Abstract
Background:
The association between changes in IGF-I and IGF binding protein (IGFBP) levels and mortality in older adults is unknown.
Study Design:
Participants were 997 persons 77 to 100 yr old enrolled in the Cardiovascular Health Study All Stars Study. Plasma levels of IGF-I, IGFBP-1, and IGFBP-3 were assessed at two study examinations (1996–1997 and 2005–2006). Mortality was assessed between 2006 and 2010.
Results:
Cumulative mortality (CM) was similar among individuals who had at least 10% decreases over time in IGF-I levels (CM = 29.6%), individuals who had at least 10% increases over time in IGF-I levels (CM = 24.7%), and individuals who had IGF-I levels remaining within ±10% over time (CM = 23.5%). Adjusted for age, sex, race, diabetes, body mass index, creatinine, albumin, and C-reactive protein, decreasing IGF-I level had no significant association with overall cancer mortality or noncancer mortality. Levels of IGFBP-1 increased markedly over time by 38% (median). Individuals with the largest increases in IGFBP-1 level over time had significantly increased risk of mortality. The adjusted hazard ratio per sd of IGFBP-1 change was 1.40 for overall cancer mortality (95% confidence interval = 1.10, 1.77; P = 0.01) and 1.14 for noncancer mortality (95% confidence interval = 1.02, 1.27; P = 0.02). Changes in IGFBP-3 levels were not significantly associated with mortality.
Conclusion:
Among older adults, decreasing IGF-I level over time does not predict subsequent all-cause mortality, whereas increasing IGFBP-1 predicts increased risk of mortality.
IGFs constitute an evolutionarily conserved system with an important physiological role during embryonic development, growth, and adulthood (1). IGF-I is a circulating hormone that mediates the effects of GH and reciprocally inhibits pituitary GH secretion. In addition to being an endocrine hormone, IGF-I is expressed locally in many tissues and acts through paracrine and autocrine mechanisms. IGF binding proteins (primarily IGFBP-3 and IGF acid-labile subunit) carry IGF-I in circulating blood and modulate interactions of IGF-I with IGF-I receptors, insulin receptors, and IGF-I/insulin hybrid receptors. Measurements of circulating IGF-I levels are used clinically to monitor patients with GH excess or deficiency and to measure responses to GH treatment.
The GH/IGF/IGFBP system plays a key role in many processes, including carbohydrate metabolism, cell differentiation and proliferation, and apoptosis, and may also be important in aging and age-related diseases such as cancer (2) and cardiovascular diseases (3, 4). Organisms with reduced insulin/insulin-like signaling have small body size and increased life span (1). On the other hand, low IGF-I activity may increase cell apoptosis and reduce tissue proliferation and repair, which is of potential importance in normal human aging; for example, increased apoptosis may produce loss of tissue mass and cellularity and impair tissue regeneration. Organisms with DNA repair defects (e.g. XPF-ERCC1 mouse, and a related human case) have decreased IGF-I and increased IGFBP-1, which may promote both accelerated dysfunction of various organ systems and development of cancer, driven by increased accumulation of somatic mutations (5).
Because circulating levels and bioactivity of IGF-I decline with aging (6), preventing or reversing age-related decreases in IGF-I is believed to have the potential to delay mortality and increase “health span” (i.e. survival free of disability and disease). Treatments that can produce up to a doubling in IGF-I levels [human GH (hGH) (7–9), oral ghrelin mimetic (10)] have shown no overall benefit in clinical trials, which has not eliminated the use of hGH for “anti-aging” purposes (11). In fact, whereas some studies, but not others, suggest that low serum IGF-I level is associated with increased mortality (12–17), the long-term health outcomes associated with declining endogenous IGF-I levels in older adults have not been assessed in prospective studies. In particular, few if any prior studies of older adults have measured IGF-I and IGFBP levels repeatedly over a long interval of time to see whether changes in the IGF axis predict risk of death.
In an observational study with long-term follow-up, we measured changes in circulating levels of IGF-I and IGFBPs over a period of 9 yr among older men and women. We then examined whether levels and changes in levels of IGF-I, IGFBP-1, and IGFBP-3 were associated with risk of subsequent mortality.
