however, only showed a gender difference for patients with papillary thyroid cancer (PTC) (1). OS was unaffected by gender in other cohorts (5, 6). Disease specific survival (DSS), examined by both univariate and multivariate analyses, was observed to favor women in five cohorts (2, 7–10). Multivariate analyses of two additional cohort studies could not confirm an effect of gender on DSS observed in univariate analyses (1, 11). A final study did not observe gender differences in DSS (12). The relationship between DTC recurrences and gender has been examined in seven studies (4, 5, 7–10, 13). Only one analysis demonstrated less disease recurrence in female patients (4), with another study noting less recurrence with univariate analyses only (9). Although the majority of these studies showed some positive impact of female gender on OS and DSS, a female survival advantage is not a universal finding, and an impact of gender on recurrences of DTC appears not to be substantiated (see Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
Worse outcomes in men could potentially be accounted for by a more aggressive biological behavior of DTC in males. Alternatively, a gender-related ascertainment bias may result if men reach medical attention at an older age with more advanced disease or if women present earlier and are screened more thoroughly for disease. For example, women use health care services (14) and participate in screening for diseases such as skin and colorectal cancer (15–18) more frequently than men. One study supported the premise of delayed DTC diagnosis in men by showing that men presented with larger tumors and more lymph node metastases than women (19). As another potential contributor to gender differences, women experience a survival advantage with some conditions, such as cardiovascular disease, when they are premenopausal, but lose that advantage with the onset of menopause (20).
To address the question of whether men come to medical attention for DTC later in the course of their disease, adjustment of outcomes for age and disease stage is necessary. To consider the possibility of female reproductive hormones affecting the behavior of DTC, the age of menopause should be considered. Ninety to 95% of women in the United States have reached menopause by age 55 yr (21, 22). In addition, because women have lower all-cause mortality rates and live longer than men (23), mortality should ideally be normalized to gender-specific OS rates.
We wanted to determine whether there were gender differences in the OS and DSS of patients with PTC in the National Thyroid Cancer Treatment Cooperative Study Group (NTCTCSG) Registry. OS rates were adjusted for the expected gender-specific OS found in the U.S. population to determine whether gender differences in OS in registry patients and the U.S. population were similar. Multivariate analyses were performed to determine whether any gender-based differences in DSS remained after accounting for gender differences in disease stage and age at diagnosis. As a surrogate for menopause, age groupings incorporating an age cutoff of 55 yr were investigated to determine the effect on DSS.
Materials and Methods
Patients and data collection
The NTCTCSG has prospectively followed a cohort of DTC patients since 1987. The data collection and analysis methods of the registry have been described previously (24–27). Patients were registered on an ongoing basis by the primary investigator at each institution after the patient's initial surgery. Data collected included patient's age at diagnosis and race/ethnicity divided into the following categories: Caucasian, Black, Asian, Jewish, Hispanic, and other. Age at menopause was not captured for female patients; medication history was not recorded for any patient. Participants received the treatment and follow-up testing that their physician deemed to be appropriate. Initial therapy included surgery, possible radioiodine administration, and use of thyroid hormone therapy. Disease stage at entry was assigned using the previously validated NTCTCSG staging system (24). Clinical status at entry was classified as either no residual disease or residual disease with documentation of sites of involvement. Follow-up data, including updated survival and disease status, mean TSH scores (26, 27), and additional therapies were prospectively collected for each patient annually. For patients lost to follow-up, date of death was obtained from the U.S. Social Security Death Index.
Recorded data were entered into a personal computer-based clinical data management system (version 2007-2; Medlog, Incline Village, NV) at each site and transmitted to a central repository. Approval was obtained from the institutional review board at each participating institution and was also obtained at M. D. Anderson Cancer Center (Houston, TX) for maintaining the central data repository and performing subsequent analysis. Data for PTC patients collected through October 2006 were included in this analysis.
Statistical analysis
OS was computed as the time in years from the date of diagnosis to death or censoring for each patient. Survival was censored at the date of last follow-up if death was not observed. To examine DSS the time to death or censoring was computed in years since the diagnosis date for each registry patient. Survival was again censored at the date of last follow-up if disease-specific death was not observed. Whenever possible, the causes of death were reviewed and confirmed. For the U.S. population, the time to death was computed in years using the general life tables of the U.S. population (23). The racial distribution for those in this population who reported one race only was 81.1% white, 12.7% Black, 3.8% Asian, 0.9% American Indian or Alaska Native, and 0.2% Hawaiian or Pacific Islander. Each PTC patient was age matched by gender with a subject from the life tables database. The expected OS for the subjects in the U.S. population from the age at diagnosis of the matched PTC patients was then calculated. Essentially, a data set of subjects with the expected OS found in the U.S. life tables, conditional on the gender and age at diagnosis of the matched PTC patient, was constructed.
Univariate Cox proportional hazard regression was used to model the association between gender and either OS or DSS for the PTC patients and OS for the subjects in the U.S. life tables data set. Multivariate Cox proportional hazards regression was used to model the association between gender and OS or DSS while adjusting for stage and age at diagnosis. With adjustment for gender, the hazard ratio (HR) for males was 1.0. Analyses were generated both by modeling gender and stage within each age group and also by modeling gender and age at diagnosis within each stage. For these models two different age stratifications were tested: 1) age younger than 45 yr (the age cutoff used in most DTC staging systems) and subsequent 15-yr intervals and 2) age younger than 55 yr [the age at which 90–95% of women have become menopausal (21, 22)] and subsequent 15-yr intervals. (Analyses using an additional cutoff of 50 yr and using 10 yr time intervals were also conducted, but the results are not reported here.)
The Kaplan-Meier product limit method was used to estimate the OS and DSS for each gender for the PTC patients and to estimate the OS for the subjects in the U.S. life tables data set. The 15-yr survival probability, the 95% confidence interval (CI) for the 15-yr survival, the HR and the 95% CI for the HR were calculated. Statistical analysis was performed using STATA/SE version 9.2 statistical software (Stata Corp. LP, College Station, TX).
