Abstract
Context:
Studies of long-term outcomes of subclinical hypothyroidism have assessed only baseline thyroid function, despite natural transitions to euthyroidism and overt hypothyroidism over time.
Objective:
We provide estimates of persistence, resolution, and progression of subclinical hypothyroidism over 4 yr, stratified by baseline TSH, anti-thyroid peroxidase antibody (TPOAb) status, age, and sex.
Design, Setting, and Participants:
Participants were 3996 U.S. individuals at least 65 yr old enrolled in the Cardiovascular Health Study. Subclinical hypothyroidism was detected at baseline in 459 individuals not taking thyroid medication.
Main Outcome Measure:
Thyroid function was evaluated at 2 and 4 yr and initiation of thyroid medication annually. Results were stratified by baseline TSH, TPOAb status, age, and sex.
Results:
Persistence of subclinical hypothyroidism was 56% at 2 and 4 yr. At 2 yr, resolution was more common with a TSH of 4.5–6.9 mU/liter (46 vs. 10% with TSH 7–9.9 mU/liter and 7% with TSH ≥10 mU/liter; P < 0.001) and with TPOAb negativity (48 vs. 15% for positive; P < 0.001). Higher TSH and TPOAb positivity were independently associated with lower likelihood of reversion to euthyroidism (P < 0.05). TSH of 10 mU/liter or higher was independently associated with progression to overt hypothyroidism (P < 0.05). Transitions between euthyroidism and subclinical hypothyroidism were common between 2 and 4 yr. Age and sex did not affect transitions.
Conclusions:
Subclinical hypothyroidism persists for 4 yr in just over half of older individuals, with high rates of reversion to euthyroidism in individuals with lower TSH concentrations and TPOAb negativity. Future studies should examine the impact of transitions in thyroid status on clinical outcomes.
Subclinical hypothyroidism is common in the elderly, with a prevalence of up to 15% in community-based studies (1–5). Controversy surrounds the issue of whether untreated, persistent subclinical hypothyroidism has sufficiently important and reversible consequences in the elderly to justify screening and treatment (6–12). In some studies, subclinical hypothyroidism has been associated with adverse health consequences in the elderly, including an increased risk of hip fracture in men (13) and the development of Alzheimer's disease in women (14). Among large prospective cohort studies, data on the associations between subclinical hypothyroidism and cardiovascular disease and mortality are conflicting (15–18), and it has been suggested that the cardiovascular risk of subclinical hypothyroidism varies by the age of the individual (12, 15). However, a recent meta-analysis using individual patient data from cohort studies demonstrates that it is the degree of TSH elevation that is associated with cardiovascular risk, independent of age or preexisting cardiovascular disease (17). A lack of data on relevant clinical outcomes from large, randomized controlled trials fuels the treatment controversy (19) and necessitates the need for further work examining the risks of untreated subclinical hypothyroidism.
All of the observational studies examining the relationship between subclinical thyroid disease and outcomes in the elderly have measured thyroid function at a single time point. These studies assume that a single measurement is representative of long-term thyroid status, despite data from smaller studies to support significant rates of resolution, from 0.43–6% per year (20–22) and progression to overt hypothyroidism, from 1.5–11.4% per year (20–22). These rates also vary depending on the anti-thyroid peroxidase antibody (TPOAb) status and the degree of elevation of TSH of study participants (20, 21, 23, 24). The clinical risks from subclinical hypothyroidism may be overestimated by the inclusion of individuals who progress to overt hypothyroidism before the outcome occurs or underestimated by the inclusion of individuals who regress to euthyroidism. Differences in transitions in thyroid status may, in part, explain the variability of risk demonstrated in analyses from different observational studies.
Using data from a large cohort study representative of community-dwelling individuals aged 65 yr and over, we sought to demonstrate the natural history of subclinical hypothyroidism over a 4-yr period including the rates of persistence, resolution, and progression of subclinical thyroid dysfunction in 2 and 4 yr. Our study is the first of the large cohorts to provide an understanding of transitions in subclinical hypothyroidism over time, key information lacking from current outcomes studies. We also evaluated the effects of the degree of baseline TSH elevation and TPOAb positivity, age, and sex within an older cohort on changes in thyroid function status over time.