Subjects and Methods
Design overview
The Cardiovascular Health Study (CHS) All Stars Study is a longitudinal cohort study of older adults living in four U.S. communities. Measurements of IGF-I, IGFBP-1, and IGFBP-3 were made using stored blood specimens collected at two time points spanning 9 yr. Levels and changes in levels of IGF-I, IGFBP-1, and IGFBP-3 were examined in relation to subsequent risk of mortality, after adjustment for potential confounders.
Setting and participants
CHS Field Centers enrolled 5888 community-dwelling adults 65 yr and older, including an original cohort of 5201 individuals recruited during 1989–1990 and an additional cohort of African-American individuals during 1992–1993. Participants were invited to repeated examinations for collection of data and blood specimens. Examinations were conducted annually through 1999. In 2005–2006, as part of the CHS All Stars Study, an additional study examination was conducted on surviving participants, who at that time were all between 77 and 100 yr of age. Follow-up of the study cohort for major health events and deaths through semiannual contacts continues through the present time. All participants provided informed consent, and institutional review board approvals were obtained at all participating institutions.
Study measurements
Physical and cognitive function tests, questionnaires, laboratory panels, and other key covariates were collected at study examinations. The present report uses data from the 1996–1997 and 2005–2006 examination cycles to define exposures and confounders. Deaths that occurred from the time of the 2005–2006 examination through February 28, 2010 were identified, and cause of death was classified based upon death certificates and medical records.
Laboratory variables
Laboratory measurements were performed using blood collected using standard procedures after an overnight fast and stored at −70 C. Measurements were performed at the Jewish General Hospital (Montreal, Canada) of IGFBP-1, IGFBP-3, and IGF-I after an extraction step using ELISA methods (Diagnostics Systems Laboratory, Webster, TX) (12). Assay coefficient of variation was 4–6% for IGF-I concentration, 3–14% for IGFBP-1 concentration, and 3–5% for IGFBP-3 concentration. Serum creatinine, albumin, C-reactive protein, IL-6, insulin, C-peptide, and glucose were measured at the CHS Central Laboratory using standard methods (18).
Clinical variables
Diabetes was defined according to measured fasting glucose levels (>126 mg/dl) or physician diagnosis with use of diabetes medication. Body mass index was calculated as the measured weight in kilograms divided by the square of measured height in meters. To control for differences in mortality by race, we classified individuals as White or Black/African-American as defined by participant self-report.
Statistical analyses
The main exposure variables of interest were repeated measurements of IGF-I, IGFBP-1, and IGFBP-3 levels at two study examinations approximately 9 yr apart, specifically the 1996–1997 and 2005–2006 examinations. Changes in IGF-I, IGFBP-1, and IGFBP-3 levels were analyzed both as continuous variables that were scaled to sd of change, as well as in categories defined as decreasing levels (10% or greater decrease), stable levels (10% decrease to 10% increase), or increasing levels (10% or greater increase). With the 2005–2006 visit defined as baseline for mortality follow-up, Cox proportional hazards regression was used to model the association between change in IGF-I or IGFBP level and hazard of death, with censoring at the end of available follow-up in 2010. We also examined whether single measurements of levels of IGF-I and IGFBPs obtained at the baseline (2005–2006) examination were associated with hazard of death. Simultaneous adjustment for level and change in levels revealed no additional associations beyond those reported here. Analyses were conducted that defined the outcome as death due to cancer (overall, at any anatomic site) with censoring at noncancer death, and as death due to causes other than cancer with censoring at cancer death.
Multivariable models were used to control for potential confounders that may have either obscured or induced an association between IGF-I or IGFBP levels and mortality. Initial models adjusted for age, sex, and race. Subsequent models included additional adjustment for diabetes, body mass index, creatinine, albumin, and C-reactive protein. Interaction terms were used to examine differences by sex in the associations of IGF-I and IGFBP levels with death; these analyses revealed no significant interactions at the P < 0.05 level. Finally, in sensitivity analyses, we confirmed that findings were similar after exclusion of individuals who had diabetes or congestive heart failure. All statistical analyses were performed using R (R Development Core Team, 2009). P < 0.05 was defined as the criterion for statistical significance.
The funders had no role in the design, collection, or analysis of study data.
Results
Mean age among 997 participants was 85.2 yr (sd = 3.6 yr) at the 2005–2006 examination, which for this study was defined as baseline for mortality follow-up (Table 1). At this time, all participants were between 77 and 100 yr of age. The cohort included 528 White women, 107 Black women, 306 White men, and 56 Black men. Deaths subsequent to the 2005–2006 baseline examination were ascertained over a median follow-up of 4.2 yr. During this period, which included 3822 person-years of follow-up, we observed 267 deaths, 39 of which were due to cancer, for a mortality incidence rate of 7.0 deaths per 100 person-years (Table 2).