Results
Cohort description
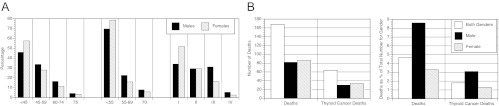
This analysis included 3,572 patients with a median follow-up of 3.8 yr for a total of 16,684 person-years of follow-up. Seventy-three percent of patients were women. The racial/ethnic breakdown of the patients was 71% Caucasian, 4% Black, 4% Asian, and 2% Other. Data were missing for 16%. Figure 1A shows the distribution of patients by gender using 1) the traditional age cutoff of younger than 45 yr with three additional age groups, 2) the menopause-based cutoff of age younger than 55 yr with two additional age groups, and 3) using disease stage. Women were significantly more likely than men to present at younger than either 45 or 55 yr of age compared with the subsequent 15-yr age groups, with 79% of women presenting at younger than 55 yr, compared with 70% of men (left and middle sections of Fig. 1A). Gender had a significant effect on both age at diagnosis and disease stage (P < 0.001). Women were more likely to present with stage I disease and less likely to present with stage III and IV disease, with 81% of women presenting with stage I-II disease, compared with 63% of men (right section of Fig. 1A). Within each advancing disease stage, there was a significantly older mean age for both male and female patients (P < 0.01).
Fig. 1.
A, Distribution of NTCTCSG registry patients by age and disease stage at diagnosis. NTCTCSG stage was not available for three men and one woman. P < 0.001 for gender differences in distribution of age groups and stages (Kruskal-Wallis test). B, Number (shown on left) and percentage (shown on right) of deaths and thyroid cancer deaths in NTCTCSG registry patients. Total number of patients was 3572, number of females was 2619, and number of males was 953.
Figure 1B shows the number of deaths and disease-specific deaths broken down by gender. PTC-related deaths accounted for 37% (30 of 82) of all deaths in men and 40% (34 of 86) of deaths in women. Men accounted for a greater percentage of deaths from any cause and deaths from thyroid cancer within our registry cohort. The subgroup of patients in the 45- to 54-yr-old age group were comprised of 445 patients (57.5%) with stage I and II disease, 294 patients (38%) with stage III disease, and 35 patients (4.5%) with stage IV disease. The number of deaths within these stages were two, eight, and seven, respectively.
Eighty-nine percent of women and 90% of men underwent a total thyroidectomy. Seventy-four percent of women and 80% of men received radioiodine therapy. The distribution of mean TSH scores (26, 27) for males was 34% with a TSH score of 1 (undetectable to subnormal); 53% with a TSH score of 2 (subnormal to normal); and 13% with a TSH score of 3 (normal to elevated). The distribution of mean TSH scores for females was 34% with a TSH score of 1; 55% with a TSH score of 2; and 11% with a TSH score of 3. The use of total thyroidectomy, radioiodine therapy, and TSH suppression was not significantly different according to gender. There were no gender differences in the median duration of follow-up, which was 3.9 yr for women vs. 3.7 yr for men (Wilcoxon test, P = 0.19). In addition, the gender composition of the cohort that was lost to follow-up was not different from the gender composition of the cohort analyzed (76% of women, 24% of men for the lost to follow-up cohort vs. 73% women and 27% for the overall cohort).
Overall survival by gender
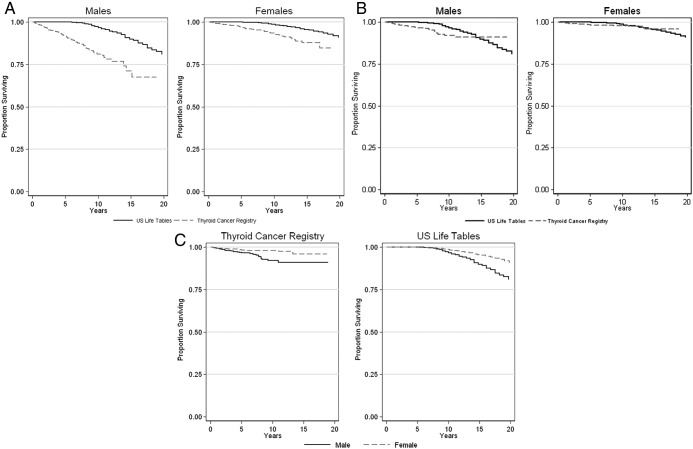
Kaplan-Meier estimates of OS and DSS from diagnosis for PTC patients and OS for the age- and gender-matched U.S. population are illustrated in Fig. 2A–C. In both genders, survival in PTC patients was worse than in the matched U.S. population (Fig. 2A). The DSS for PTC patients overlaps with the OS of the U.S. population for each gender (Fig. 2B). The gender difference in DSS seen in PTC patients mirrors the gender difference in OS for the U.S. population, with greater survival of females in both cases (Fig. 2C). DSS appears to plateau after 10 yr in both genders, although fewer patients contributed to the data as the duration of follow-up lengthened.
Fig. 2.
A, Overall survival of United States population compared with overall survival of thyroid cancer registry patients, with survival displayed separately for males (left panel) and females (right panel). B, Overall survival of United States population compared with disease-specific survival of thyroid cancer registry patients, with survival displayed separately for males (left panel) and females (right panel). C, Disease-specific survival of thyroid cancer registry patients by gender (left panel) and overall survival of United States population by gender (right panel). [Source for U.S. population is United States life tables, 2003 (23).]
Fifteen-year OS probabilities and HRs for the U.S. population and PTC patients are shown in Table 1. The analysis of the OS of the U.S. population subjects showed that females were at less risk of dying than males (HR 0.55, CI 0.51–0.59, P < 0.001). In univariate analysis, women with PTC were at less risk of dying than men with PTC (HR 0.36, CI 0.26–0.48, P < 0.001) and also at less risk of dying compared with men than their U.S. population counterparts. In multivariate analyses incorporating age at diagnosis and stage, women with PTC still had an OS that remained better than men with PTC, but their OS with respect to men was now similar to the women in the U.S. population (HR 0.56, CI 0.41–0.77, P < 0.001).
Table 1.
