Abstract
Arteriovenous fistulas can rarely occur in patients with neurofibromatosis type 1. These lesions typically result from traumatic insult to the dysplastic parent artery. The damaged artery forms abnormal connections with nearby paraspinal and epidural venous structures. Surgical treatment of these lesions can be extremely challenging given the proximity to the spinal cord and the ability of the fistula to recruit vessels from adjacent vascular structures. A 29-year-old woman with neurofibromatosis type 1 and a motor vehicle collision 2 years earlier presented with gait difficulty, lower extremity spasticity and neck and arm pain. Her investigation revealed a giant cervical vertebral arteriovenous fistula. The fistula was successfully treated in multiple stages using all endovascular techniques including detachable coils, stents and glue embolisation. Reduction in flow and improvement in symptoms are reasonable goals in this specific rare subgroup of complex cervical arteriovenous fistulae.
Background
This is a very complex and unusual vascular case that required all the techniques of endovascular for satisfactory treatment.
Case presentation
The patient is a 29-year-old woman with neurofibromatosis type 1 (NF-1) who presented with a 1-month history of gait difficulty and spasticity. She also reported neck and upper extremity pain and decreased lower extremity sensation for 2 weeks. Her strength in the lower extremities and left upper extremity was 4/5 and 4+/5, respectively. She had bilateral Hoffman’s sign and sustained clonus in the lower extremities. She required a walker for ambulation at presentation. On further history, the patient reported having been in motor vehicle collision 2 years previously.
Investigations
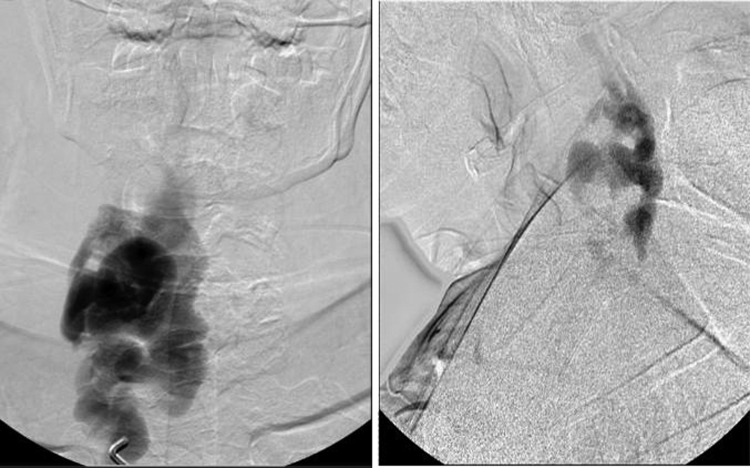
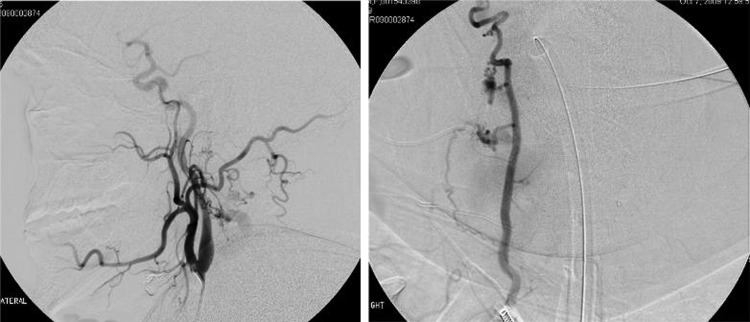
