Abstract
Tuberculous pericarditis is an exceedingly rare but a well-described extra-pulmonary manifestation of tuberculosis (TB) infection in Hong Kong. An 82-year-old woman with a known history of diabetes mellitus, hypertension and hyperlipidaemia was admitted for congestive heart failure. Routine echocardiographic study during admission revealed a massive pericardial effusion (~4 cm in thickness) but with no tamponade effect. Pericardiocentesis was performed and 1.6 L of heavily blood stained fluid was drained. All tumour and auto-immune markers were unremarkable. A whole body positron emission tomography-computer tomography (PET-CT) scan was then performed and showed an increased fluorodeoxyglucose uptake in the entire pericardium with no sources of possible malignancy identified. Rapid acid fast bacilli culture and Genprobe examination of the pericardial fluid then demonstrated the growth of Mycobacterium tuberculosis. She was started on anti-TB medications and tolerated them well. Follow-up echocardiographic study showed no re-accumulation of pericardial fluid.
Background
Tuberculosis (TB) is an important airborne infectious disease causing an epidemic worldwide. There were around 9.4 million new or relapse TB cases diagnosed in 2009.1 An estimated 1.7 million deaths were caused by TB, 0.38 million of those were HIV infected.
Hong Kong is a TB endemic region. As compared with other developed countries, the rate of TB in Hong Kong is about seven times higher. According to the Centre for Health Protection of the Department of Health in Hong Kong, 5132 cases of TB in all forms were notified in 2010 with a notification rate and death rate of 72.61 and 2.7 per 100 000 population, respectively.2 Extra-pulmonary TB accounted for 15% of the cases3 and can affect any organ systems. There are no available data in Hong Kong on the prevalence of tuberculous pericarditis. In developed countries, TB accounts for 4% of cases of acute pericarditis.4 As for developing countries such as Africa, tuberculous pericarditis is the commonest cause of pericardial effusion accounting for 69.5% of patients referred for diagnostic pericardiocentesis in one case series.5 This case hopes to illustrate the importance of recognising and diagnosing tuberculous pericarditis in a TB endemic region such as Hong Kong and the need to be aware of atypical presentations.
Case presentation
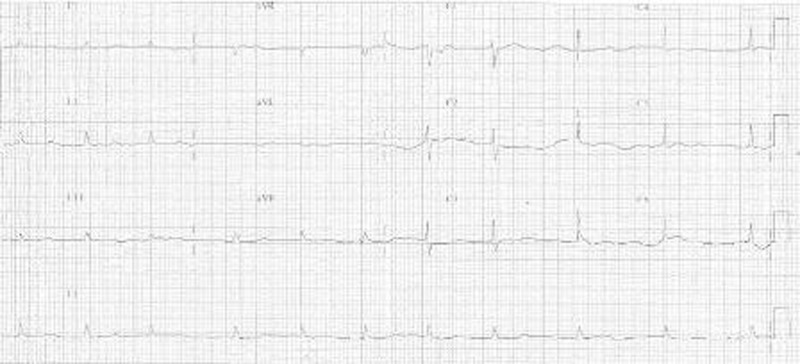
An 82-year-old physically active lady presented to our medical clinic on 18 July 2011 for progressively worsening shortness of breath on exertion and bilateral ankle oedema. She has known history of hypertension, diabetes mellitus and hyperlipidaemia and was seen regularly in our clinic. She did not complain of chest pain. Clinical examination confirmed the diagnosis of heart failure and she was hence admitted into the general medical ward. Preliminary blood tests were unremarkable (table 1) except for mild iron deficiency anaemia with haemoglobin level of 10 g/dl. Serial troponin I testing was negative. ECG showed sinus rhythm of 53/min with low voltage, no acute ST-T wave changes were noted (figure 1). Chest x-ray showed cardiomegaly, pulmonary congestion and bilateral pleural effusion (figure 2).
Table 1.
Laboratory values
| Variable | Value | Normal range |
|---|---|---|
| Haemoglobin (g/dl) | 10 | 12–15 |
| Creatinine (µmol/l) | 120 | 53–105 |
| Erythrocyte sedimentation rate (mm/h) | >130 | <35 |
| C reactive protein (mg/l) | 50 | <8 |
| D-dimer (ng/ml FEU) | 2339 | <500 |
| Thyroid stimulating hormone (mIU/l) | 1.10 | 0.29–4.00 |
| Autoimmune markers | ||
| Antinuclear antibodies | Negative | <80 |
| Rheumatoid factor | Negative | |
| Complement 3 (g/l) | 1.75 | 0.79–1.52 |
| Complement 4 | Normal | |
| Immunoglobulin G, A, M | Normal | |
| Tumour markers | ||
| α-fetal protein | 3 | <20 |
| Carcinoembryonic antigen (ug/l) | 0.9 | <3 – non-smoker |
| Cancer antigen 125 (kU/l) | 316 | <35 |
Figure 1.