Cox proportional hazards regression for OS and DSS, with P value for gender differences shown
| Population | Patients, n | Deaths, n | 15-yr survival (%) | 95% CI for 15-yr survival | HR | 95% CI for HR | P value |
|---|---|---|---|---|---|---|---|
| OS U.S. life tables | |||||||
| Males | 953 | 90 | 0.88–0.92 | 1.00 | |||
| Females | 2619 | 95 | 0.95–0.96 | 0.55 | 0.51–0.59 | <0.001 | |
| OS registry population/univariate | |||||||
| Males | 953 | 82 | 71 | 0.61–0.79 | 1.00 | ||
| Females | 2619 | 86 | 88 | 0.84–0.91 | 0.36 | 0.26–0.48 | <0.001 |
| OS registry population/multivariate | |||||||
| Males | 953 | 82 | 71 | 0.61–0.79 | 1.00 | ||
| Females | 2619 | 86 | 88 | 0.84–0.91 | 0.56 | 0.41–0.77 | <0.001 |
| DSS registry population/univariate | |||||||
| Males | 953 | 30 | 91 | 0.86–0.94 | 1.00 | ||
| Females | 2619 | 34 | 96 | 0.93–0.98 | 0.40 | 0.24–0.65 | <0.001 |
| DSS registry population/multivariate | |||||||
| Males | 953 | 30 | 91 | 0.86–0.94 | 1.00 | ||
| Females | 2619 | 34 | 96 | 0.93–0.98 | 0.73 | 0.44–1.20 | 0.215 |
Association between gender and survival was adjusted for stage and age at diagnosis.
Disease-specific survival by gender
Fifteen-year DSS probabilities and HRs are also shown for PTC patients in Table 1. Univariate analyses suggested that female patients were at significantly less risk of dying from PTC than male patients (HR 0.40, CI 0.24–0.65, P < 0.001), likely reflecting that 79% of women presented at younger than 55 yr, compared with 70% of men, and 81% of women presented with stage I-II disease, compared with 63% of men. Because of gender differences in stage and age at diagnosis (see Fig. 1A), these factors were included in two models to determine whether the observed gender differences in DSS could be explained by differences in stage and age at diagnosis. Gender was no longer significant in either of these models. In a model of DSS for the PTC patients using age as a continuous variable, there were significant differences in stage and age but not in gender (P < 0.001, P < 0.001, and P = 0.203, respectively). In a model of DSS for PTC patients using age grouped as a categorical variable, there were significant differences in stage (P < 0.001) and age (P < 0.012) but not gender (P = 0.142). Consequently, with a multivariate model incorporating gender, age at diagnosis, and disease stage, female patients no longer had a survival advantage over their male counterparts (HR 0.73, CI 0.44–1.20, P = 0.215) (see Table 1). In addition, their HR was less favorable to women than any of the HRs for OS.
Comparison of HR for DSS using two different age cutoffs
Additional analyses (Table 2) examined the effect of gender and adjusted for stage within each age group using the two different initial age cutoffs of less than 45 and 55 yr (reflecting the usual age cutoff used in staging systems and a surrogate for age of completion of menopause, respectively). When the initial age cutoff was less than 45 yr, the differences in risk of death from disease between genders changed with age (HR 0.39 for < 45 yr, HR 0.55 for 45–59 yr, HR 0.73 for 60–74 yr, and HR 1.19 for ≥ 75 yr).
Table 2.
Multivariate Cox proportional hazards regression for the effect of gender on disease-specific survival using two different initial age cutoffs while adjusting for stage within each age group, with P value for gender differences shown
| Population | Patients, n | Deaths, n | 15-yr survival (%) | 95% CI for 15-yr survival | HR | 95% CI for HR | P value |
|---|---|---|---|---|---|---|---|
| All ages | |||||||
| Males | 953 | 30 | 91 | 0.86–0.94 | 1.00 | ||
| Females | 2619 | 34 | 96 | 0.93–0.98 | 0.73 | 0.44–1.20 | 0.215 |
| Initial age cutoff of <45 yr | |||||||
| Age group <45 yr | |||||||
| Males | 437 | 2 | 99 | 0.96–1.00 | 1.00 | ||
| Females | 1507 | 2 | 100 | 0.98–1.00 | 0.39 | 0.05–2.88 | 0.356 |
| Age group 45–59 yr | |||||||
| Males | 320 | 13 | 87 | 0.75–0.93 | 1.00 | ||
| Females | 731 | 11 | 91 | 0.81–0.96 | 0.55 | 0.24–1.26 | 0.159 |
| Age group 60–74 yr | |||||||
| Males | 156 | 10 | 81 | 0.64–0.90 | 1.00 | ||
| Females | 293 | 12 | 93 | 0.88–0.96 | 0.73 | 0.31–1.70 | 0.466 |
| Age group ≥75 yra | |||||||
| Males | 40 | 5 | 65 | 0.27–0.86 | 1.00 | ||
| Females | 88 | 9 | 85 | 0.72–0.92 | 1.19 | 0.38–3.66 | 0.767 |
| Initial age cutoff of <55 yr | |||||||
| Age group <55 yr | |||||||
| Males | 663 | 13 | 94 | 0.89–0.97 | 1.00 | ||
| Females | 2055 | 8 | 98 | 0.94–0.99 | 0.33 | 0.13–0.81 | 0.016 |
| Age group 55–69 yr | |||||||
| Males | 214 | 9 | 87 | 0.75–0.94 | 1.00 | ||
| Females | 416 | 13 | 91 | 0.78–0.96 | 1.01 | 0.42–2.37 | 0.983 |
| Age group >70 yra | |||||||
| Males | 76 | 8 | 67 | 0.38–0.85 | 1.00 | ||
| Females | 148 | 13 | 85 | 0.75–0.91 | 1.17 | 0.48–2.85 | 0.737 |
Initial age cutoff of less than 45 yr shows the usual staging age cutoff of less than 45 yr; the initial age cutoff of less than 55 yr shows a cutoff of less than 55 yr based on menopause. Patients younger than either 45 or 55 yr were combined as a group because there were very few deaths in these age groups. HR = 1.0 for stage I and II.
Survival probability and 95% CI were based on 10 yr, rather than 15 yr.