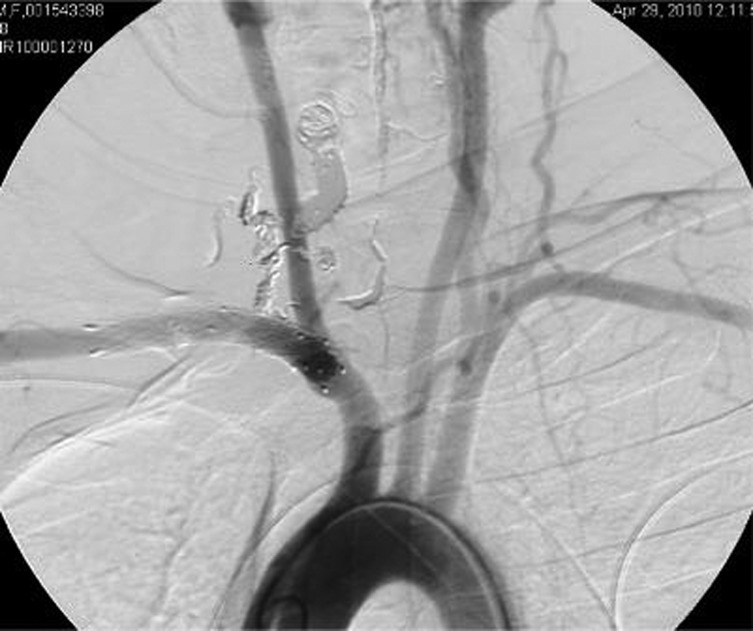
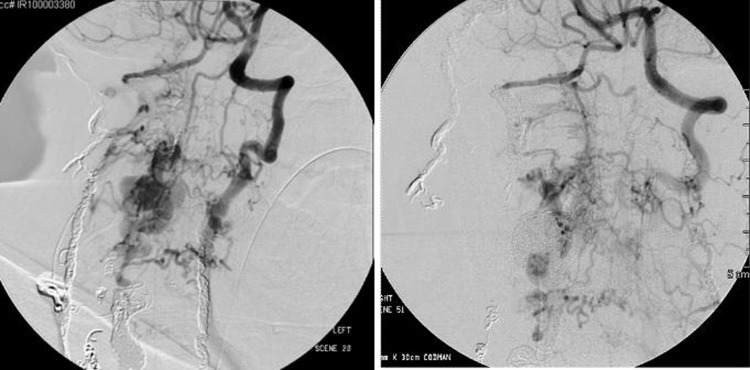
CT angiography revealed a profound cervical kyphoscoliosis and a massive arteriovenous fistula (AV) that extended from C2 to C7 in the spinal canal. The fistula included an enlarged right vertebral artery that drained into a massively enlarged perivertebral ectactic venous plexus that displaced the spinal cord laterally and extended through the neural foramen at multiple levels (figure 1A,B). The fistula appeared centered at C5 as the neural foramen was obliterated. The angiogram revealed that the fistula had recruited feeding vessels from the right external carotid, subclavian, costocervical trunk and thyrocervical trunk (figure 2A,B). The left external carotid and left vertebral artery also provided smaller afferent vessels. The MRI showed cord signal change, worse in the posterior columns at C5/6.
Figure 1.
Right vertebral injection, AP view (A) and lateral view (B), shows major afferent flow to fistula.
Figure 2.
Right external carotid injection, lateral view (A), and right thyrocervical trunk, AP view (B), provides feeders to the fistula.
Treatment
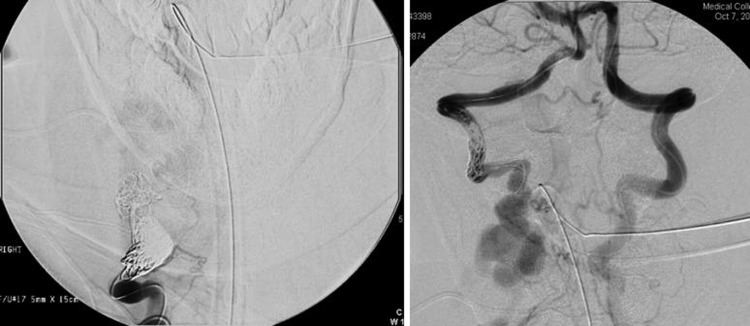
The arterial supply to the fistula was addressed in a six-stage procedure and a transvenous approach performed in a seventh stage. In the first stage the proximal and distal right vertebral artery were occluded using 23 assorted Cordis endovascular coils (figure 3A,B). The coils in the proximal vertebral artery were placed at the site of the fistula. The right thyrocervical trunk was occluded using 13 assorted Cordis endovascular coils. The right costocervical trunk was occluded using assorted Cordis endovascular coils. Multiple smaller third order feeding branches originating from the right subclavian artery were also occluded with nine coils.
Figure 3.