ECG upon admission showing low voltage QRS complexes.
Figure 2.
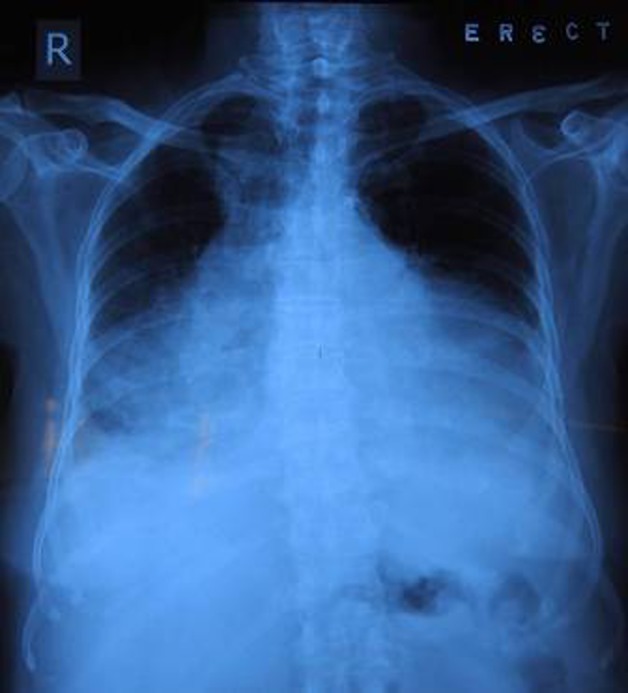
Chest radiograph upon admission showing cardiomegaly, pulmonary congestion and bilateral pleural effusion.
Despite initiation of anti-heart failure treatment including intravenous frusemide and oxygen therapy on the patient, her condition deteriorated. She was subsequently transferred to the special care unit for acute pulmonary oedema where non-invasive positive pressure ventilation (NIPPV) was given. She later stabilised and improved.
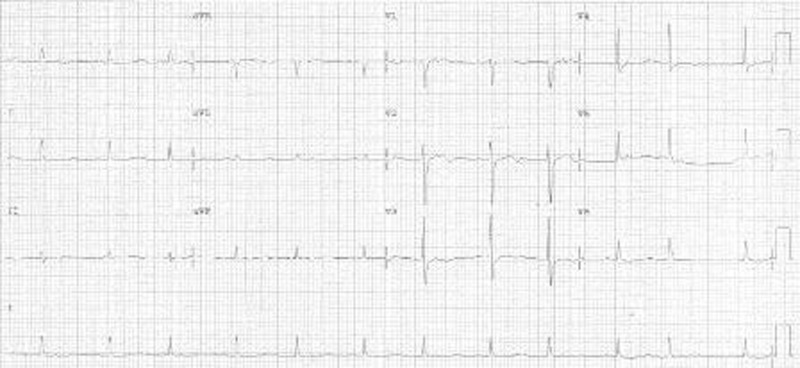
Routine echocardiogram was performed which showed a massive pericardial effusion (~4 cm in thickness) without tamponade effect, mild mitral regurgitation and fair left ventricular systolic function. Pericardiocentesis drained 1.6 litres of heavily blood stained fluid. The pericardial fluid was sent for various tests. Repeated ECG showed sinus rhythm with voltage returning to normal (figure 3).
Figure 3.
ECG after drainage of pericardial effusion with normal voltage QRS complexes.