Table 1.
Characteristics of 997 Cardiovascular Health Study All Stars Study participants at the baseline (2005–2006) examination
| Meana | sda | n (%) | |
|---|---|---|---|
| Age (yr) | 85.2 | 3.6 | |
| Male | 362 (36%) | ||
| Black race | 163 (16%) | ||
| Body mass index (kg/m2) | 26.7 | 4.6 | |
| Serum creatinine (mg/dl) | 1.1 | 0.5 | |
| C-reactive protein (mg/liter)a | 2.0 | 4.6 | |
| Serum albumin (mg/dl) | 3.8 | 0.3 | |
| Diabetes | 161 (16%) | ||
| IGF-I (μg/liter) | 152.7 | 60.9 | |
| IGFBP-1 (μg/liter) | 54.4 | 37.0 | |
| IGFBP-3 (μg/liter) | 3075.0 | 912.4 |
For each variable, fewer than 5% of subjects had missing values. Data were missing for 11 subjects for IGF-I and two subjects for IGFBP-3. Conversion factors to change published units to SI units are: creatinine, mg/dl × 88.4 = μmol/liter; C-reactive protein, mg/liter × 9.524 = nmol/liter; IGF-I, μg/liter × 0.131 = nmol/liter; IGFBP-1, μg/liter × 0.22 = nmol/liter; and IGFBP-3, μg/liter × 0.035 = nmol/liter.
Values shown for C-reactive protein are the median and 75th percentile.
Table 2.
Occurrence of death among Cardiovascular Health Study All Stars Study participants with increasing, stable, or decreasing levels of IGF-I, IGFBP-1, or IGFBP-3
| IGF-I |
IGFBP-1 |
IGFBP-3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of deaths/total | % of total | CM (%) | No. of deaths/total | % of total | CM (%) | No. of deaths/total | % of total | CM (%) | |
| Increasing levels (+10% or more) | 61/247 | 24.8 | 24.7 | 191/650 | 65.9 | 29.4 | 37/147 | 14.8 | 25.2 |
| Stable levels (±10%) | 61/260 | 26.1 | 23.5 | 29/124 | 12.6 | 23.4 | 93/376 | 37.8 | 24.7 |
| Decreasing levels (−10% or more) | 145/490 | 49.1 | 29.6 | 46/212 | 21.5 | 21.7 | 137/472 | 47.4 | 29.0 |
| Total | 267/997 | 266/986 | 267/995 | ||||||
Follow-up for mortality was conducted through 2/28/2010. Follow-up time ranged from 0.1 to 4.9 yr, with a median of 4.2 yr, and a total of 3822 person- years. CM, Cumulative mortality.
Change in circulating IGF levels over 9 yr
At the 1996–1997 examination, mean (sd) IGF-I levels were 167.0 (61.4) μg/liter. At the 2005–2006 study examination, IGF-I levels had decreased by an average of 14.4 μg/liter (sd for change in IGF-I level = 48.4 μg/liter). This represented a median 9% decrease in IGF-I levels between the repeat visits, or a decline of approximately 1%/yr. Approximately half of participants had decreases in IGF-I levels of 10% or greater, whereas one fourth had IGF-I levels that were stable over time within ±10%. IGFBP-1 levels increased markedly by 38% (median) between the 1996–1997 study examination and the 2005–2006 study examination. Levels of IGFBP-3 decreased by 9% over time (median). The direction and magnitude of change in IGFBP-3 level was substantially correlated with contemporaneous change in IGF-I level [r = 0.65; 95% confidence interval (CI) = 0.61, 0.68].
Circulating IGF-I levels and mortality
Among 490 individuals with a 10% or greater decrease in IGF-I levels between repeat visits, we observed 145 deaths (cumulative mortality = 29.6%), whereas among 260 individuals whose IGF-I levels remained stable within ±10%, we observed 61 deaths (cumulative mortality = 23.5%) (Table 2). In multivariable adjusted analyses, we found no significant association between risk of overall mortality and change in IGF-I levels between the repeat study visits (Fig. 1). When analyzed as a continuous variable, change in IGF-I level did not have a significant association with overall mortality. Change in IGF-I level was not associated with either overall cancer mortality or noncancer mortality (Table 3).