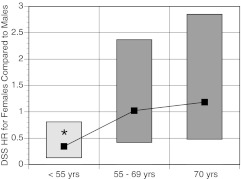
When the initial age cutoff was less than 55 yr, the differences in risk of thyroid cancer death between genders also changed with age (HR 0.33 for < 55 yr, HR 1.01 for 55–69 yr, and HR 1.17 for ≥ 70 yr). A pronounced step-up in risk of female death from thyroid cancer after age 55 yr was observed (see Fig. 3). Before age 55 yr, females were at significantly less risk of dying from thyroid cancer than males (HR 0.33, 95% CI 0.13–0.81, P = 0.016), but the risk was similar for both genders after age 55 yr. In fact, the younger age group retained the significant female advantage that was already shown for OS. Thus, gender differences favoring the OS of women were present in the U.S. population and the registry population, but gender differences favoring the DSS of registry women were present only in multivariate analyses in the age group of less than 55 yr.
Fig. 3.
HR (shown as black squares) and 95% CI for HR (shown as top and bottom of rectangle) for DSS for females compared with males. *, P value for gender difference = 0.016.
Additional analyses conducted using 10-yr intervals instead of 15-yr intervals and also using a 50-yr cutoff showed similar age-related trends. These analyses suggested that the step-up in female risk was between the 45- to 54-yr and 55- to 64-yr intervals.
Comparison of HR using a 55-year cutoff within stage
Table 3 shows the result of modeling gender and adjusting for age with an initial age cutoff of younger than 55 yr within each stage. For all stages together, there was a survival advantage for women with a HR of 0.48. Within the low-risk group of stage I and II patients, there was a survival advantage for women with a HR of 0.10. Thus, women have a significantly lower risk of dying from stage I and II thyroid cancer than do men. However, within more advanced disease stages, the confidence intervals for the HR include unity, and women have the same risk of death from thyroid cancer as men. The specific age and gender characteristics of patients with either stage I or stage II disease who died of their thyroid cancer are shown in Table 4.
Table 3.
Multivariate Cox proportional hazards regression for the effect of gender on disease-specific survival using an initial age cutoff of 55 yr within each stage while adjusting for age, with P value for gender difference shown
| Population | Patients, n | Deaths, n | 15-yr survival (%) | 95% CI for 15-yr survival | HR | 95% CI for HR | P value |
|---|---|---|---|---|---|---|---|
| All stages | |||||||
| Males | 953 | 30 | 91 | 0.86–0.94 | 1.00 | ||
| Females | 2619 | 34 | 96 | 0.93–0.98 | 0.48 | 0.29–0.79 | 0.004 |
| Stages I and II | |||||||
| Males | 603 | 5 | 98 | 0.94–0.99 | 1.00 | ||
| Females | 2125 | 2 | 100 | 0.99–1.00 | 0.10 | 0.02–0.51 | 0.006 |
| Stage III | |||||||
| Males | 296 | 10 | 86 | 0.73–0.93 | 1.00 | ||
| Females | 428 | 16 | 87 | 0.75–0.94 | 0.95 | 0.43–2.11 | 0.908 |
| Stage IV | |||||||
| Males | 51 | 15 | 50 | 0.26–0.70 | 1.00 | ||
| Females | 65 | 16 | 62 | 0.44–0.76 | 0.84 | 0.41–1.71 | 0.632 |
Stages I and II patients were combined into one low-risk group because they accounted for very few deaths. HR = 1.0 for <55 yr.
Table 4.
Characteristics of the seven patients with stage I or II disease who died of thyroid cancer
| Patient | Stage | Age (yr) | Gender | Tumor size (cm) | Tumor features* | Survival (yr) |
|---|---|---|---|---|---|---|
| 1 | II | 32 | M | >4 | Multifocal, ETE, CN | 1.5 |
| 2 | II | 44 | M | 1–4 | Multifocal, ETE | 4.7 |
| 3 | II | 51 | M | 1–4 | 8.2 | |
| 4 | II | 53 | M | 1–4 | Multifocal | 3.3 |
| 5 | II | 62 | M | 1–4 | 6.9 | |
| 6 | I | 78 | F | <1 | 1.9 | |
| 7 | II | 82 | F | 1–4 | 3.5 |
All tumors were papillary or mixed papillary and follicular, with no unusual variants. M, Male; F, female; ETE, extrathyroidal extension; CN, cervical nodes involved.
Discussion
With a cohort typical of other studies of PTC (1, 2, 8, 9, 11, 19), our analyses identify three new observations about gender differences in PTC. First, we conclude that a gender advantage in survival for women with PTC reflects a generalized gender advantage seen in the U.S. population. Second, we note that once women have been diagnosed with PTC, their outcomes are similar to men, after adjustment has been made for their presenting disease stage and age. And third, we find that, although with adjustments for stage women as a group fare similarly to men, women with stage I and II disease have better outcomes than men when comparing individuals diagnosed before age 55 yr.
Women with PTC initially appeared to have better OS when compared with men. However evaluation of a matched control population showed that mortality rates in the U.S. population were also lower for women than their male counterparts. Without these values, it could be erroneously concluded that women with PTC have a better OS than men with PTC, when in fact their survival advantage simply mirrors their gender advantage documented in the U.S. population (see Fig. 2C).
Examining gender differences in DSS, univariate analyses suggested that women had better DSS than men. However, in multivariate analyses including both age and disease stage, women did not have better DSS than men but actually lost their gender advantage so that their DSS was not different than the DSS of men (see Table 1). The impression that women with DTC have better outcomes than men is likely accounted for by their being diagnosed with DTC at a lesser stage and younger age than men, confirming what Machens et al. (19) termed a gender divide.
Perhaps the most fascinating aspect of this analysis is the impact of an age cutoff of 55 yr, corresponding to the age at which most women have attained menopause (21, 22). Women diagnosed at younger than 55 yr had a better DSS than men, whereas women diagnosed at older ages had a DSS indistinguishable from men. This age subdivision resulted in a clear step-up in risk for women in older age groups (see Fig. 3). It is possible that PTC cases that come to attention in an estrogen-deficient environment have a worse prognosis than those diagnosed when the patient is exposed to female hormones or, conversely, that PTC diagnosed in the presence of female hormones has a better prognosis. This altered prognosis for women at around the age of menopause was previously described in a small group of 631 DTC patients (28). We hypothesize that the better DSS of women with stage I and II disease compared with men (see Table 3) is due to the intimate relationship between stage and age, which probably resulted in more of these low-risk women also being under the age 55 yr.