Subjects and Methods
Study population
These analyses are based on data from the Cardiovascular Health Study (CHS) (25). The CHS is a population-based, longitudinal study of risk factors for the development of cardiovascular disease in 5888 adults 65 yr and older. Enrollment of an original cohort of 5201 adults occurred between May 1989 and June 1990, and an additional cohort of 687 African-Americans was enrolled in 1992–1993. Eligible individuals were identified from an age- and gender-stratified random sample of the Medicare eligibility rosters in four U.S. communities: Washington County, MD; Pittsburgh, PA; Sacramento County, CA; and Forsyth County, NC. To be eligible, individuals had to be noninstitutionalized, expecting to remain in the area for the following 3 yr, not under active treatment for cancer, not wheelchair-bound in the home, not requiring a proxy respondent at entry, and capable of providing consent. Household members of the sampled individual were recruited, if eligible. The institutional review boards of all four sites and the coordinating center at the University of Washington in Seattle approved the study. All participants gave informed consent.
Data from the 1992–1993, 1994–1995, and 1996–1997 study visits from both cohorts are included in this analysis and are designated as baseline, yr 2, and yr 4 in this report. These visits included a detailed medical history, physical examination, and assessment of health status. Thyroid hormone medication use was assessed via medication bottle examination during annual study visits. Blood was drawn after a 12-h fast, and serum was processed and stored in −70 C freezers for future investigations.
Thyroid hormone testing
Thyroid function assays were performed in 2010 at the CHS Central Blood Analysis Laboratory at the University of Vermont on banked samples from 3996 individuals with available serum for analysis at the 1992–1993 visit, 4005 individuals at the 1994–1995 visit, and 3371 individuals at the 1996–1997 visit. Serum TSH, free T4, total T3, and TPOAb concentrations were measured using chemiluminescent immunoassays on the Elecsys 2010 analyzer (Roche Diagnostics, Indianapolis, IN). TSH concentrations were measured at each visit with a third-generation assay with a functional sensitivity of 0.005 mU/liter, reference range 0.27–4.2 mU/liter, and 2.1% intrassay and 3.1% interassay coefficients of variation (CV). Free T4 concentrations were measured at each visit in individuals with serum TSH levels below 0.10 or above 4.50 mU/liter. The free T4 assay had a functional sensitivity of 0.23 ng/dl, reference range of 0.7–1.7 ng/dl (9–22 pmol/liter), and 1.7% intraassay and 3.3% interassay CV. Total T3 concentrations were measured in samples with a TSH below 0.10 mU/liter. The assay had a functional sensitivity of 0.3 nmol/liter, reference range of 1.3–3.1 nmol/liter, and 4.2% intrassay and 4.7% interassay CV. The TPOAb assay was performed in 3920 individuals with available serum at the baseline visit. This assay had a functional sensitivity of 5.0 IU/liter, a threshold of 37 IU/liter to define positivity (26), and 2.5% intrassay and 9.2% interassay CV.
Classification by thyroid status
For data analysis, individuals at baseline, yr 2, and yr 4 were classified into one of the following five groups based on their thyroid function tests. 1) Overt hyperthyroidism was defined as a TSH concentration below 0.10 mU/liter with an elevated free T4, or total T3. 2) Subclinical hyperthyroidism was defined as a TSH concentration below 0.45 mU/liter with normal free T4 and total T3 concentrations. 3) Euthyroidism was defined as a normal TSH concentration (0.45–4.49 mU/liter). 4) Subclinical hypothyroidism was defined as a TSH 4.5–19.9 mU/liter with a normal free T4 concentration. 5) Overt hypothyroidism was defined as a TSH concentration over 4.5 mU/liter with a free T4 concentration level below normal (0.7 ng/dl) or a TSH of 20 mU/liter or higher. All participants with TSH levels of 20 mU/liter or higher had low free T4 values except for two participants with free T4 values at the assay lower bound.
Statistical analysis
Baseline characteristics between participants with subclinical hypothyroidism (n = 459) and participants who were euthyroid (n = 3057) were compared using a t test or χ2 test as appropriate. In the group with subclinical hypothyroidism, thyroid status at 2 and 4 yr after baseline was determined and stratified by baseline TSH level (4.5–6.9, 7.0–9.9, or 10.0–19.9 mU/liter), TPOAb status (negative or positive) in the 451 individuals for whom TPOAb were obtained, age (65.0–74.9 or 75.0 yr and older), and sex. The χ2 tests were used to compare follow-up status across strata. A multinomial logistic regression model was used to assess associations between age, sex, race, TSH strata, and TPOAb status and independent transitions from subclinical hypothyroidism to each thyroid category (euthyroidism, overt hypothyroidism, or thyroid medication initiation) at 2 yr.