Fig. 1.
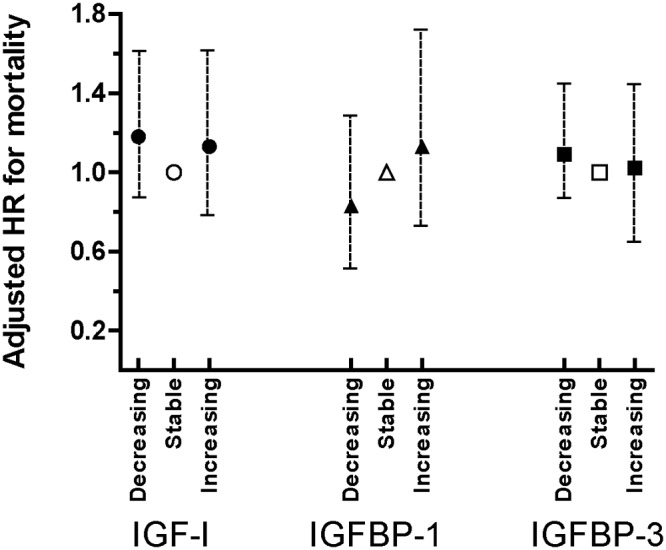
Multivariate adjusted analyses of death in relation to change in IGF-I, IGFBP-1, and IGFBP-3 levels. The reference groups, depicted with open symbols, are individuals who had stable levels of IGF-I (circles), IGFBP-1 (triangles), or IGFBP-3 (squares) between 1996–1997 and 2005–2006 visits. Decreasing level was defined as at least a 10% decrease between repeated visits, and increasing level was defined as at least a 10% increase between repeated visits. Analyses are adjusted for age, sex, race, diabetes, body mass index, creatinine, albumin, and C-reactive protein. HR, Hazard ratio.
Table 3.
Multivariable analyses of noncancer death and cancer death in relation to levels and longitudinal changes in IGF-I, IGFBP-1, and IGFBP-3 among Cardiovascular Health Study All Stars Study participants
| Noncancer death (228 events) |
Overall cancer death (39 events) |
|||||
|---|---|---|---|---|---|---|
| Adjusted HR | 95% CI | P | Adjusted HR | 95% CI | P | |
| IGF-I, change in level | 0.91 | 0.79, 1.06 | 0.23 | 1.03 | 0.72, 1.47 | 0.87 |
| IGF-I, level at baseline visit | 0.95 | 0.83, 1.10 | 0.51 | 0.71 | 0.49, 1.03 | 0.07 |
| IGFBP-1, change in level | 1.14 | 1.02, 1.27 | 0.02 | 1.40 | 1.10, 1.77 | 0.01 |
| IGFBP-1, level at baseline visit | 1.16 | 1.04, 1.30 | 0.01 | 1.23 | 0.92, 1.67 | 0.17 |
| IGFBP-3, change in level | 0.89 | 0.77, 1.03 | 0.13 | 1.09 | 0.76, 1.55 | 0.65 |
| IGFBP-3, level at baseline visit | 0.91 | 0.79, 1.05 | 0.19 | 0.72 | 0.49, 1.05 | 0.09 |
Change in IGF-I, IGFBP-1, and IGFBP-3 levels defined as percentage change between 1996–1997 visit and 2005–2006 visit, rescaled as sd of change. Baseline level refers to measurement obtained at the 2005–2006 visit, rescaled as sd of level. Hazard ratios represent relative hazard of death per sd increase in change or baseline level of IGF-I, IGFBP-1, or IGFBP-3. Models were adjusted for age, sex, race, diabetes, body mass index, creatinine, albumin, and C-reactive protein. The sd values were: 48.4 μg/liter for change in IGF-I level, 36.2 μg/liter for change in IGFBP-1 level, and 618.6 μg/liter for change in IGFBP-3 level. Table 1 displays sd values for IGF-I, IGFBP-1, and IGFBP-3 levels at baseline. HR, Hazard ratio.