Rigorous statistical analyses are indicated to ensure that claims of gender differences or menopausal influences are not spurious (29). Limitations of our study include our restricted data capture. Confounding factors associated with gender, such as body mass index (30), smoking (31), alcohol consumption, use of health care (14), educational level, marital status, and income (32), should ideally be considered in any study of gender-based differences. For example, it is possible that the observed lower disease stage at diagnosis and more favorable DSS in women aged 55 yr or younger could be attributable to their well-described greater use of health care resources and contact with health care providers (14). An optimized understanding of hormonal influences requires documentation of menstrual history, age of menopause, parity, and use of estrogen-containing medications. However, to maintain a manageable data collection process, the NTCTCSG does not collect such information. Moreover, the hypothesis that the premenopausal period is associated with protection from cancer growth and spread would need to be tested in a prospective study in which age of menopause was actually documented.
Literature examining the impact of estrogen on thyroid cancer is inconsistent and partly based on epidemiological studies or anecdotal reports. Numerous studies report conflicting relationships between estrogen status and both incidence and aggressiveness of DTC, including during the estrogen-rich environment of pregnancy (33–48). Data regarding thyroid cancer behavior after menopause are also conflicting. The incidence of thyroid cancer decreases after the age of menopause (23, 47), but there may be a higher risk of thyroid cancer associated with artificial menopause (34, 49) or surgical menopause (33). It has also been suggested that DTC recurrence may be more likely to occur after menopause (50).
Thus, some literature suggests that estrogen presence is associated with an increased incidence of DTC. However, our current data are consistent with the hypothesis that there is more indolent behavior of DTC when it is diagnosed during a time of estrogen exposure. To incorporate both of these observations, one could postulate that estrogen is associated with induction or growth of DTC but is also associated with a more treatable, and possibly more differentiated, cancer. This could be analogous to the observation that breast carcinoma is more frequent in women taking hormone replacement but is associated with longer survival than breast cancer arising in women not taking hormone replacement (51). Thyroid carcinoma cells have both estrogen-α and estrogen-β receptors, affecting both proliferation and apoptosis (39, 52–57). Possibly different balances between estrogen receptors may explain both the increased incidence of DTC in premenopausal women and the worse prognosis of DTC in menopausal women.
Older age is known to adversely affect thyroid cancer prognosis (2, 5, 10, 13, 58, 59), and prior studies have implicated diverse age cutoffs as prognostic factors (see Supplemental Table 2). Several staging systems reflect the worsening prognosis of DTC in older patients by advancing disease stage for patients 45 yr old or older (24, 60). However, one staging system advances stage above the age of 40 yr (61). Based on our data, the effect of age appears to be modified according to gender. Both sexes may have gradually worsening prognosis with age, but superimposed on this may be an additional step-up in female risk at an age approximately within the mid-50s. An increased risk of death from thyroid cancer in women older than 55 yr old compared with younger women was reported in an analysis of a European cancer registry (3). Such a dramatic increase in risk at a specific age was not seen for any other malignancies examined. The predictive value of staging systems with an age cutoff may primarily reflect the behavior of DTC in women, who account for the majority of DTC cases. One staging system does use a gender-specific age cutoff: the AMES system uses a cutoff of 41 yr for men, compared with 51 yr in women (28, 62). Another staging system also considers gender as a prognostic factor (58). Use of a staging system that incorporates different cutoffs based on gender is biologically plausible but may be more cumbersome to use. It is possible that an age cutoff of 55 yr may be sufficiently predictive for both genders and that a uniform age cutoff would facilitate use of the staging system. Analyses are being planned to restage our NTCTCSG cohort to test this hypothesis and assess the impact of various age cutoffs on product-limit survival. We hope to then validate this revised staging system using an external, independent database.
In summary, although the overall outcome of women with PTC is similar to that of men, this composite outcome is composed of two periods with different outcomes. The first is a period with better outcomes for women than men diagnosed at ages younger than 55 yr, whereas the second is a period with similar outcomes for both women and men diagnosed at ages older than 55 yr. The age group with the most favorable outcome also corresponds to the age group with the highest incidence of thyroid cancer. It is possible that incorporating an age cutoff of 55 yr may refine the performance of some DTC staging systems, and we plan to test this hypothesis in a future analysis.
Supplementary Material
Acknowledgments
We, as principal investigators at each NTCTCSG institution, thank the physicians and staff members who participated in the management and follow-up of these patients. We acknowledge the substantial contributions of the institutional research staff who collected and submitted the data, including Shehnaz Bana; Marge E. Ewertz, R.N.; and Beverly McLaughlin, R.M.A. We also appreciate the considerable assistance provided by Jeffrey Cui for the management of NTCTCSG's databases. Finally, we acknowledge the efforts of the numerous physicians and scientists whose contributions were critical in the early years of the registry.
Presented in part at the 79th Annual Meeting of the American Thyroid Association, Chicago, Illinois, 2008.
The NTCTCSG is supported in part by research grants from Genzyme Corporation and Pfizer, and by the University of Texas M.D. Anderson Cancer Center Support Grant (NCI Grant P30 CA016672). J.J. is supported by Grant 1UL1RR031975 from the National Center for Research Resources, National Institutes of Health.
Present address for S.T.B.: Veterans Affairs Medical Center, Portland, Maine.
Disclosure Summary: J.J., G.N.-G., M.M., D.L., K.B.A., S.T.B., J.D.B., D.S.C., P.W.L., M.C.S., and H.R.M. have nothing to declare. B.R.H. receives lecture fees from Genzyme Corp., J.M. is an employee and shareholder of Genzyme Corp., D.S.R. has consulted for Genzyme Corp., D.L.S. receives research funding from Veracyte, and S.I.S. consults for Bayer, Exelixis, Eisai, Pfizer, Eli Lilly, NovoNordisk, Roche, and AstraZeneca and is a member of the advisory board of Veracyte.