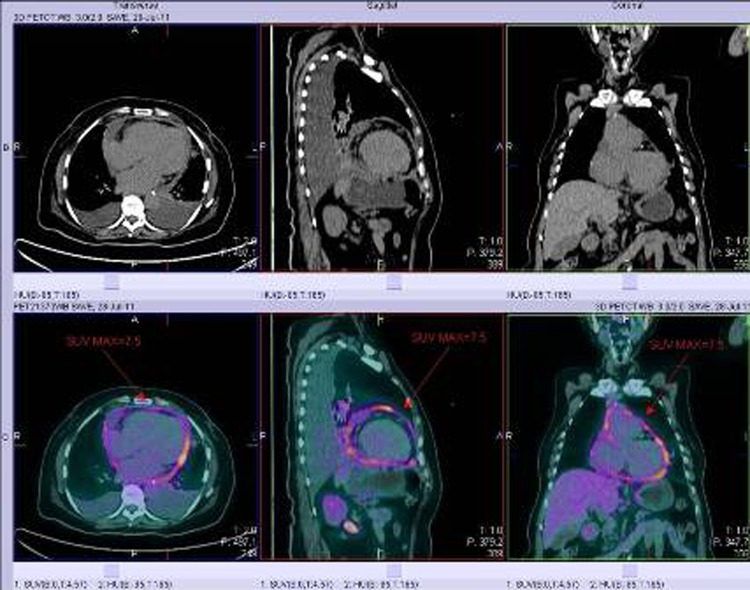
Further investigation was performed to search for the underlying cause of this massive haemorrhagic pericardial effusion. Physical examination showed no lymphadenopathy and no palpable breast or abdominal masses. Whole body PET-CT showed diffuse inflammation of the pericardium, and hypermetabolic lymph nodes at the left hilum and precarinal region which were likely to be reactive in nature. No evidence of occult primary cancer was seen (figure 4). The initial pericardial fluid examination results came back negative for malignant cells, acid fast bacilli (AFB) smear and Mycobacterium tuberculosis PCR.
Figure 4.
PET-CT images showing diffuse inflammation of the pericardium.
Her condition gradually improved and NIPPV and oxygen were weaned off. She was transferred back to general ward and a diagnosis of diastolic heart failure was made. A predischarge echocardiogram was performed and revealed only a thin rim of pericardial effusion. She was subsequently discharged while awaiting the remaining investigation results. Follow-up echocardiogram was arranged 2 weeks after discharge.
Repeated blood testing on 8 August 2011 showed persistent mild anaemia and static elevation of inflammatory markers. Later, the rapid AFB culture yielded Mycobacterium species, and subsequently, Genprobe examination also detected M tuberculosis in the pericardial fluid. Hepatitis B surface antigen and anti-HIV antibodies were negative.
She was readmitted to our department on 16 August 2011 for assessment by the respiratory physician. She had on and off nocturnal cough treated as asthma by chest clinic. Otherwise, she did not complain of any haemoptysis, weight loss, decreased appetite or night sweats. She has no family history of TB. She immigrated to Hong Kong from China when she was young and occasionally travels back to China. In the past year, she only travelled to Macau for a few days. Anti-TB medication (rifampicin, isoniazid, pyrazinamide, ethambutol), pyridoxine, and a 4-week course of steroids were commenced. She was subsequently discharged and referred to chest clinic for directly observed treatment short course (DOTS) for continuation of TB treatment. A notification was made to the Department of Health and contact tracing was performed for screening. Repeated chest radiograph showed an enlarged cardiac shadow, moderate left pleural effusion and no active lung lesions were seen in the right lung and upper zone of the left lung.
Outcome and follow-up
We continued to follow-up this patient at our cardiac clinic and the patient remained well after discharge.
Discussion
The clinical presentation of tuberculous pericarditis is variable and non-specific with symptoms including fever, weight loss, fatigue, night sweats. More common symptoms are cough, dyspnoea and chest pain.6 Patients can present as heart failure from chronic cardiac compression,6 but may also present acutely with cardiac tamponade.7 Its varied presentation and sometimes vague symptoms make the condition difficult to recognise.
Four stages of tuberculous pericarditis have been described: (1) dry stage with exudation of fibrinous material and early immune response; (2) effusive stage of serosanguineous fluid; (3) absorptive stage with organisation of granulomatous caseation and pericardial thicking; (4) constrictive stage caused by scarring. Our patient was in the effusive stage, however, very heavily blood stained pericardial fluid was drained which is atypical as compared with the well-documented serosanguineous effusion found in tuberculous pericarditis.6 7 Our initial suspicion was malignancy due to the nature of the fluid drained, thus other causes of haemorrhagic effusion including malignancy and trauma needed to be excluded. Extensive literature search was performed on Medline and only a handful of cases reports mentioned the presence of massive amount of effusion of up to 1.5 L or 4 cm thickness, or bloody or haemorrhagic pericardial fluid.8–10 The majority of cases yielded straw-coloured or serosanguinous fluid. Our case might be one of the first case reports to document tuberculous pericarditis causing massive heavily haemorrhagic pericardial effusion. Since the pericardial fluid accumulated slowly, no tamponade effect was seen in our patient.