Results
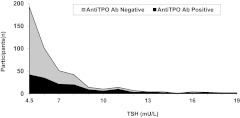
At baseline, of the 3996 individuals on whom thyroid tests were performed, 402 (10%) were excluded due to use of thyroid medication. Of the remaining 3594 individuals, 85% (n = 3057) were euthyroid and 12.8% (n = 459) had subclinical hypothyroidism, representing the highest prevalence of any thyroid testing abnormality. Far fewer participants were overtly hypothyroid (0.61%; n = 22), overtly hyperthyroid (0.33%; n = 12), or subclinically hyperthyroid (1.2%; n = 44). The incidence of subclinical hypothyroidism in those who were euthyroid at baseline was 2.7% (84 of 3057) over a 2-yr period. Compared with the euthyroid group, those with subclinical hypothyroidism at baseline were slightly older and more likely to be female, Caucasian, and nonsmokers, but they did not differ in educational level, income, or body mass index (Table 1). As expected, TSH levels were higher, free T4 levels were lower, and TPOAb prevalence was higher in the subclinical hypothyroid group. The distribution of TSH levels by TPOAb is shown in those with subclinical hypothyroidism at baseline in Fig. 1.
Table 1.
Characteristics of individuals with subclinical hypothyroidism and euthyroidism at baseline
| Characteristic | Subclinical hypothyroidism, n = 459 | Euthyroidism, n = 3057 | P value |
|---|---|---|---|
| Age (yr) | 75.6 (5.7) | 74.6 (5.2) | <0.001 |
| Male | 177 (38.6) | 1344 (44.0) | 0.03 |
| Caucasian | 412 (89.8) | 2472 (80.9) | <0.001 |
| High school graduate | 346 (75.6) | 2185 (71.6) | 0.08 |
| Income ≥$25,000 | 178 (41.3) | 1132 (39.6) | 0.50 |
| Current smoker | 30 (6.6) | 318 (10.5) | 0.01 |
| BMI (kg/m2) | 26.8 (4.8) | 26.7 (4.7) | 0.83 |
| TSH (mU/liter) | 6.7 (2.6) | 2.1 (0.96) | NAa |
| Free T4 (ng/dl) | 1.08 (0.17) | 1.22 (0.17) | <0.001 |
| TPOAb positive | 161 (35.7) | 237 (8.0) | <0.001 |
Entries are shown as n (percent), except for age, BMI, TSH, and free T4, which are shown as mean (sd). NA, Not applicable.
Groups defined by this measure.
Fig. 1.
TSH distribution in subjects with subclinical hypothyroidism at baseline, stratified by TPOAb status.
Of the 459 individuals with subclinical hypothyroidism at baseline, 48 did not have adequate sera for testing and were not taking medications 2 yr later, 15 had no clinic visit, and 27 died. Mean TSH levels were similar at baseline between the 369 individuals with and the 90 without follow-up thyroid testing at yr 2 (TSH 6.8 vs. 6.3 mU/liter). Mortality did not differ between those who were euthyroid and those who had subclinical hypothyroidism at baseline at either 2 yr (5.3 vs. 5.9%, P = 0.63) or 4 yr (12.9 vs. 12.6%, P = 0.88) of follow-up.
At yr 2, of the 369 available for evaluation, 56% (n = 208) remained subclinically hypothyroid (persistent subclinical hypothyroidism), 35% (n = 128) reverted to euthyroidism, 2% (n = 8) became overtly hypothyroid, and 7% (n = 25) initiated treatment with thyroid hormone replacement (Table 2). In those with persistent subclinical hypothyroidism, the median baseline TSH was 6.3 mU/liter, and the median TSH at yr 2 was 6.2 mU/liter. In those who reverted to euthyroidism (transient subclinical hypothyroidism), the median baseline TSH was 5.2 mU/liter, and the median TSH at yr 2 was 3.5 mU/liter. TSH decreased by greater than 1 mU/liter in more than 85% of those whose subclinical hypothyroidism resolved.
Table 2.