This work is dedicated to Jack Robbins, who contributed to the preparation of this manuscript prior to his unexpected death on May 12, 2008. He epitomized the wisdom of his age and the best qualities of his gender.
- CI
- Confidence interval
- DSS
- disease-specific survival
- DTC
- differentiated thyroid cancer
- HR
- hazard ratio
- NTCTCSG
- National Thyroid Cancer Treatment Cooperative Study Group
- OS
- overall survival
- PTC
- papillary thyroid cancer.
References
- 1. Akslen LA, Haldorsen T, Thoresen SO, Glattre E. 1991. Survival and causes of death in thyroid cancer: a population-based study of 2479 cases from Norway. Cancer Res 51:1234–1241 [PubMed] [Google Scholar]
- 2. Cunningham MP, Duda RB, Recant W, Chmiel JS, Sylvester JA, Fremgen A. 1990. Survival discriminants for differentiated thyroid cancer. Am J Surg 160:344–347 [DOI] [PubMed] [Google Scholar]
- 3. Micheli A, Ciampichini R, Oberaigner W, Ciccolallo L, de Vries E, Izarzugaza I, Zambon P, Gatta G, De Angelis R. 2009. The advantage of women in cancer survival: an analysis of EUROCARE-4 data. Eur J Cancer 45:1017–1027 [DOI] [PubMed] [Google Scholar]
- 4. Tubiana M, Schlumberger M, Rougier P, Laplanche A, Benhamou E, Gardet P, Caillou B, Travagli JP, Parmentier C. 1985. Long-term results and prognostic factors in patients with differentiated thyroid carcinoma. Cancer 55:794–804 [DOI] [PubMed] [Google Scholar]
- 5. Carcangiu ML, Zampi G, Pupi A, Castagnoli A, Rosai J. 1985. Papillary carcinoma of the thyroid. A clinicopathologic study of 241 cases treated at the University of Florence, Italy. Cancer 55:805–828 [DOI] [PubMed] [Google Scholar]
- 6. Elisei R, Molinaro E, Agate L, Bottici V, Masserini L, Ceccarelli C, Lippi F, Grasso L, Basolo F, Bevilacqua G, Miccoli P, Di Coscio G, Vitti P, Pacini F, Pinchera A. 2010. Are the clinical and pathological features of differentiated thyroid carcinoma really changed over the last 35 years? Study on 4187 patients from a single Italian institution to answer this question. J Clin Endocrinol Metab 95:1516–1527 [DOI] [PubMed] [Google Scholar]
- 7. Akslen LA, Myking AO, Salvesen H, Varhaug JE. 1992. Prognostic importance of various clinicopathological features in papillary thyroid carcinoma. Eur J Cancer 29A:44–51 [DOI] [PubMed] [Google Scholar]
- 8. Mazzaferri EL, Jhiang SM. 1994. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med 97:418–428 [DOI] [PubMed] [Google Scholar]
- 9. Samaan NA, Schultz PN, Hickey RC, Goepfert H, Haynie TP, Johnston DA, Ordonez NG. 1992. The results of various modalities of treatment of well differentiated thyroid carcinomas: a retrospective review of 1599 patients. J Clin Endocrinol Metab 75:714–720 [DOI] [PubMed] [Google Scholar]
- 10. Mazzaferri EL, Kloos RT. 2001. Clinical review 128: current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab 86:1447–1463 [DOI] [PubMed] [Google Scholar]
- 11. Shah JP, Loree TR, Dharker D, Strong EW, Begg C, Vlamis V. 1992. Prognostic factors in differentiated carcinoma of the thyroid gland. Am J Surg 164:658–661 [DOI] [PubMed] [Google Scholar]
- 12. Mihailovic J, Stefanovic L, Malesevic M. 2007. Differentiated thyroid carcinoma with distant metastases: probability of survival and its predicting factors. Cancer Biother Radiopharm 22:250–255 [DOI] [PubMed] [Google Scholar]
- 13. Toniato A, Boschin I, Casara D, Mazzarotto R, Rubello D, Pelizzo M. 2008. Papillary thyroid carcinoma: factors influencing recurrence and survival. Ann Surg Oncol 15:1518–1522 [DOI] [PubMed] [Google Scholar]
- 14. Bertakis KD, Azari R, Helms LJ, Callahan EJ, Robbins JA. 2000. Gender differences in the utilization of health care services. J Fam Pract 49:147–152 [PubMed] [Google Scholar]
- 15. Blom J, Yin L, Lidén A, Dolk A, Jeppsson B, Påhlman L, Holmberg L, Nyrén O. 2008. Toward understanding nonparticipation in sigmoidoscopy screening for colorectal cancer. Int J Cancer 122:1618–1623 [DOI] [PubMed] [Google Scholar]
- 16. Geiger TM, Miedema BW, Geana MV, Thaler K, Rangnekar NJ, Cameron GT. 2008. Improving rates for screening colonoscopy: analysis of the health information national trends survey (HINTS I) data. Surg Endosc 22:527–533 [DOI] [PubMed] [Google Scholar]
- 17. Streetly A, Markowe H. 1995. Changing trends in the epidemiology of malignant melanoma: gender differences and their implications for public health. Int J Epidemiol 24:897–907 [DOI] [PubMed] [Google Scholar]
- 18. Oliveria SA, Christos PJ, Halpern AC, Fine JA, Barnhill RL, Berwick M. 1999. Evaluation of factors associated with skin self-examination. Cancer Epidemiol Biomarkers Prev 8:971–978 [PubMed] [Google Scholar]
- 19. Machens A, Hauptmann S, Dralle H. 2006. Disparities between male and female patients with thyroid cancers: sex difference or gender divide? Clin Endocrinol (Oxf) 65:500–505 [DOI] [PubMed] [Google Scholar]
- 20. van der Schouw YT, van der Graaf Y, Steyerberg EW, Eijkemans JC, Banga JD. 1996. Age at menopause as a risk factor for cardiovascular mortality. Lancet 347:714–718 [DOI] [PubMed] [Google Scholar]
