Abstract
Objectives
The purpose of this study was to: 1) investigate the feasibility of performing mucosal elevation of a vocal fold microflap in a rabbit model, and 2) measure the acute healing of rabbit microflap incisions compared to control vocal folds.
Study Design
Prospective animal study
Methods
Ten New Zealand white rabbits were used in this study. All rabbits received a 3mm incision through the epithelium of one vocal fold using a sickle knife and mucosal elevation through this incision using a microlaryngeal fine angled spatula. The contralateral vocal fold was left intact to serve as an internal control. Student t tests were used to investigate differences in epithelial thickness, immunohistochemical staining of CD 45, and inflammatory and pro-fibrotic gene expression between vocal folds undergoing microflap and control.
Results
Exposure of the rabbit larynx was achieved, allowing for the identification of a surgical plane and the creation of a microflap and elevation of the vocal fold mucosa. Hematoxylin-eosin staining revealed no significant differences in epithelial thickness, immunohistochemistry for CD 45 showed no significant differences in CD 45 positive cells, and quantitative PCR revealed no significant differences in IL-1β, TGFβ-1, or COX-2 gene expression between vocal folds undergoing microflap and control.
Conclusions
We demonstrate the feasibility of vocal fold microflap surgery in a rabbit model. With the advantage of greater access to primers and antibodies for molecular biological studies, the application of the microflap technique in a small animal model such as rabbit has broad implications for future experimental investigations in laryngology.
Level of Evidence
Animal Research.
Keywords: microflap, vocal fold, rabbit, wound healing, gene expression
INTRODUCTION
Optimal post-operative voice outcomes are dependent on preservation of the normal structure of the vocal fold lamina propria. Minimal disruption of the epithelium and surrounding tissues is critical to minimizing the inflammatory response and scar formation.1 The microflap is a well-established technique used in the surgical management of benign vocal fold lesions (e.g. vocal fold polyp.). Studies have demonstrated the usefulness of the microflap technique in preserving the vocal fold mucosa and yielding exceptional voice outcomes after phonomicrosurgery. 2, 3 Experimental studies have been performed in large animals such as canine, swine, monkey, and sheep using the microflap technique to investigate the effects of various interventions on post-operative wound healing outcomes.4–8 However, the availability of primers and antibodies for molecular biological assays in these animals is limited.
The application of the microflap technique in a small animal such as rabbit poses challenges due to the small size of the larynx, but also a unique opportunity with the advantage of greater access to primers and antibodies for molecular biology experiments. The development of in-vivo phonation procedures in rabbits also provides a unique opportunity to investigate the effects of phonation on microflap healing at the molecular level. 9, 10 For example, post-operative voice rest is frequently used in patients following microflap for benign vocal fold lesions; however, the type of voice rest, (i.e. absolute versus relative), and the duration of voice rest (i.e. 3, 7, 14 days) used to manage patients remains controversial. Thus, biochemical investigations are needed and would provide an extremely valuable approach to determine the basis for early versus delayed tissue mobilization after vocal fold microflap procedures. Because the vocal folds are a highly-specialized tissue, the effects of mobilization and wound healing principles from other biological tissues may not be applicable. To address these questions, our laboratory has developed an in vivo rabbit phonation model to investigate the effects of phonation on vocal fold tissue repair, however, the feasibility of the vocal fold microflap technique in the rabbit has yet to be demonstrated. 9–12
Therefore, the purpose of the present study was to: 1) Demonstrate the feasibility of performing mucosal elevation through vocal fold microflap incisions in a rabbit model, 2) Measure the thickness of the epithelium in vocal folds undergoing microflap compared to control vocal folds, and 3) Investigate the acute inflammatory response using immunohistochemistry to detect the presence of CD 45 positive cells and quantitative polymerase chain reaction for inflammatory and pro-fibrotic gene expression (IL-1β, TGFβ-1, and COX-2). If successful, the use of the microflap technique in animals undergoing in vivo phonation procedures will provide a unique opportunity to investigate the effects of phonation on microflap healing in future studies.
MATERIALS AND METHODS
Animals
Ten New Zealand white breeder rabbits weighing 3 to 5 kg were used in this study. Five of the ten rabbits were used for immunohistochemical and histological procedures and the remaining five were used for gene expression assays. This study was performed in accordance with the PHS Policy on Humane Care and Use of Laboratory Animals, the NIH Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act (7 U.S.C. et seq.); the animal use protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Vanderbilt University Medical Center.