The yr 2 thyroid status of individuals with subclinical hypothyroidism at baseline, stratified by baseline TSH and age
| Baseline status | Thyroid status yr 2 [n (%)] |
||||
|---|---|---|---|---|---|
| Euthyroid | Subclinical hypothyroid | Overt hypothyroid | Started l-T4 | Total | |
| TSH strata | |||||
| TSH 4.5–6.9 mU/liter | 118 (46) | 125 (49) | 3 (1) | 10 (4) | 256 (100) |
| TSH 7.0–9.9 mU/liter | 7 (10) | 58 (81) | 1 (1) | 6 (8) | 72 (100) |
| TSH ≥10 mU/liter | 3 (7) | 25 (61) | 4 (10) | 9 (22) | 41 (100) |
| Age strata | |||||
| Age 65–74 yr | 68 (35) | 111 (56) | 5 (2) | 13 (7) | 197 (100) |
| Age ≥75 yr | 60 (35) | 97 (56) | 3 (2) | 12 (7) | 172 (100) |
| Sex strata | |||||
| Women | 84 (36) | 129 (55) | 3 (1) | 18 (8) | 234 (100) |
| Men | 44 (33) | 79 (59) | 5 (4) | 7 (5) | 135 (100) |
| Total | 128 (35) | 208 (56) | 8 (2) | 25 (7) | 369 (100) |
Stratification by TSH
At baseline, 69% of individuals with subclinical hypothyroidism had a TSH between 4.5 and 6.9 mU/liter, 20% had a TSH between 7.0 and 10 mU/liter, and 11% had a TSH of 10 mU/liter or higher. The majority in each TSH stratum remained subclinically hypothyroid over 2 yr of follow-up. However, the rates of resolution, progression, and medication initiation differed by baseline TSH (Table 2). Resolution of subclinical hypothyroidism by yr 2 was more common in those with a baseline TSH of 4.5–6.9 mU/liter (46%) compared with those with higher baseline TSH levels (10% for TSH of 7–9.9 mU/liter, and 7% for TSH ≥10 mU/liter; P < 0.001). In contrast, progression to overt hypothyroidism was more likely in those with higher baseline TSH concentrations, at 10% in those with a TSH of 10 mU/liter or higher compared with 1% in each of the lower TSH strata (P < 0.001). In addition, thyroid hormone initiation was more common in individuals in the highest TSH stratum at baseline (P = 0.002).
Stratification by age and sex
There was no difference in the proportion of patients who were euthyroid, subclinically hypothyroid, or overtly hypothyroid at yr 2 comparing participants aged 65–74 yr with those aged 75 yr and older (P = 0.96, Table 2). In addition, similar proportions of patients initiated thyroid hormone replacement by yr 2 in the two age groups (P = 0.89). There was also no difference by sex (P = 0.31).
Stratification by TPOAb status
Overall, 35% of subclinically hypothyroid individuals were TPOAb positive. As expected, the proportion of individuals with TPOAb positivity was greater at higher TSH concentrations (Fig. 1). Among the 451 individuals with TPOAb status who had follow-up TSH or who initiated thyroid medications (n = 364), those who were TPOAb positive were significantly less likely to revert to euthyroidism (15 vs. 48%; P < 0.001) within 2 yr than those who were TPOAb negative (Table 3). A higher proportion of those who were TPOAb positive remained subclinically hypothyroid compared with those who were TPOAb negative (73 vs. 45%; P < 0.001).
Table 3.
The yr 2 thyroid status of individuals with subclinical hypothyroidism at baseline, stratified by baseline TPOAb status
| Baseline TPOAb status | Thyroid status yr 2 [n (%)] |
||||
|---|---|---|---|---|---|
| Euthyroid | Subclinical hypothyroid | Overt hypothyroid | Started l-T4 | Total | |
| Negative (≤37 IU/liter) | 108 (48) | 102 (45) | 4 (2) | 12 (5) | 226 (100) |
| Positive (>37 IU/liter) | 20 (15) | 101 (73) | 4 (3) | 13 (9) | 138 (100) |
Regression model
We performed a multinomial logistic regression model to determine factors independently associated with the risk of resolution or progression. Age (P = 0.69), sex (P = 0.13), and race (P = 0.14) were not significantly associated with transitions from subclinical hypothyroidism to other states, whereas both levels of TSH at baseline (P < 0.0001) and TPOAb status (P < 0.0001) were. Due to small numbers in some groups, results are reported for the most parsimonious model (Table 4). Those with TSH levels of 7.0–9.9 mU/liter were 84% less likely to revert to euthyroidism, and those with TSH levels of 10.0 mU/liter or higher were 82% less likely to revert to euthyroidism than those with levels of 4.5–6.9 mU/liter (P < 0.05 for each). Likewise, those who were TPOAb positive were 76% less likely to revert to euthyroidism than their TPOAb-negative counterparts (P < 0.05). TSH levels of 10 mU/liter or greater were positively associated with progression to overt hypothyroidism and to initiation of thyroid medications (P < 0.05 for each), although the effect estimates are unreliable due to small numbers of participants.
Table 4.