- 21. Weinstein M, Gorrindo T, Riley A, Mormino J, Niedfeldt J, Singer B, Rodríguez G, Simon J, Pincus S. 2003. Timing of menopause and patterns of menstrual bleeding. Am J Epidemiol 158:782–791 [DOI] [PubMed] [Google Scholar]
- 22. McKinlay SM, Brambilla DJ, Posner JG. 1992. The normal menopause transition. Maturitas 14:103–115 [DOI] [PubMed] [Google Scholar]
- 23. 2003. United States life tables, 2003. www.cdc.gov/nchs/data/statab/lewk3 pdf Accessed January 7, 2007
- 24. Sherman SI, Brierley JD, Sperling M, Ain KB, Bigos ST, Cooper DS, Haugen BR, Ho M, Klein I, Ladenson PW, Robbins J, Ross DS, Specker B, Taylor T, Maxon HR., 3rd 1998. Prospective multicenter study of thyroid carcinoma treatment: initial analysis of staging and outcome. National Thyroid Cancer Treatment Cooperative Study Registry Group. Cancer 83:1012–1021 [DOI] [PubMed] [Google Scholar]
- 25. Taylor T, Specker B, Robbins J, Sperling M, Ho M, Ain K, Bigos ST, Brierley J, Cooper D, Haugen B, Hay I, Hertzberg V, Klein I, Klein H, Ladenson P, Nishiyama R, Ross D, Sherman S, Maxon HR. 1998. Outcome after treatment of high-risk papillary and non-Hurthle-cell follicular thyroid carcinoma. Ann Intern Med 129:622–627 [DOI] [PubMed] [Google Scholar]
- 26. Cooper DS, Specker B, Ho M, Sperling M, Ladenson PW, Ross DS, Ain KB, Bigos ST, Brierley JD, Haugen BR, Klein I, Robbins J, Sherman SI, Taylor T, Maxon HR., 3rd 1998. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid 8:737–744 [DOI] [PubMed] [Google Scholar]
- 27. Jonklaas J, Sarlis NJ, Litofsky D, Ain KB, Bigos ST, Brierley JD, Cooper DS, Haugen BR, Ladenson PW, Magner J, Robbins J, Ross DS, Skarulis M, Maxon HR, Sherman SI. 2006. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid 16:1229–1242 [DOI] [PubMed] [Google Scholar]
- 28. Cady B, Sedgwick CE, Meissner WA, Wool MS, Salzman FA, Werber J. 1979. Risk factor analysis in differentiated thyroid cancer. Cancer 43:810–820 [DOI] [PubMed] [Google Scholar]
- 29. Patsopoulos NA, Tatsioni A, Ioannidis JP. 2007. Claims of sex differences: an empirical assessment in genetic associations. JAMA 298:880–893 [DOI] [PubMed] [Google Scholar]
- 30. Engeland A, Tretli S, Akslen LA, Bjørge T. 2006. Body size and thyroid cancer in two million Norwegian men and women. Br J Cancer 95:366–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mack WJ, Preston-Martin S, Dal Maso L, Galanti R, Xiang M, Franceschi S, Hallquist A, Jin F, Kolonel L, La Vecchia C, Levi F, Linos A, Lund E, McTiernan A, Mabuchi K, Negri E, Wingren G, Ron E. 2003. A pooled analysis of case-control studies of thyroid cancer: cigarette smoking and consumption of alcohol, coffee, and tea. Cancer Causes Control 14:773–785 [DOI] [PubMed] [Google Scholar]
- 32. Ghori FY, Gutterman-Litofsky DR, Jamal A, Yeung SC, Arem R, Sherman SI. 2002. Socioeconomic factors and the presentation, management, and outcome of patients with differentiated thyroid carcinoma. Thyroid 12:1009–1016 [DOI] [PubMed] [Google Scholar]
- 33. Mack WJ, Preston-Martin S, Bernstein L, Qian D, Xiang M. 1999. Reproductive and hormonal risk factors for thyroid cancer in Los Angeles County females. Cancer Epidemiol Biomarkers Prev 8:991–997 [PubMed] [Google Scholar]
- 34. Negri E, Dal Maso L, Ron E, La Vecchia C, Mark SD, Preston-Martin S, McTiernan A, Kolonel L, Yoshimoto Y, Jin F, Wingren G, Rosaria Galanti M, Hardell L, Glattre E, Lund E, Levi F, Linos D, Braga C, Franceschi S. 1999. A pooled analysis of case-control studies of thyroid cancer. II. Menstrual and reproductive factors. Cancer Causes Control 10:143–155 [DOI] [PubMed] [Google Scholar]
- 35. La Vecchia C, Ron E, Franceschi S, Dal Maso L, Mark SD, Chatenoud L, Braga C, Preston-Martin S, McTiernan A, Kolonel L, Mabuchi K, Jin F, Wingren G, Galanti MR, Hallquist A, Lund E, Levi F, Linos D, Negri E. 1999. A pooled analysis of case-control studies of thyroid cancer. III. Oral contraceptives, menopausal replacement therapy and other female hormones. Cancer Causes Control 10:157–166 [DOI] [PubMed] [Google Scholar]
- 36. Galanti MR, Lambe M, Ekbom A, Sparén P, Pettersson B. 1995. Parity and risk of thyroid cancer: a nested case-control study of a nationwide Swedish cohort. Cancer Causes Control 6:37–44 [DOI] [PubMed] [Google Scholar]
- 37. Rossing MA, Voigt LF, Wicklund KG, Williams M, Daling JR. 1998. Use of exogenous hormones and risk of papillary thyroid cancer (Washington, United States). Cancer Causes Control 9:341–349 [DOI] [PubMed] [Google Scholar]
- 38. McTiernan AM, Weiss NS, Daling JR. 1984. Incidence of thyroid cancer in women in relation to reproductive and hormonal factors. Am J Epidemiol 120:423–435 [DOI] [PubMed] [Google Scholar]
- 39. Rajoria S, Suriano R, Shanmugam A, Wilson YL, Schantz SP, Geliebter J, Tiwari RK. 2010. Metastatic phenotype is regulated by estrogen in thyroid cells. Thyroid 20:33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Parlea L, Fahim L, Munoz D, Hanna A, Anderson J, Cusimano M, Kovacs K, Gardiner G. 2006. Follicular carcinoma of the thyroid with aggressive metastatic behavior in a pregnant woman: report of a case and review of the literature. Hormones (Athens) 5:295–302 [DOI] [PubMed] [Google Scholar]