Surgical Procedure
The rabbits were anesthetized using intramuscular injections of Ketamine (35mg/kg), Xylazine (5mg/kg), and Acepromazine (0.75mg/kg). Heart rate, body temperature, and oxygen saturation levels were monitored throughout the experiment to monitor the animal’s state of anesthesia and general well-being. Transoral direct suspension microlaryngoscopy was performed using an 11 cm Holinger-Tucker pediatric anterior commissure side-slotted laryngoscope (Karl Storz Endoscopy America, Inc., El Segundo, CA.) (Figure 1). Video documentation of the surgical procedures was accomplished using a 30° 2.7mm rigid endoscope (Karl Storz Endoscopy, Endoscopy–America, Inc., El Segundo, CA) coupled to a Telecam-C camera (Karl Storz Endoscopy, Endoscopy–America, Inc., El Segundo, CA). A 3mm incision was made through the epithelium and mucosal cover of one vocal fold and elevation was performed in an easily located surgical plane (Figure 2). The flap was thin enough that the microlaryngeal fine angled spatula could be visualized through the mucosa. The contralateral vocal fold was left intact to serve as an internal control.
Fig 1.
Representative photograph of transoral direct suspension microlaryngoscopy performed in rabbit.
Fig 2.
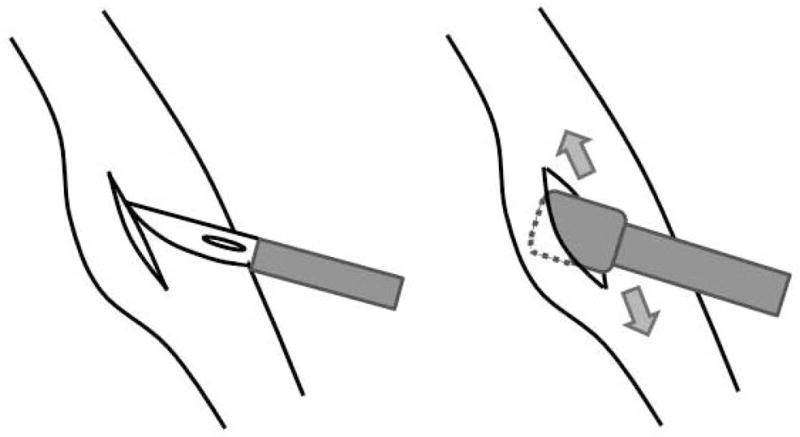
Schematic of microflap procedure showing an incision being made through the epithelium and mucosal cover of one vocal fold and elevation being performed using a microlaryngeal fine angled spatula.
Histological Analysis
Five of the ten rabbits were euthanized two weeks after the surgical procedure. Whole larynges were harvested from animals following an overdose intracardiac injection of pentobarbital sodium (150 mg/kg). The specimens were soaked in 15% sucrose solution for 3 hours and in 30% Sucrose solution for 18 hours at 4°C for dehydration. Subsequently, the larynges were transferred into embedding medium (OCT compound, Tissue-Tek, Torrance, CA), and frozen quickly using a combination of acetone and dry ice, and stored in a deep freezer (−80°C) for later analysis. In preparation for histology, 10-μm-thick frozen sections were made using a cryostat (Leica, Bannockburn, IL). To investigate the degree of epithelial hypertrophy, thickness of the epithelium was measured using Hematoxylin-Eosin (H & E) staining. Immunohistochemical staining for CD 45 was performed to investigate the inflammatory response. After performing the blocking procedure for 1 hour at room temperature, sections were incubated in primary antibody solution for 18 hours at 4°C. The primary antibody used in this study was mouse anti-CD45 (Santa Cruz, Santa Cruz, CA) at 1:100. After washing, sections were incubated in the secondary antibody for 1 hour at room temperature. The secondary antibody used in this study was DyLight594 conjugated anti-mouse goat antibody (Abcam, Cambridge, MA) at 1:150. 20ng/μl of DAPI was used for nuclear staining. After washing, the sections were mounted on coverslips in Vectashield (Vector Laboratories, Burlingame, CA). Image capturing was performed using a NIKON Eclipse 90i microscope (NIKON, Tokyo, Japan). Image analysis was performed using NIS-Elements software (NIKON, Tokyo, Japan). Intensity of Dylight594 in each section was examined and measurements were made between the microflap and control vocal fold.