Associations of TSH category and TPOAb positivity with transitions in thyroid status at 2 yr in individuals with subclinical hypothyroidism at baseline
| Risk factor | Euthyroid (n = 128) | Overt hypothyroid (n = 8) | Started l-T4 (n = 25) | P value |
|---|---|---|---|---|
| TSH at baseline | <0.0001 | |||
| 4.5–6.9 mU/liter | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| 7.0–9.9 mU/liter | 0.16 (0.07–0.36) | 0.75 (0.08–7.47) | 1.31 (0.45–3.82) | |
| 10–19.9 mU/liter | 0.18 (0.05–0.63) | 7.11 (1.41–35.9) | 4.56 (1.63–1.28) | |
| TPOAb positive | 0.24 (0.14–0.43) | 0.71 (0.16–3.18) | 0.85 (0.35–2.04) | <0.0001 |
Reference group is persistent subclinical hypothyroidism (n = 208). Statistically significant comparisons are indicated in bold.
Year 4 transitions
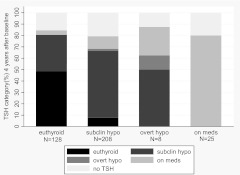
We then examined the status at yr 4 of the 369 individuals with thyroid function testing or thyroid medication use at yr 2 (Fig. 2). Of the 128 who had reverted to euthyroidism at yr 2, 62 (48%) remained euthyroid at yr 4, 41 (32%) were again subclinically hypothyroid, five started thyroid medications, nine died, and the remainder had no TSH measured. Of the 208 who were subclinically hypothyroid at baseline and yr 2, 122 (59%) remained so at yr 4, only 16 (8%) resolved to euthyroid, four became overtly hypothyroid, 23 initiated thyroid medications, 11 died, and the remainder had no TSH. If the denominator is limited to those who had thyroid testing at yr 4, 58% (62 of 107) of those who were euthyroid at yr 2 remained euthyroid at yr 4, 38% (41 of 107) of those who were euthyroid at yr 2 returned to subclinical hypothyroidism at yr 4, and 76% (122 of 161) of those with persistent subclinical hypothyroidism at yr 2 remained so at the third testing period.
Fig. 2.
Thyroid status at yr 4 (y-axis) by thyroid status at yr 2 (x-axis) among participants who had subclinical hypothyroidism (subclin hypo) at baseline, were not taking thyroid medications at baseline, and who had follow-up data at 2 yr.
Discussion
This is the largest longitudinal cohort study to examine the natural history of subclinical hypothyroidism in an elderly, community-dwelling population. We performed thyroid function testing at three time points to determine the rates of regression, persistence, and progression over a 4-yr period. We found that subclinical hypothyroidism is frequently transient, with over a third of those with follow-up measures becoming euthyroid, and a 10th progressing to overt hypothyroidism or initiating thyroid hormone replacement over a 2-yr period. There was an increased likelihood of reversion to euthyroidism with a lower baseline TSH and TPOAb negativity, but age and sex were not significant factors in this cohort of men and women aged 65 and older.
Our 12.8% prevalence of subclinical hypothyroidism is within the range of 3.4–15% reported in community based cohorts of elderly individuals (1–5, 27). In addition, the overall proportion of TPOAb positive individuals in our cohort, 11.9%, is comparable to that observed in National Health and Nutrition Examination Survey III, where 10.4% of those aged 70–79 yr old were TPOAb positive (28). These data support the generalizability of our findings to older U.S. adults.
Persistence of subclinical hypothyroidism over 2 yr was seen in 56% of individuals who had repeat TSH measures. Huber et al. (22) reported a slightly higher degree of persistence, at 68%, in an endocrinology clinic-based study of 82 younger women (mean age 50 yr) with an average of 9.2 yr of follow-up, whereas Díez et al. (20) found lower persistence, at 36%, in their endocrinology clinic-based study of 107 men and women aged 55 and older with an average follow-up of less than 3 yr. Of note, these studies required confirmation of subclinical hypothyroidism for study inclusion with a second set of thyroid function tests within 1–3 months, which in one study resulted in immediate exclusion of 20% of individuals (22), and both had a higher mean baseline TSH level than ours, likely reflecting referral practices. A third study by Parle et al. (21) screened individuals aged 60 and older in a general medical practice and followed 73 with subclinical hypothyroidism for 1 yr, with a reported persistence of 77%. Similar to our study, persistence varied by baseline TSH and antibody status in this study, ranging from 64% in those who were antibody positive with TSH concentrations of over 10 mU/liter to 82% in those who were antibody negative with TSH concentrations of 5–10 mU/liter.