- 41. Leboeuf R, Emerick LE, Martorella AJ, Tuttle RM. 2007. Impact of pregnancy on serum thyroglobulin and detection of recurrent disease shortly after delivery in thyroid cancer survivors. Thyroid 17:543–547 [DOI] [PubMed] [Google Scholar]
- 42. Yasmeen S, Cress R, Romano PS, Xing G, Berger-Chen S, Danielsen B, Smith LH. 2005. Thyroid cancer in pregnancy. Int J Gynaecol Obstet 91:15–20 [DOI] [PubMed] [Google Scholar]
- 43. Vannucchi G, Perrino M, Rossi S, Colombo C, Vicentini L, Dazzi D, Beck-Peccoz P, Fugazzola L. 2010. Clinical and molecular features of differentiated thyroid cancer diagnosed during pregnancy. Eur J Endocrinol 162:145–151 [DOI] [PubMed] [Google Scholar]
- 44. Moosa M, Mazzaferri EL. 1997. Outcome of differentiated thyroid cancer diagnosed in pregnant women. J Clin Endocrinol Metab 82:2862–2866 [DOI] [PubMed] [Google Scholar]
- 45. Navarro Silvera SA, Miller AB, Rohan TE. 2005. Risk factors for thyroid cancer: a prospective cohort study. Int J Cancer 116:433–438 [DOI] [PubMed] [Google Scholar]
- 46. Truong T, Orsi L, Dubourdieu D, Rougier Y, Hemon D, Guénel P. 2005. Role of goiter and of menstrual and reproductive factors in thyroid cancer: a population-based case-control study in New Caledonia (South Pacific), a very high incidence area. Am J Epidemiol 161:1056–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sakoda LC, Horn-Ross PL. 2002. Reproductive and menstrual history and papillary thyroid cancer risk: the San Francisco Bay Area thyroid cancer study. Cancer Epidemiol Biomarkers Prev 11:51–57 [PubMed] [Google Scholar]
- 48. Rossing MA, Voigt LF, Wicklund KG, Daling JR. 2000. Reproductive factors and risk of papillary thyroid cancer in women. Am J Epidemiol 151:765–772 [DOI] [PubMed] [Google Scholar]
- 49. Levi F, Franceschi S, Gulie C, Negri E, La Vecchia C. 1993. Female thyroid cancer: the role of reproductive and hormonal factors in Switzerland. Oncology 50:309–315 [DOI] [PubMed] [Google Scholar]
- 50. Candanedo-Gonzalez FA, Gamboa-Dominguez A. 2007. Postmenopause is associated with recurrence of differentiated papillary thyroid carcinoma. Med Hypotheses 69:209–213 [DOI] [PubMed] [Google Scholar]
- 51. Sener SF, Winchester DJ, Winchester DP, Du H, Barrera E, Bilimoria M, Krantz S, Rabbitt S. 2009. The effects of hormone replacement therapy on postmenopausal breast cancer biology and survival. Am J Surg 197:403–407 [DOI] [PubMed] [Google Scholar]
- 52. Lee ML, Chen GG, Vlantis AC, Tse GM, Leung BC, van Hasselt CA. 2005. Induction of thyroid papillary carcinoma cell proliferation by estrogen is associated with an altered expression of Bcl-xL. Cancer J 11:113–121 [DOI] [PubMed] [Google Scholar]
- 53. Kawabata W, Suzuki T, Moriya T, Fujimori K, Naganuma H, Inoue S, Kinouchi Y, Kameyama K, Takami H, Shimosegawa T, Sasano H. 2003. Estrogen receptors (α and β) and 17β-hydroxysteroid dehydrogenase type 1 and 2 in thyroid disorders: possible in situ estrogen synthesis and actions. Mod Pathol 16:437–444 [DOI] [PubMed] [Google Scholar]
- 54. Bonacci R, Pinchera A, Fierabracci P, Gigliotti A, Grasso L, Giani C. 1996. Relevance of estrogen and progesterone receptors enzyme immunoassay in malignant, benign and surrounding normal thyroid tissue. J Endocrinol Invest 19:159–164 [DOI] [PubMed] [Google Scholar]
- 55. Dalla Valle L, Ramina A, Vianello S, Fassina A, Belvedere P, Colombo L. 1998. Potential for estrogen synthesis and action in human normal and neoplastic thyroid tissues. J Clin Endocrinol Metab 83:3702–3709 [DOI] [PubMed] [Google Scholar]
- 56. Manole D, Schildknecht B, Gosnell B, Adams E, Derwahl M. 2001. Estrogen promotes growth of human thyroid tumor cells by different molecular mechanisms. J Clin Endocrinol Metab 86:1072–1077 [DOI] [PubMed] [Google Scholar]
- 57. Chen GG, Vlantis AC, Zeng Q, van Hasselt CA. 2008. Regulation of cell growth by estrogen signaling and potential targets in thyroid cancer. Curr Cancer Drug Targets 8:367–377 [DOI] [PubMed] [Google Scholar]
- 58. Byar DP, Green SB, Dor P, Williams ED, Colon J, van Gilse HA, Mayer M, Sylvester RJ, van Glabbeke M. 1979. A prognostic index for thyroid carcinoma. A study of the E.O.R.T.C. Thyroid Cancer Cooperative Group. Eur J Cancer 15:1033–1041 [DOI] [PubMed] [Google Scholar]
- 59. Brierley J, Tsang R, Panzarella T, Bana N. 2005. Prognostic factors and the effect of treatment with radioactive iodine and external beam radiation on patients with differentiated thyroid cancer seen at a single institution over 40 years. Clin Endocrinol (Oxf) 63:418–427 [DOI] [PubMed] [Google Scholar]
- 60. Greene F, Page DL, Fleming ID, eds. 2002. American Joint Committee on Cancer staging manual. New York: Springer-Verlag [Google Scholar]
- 61. Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. 1993. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery 114:1050–1057; discussion 1057–1058 [PubMed] [Google Scholar]
- 62. Cady B, Rossi R. 1988. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery 104:947–953 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.