Right vertebral artery injection, AP view (A), and left vertebral artery injection, AP view (B), shows proximal and distal coil occlusion of the right vertebral artery.
At the second stage the right external carotid artery branches (occipital and posterior auricular) were occluded using Onyx glue. At the third stage an attempt was made to advance the microcatheter into several tiny feeders from the left vertebral artery but only a single small coil could be placed. After this stage the patient was seen 1 month later with subjective improvement in ambulation but still required a walker. Her strength was full throughout but she remained hyperreflexic.
A follow-up study 4 months after the initial procedure showed recruitment of a plexus of vessels from the costocervical trunk (figure 4) and at a fourth stage procedure, further coils were placed leaving only a fine fibrillar mesh of feeding vessels to the fistula. Given the risk of further recruitment and enlargement of these vessels over time, we placed two covered stents (fluency 8×60 mm and 10×40 mm) in the right subclavian artery just distal to the right common carotid artery (fifth stage) with good result (figure 5).
Figure 4.
Right subclavian artery injection shows recruitment of vessels from the costocervical trunk.
Figure 5.
Aortic arch injection shows two covered stents in the right subclavian artery.
A follow-up angiogram 8 months after initial treatment showed continued occlusion of the embolised vessels with the majority of filling from branches from the left thyrocervical and external carotid. Eleven coils (Cordis DCS) were used to obliterate the thyrocervical trunk and two coils used in the small third-order branch from the external carotid (sixth stage).
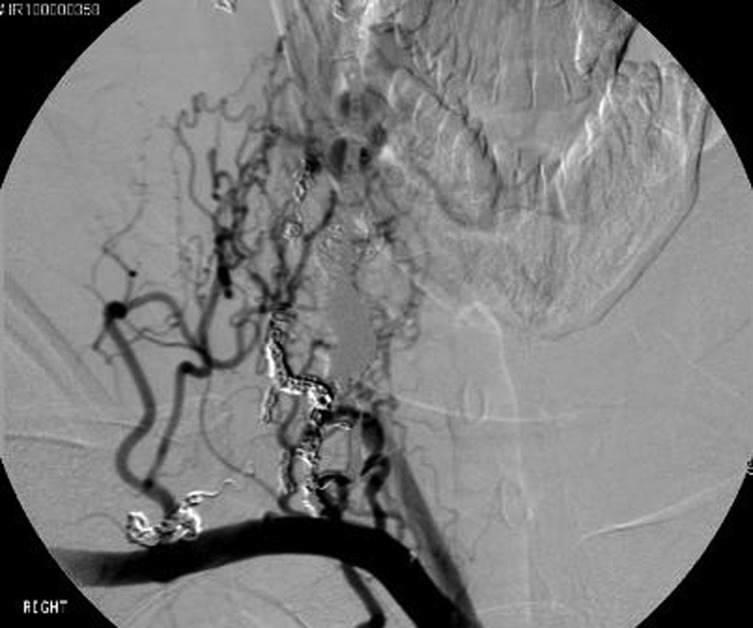
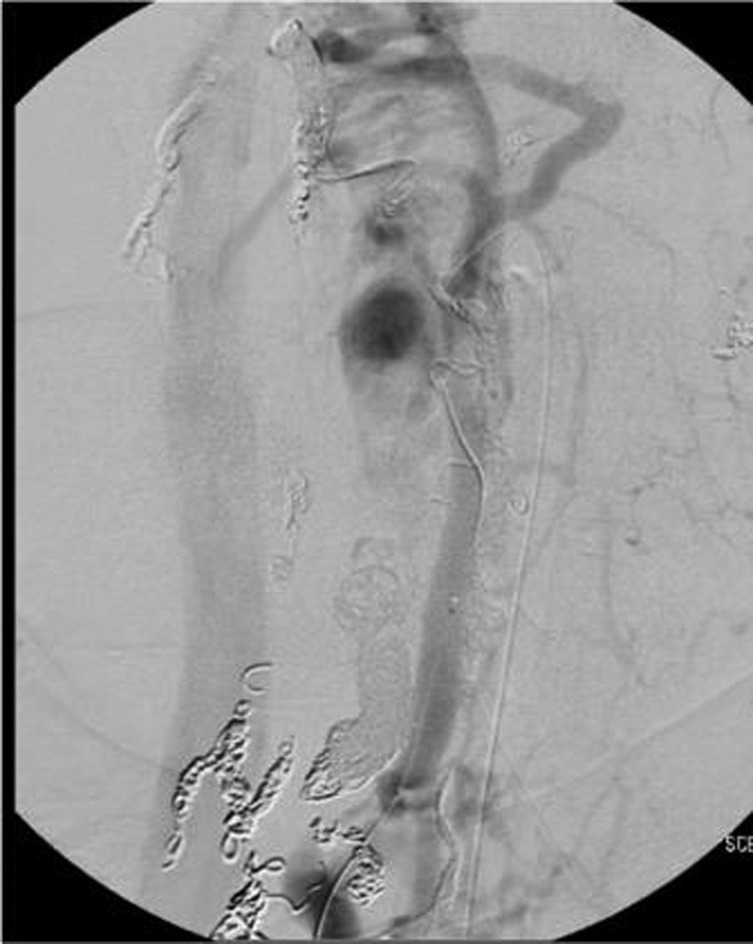
By 1 year after initial treatment the patient remained stable clinically but a follow-up angiogram showed markedly slowed but persistent filling of the fistula primarily from a myriad of mesh-like vessels from the left vertebral artery. A month later she was taken for a transvenous approach after extensive discussion of the risks including worsening spinal cord compression with resultant symptoms. The perimedullary venous ectatic pouch was accessed from the vena cava (figure 6). A combination of twenty fibre push coils and bare platinum coils (Codman DCS and Axium) were deployed (seventh stage). Considerable reduction in flow was noted on the follow-up angiogram and one done the next day (figure 7A,B).
Figure 6.
External jugular venogram, AP view, shows ectatic venous aneurysm.
Figure 7.
Pre- (A) and post (B) embolisation images, AP views, showing coil mass in venous aneurysm.
Outcome and follow-up
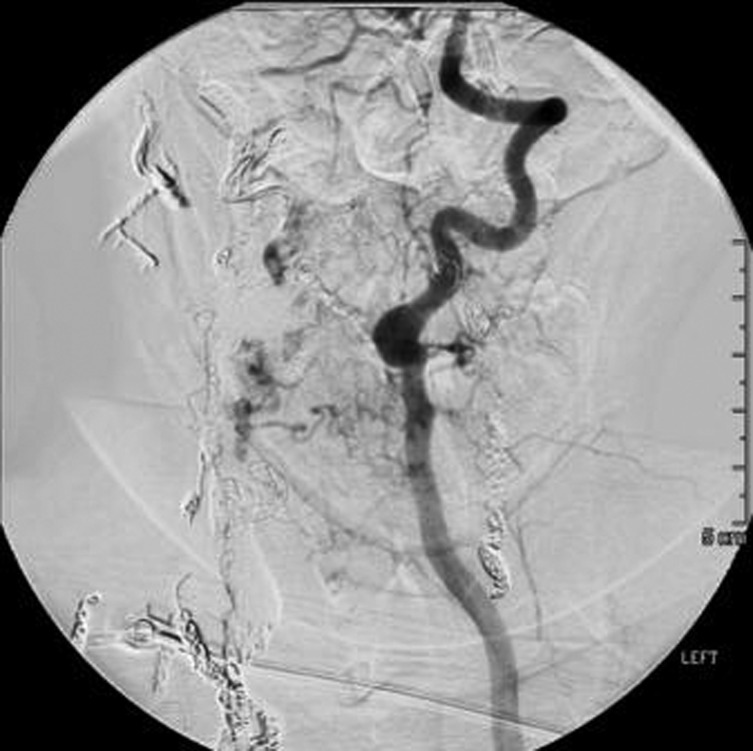
A follow-up CT angiography performed 17 months after initial treatment showed stable coil masses and embolysate without new vessel recruitment (figure 8). Her neurological exam was stable.
Figure 8.
Left vertebral artery injection, AP view, shows no new vessel recruitment.