In our community based-population, we demonstrate a similar percentage, 35%, who normalize over 2 yr compared with the clinic-based study of Díez et al. (20) in which 37% normalized over slightly less than 3 yr. In a more detailed analysis of the 40 patients from this study who normalized, the median time to normalization was 18 months, with 67% normalizing within 2 yr (29). In the clinic-based study of Huber et al. (22), a much lower proportion became euthyroid, at 4%, and 5.5% normalized in the older population studied by Parle et al. (21). The only prospective cohort study other than ours to report rates of normalization is the Leiden 85-Plus study, in which Gussekloo et al. (30) reported that 11 of 21 participants with subclinical hypothyroidism normalized their values within 3 yr (30). We also noted more normalization at lower TSH levels, with 46% of those with a baseline TSH of 4.5–6.9 mU/liter regressing to euthyroidism within a 2-yr period. There was little change at more extreme values of TSH, arguing against significant regression to the mean, and the amount of TSH change was too large to suggest that simply repeating the thyroid testing was responsible for the observed changes.
As expected, progression to overt hypothyroidism and initiation of thyroid hormone replacement were more common in those with a higher baseline TSH. Huber et al. (22) demonstrated a 3.3% annual incidence of hypothyroidism in their patients with subclinical hypothyroidism with TSH 6–12 mU/liter and an 11.4% annual incidence with TSH higher than 12 mU/liter, although their study had a higher prevalence of TPOAb positivity, at 51%, than the 36% seen in our participants with subclinical hypothyroidism. Likewise, in the study by Díez et al. (20), 1.8% of patients with initial TSH values between 5.0 and 9.9 mU/liter, 20% of those with TSH 10.0–14.9 mU/liter, and 74% of those with TSH 15.0 mU/liter or higher developed overt hypothyroidism, with an overall prevalence of TPOAb positivity of 76% (20). The incidence of hypothyroidism was highest in the study of Parle et al. (21) with an 18% incidence over 1 yr. In a 20-yr follow-up evaluation of the Whickham Survey, the annual risk of developing hypothyroidism was 2.6% with elevated serum TSH alone and 4.3% with both elevated TSH and positive antithyroid antibodies (24). In the Busselton Health Survey, the incidence of overt hypothyroidism, defined as TSH over 10 mU/liter or initiating levothyroxine treatment, was 3.5% in the overall population over a 13-yr period, although the incidence in those with subclinical hypothyroidism at baseline was not reported (23).
Our study does not reveal a difference in the course of subclinical hypothyroidism with age within our cohort of individuals aged 65 and older. The prevalence of subclinical hypothyroidism increases with increasing age, with 13% of those in National Health and Nutrition Examination Survey III over the age of 80 without thyroid disease having a TSH above the reference range developed in younger adults (28). This higher TSH with aging may be independent of antithyroid antibodies, because the prevalence of antithyroid antibodies in those with a TSH greater than 4.5 mU/liter has been shown to increase with age until age 70, then decline (31). The Leiden 85-Plus study is the only other study to include participants in their 80s, and although their number with subclinical hypothyroidism with follow-up testing is small (n = 21), their data also support a high rate of reversion to euthyroidism and a low rate of progression to hypothyroidism (30). Additional studies are needed to determine the clinical relevance of the age-associated upward shift in the distribution of TSH levels with increasing age and therapeutic targets in the elderly.
A major strength of our study is the use of a large population-based cohort of older men and women followed over 4 yr. We provide better estimates of subclinical hypothyroidism than previous studies using clinic-based populations because we were able to avoid a referral bias of individuals with more severe testing abnormalities. In addition, thyroid function testing was performed using banked samples, and results were not provided to study participants or their physicians. However, our study has several limitations. These include a lack of thyroid testing at the time of thyroid hormone initiation. We do not know whether individuals who started levothyroxine were subclinically or overtly hypothyroid at the time of medication initiation, leading to some uncertainty in our estimates of overt hypothyroidism. Also, a number of individuals were not available for follow-up, although we demonstrate no difference in baseline TSH between those who had TSH measurements and those who were not included in follow-up, suggesting that the two groups were similar. Finally, our results may not apply to populations under the age of 65 yr.