Gene Expression Analysis
The remaining five rabbits were used to investigate inflammatory and pro-fibrotic gene expression in microflap and control vocal folds. Vocal folds were dissected away from the larynx and immediately stabilized in RNAlater (Ambion, Austin, TX). An ultrasonic homogenizer (Fisher Scientific, Pittsburgh, PA) was used to homogenize the vocal fold tissue. Total RNA was extracted using an RNeasy Mini Kit (Qiagen, Valencia, CA) and treated with deoxyribonuclease I (Qiagen, Valencia, CA) to minimize contamination from genomic DNA. The quantity of total RNA was determined using the A260/A280 ratio and electrophoresis was used to evaluate the quantity based on the appearance of the 18S and 28S ribosomal RNA bands. Reverse transcription was performed using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) per the manufacturer’s recommended reaction protocol. Reactions were performed using a Veriti Thermal Cycler (Applied Biosystems) with the following parameters: 25°C for 10 minutes, 37°C for 120 minutes, and 85°C for 5 minutes. Primers for IL-1β, TGFβ-1, COX-2, and glyceraldehydes-3-phosphate dehydrogenase (GAPDH) were synthesized by Sigma-Aldrich Corp. (St. Louis, MO). Sequences of the primers are shown in Table I. Real-time polymerase chain reaction was performed in a final volume of 20 μl reaction mixture composed of 2 μl of template complementary DNA, 0.9 μl of forward primer (20pmol/μl), 0.9 μl of reverse primer (20 pmol/μl), 10ml of Power SYBR Green PCR Master Mix (Applied Biosystems, Carlsbad, CA.), and 6.2 ml of ribonuclease-free water. The following protocol was used for real-time PCR: 95°C for 10 minutes, 40 cycles at 95°C for 15 seconds, and 60°C for 1 minute. Fluorescence was detected using a StepOnePlus Real-time PCR System (Applied Biosystems, Carlsbad, CA.). The 33 Ct method was used to determine the relative ratio of gene expression. Target gene ratios from each sample were normalized using expression ratios of the internal control gene (GAPDH). Normalized gene expression ratios were then compared between the microflap and control vocal folds.
Table I.
Primer Sequences
| Primer Sequences (5′-3′) | ||
|---|---|---|
| IL-1β | Forward Reverse |
GGA TAT GGA GCA ACA AGT GG ATG TAC CAG TTG GGG AAC TG |
| TGF-β1 | Forward Reverse |
GGG ACT ATC CAC CTG CAA GA CTC CTT GGC GTA GTA GTC GG |
| COX-2 | Forward Reverse |
GAA TCA TTC ACC AGG CAA ATT G TCT GTA CTG CGG GTG GAA CA |
| GAPDH | Forward Reverse |
ACC ACA GTC CAT GCC ATC AC TCC ACC ACC CTG TTG CTG TA |
Statistical Analysis
Student t tests were used to investigate differences in epithelial thickness and intensity of CD 45 immunohistochemical staining between microflap and control vocal folds. An adjusted alpha level of 0.025 (0.05/2) was used to control for type I error. For analysis of gene expression, Student t tests were used to investigate differences in IL-1β, TGFβ-1, and COX-2 between the microflap and control vocal fold. An adjusted alpha level of 0.017 (0.05/3) was used to control for type I error.