We next examined baseline levels of IGF-I at the 2005–2006 examination as a predictor of subsequent mortality. In models adjusted for age, sex, and race, we found an association between higher IGF-I levels and lower risk of death due to cancer (Table 3). This association fell short of statistical significance in further multivariable adjusted analyses. For the outcome of overall cancer mortality, after adjustment for age, sex, and race, the hazard ratio for each sd increase in IGF-I levels was 0.68 (95% CI = 0.47, 0.98; P = 0.04). This association was attenuated slightly and was not statistically significant after further adjustment for body mass index, creatinine, albumin, and C-reactive protein (hazard ratio = 0.71; 95% CI = 0.49, 1.03; P = 0.07). Baseline IGF-I levels had no association with noncancer mortality (Table 3). Although the tabular data considered IGF-I level as a continuous variable, we also found no evidence of association between low or high IGF-I level when compared across tertiles. Compared with a middle IGF-I tertile of 120–171 μg/liter, hazard ratios of death were 1.2 (95% CI = 0.89, 1.6) for the lowest IGF-I tertile and 0.97 (95% CI = 0.70, 1.35) for the highest IGF-I tertile.
Circulating IGFBP-1 levels and mortality
Individuals with increasing levels of IGFBP-1 between the repeat visits had significantly elevated risk of overall cancer and noncancer mortality (Table 3 and Fig.1). In multivariable-adjusted models, the hazard ratio for overall cancer mortality for each sd increase in IGFBP-1 change was 1.40 (95% CI = 1.10, 1.77; P = 0.01). The hazard ratio for noncancer mortality for each sd increase in IGFBP-1 change was 1.14 (95% CI = 1.02, 1.27; P = 0.02). Those with higher levels of IGFBP-1 at the baseline examination also had significantly increased risk of noncancer mortality (multivariate adjusted hazard ratio for each sd increase in baseline IGFBP-1 = 1.16; 95% CI = 1.04, 1.30; P = 0.01). In contrast to the results for noncancer mortality, overall cancer mortality was not significantly associated with baseline IGFBP-1 levels (P = 0.17).
Circulating IGFBP-3 levels and mortality
Change in IGFBP-3 levels between repeat visits was not associated with overall mortality (Table 3 and Fig. 1), cancer mortality (Table 3), or noncancer mortality (Table 3). Higher baseline levels of IGFBP-3 tended to be associated with lower risk of overall cancer mortality, although the association was not statistically significant after adjustment for age, sex, race, body mass index, creatinine, albumin, and C-reactive protein (hazard ratio = 0.72; 95% CI = 0.49, 1.05; P = 0.09) (Table 3). There was no evidence for association between baseline levels of IGFBP-3 and risk of noncancer mortality (Table 3).
Correlates of change in IGFBP-1 level
As compared with individuals with decreasing IGFBP-1 levels between repeated visits, those with stable or increasing IGFBP-1 levels tended to have lower levels of albumin, IL-6, insulin, and C-peptide (Table 4). Those with decreasing IGFBP-1 levels also tended to have higher levels of IGF-I (P < 0.05), and change in IGFBP-1 and change in IGF-I were inversely correlated (r = −0.29; 95% CI = −0.34, −0.23).
Table 4.
Variables associated with longitudinal change in IGFBP-1 level
| Decreasing IGFBP-1 | Stable IGFBP-1 (±10%) | Increasing IGFBP-1 | P | |
|---|---|---|---|---|
| Creatinine (mg/dl) | 1.1 ± 0.57 | 1.0 ± 0.33 | 1.0 ± 0.52 | 0.07 |
| C-reactive protein (mg/liter) | 4.8 ± 8.9 | 3.2 ± 4.7 | 3.6 ± 7.4 | 0.05 |
| Albumin (mg/dl) | 3.86 ± 0.29 | 3.82 ± 0.28 | 3.76 ± 0.29 | <0.01 |
| IL-6 (mg/dl) | 4.3 ± 2.4 | 3.7 ± 2.3 | 3.7 ± 2.3 | <0.01 |
| Insulin (μU/ml) | 11.1 ± 17.1 | 8.0 ± 4.8 | 8.5 ± 8.2 | 0.02 |
| C-peptide (ng/ml) | 2.6 ± 1.3 | 2.2 ± 0.88 | 2.3 ± 0.95 | <0.01 |
| IGF-I (μg/liter) | 148.8 ± 59.6 | 155.1 ± 58.2 | 160.3 ± 63.7 | 0.05 |
| Change in IGF-I (μg/liter) | −22.0 ± 47.1 | −9.0 ± 41.1 | 6.9 ± 49.1 | <0.01 |
Data are presented as mean ± sd. P values were calculated using F test. Categories were defined as 10% or greater decline in IGFBP-1 levels (“decreasing”), 10% or greater increase in IGFBP-1 levels (“increasing”), or stable IGFBP-1 defined as levels within ± 10% between repeat visits.