Based on our findings, using one set of thyroid function tests to define subclinical hypothyroidism would lead to misclassification of more than 40% of elderly individuals, even if those who initiated thyroid hormone during follow-up were censored from analysis. Knowledge of transitions in thyroid function tests over time may be important in understanding the relationship between subclinical hypothyroidism and clinical outcomes. For example, using CHS data, we have previously demonstrated no relationship between subclinical hypothyroidism, defined at a single time point, and cardiovascular disease or death (1). However, inclusion of individuals with transient subclinical hypothyroidism may have biased these results toward the null. Our data also suggest that a significantly large subset of older individuals with subclinical hypothyroidism may not require treatment, because their TSH may normalize within 2 yr. Conversely, it has been shown that those with subclinical hypothyroidism with a TSH of at least 10 mU/liter are at increased risk of both coronary heart disease events and mortality (18), although this is a group that we and others have shown has a high rate of progression to overt hypothyroidism (20–22). Our findings suggest that requiring more than one set of thyroid function tests to establish subclinical hypothyroidism, aside from mirroring clinical practice, would improve the quality of risk estimates from cohort studies. This, in turn, would provide a better definition of the subpopulations at risk for the long-term consequences of subclinical hypothyroidism and guide the design of randomized clinical trials for this condition.
Acknowledgments
This work was supported by R01AG-032317 from the National Institute on Aging (NIA), contracts N01-HC-85239, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, and Grant HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided through AG-023629, AG-15928, AG-20098, and AG-027058 from the NIA.
A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CHS
- Cardiovascular Health Study
- CV
- coefficients of variation
- TPOAb
- anti-thyroid peroxidase antibody.
References
- 1. Cappola AR, Fried LP, Arnold AM, Danese MD, Kuller LH, Burke GL, Tracy RP, Ladenson PW. 2006. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA 295:1033–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rodondi N, Newman AB, Vittinghoff E, de Rekeneire N, Satterfield S, Harris TB, Bauer DC. 2005. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med 165:2460–2466 [DOI] [PubMed] [Google Scholar]
- 3. Kanaya AM, Harris F, Volpato S, Pérez-Stable EJ, Harris T, Bauer DC. 2002. Association between thyroid dysfunction and total cholesterol level in an older biracial population: the health, aging and body composition study. Arch Intern Med 162:773–779 [DOI] [PubMed] [Google Scholar]
- 4. Sawin CT, Chopra D, Azizi F, Mannix JE, Bacharach P. 1979. The aging thyroid. Increased prevalence of elevated serum thyrotropin levels in the elderly. JAMA 242:247–250 [DOI] [PubMed] [Google Scholar]
- 5. Bagchi N, Brown TR, Parish RF. 1990. Thyroid dysfunction in adults over age 55 years. A study in an urban US community. Arch Intern Med 150:785–787 [PubMed] [Google Scholar]
- 6. Ladenson PW, Singer PA, Ain KB, Bagchi N, Bigos ST, Levy EG, Smith SA, Daniels GH, Cohen HD. 2000. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med 160:1573–1575 [DOI] [PubMed] [Google Scholar]
- 7. Helfand M. 2004. Screening for subclinical thyroid dysfunction in nonpregnant adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 140:128–141 [DOI] [PubMed] [Google Scholar]
- 8. Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, Franklyn JA, Hershman JM, Burman KD, Denke MA, Gorman C, Cooper RS, Weissman NJ. 2004. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA 291:228–238 [DOI] [PubMed] [Google Scholar]
- 9. Gharib H, Tuttle RM, Baskin HJ, Fish LH, Singer PA, McDermott MT. 2005. Subclinical thyroid dysfunction: a joint statement on management from the American Association of Clinical Endocrinologists, the American Thyroid Association, and the Endocrine Society. J Clin Endocrinol Metab 90:581–585; discussion 586–587 [DOI] [PubMed] [Google Scholar]
- 10. Chu JW, Crapo LM. 2001. The treatment of subclinical hypothyroidism is seldom necessary. J Clin Endocrinol Metab 86:4591–4599 [DOI] [PubMed] [Google Scholar]
- 11. McDermott MT, Ridgway EC. 2001. Subclinical hypothyroidism is mild thyroid failure and should be treated. J Clin Endocrinol Metab 86:4585–4590 [DOI] [PubMed] [Google Scholar]