RESULTS
Instruments and Procedures
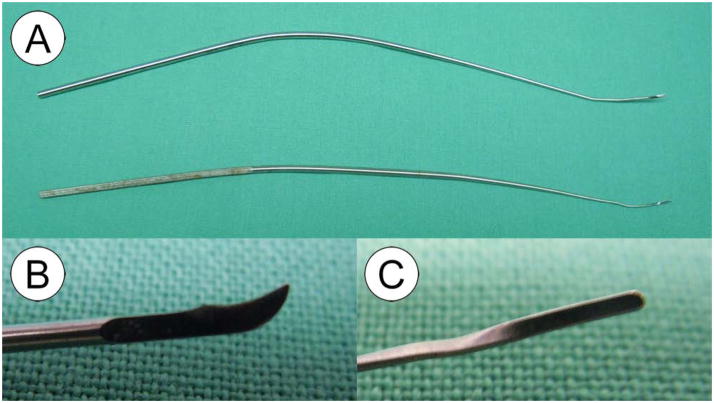
In order to obtain adequate visualization and to provide sufficient working space for the introduction of microlaryngeal instruments, a side-slotted pediatric laryngoscope with a wide distal aspect was used to allow direct suspension microlaryngoscopy (Holinger-Tucker 8574B, Karl Storz Endoscopy America, Inc., El Segundo, CA.) (Figure 3). Examination of the larynx was performed using a 30 degree 2.7mm operating telescope (Karl Storz Endoscopy, Endoscopy–America, Inc., El Segundo, CA). An incision was created through the epithelium of the vocal fold using a 3mm curved blade sickle knife (Pilling, Durham, NC) under direct endoscopic vision. Elevation of the mucosal cover was then performed using a microlaryngeal fine angled spatula (Instrumentarium, Terrebonne, Canada). The elevator easily fell into a surgical plane within the lamina propria. The instrument handles were bent to provide sufficient working space for the introduction of both the microsurgical instruments and the operating telescope into the narrow aperture of the Holinger-Tucker pediatric laryngoscope (Figure 4).
Fig 3.
Holinger-Tucker pediatric anterior commissure side-slotted laryngoscope.
Fig 4.
Curved blade sickle-knife (A/B) and microlaryngeal fine angled spatula (A/C).
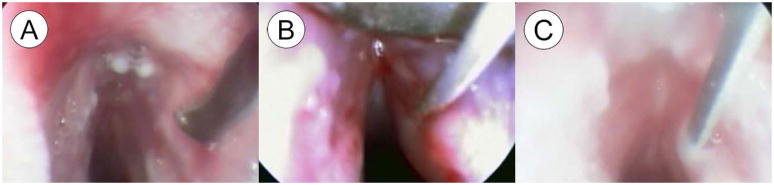
Figure 5(A–C) shows the endoscopic findings during creation of the vocal fold microflap. The curved blade sickle knife was used to create a 3 mm superficial incision along the medial aspect of the vocal fold (Figure 5AB) and the mucosa was elevated using the microlaryngeal fine angled spatula (Fig 5C). The flap was redraped and allowed to heal by secondary intention without the need for suture or glue fixation. Procedure duration was less than 10 minutes.
Fig 5.
Representative endoscopic images of microflap procedure showing: (A) Introduction of the curved blade sickle knife, (B) A 3 mm incision being made along the medial aspect of the vocal fold, and (C) The mucosa being elevated using a microlaryngeal fine angled spatula.
Histological Analysis
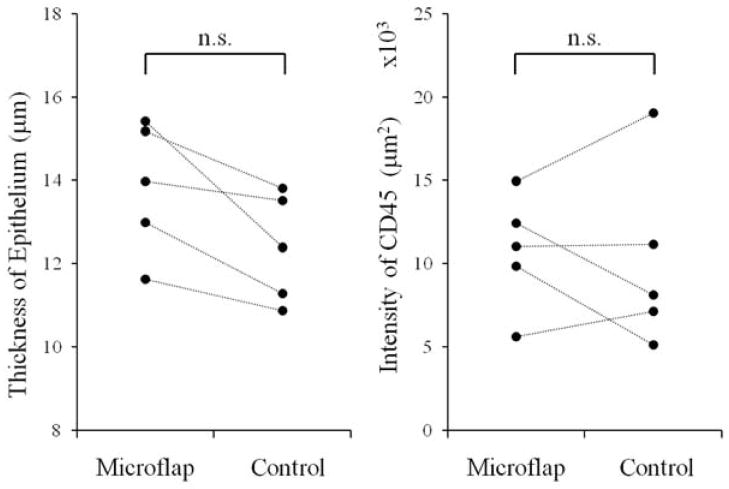
Figure 6A shows a representative H & E stained section of a vocal fold after microflap. Incision lines were undetectable in all sections. The thickness of the epithelium was measured in each section (Figure 6B). The epithelial thickness was 13.84 μm (S.D. ±1.57 μm) for vocal folds undergoing microflap and 12.37 μm (S.D. ±1.30 μm) for control vocal folds. Figure 6C shows a representative immunohistochemical stained section for CD45. Intensity of Dylight594 conjugated to CD45 was 10.77 ×103 (S.D. ±3.46 ×103) for vocal folds undergoing microflap and 10.12 ×103 (S.D. ±5.45 ×103) for the control vocal folds. There were no significant differences in epithelial thickness or CD45 staining between the microflap and control vocal folds (p>0.025) (Figure 7).