Discussion
This study did not confirm the hypothesis that declining IGF-I level is a mortality risk factor. Several prior studies have addressed this question, with conflicting results (12–15), although our study is the first, to our knowledge, that measured changes in IGF-I levels over nearly a decade. If the GH/IGF axis is important in longevity and diseases of aging, how can the lack of association between circulating IGF-I and mortality be explained? IGF-I has important paracrine and autocrine activity, as well as endocrine functions, and circulating levels of IGF-I may not accurately reflect IGF-I bioactivity. In addition, total IGF-I levels typically correlate weakly, if at all, with phenotypes that are believed to be influenced by the insulin-like growth system axis. For example, patients with extremely low constitutive levels of circulating IGF-I, such as those with IGFALS gene mutations, can survive to adulthood and have only modest impairments in growth and glucose metabolism (19). On the other hand, circulating IGF-I levels are high in centenarians with specific IGF1R mutations, and in these individuals IGF-I receptor signaling is defective and height may be reduced (20). The Zoetermeer study recently reported that low IGF-I bioactivity, as measured by the IGF-I kinase receptor activation assay, predicted increased mortality among older adults, whereas circulating concentrations of total and free IGF-I did not (14). Measurements of IGF-I bioactivity were unavailable in the present study, which is a limitation.
On average, older adults in this study had substantial increases in circulating levels of IGFBP-1 over 9 yr of follow-up. Those with the greatest increases over time in circulating IGFBP-1 level had the highest mortality. This finding was consistent across subgroups of cancer-related deaths and non-cancer-related deaths. By obtaining repeated measurements of IGFBP-1 in an elderly cohort over time, this study further extends the evidence that a high level of IGFBP-1 is a robust mortality risk factor (12, 13). Notably, the association of increasing IGFBP-1 with mortality was independent of possible confounders and conditions including heart failure, high or low body mass index, inflammation, diabetes mellitus, impaired renal function (creatinine), and malnutrition (albumin), which are common in the elderly and also associated with IGF-axis abnormalities. Hepatic IGFBP-1 production is under strong inverse regulation by insulin and may be stimulated by proinflammatory cytokines (21). Paradoxically, however, we observed that increasing IGFBP-1 levels, whereas predictive of higher future mortality, was also associated with more favorable levels of IL-6 and glucose metabolism markers (C-peptide and fasting insulin). The finding that high IGFBP-1 and low albumin levels were correlated suggests the hypothesis that covariability between these two analytes may be informative for determining nutritional status in older adults. Therefore, further research is warranted to identify the factors, beyond insulin, that predict, modify, and mediate this phenotype of increasing IGFBP-1 levels. High circulating IGFBP-1 level correlates with low IGF-I bioactivity (14), and experimental models suggest that IGFBP-1 has IGF-I-inhibitory effects (22). Rising levels of circulating IGFBP-1 in an older person may therefore reflect a negative regulatory response to dampen anabolic IGF-I-mediated effects. IGFBP-1 may also have IGF-I-independent effects on cell survival (23).
Strengths of this study include its prospective design and long follow-up period. Because levels and changes in IGFs are highly age-dependent, when generalizing these findings it should be noted that the population had a mean age of 85 yr (range, 77 to 100 yr) at the time that we began mortality follow-up. Limitations include an observational study design, which makes it impossible to attribute causality to the association between IGF system proteins and mortality, but which nonetheless permits us to draw conclusions about the ability of circulating biomarkers of IGF status to predict mortality. Mortality risk factors are known to vary by cause of death (24). Here, we classified events according to cancer and noncancer causes of death, but without specific information on cancer type and with relatively few cancer deaths, which limited our ability to understand cause-specific mortality risks (n = 39).
In conclusion, our study found no evidence that older adults with decreases in IGF-I levels over a period of years have diminished likelihood of long-term survival. A possible increase in overall cancer mortality, but no difference in noncancer mortality, was found comparing individuals with low vs. high IGF-I levels. Although this finding was of borderline statistical significance and is only partially consistent with prior studies (15, 16), it may be of concern among elderly patients with low IGF-I levels who are treated with hGH because of the drug's potential cancer-promoting potential (25). We also showed that changes in IGFBP-1 levels with aging are pronounced, and IGFBP-1 may have important IGF-I-dependent or IGF-I-independent mechanisms that influence survival into old age.