- 12. Biondi B, Cooper DS. 2008. The clinical significance of subclinical thyroid dysfunction. Endocr Rev 29:76–131 [DOI] [PubMed] [Google Scholar]
- 13. Lee JS, Buzková P, Fink HA, Vu J, Carbone L, Chen Z, Cauley J, Bauer DC, Cappola AR, Robbins J. 2010. Subclinical thyroid dysfunction and incident hip fracture in older adults. Arch Intern Med 170:1876–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tan ZS, Beiser A, Vasan RS, Au R, Auerbach S, Kiel DP, Wolf PA, Seshadri S. 2008. Thyroid function and the risk of Alzheimer disease: the Framingham Study. Arch Intern Med 168:1514–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ochs N, Auer R, Bauer DC, Nanchen D, Gussekloo J, Cornuz J, Rodondi N. 2008. Meta-analysis: subclinical thyroid dysfunction and the risk for coronary heart disease and mortality. Ann Intern Med 148:832–845 [DOI] [PubMed] [Google Scholar]
- 16. Razvi S, Weaver JU, Vanderpump MP, Pearce SH. 2010. The incidence of ischemic heart disease and mortality in people with subclinical hypothyroidism: reanalysis of the Whickham Survey cohort. J Clin Endocrinol Metab 95:1734–1740 [DOI] [PubMed] [Google Scholar]
- 17. Völzke H, Schwahn C, Wallaschofski H, Dörr M. 2007. Review: The association of thyroid dysfunction with all-cause and circulatory mortality: is there a causal relationship? J Clin Endocrinol Metab 92:2421–2429 [DOI] [PubMed] [Google Scholar]
- 18. Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, Asvold BO, Iervasi G, Imaizumi M, Collet TH, Bremner A, Maisonneuve P, Sgarbi JA, Khaw KT, Vanderpump MP, Newman AB, Cornuz J, Franklyn JA, Westendorp RG, Vittinghoff E, Gussekloo J. 2010. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 304:1365–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Villar HC, Saconato H, Valente O, Atallah AN. 2007. Thyroid hormone replacement for subclinical hypothyroidism. Cochrane Database Syst Rev 3:CD003419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Díez JJ, Iglesias P. 2004. Spontaneous subclinical hypothyroidism in patients older than 55 years: an analysis of natural course and risk factors for the development of overt thyroid failure. J Clin Endocrinol Metab 89:4890–4897 [DOI] [PubMed] [Google Scholar]
- 21. Parle JV, Franklyn JA, Cross KW, Jones SC, Sheppard MC. 1991. Prevalence and follow-up of abnormal thyrotrophin (TSH) concentrations in the elderly in the United Kingdom. Clin Endocrinol (Oxf) 34:77–83 [DOI] [PubMed] [Google Scholar]
- 22. Huber G, Staub JJ, Meier C, Mitrache C, Guglielmetti M, Huber P, Braverman LE. 2002. Prospective study of the spontaneous course of subclinical hypothyroidism: prognostic value of thyrotropin, thyroid reserve, and thyroid antibodies. J Clin Endocrinol Metab 87:3221–3226 [DOI] [PubMed] [Google Scholar]
- 23. Walsh JP, Bremner AP, Feddema P, Leedman PJ, Brown SJ, O'Leary P. 2010. Thyrotropin and thyroid antibodies as predictors of hypothyroidism: a 13-year, longitudinal study of a community-based cohort using current immunoassay techniques. J Clin Endocrinol Metab 95:1095–1104 [DOI] [PubMed] [Google Scholar]
- 24. Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, Grimley EJ, Hasan DM, Rodgers H, Tunbridge F. 1995. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf) 43:55–68 [DOI] [PubMed] [Google Scholar]
- 25. Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A. 1991. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1:263–276 [DOI] [PubMed] [Google Scholar]
- 26. Kratzsch J, Fiedler GM, Leichtle A, Brügel M, Buchbinder S, Otto L, Sabri O, Matthes G, Thiery J. 2005. New reference intervals for thyrotropin and thyroid hormones based on National Academy of Clinical Biochemistry criteria and regular ultrasonography of the thyroid. Clin Chem 51:1480–1486 [DOI] [PubMed] [Google Scholar]
- 27. Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. 2000. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam Study. Ann Intern Med 132:270–278 [DOI] [PubMed] [Google Scholar]
- 28. Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. 2002. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 87:489–499 [DOI] [PubMed] [Google Scholar]
- 29. Díez JJ, Iglesias P, Burman KD. 2005. Spontaneous normalization of thyrotropin concentrations in patients with subclinical hypothyroidism. J Clin Endocrinol Metab 90:4124–4127 [DOI] [PubMed] [Google Scholar]
- 30. Gussekloo J, van Exel E, de Craen AJ, Meinders AE, Frölich M, Westendorp RG. 2004. Thyroid status, disability and cognitive function, and survival in old age. JAMA 292:2591–2599 [DOI] [PubMed] [Google Scholar]
- 31. Surks MI, Hollowell JG. 2007. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab 92:4575–4582 [DOI] [PubMed] [Google Scholar]