Fig 6.
(A) H & E stained coronal section of a vocal fold after microflap (bar represents 250μm), (B) Magnified image of vocal fold epithelium (bar represents 25μm), (C) Immunohistochemical staining of CD45. Red intensity (DyLight594) represents CD45 positive cells. Blue counterstain (DAPI) represents staining of cell nuclei.
Fig 7.
Epithelial thickness and CD45 staining between vocal folds undergoing microflap and control vocal folds.
Gene expression Analyses
Figure 8 shows the result of gene expression analysis for IL-1β, TGFβ-1 and COX-2. The gene expression ratio of IL-1β normalized by GAPDH was 0.314 (S.D. ±0.404) in vocal folds undergoing microflap and 0.242 (S.D. ±0.349) in control vocal folds. The gene expression ratio of TGFβ-1 normalized by GAPDH was 0.696 (S.D. ±0.254) in vocal folds undergoing microflap and 0.463 (S.D. ±0.123) in control vocal folds. The gene expression ratio of COX-2 normalized by GAPDH was 0.647 (S.D. ±0.389) in vocal folds undergoing microflap and 0.348 (S.D. ±0.252) in control vocal folds. Statistical analysis revealed no significant differences in inflammatory and pro-fibrotic gene expression between vocal folds undergoing microflap and control (p>0.017).
Fig 8.
Normalized gene expression ratios for IL-1β, TGFβ-1 and COX-2 between vocal folds undergoing microflap and control vocal folds.
DISCUSSION
Over the past three decades, basic science and clinical research has provided important information regarding the importance of the structure of the vocal fold, the etiology of vocal fold lesions, and improved treatment strategies for benign vocal fold disease. It is generally accepted that the quality of the voice after phonomicrosurgery is very much dependent on preservation of normal vocal fold microarchitecture. The goal of phonomicrosurgery is to take advantage of the vocal fold’s natural histologic planes to carefully remove vocal fold disease with minimal disruption to vibratory mechanics.3 The microflap technique is a well-established method used in the surgical management of benign vocal fold lesions. Because dissection within the vocal ligament is avoided, inflammation and scar formation is minimized.
Animal models have been used to investigate the effects of various types of interventions on post-operative microflap healing. These studies have been performed in larger animals, such as canine, swine, monkey, and sheep. While the size of the larynx in these animals makes the procedure of creating a microflap much easier than performing the same in small animals, animal procurement and maintenance costs are often prohibitive, and it is increasingly difficult to find the primers and antibodies necessary for performing common molecular biological assays. While the application of the microflap technique in rabbits has the potential to obviate the aforementioned challenges, the microflap technique has yet to be performed in a small animal. Thus, the purpose of the present study was two-fold: 1) to investigate the feasibility of performing mucosal elevation of a vocal fold microflap in a rabbit model, and 2) to investigate the acute healing of rabbit vocal fold microflap incisions.
Results of the present study revealed that adequate exposure of the larynx, visualization of the vocal folds, and suitable working space for the introduction of a 30° 2.7mm rigid endoscope and microlaryngeal instruments necessary for the creation of a microflap and undermining of the mucosa is feasible using an 11 cm Holinger-Tucker pediatric side-slotted laryngoscope. We found the wide distal aspect of the Holinger-Tucker laryngoscope useful for pinning back the epiglottis during the procedure, allowing for optimal visualization of the anterior commissure. The passage of a 3mm curved blade sickle knife and microlaryngeal fine angled spatula was introduced through the side-slotted channel of the laryngoscope. The instrument handles were bent as the narrow aperture of the pediatric laryngoscope makes it quite challenging to maneuver a surgical instrument and the operating telescope in the rabbit larynx at the same time. Attempts were made to do this with an operating microscope to establish binocular visualization for surgery, but due to the small size of the rabbit larynx and laryngoscope, binocular visualization was not possible with any instrument in the larynx.