Acknowledgments
The authors gratefully acknowledge Drs. Kenneth Mukamal, Luc Djoussé, Joachim Ix, Susan Zieman, and Jorge Kizer for measurement of insulin and C-peptide data.
The research reported in this article was supported by HL094555 (from National Heart, Lung, and Blood Institute) contract no. N01-HC-85239, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133; Grant HL080295 from the National Heart, Lung, and Blood Institute; and Grant AG-023269 from the National Institute on Aging, with an additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided through Grants AG-15928, AG-20098, and AG-027058 and 2R01AG023629-05A2 from the National Institute on Aging, HL-075366 from the National Heart, Lung and Blood Institute, 1R01AG031890 from the National Institute of Aging (to R.C.K.), and the University of Pittsburgh Claude. D. Pepper Older Americans Independence Center Grant P30-AG-024827.
R.C.K. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The funding agencies had no role in the conduct of the study or preparation of the manuscript.
A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
Disclosure Summary: None of the authors has a conflict of interest.
Footnotes
- CI
- Confidence interval
- hGH
- human GH
- IGFBP
- IGF binding protein.
References
- 1. Le Roith D. 1997. Seminars in medicine of the Beth Israel Deaconess Medical Center. Insulin-like growth factors. N Engl J Med 336:633–640 [DOI] [PubMed] [Google Scholar]
- 2. Renehan AG, Zwahlen M, Minder C, O'Dwyer ST, Shalet SM, Egger M. 2004. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet 363:1346–1353 [DOI] [PubMed] [Google Scholar]
- 3. Vasan RS, Sullivan LM, D'Agostino RB, Roubenoff R, Harris T, Sawyer DB, Levy D, Wilson PW. 2003. Serum insulin-like growth factor I and risk for heart failure in elderly individuals without a previous myocardial infarction: the Framingham Heart Study. Ann Intern Med 139:642–648 [DOI] [PubMed] [Google Scholar]
- 4. Kaplan RC, McGinn AP, Pollak MN, Kuller L, Strickler HD, Rohan TE, Cappola AR, Xue X, Psaty BM. 2008. High insulinlike growth factor binding protein 1 level predicts incident congestive heart failure in the elderly. Am Heart J 155:1006–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Niedernhofer LJ, Garinis GA, Raams A, Lalai AS, Robinson AR, Appeldoorn E, Odijk H, Oostendorp R, Ahmad A, van Leeuwen W, Theil AF, Vermeulen W, van der Horst GT, Meinecke P, Kleijer WJ, Vijg J, Jaspers NG, Hoeijmakers JH. 2006. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature 444:1038–1043 [DOI] [PubMed] [Google Scholar]
- 6. Hilding A, Brismar K, Degerblad M, Thorén M, Hall K. 1995. Altered relation between circulating levels of insulin-like growth factor-binding protein-1 and insulin in growth hormone-deficient patients and insulin-dependent diabetic patients compared to that in healthy subjects. J Clin Endocrinol Metab 80:2646–2652 [DOI] [PubMed] [Google Scholar]
- 7. Liu H, Bravata DM, Olkin I, Nayak S, Roberts B, Garber AM, Hoffman AR. 2007. Systematic review: the safety and efficacy of growth hormone in the healthy elderly. Ann Intern Med 146:104–115 [DOI] [PubMed] [Google Scholar]
- 8. Papadakis MA, Grady D, Black D, Tierney MJ, Gooding GA, Schambelan M, Grunfeld C. 1996. Growth hormone replacement in healthy older men improves body composition but not functional ability. Ann Intern Med 124:708–716 [DOI] [PubMed] [Google Scholar]
- 9. Blackman MR, Sorkin JD, Münzer T, Bellantoni MF, Busby-Whitehead J, Stevens TE, Jayme J, O'Connor KG, Christmas C, Tobin JD, Stewart KJ, Cottrell E, St Clair C, Pabst KM, Harman SM. 2002. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA 288:2282–2292 [DOI] [PubMed] [Google Scholar]