For the diagnosis of tuberculous pericarditis, pericardial fluid should be obtained for analysis. Typical TB effusions are exudative. Adenosine deaminase (ADA) activity and interferon (IFN)-γ levels are useful tools to aid diagnosis. Elevation of ADA activity ≥40 U/l had 87% sensitivity and 89% specificity for diagnosing tuberculous pericarditis and IFN-γ ≥50 pg/ml concentration had 92% sensitivity, 100% specificity and a positive predictive value (PPV) of 100%.11
Direct examination including AFB smears, cultures and PCR have variable detection rates. Direct Ziehl–Neelsen stain has a poor detection rate of 0% to 42% only.6 Culture of pericardial effusion provided positive results in 55% of patients with tuberculous pericarditis in one study.4 In another study,12 comparing the use of PCR, culture and histopathology in diagnosing tuberculous pericarditis, TB was correctly diagnosed by culture in 93% of patients as compared with 81% by PCR and 87% by histology. They found that with the use of PCR, the sensitivity was higher for tissue specimens as compared with fluid specimens. Despite PCR being much faster, it does have its pitfalls including false-positive results and being prone to contamination.
PET-CT is generally used for investigation of malignancies but it can also detect infection and inflammation. It is currently not a routine investigation for TB due to its cost. However, in a few recent studies and case reports in Japan and Korea, it has been shown to play an important role in the detection and monitoring of TB extent and activity, and can guide and plan site of biopsies. The use of specific isotopes such as 18F-2-deoxyglucose or 11C-choline currently cannot consistently differentiate between TB and malignant lesions, but the future development of TB specific radiotracers may overcome this problem.13 14 It has also been shown to be useful in evaluating tuberculous pericarditis and monitoring treatment response.15 16 In a study by Demura et al17 evaluating disease activity and treatment response in pulmonary TB, in all 14 patients who received anti-TB medication, positive treatment response was seen as evidenced by a significantly decreased 18F-2-deoxyglucose uptake after treatment.
It has been recommended that a 6-month course of anti-TB medication is adequate for the treatment of tuberculous pericarditis or pericardial effusion.18 This is also supported by a review paper on tuberculous pericarditis by Moyasi et al6 published in 2005. For patients with diabetes, treatment should be extended to 9 months (an addition of 3 months on top of standard regimen) in view of the relative immune suppression in this patient group.19
As for the role of steroids in the treatment of tuberculous pericarditis, current evidence remains controversial or inconclusive. A review on the use of corticosteroids in patients with tuberculous pericarditis20 found that the use of anti-TB medication as well as steroids reduced case-death rate and the likelihood of pericardial effusion reaccumulation and a more favourable clinical status may be seen after 18–24 months of follow-up. However, the small sample size renders the results inconclusive. Choi et al21 tried to identify predictors of subsequent constrictive pericarditis and the need for pericardiectomy and found that the presence of pericardial constriction and echogenic pericardial effusion on initial presentation may be poor prognostic factors. In cases of reaccumlation of pericardial effusion, pericardial window should be considered to relieve and prevent cardiac tamponade.22 In those with constrictive pericarditis, a serious consequence of tuberculous pericarditis, pericardiectomy is the treatment of choice to relieve constriction. In our patient, no reaccumulation of pericardial effusion was noted after starting steroids. However, regular follow-up echocardiography is needed to monitor for any increase in pericardial effusion or development of constrictive pericarditis which may require further drainage or surgical intervention.
Learning points.
Tuberculous pericarditis is a well-described manifestation of extra-pulmonary TB with varied presentation.
Physicians should be aware of this condition and possible atypical presentations (in this case, the presence of a massive haemorrhagic pericardial effusion – which almost always point to underlying malignancy, is indeed a rare and atypical presentation of tuberculous pericarditis) to ensure timely diagnosis and treatment.
PET-CT maybe considered as a non-invasive investigation to aid the diagnosis of tuberculous pericarditis and in evaluating the extent of TB.
Anti-TB medication for 6 months is generally adequate for the treatment of tuberculous pericarditis, but the role of steroids in reducing pericardial effusion re-accumulation remains controversial.