The acute healing of vocal fold microflap incisions was investigated for the presence of CD45 positive inflammatory cells in microflap and control vocal folds using routine immunohistochemistry, inflammatory and pro-fibrotic gene expression for interleukin-1beta (IL-1β), transforming growth factor beta-1 (TGFβ-1), and cyclooxygenase-2 (COX-2) using quantitative polymerase chain reaction, and epithelial hypertrophy using measurements of epithelial thickness from H&E stained microflap and control vocal folds. Results of immunohistochemistry and gene expression analysis revealed no significant differences in the presence of CD45 positive inflammatory cells and IL-1β, TGFβ-1, or COX-2 inflammatory and pro-fibrotic gene expression in vocal folds undergoing microflap versus control vocal folds. While no differences in the IL-1β, TGFβ-1, and COX-2 dependent measures were found, the expression ratios for these three genes in the microflap observations was generally higher than the same in the control observations. Thus, the possibility of finding these differences to be significant in a larger number of microflap and control observations cannot be ruled out, given the small number of microflap and control observations in the present study and the strict alpha level used to control for type I error [adjusted alpha level of 0.017 (0.05/3)].
H&E staining revealed that the presence of microflap incisions was undetectable two-weeks after surgery and thickness of the epithelium was preserved with no significant epithelial hypertrophy in vocal folds undergoing microflap compared to control. In contrast to microflap incisions, it has been shown that injury by microcup forceps, another common form of injury used in animal wound healing investigations, results in a significant and immediate inflammatory response with corresponding changes in extracellular matrix production and epithelial hypertrophy. 13–17 In the microcup forcep injury procedure, penetration of the injury is much deeper and involves denuding the epithelium and lamina propria along the entire length of the membranous vocal fold. Accordingly, differences in the healing response between these two types of injury are thought to be related to the extent and involvement of the deeper layers of the lamina propria and vocal ligament. These investigations have been extremely valuable in providing fundamental information regarding the process of vocal fold injury and repair. Yet, information regarding the cellular and molecular events underlying modern-day phonomicrosurgical procedures, such as the microflap technique in animal wound healing studies has received less attention. The application of the microflap technique in a small animal is certainly challenging, but the potential benefits of creating a small animal microflap model are significant, and may provide an opportunity to investigate the wound healing and repair process using techniques that are germane to modern-day phonomicrosurgery. While there are likely to be some differences in the healing response between rabbits and humans secondary to phonatory forces and variations in tissue layered structure, with the advantage of greater access to primers and antibodies for molecular biological assays relative to larger animal models (e.g. canine, pig, sheep), the application of the microflap technique in a small animal model such as rabbit will have broad implications for future experimental investigations in laryngology. With feasibility of the microflap technique now demonstrated in rabbits, it will be possible in future studies to systematically investigate the effects of experimentally induced phonation on acute microflap healing, using the in vivo rabbit phonation model described in previous studies. 9–12
CONCLUSIONS
We demonstrate the feasibility of mucosal elevation and the creation of microflap incisions in a rabbit model. Adequate exposure of the larynx can be achieved using an 11cm pediatric laryngoscope and 2.7mm 30 degree endoscope. With adequate exposure and access to the vocal folds, a 3mm curved blade sickle knife and microlaryngeal fine angled spatula can be used to perform an incision through the epithelium of the vocal fold and mucosal elevation can be performed through this microflap incision. The microflap is redraped and allowed to heal by secondary intention without the need for sutures and/or glue to close the defect. Microflap incisions are undetectable two-weeks after surgery and thickness of the epithelium is preserved with no evidence of inflammation as measured using immunohistochemistry for CD45 positive inflammatory cells and IL-1β, TGFβ-1, and COX-2 inflammatory and pro-fibrotic gene expression.
Acknowledgments
Research supported by NIH grants R03 DC 008400 and R01 DC 011338 from the National Institute of Deafness and other Communication Disorders.