- 10. Nass R, Pezzoli SS, Oliveri MC, Patrie JT, Harrell FE, Jr, Clasey JL, Heymsfield SB, Bach MA, Vance ML, Thorner MO. 2008. Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized trial. Ann Intern Med 149:601–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Perls TT, Reisman NR, Olshansky SJ. 2005. Provision or distribution of growth hormone for “antiaging”: clinical and legal issues. JAMA 294:2086–2090 [DOI] [PubMed] [Google Scholar]
- 12. Kaplan RC, McGinn AP, Pollak MN, Kuller L, Strickler HD, Rohan TE, Xue X, Kritchevsky SB, Newman AB, Psaty BM. 2008. Total insulinlike growth factor 1 and insulinlike growth factor binding protein levels, functional status, and mortality in older adults. J Am Geriatr Soc 56:652–660 [DOI] [PubMed] [Google Scholar]
- 13. Hu D, Pawlikowska L, Kanaya A, Hsueh WC, Colbert L, Newman AB, Satterfield S, Rosen C, Cummings SR, Harris TB, Ziv E. 2009. Serum insulin-like growth factor-1 binding proteins 1 and 2 and mortality in older adults: the Health, Aging, and Body Composition Study. J Am Geriatr Soc 57:1213–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brugts MP, van den Beld AW, Hofland LJ, van der Wansem K, van Koetsveld PM, Frystyk J, Lamberts SW, Janssen JA. 2008. Low circulating insulin-like growth factor I bioactivity in elderly men is associated with increased mortality. J Clin Endocrinol Metab 93:2515–2522 [DOI] [PubMed] [Google Scholar]
- 15. van Bunderen CC, van Nieuwpoort IC, van Schoor NM, Deeg DJ, Lips P, Drent ML. 2010. The association of serum insulin-like growth factor-I with mortality, cardiovascular disease, and cancer in the elderly: a population-based study. J Clin Endocrinol Metab 95:4616–4624 [DOI] [PubMed] [Google Scholar]
- 16. Friedrich N, Haring R, Nauck M, Lüdemann J, Rosskopf D, Spilcke-Liss E, Felix SB, Dörr M, Brabant G, Völzke H, Wallaschofski H. 2009. Mortality and serum insulin-like growth factor (IGF)-I and IGF binding protein 3 concentrations. J Clin Endocrinol Metab 94:1732–1739 [DOI] [PubMed] [Google Scholar]
- 17. Laughlin GA, Barrett-Connor E, Criqui MH, Kritz-Silverstein D. 2004. The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: the Rancho Bernardo Study. J Clin Endocrinol Metab 89:114–120 [DOI] [PubMed] [Google Scholar]
- 18. Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. 1995. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem 41:264–270 [PubMed] [Google Scholar]
- 19. Domené HM, Bengolea SV, Martínez AS, Ropelato MG, Pennisi P, Scaglia P, Heinrich JJ, Jasper HG. 2004. Deficiency of the circulating insulin-like growth factor system associated with inactivation of the acid-labile subunit gene. N Engl J Med 350:570–577 [DOI] [PubMed] [Google Scholar]
- 20. Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P. 2008. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci USA 105:3438–3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Samstein B, Hoimes ML, Fan J, Frost RA, Gelato MC, Lang CH. 1996. IL-6 stimulation of insulin-like growth factor binding protein (IGFBP)-1 production. Biochem Biophys Res Commun 228:611–615 [DOI] [PubMed] [Google Scholar]
- 22. Lang CH, Vary TC, Frost RA. 2003. Acute in vivo elevation of insulin-like growth factor (IGF) binding protein-1 decreases plasma free IGF-I and muscle protein synthesis. Endocrinology 144:3922–3933 [DOI] [PubMed] [Google Scholar]
- 23. Leu JI, George DL. 2007. Hepatic IGFBP1 is a prosurvival factor that binds to BAK, protects the liver from apoptosis, and antagonizes the proapoptotic actions of p53 at mitochondria. Genes Dev 21:3095–3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Newman AB, Sachs MC, Arnold AM, Fried LP, Kronmal R, Cushman M, Psaty BM, Harris TB, Robbins JA, Burke GL, Kuller LH, Lumley T. 2009. Total and cause-specific mortality in the cardiovascular health study. J Gerontol A Biol Sci Med Sci 64:1251–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Swerdlow AJ, Higgins CD, Adlard P, Preece MA. 2002. Risk of cancer in patients treated with human pituitary growth hormone in the UK, 1959–85: a cohort study. Lancet 360:273–277 [DOI] [PubMed] [Google Scholar]