In patients with cardiac tamponade or constrictive pericarditis, surgical intervention is warranted.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.World Health Organization. Global Health Observatory. MDG6 Tuberculosis [Internet]. 2011 [cited 2011 Sep 15] Available from: http://www.who.int/gho/tb/epidemic/cases_deaths/en/index.html
- 2.Centre for Health Protection. Notification and death rate of tuberculosis (all forms), 1947–2010. Department of Health, The Government of Hong Kong SAR, 2010 [Internet] [cited 2011 Sep 15]. Available from: http://www.chp.gov.hk/en/data/4/10/26/43/88.html
- 3.World Health Organisation. Tuberculosis profile for China, Hong Kong SAR, 2010 [Internet] [cited 2011 Sep 15]. Available from: https://extranet.who.int/sree/Reports?op=Replet&name=%2FWHO_HQ_Reports%2FG2%2FPROD%2FEXT%2FTBCountryProfile&ISO2=HK&outtype=pdf
- 4.Sagristà-Sauleda J, Permanyer-Miralda G, Soler-Soler J. Tuberculous pericarditis: ten year experience with a prospective protocol for diagnosis and treatment. J Am Coll Cardiol 1988;11:724–8. [DOI] [PubMed] [Google Scholar]
- 5.Reuter H, Burgess LJ, Doubell AF. Epidemiology of pericardial effusions at a large academic hospital in South Africa. Epidemiol Infect 2005;133:393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayosi BM, Burgess LJ, Doubell AF. Tuberculous pericarditis. Circulation 2005;112:3608–16. [DOI] [PubMed] [Google Scholar]
- 7.Sharma SK, Mohan A. Extrapulmonary tuberculosis. Indian J Med Res 2004;120:316–53. [PubMed] [Google Scholar]
- 8.Heller T, Lessells RJ, Wallrauch C, et al. Tuberculosis pericarditis with cardiac tamponade: management in the resource-limited setting. Am J Trop Med Hyg 2010;83:1311–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massoure PL, Boddaert G, Caumes JL, et al. Porridge-like tuberculous cardiac tamponade: treatment difficulties in the Horn of Africa. Gen Thorac Cardiovasc Surg 2010;58:276–8. [DOI] [PubMed] [Google Scholar]
- 10.Avşar A, Günay NK, Celik A, et al. A case of cardiac tamponade caused by tuberculous pericarditis. Turk Kardiyol Dern Ars 2008;36:482–4. [PubMed] [Google Scholar]
- 11.Reuter H, Burgess L, van Vuuren W, et al. Diagnosing tuberculous pericarditis. QJM 2006;99:827–39. [DOI] [PubMed] [Google Scholar]
- 12.Cegielski JP, Devlin BH, Morris AJ, et al. Comparison of PCR, culture, and histopathology for diagnosis of tuberculous pericarditis. J Clin Microbiol 1997;35:3254–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harkirat S, Anand SS, Indrajit IK, et al. Pictorial essay: PET/CT in tuberculosis. Indian J Radiol Imaging 2008;18:141–7. [Google Scholar]
- 14.Jeong YJ, Lee KS. Pulmonary tuberculosis: up-to-date imaging and management. AJR Am J Roentgenol 2008;191:834–44. [DOI] [PubMed] [Google Scholar]
- 15.Ha JW, Lee JD, Ko YG, et al. Images in cardiovascular medicine. Assessment of pericardial inflammation in a patient with tuberculous effusive constrictive pericarditis with 18F-2-deoxyglucose positron emission tomography. Circulation 2006;113:e4–5. [DOI] [PubMed] [Google Scholar]
- 16.Testempassi E, Kubota K, Morooka M, et al. Constrictive tuberculous pericarditis diagnosed using 18F-fluorodeoxyglucose positron emission tomography: a report of two cases. Ann Nucl Med 2010;24:421–5. [DOI] [PubMed] [Google Scholar]
- 17.Demura Y, Tsuchida T, Uesaka D, et al. Usefulness of 18F-fluorodeoxyglucose positron emission tomography for diagnosing disease activity and monitoring therapeutic response in patients with pulmonary mycobacteriosis. Eur J Nucl Med Mol Imaging 2009;36:632–9. [DOI] [PubMed] [Google Scholar]
- 18.Chan SL. Tuberculosis in Hong Kong – Update. Journal of the Hong Kong Medical Association 1990;42. [Google Scholar]
- 19.USAPI Standards for the Management of Tuberculosis and Diabetes. Draft Interim: 1 December 2010. [Internet] [cited 2011 Nov 27]. Available from: http://www.currytbcenter.ucsf.edu/abouttb/TB_DM_USAPI_Standards_document_12_01_2010.pdf
- 20.Ntsekhe M, Wiysonge C, Volmink JA, et al. Adjuvant corticosteroids for tuberculous pericarditis: promising, but not proven. QJM 2003;96:593–9. [DOI] [PubMed] [Google Scholar]
- 21.Choi HO, Song JM, Shim TS, et al. Prognostic value of initial echocardiographic features in patients with tuberculous pericarditis. Korean Circ J 2010;40:377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strang JI, Nunn AJ, Johnson DA, et al. Management of tuberculous constrictive pericarditis and tuberculous pericardial effusion in Transkei: results at 10 years follow-up. QJM 2004;97:525–35. [DOI] [PubMed] [Google Scholar]