Footnotes
Conflicts of Interest: None
References
- 1.Courey MS, Shohet JA, Scott MA, Ossoff RH. Immunohistochemical characterization of benign laryngeal lesions. Ann Otol Rhinol Laryngol. 1996 Jul;105(7):525–531. doi: 10.1177/000348949610500706. [DOI] [PubMed] [Google Scholar]
- 2.Courey MS, Gardner GM, Stone RE, Ossoff RH. Endoscopic vocal fold microflap: a three-year experience. Ann Otol Rhinol Laryngol. 1995 Apr;104(4 Pt 1):267–273. doi: 10.1177/000348949510400402. [DOI] [PubMed] [Google Scholar]
- 3.Courey MS, Garrett CG, Ossoff RH. Medial microflap for excision of benign vocal fold lesions. Laryngoscope. 1997 Mar;107(3):340–344. doi: 10.1097/00005537-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Fleming DJ, McGuff S, Simpson CB. Comparison of microflap healing outcomes with traditional and microsuturing techniques: initial results in a canine model. Ann Otol Rhinol Laryngol. 2001 Aug;110(8):707–712. doi: 10.1177/000348940111000802. [DOI] [PubMed] [Google Scholar]
- 5.Garrett CG, Coleman JR, Reinisch L. Comparative histology and vibration of the vocal folds: implications for experimental studies in microlaryngeal surgery. Laryngoscope. 2000 May;110(5 Pt 1):814–824. doi: 10.1097/00005537-200005000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Garrett CG, Soto J, Riddick J, Billante CR, Reinisch L. Effect of mitomycin-C on vocal fold healing in a canine model. Ann Otol Rhinol Laryngol. 2001 Jan;110(1):25–30. doi: 10.1177/000348940111000105. [DOI] [PubMed] [Google Scholar]
- 7.Coleman JR, Jr, Smith S, Reinisch L, et al. Histomorphometric and laryngeal videostroboscopic analysis of the effects of corticosteroids on microflap healing in the dog larynx. Ann Otol Rhinol Laryngol. 1999 Feb;108(2):119–127. doi: 10.1177/000348949910800203. [DOI] [PubMed] [Google Scholar]
- 8.Franco RA, Dowdall JR, Bujold K, et al. Photochemical repair of vocal fold microflap defects. Laryngoscope. 2011 Jun;121(6):1244–1251. doi: 10.1002/lary.21797. [DOI] [PubMed] [Google Scholar]
- 9.Rousseau B, Ge P, French LC, Zealear DL, Thibeault SL, Ossoff RH. Experimentally induced phonation increases matrix metalloproteinase-1 gene expression in normal rabbit vocal fold. Otolaryngol Head Neck Surg. 2008;138:62–68. doi: 10.1016/j.otohns.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swanson ER, Ohno T, Abdollahian D, Garrett CG, Rousseau B. Effects of raised-intensity phonation on inflammatory mediator gene expression in normal rabbit vocal fold. Otolaryngol Head Neck Surg. 2010 Oct;143(4):567–572. doi: 10.1016/j.otohns.2010.04.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ge PJ, French LC, Ohno T, Zealear DL, Rousseau B. Model of evoked rabbit phonation. Ann Otol Rhinol Laryngol. 2009 Jan;118(1):51–55. doi: 10.1177/000348940911800109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swanson ER, Abdollahian D, Ohno T, Ge P, Zealear DL, Rousseau B. Characterization of raised phonation in an evoked rabbit phonation model. Laryngoscope. 2009 Jul;119(7):1439–1443. doi: 10.1002/lary.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welham NV, Lim X, Tateya I, Bless DM. Inflammatory Factor Profiles One Hour Following Vocal Fold Injury. Ann Otol Rhinol Laryngol. 2008;117(2):145–152. doi: 10.1177/000348940811700213. [DOI] [PubMed] [Google Scholar]
- 14.Lim X, Tateya I, Tateya T, Munoz-Del-Rio A, Bless DM. Immediate inflammatory response and scar formation in wounded vocal folds. Ann Otol Rhinol Laryngol. 2006 Dec;115(12):921–929. doi: 10.1177/000348940611501212. [DOI] [PubMed] [Google Scholar]
- 15.Branski RC, Rosen CA, Verdolini K, Hebda PA. Acute vocal fold wound healing in a rabbit model. Ann Otol Rhinol Laryngol. 2005 Jan;114(1 Pt 1):19–24. doi: 10.1177/000348940511400105. [DOI] [PubMed] [Google Scholar]
- 16.Ohno T, Hirano S, Rousseau B. Extracellular matrix gene expression during wound healing of the injured rat vocal fold. Otolaryngol Head Neck Surg. 2009 May;140(5):757–761. doi: 10.1016/j.otohns.2008.12.065. [DOI] [PubMed] [Google Scholar]
- 17.Rousseau B, Ge PJ, Ohno T, French LC, Thibeault SL. Extracellular matrix gene expression after vocal fold injury in a rabbit model. Ann Otol Rhinol Laryngol. 2008 Aug;117(8):598–603. doi: 10.1177/000348940811700809. [DOI] [PMC free article] [PubMed] [Google Scholar]