Discussion
NF-1 is an autosomal dominant syndrome resulting from abnormality of 17q11.2 and affects mesodermic tissues including blood vessels.1–3 This results in vascular abnormalities including occlusive diseases, aneurysms, arteriovenous malformations and AVs. Vascular changes occurring in microvessels include intimal proliferation with vessel occlusion, fibrohyaline thickening of the intima with fragmentation of the muscularis and elastica, and a nodular form with spindle or epithelioid cells with vessel wall disruption.2 3 AVFs can be congenital or can result from spontaneous rupture of saccular aneurysms.4–10 Trauma is the most common aetiology of AVFs especially in the cervical spine. These can be postoperative or can result after blunt trauma. The recent history of motor vehicle collision and progressive neurological course suggests a traumatic etiology in this patient.
Saito et al2 described 28 cases (6 men, 22 women) of AVF in NF. The majority was from the vertebral artery and only seven were supplied by external carotid artery branches. Surgical ligation of feeding artery was performed in 10 cases. Only four of these procedures were successful but symptoms improved in all 10 cases. Embolisation procedures were performed in eight cases (balloons in three patients, coils in five patients) and were successful in seven. Symptoms, however, improved in all eight. A combination of surgery and embolisation was performed in six cases, four of which were successful. Again, symptoms improved in all six patients.
Learning points.
Detachable coils and balloons as well as advanced embolic agents and stents have given treating physicians multiple possibilities for reducing and hopefully eliminating blood flow to the lesion.
A combination of endovascular techniques was instrumental in reducing flow and improving symptoms in our patient. The complexity, size, diverse blood supply and proximity to critical neural structures were all factors that led to the use of endovascular techniques over open surgical approaches to this lesion.
Reduction in flow and improvement in symptoms are reasonable goals in this specific subgroup of complex cervical AV.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Deans WR, Bloch S, Leibrock L, et al. Arteriovenous fistula in patients with neurofibromatosis. Radiology 1982;144:103–7. [DOI] [PubMed] [Google Scholar]
- 2.Saito A, Takahashi T, Ezura M, et al. Intercostal arteriovenous fistula associated with neurofibromatosis manifesting as congestive myelopathy: case report. Neurosurgery 2007;61:E656–7; discussion E657. [DOI] [PubMed] [Google Scholar]
- 3.Schievink WI, Piepgras DG. Cervical vertebral artery aneurysms and arteriovenous fistulae in neurofibromatosis type 1: case reports. Neurosurgery 1991;29:760–5. [DOI] [PubMed] [Google Scholar]
- 4.Chou SN, French LA. Arteriovenous fistula of vertebral vessels in the neck. J Neurosurg 1965;22:77–80. [DOI] [PubMed] [Google Scholar]
- 5.Kāhārā V, Lehto U, Ryymin P, et al. Vertebral epidural arteriovenous fistula and radicular pain in neurofibromatosis type I. Acta Neurochir (Wien) 2002;144:493–6. [DOI] [PubMed] [Google Scholar]
- 6.Miyazaki T, Ohta F, Daisu M, et al. Extracranial vertebral artery aneurysm ruptured into the thoracic cavity with neurofibromatosis type 1: case report. Neurosurgery 2004;54:1517–20; discussion 1520–1. [DOI] [PubMed] [Google Scholar]
- 7.Nagashima C, Iwasaki T, Kawanuma S, et al. Traumatic arteriovenous fistula of the vertebral artery with spinal cord symptoms. Case report. J Neurosurg 1977;46:681–7. [DOI] [PubMed] [Google Scholar]
- 8.Paolini S, Colonnese C, Galasso V, et al. Extradural arteriovenous fistulas involving the vertebral artery in neurofibromatosis Type 1. J Neurosurg Spine 2008;8:181–5. [DOI] [PubMed] [Google Scholar]
- 9.Peyre M, Ozanne A, Bhangoo R, et al. Pseudotumoral presentation of a cervical extracranial vertebral artery aneurysm in neurofibromatosis type 1: case report. Neurosurgery 2007;61:E658; discussion E658. [DOI] [PubMed] [Google Scholar]
- 10.Reddy SV, Karnes WE, Earnest F, 4th, et al. Spontaneous extracranial vertebral arteriovenous fistula with fibromuscular dysplasia. Case report. J Neurosurg 1981;54:399–402. [DOI] [PubMed] [Google Scholar]