Abstract
The authors present the case of a 68-year-old male patient with metastatic rectal cancer. A pelvic recurrence resulted in neuropathic pain, radiating down his left leg. The pain was resistant to standard treatments. However, after nearly 3 years of debilitating pain, the patient experienced dramatic relief just hours after an infusion of the antiepidermal growth factor receptor antibody cetuximab. The analgesic effect lasted for 10–12 days and was repeated roughly every 12 days for three and a half years. To test for placebo effect, the patient received (unknown to him) 20% of his usual cetuximab dose and experienced no pain relief. The dramatic analgesic effect was documented in clinical notes, medication lists and in numeric rating scales even while his cancer was in radiological progression. Mitogen-activated protein kinase (MAPK)-signalling is believed to be an important driver of neuropathic pain and therefore, the authors hypothesise a direct inhibition of MAPK-signalling by cetuximab in neuronal or glial cells.
Background
Neuropathic pain (NP) is defined as ‘pain caused by a primary lesion or disease of the somatosensory system’.1 It is commonly characterised by patients as burning, tingling or electric shock-like and is often associated with other neurological symptoms or deficits.2 Due to its severity, chronicity and the poor side effect to benefit ratio of current pharmacotherapy,3 4 NP often causes psychological distress, sleep deprivation, functional impairment and overall poor quality of life.5 With an annual incidence of up to 1% in the general population6 and a rising prevalence,3 NP is a common and formidable health problem worldwide.
NP has numerous aetiologies, including mechanical nerve injury, toxic and ischaemic effects, infections and immune-mediated damage. The mechanism of perpetuation of NP, regardless of origin, involves the interaction of neuronal, glial and immune cells.7 However, a better pathophysiological characterisation of NP is needed in order to promote the development of more individualised, targeted therapies.8
Communication between the involved cells is complex and depends partly on signalling via the family of mitogen-activated protein kinase (MAPK) proteins which have been proposed as targets for therapies directed against NP, as well as other chronic neurological diseases.9–12 Several small molecules designed to inhibit the intracellular MAPK-signalling pathway are currently undergoing clinical phase I and II studies.13 To date, we are not aware of any reports of extracellular epidermal growth factor receptor (EGFR) inhibition to target MAPK-signalling upstream of RAS, extracellular-signal-regulated kinase, p38 and c-Jun N-terminal kinases. However, several other ligands with the potential to signal via MAPK have been proposed as possible pharmaceutical targets.14
We describe a patient’s remarkable analgesic response to treatment with the EGFR-inhibitor cetuximab. The drug was initially given to treat metastatic rectal cancer, but serendipitously served as an effective analgesic. The treatment was well tolerated and allowed the patient to maintain a quality of life that otherwise would seem impossible.
If this observation can be repeated and its mechanism understood, it could potentially have important therapeutic consequences for a large group of patients with chronic NP.
Case presentation
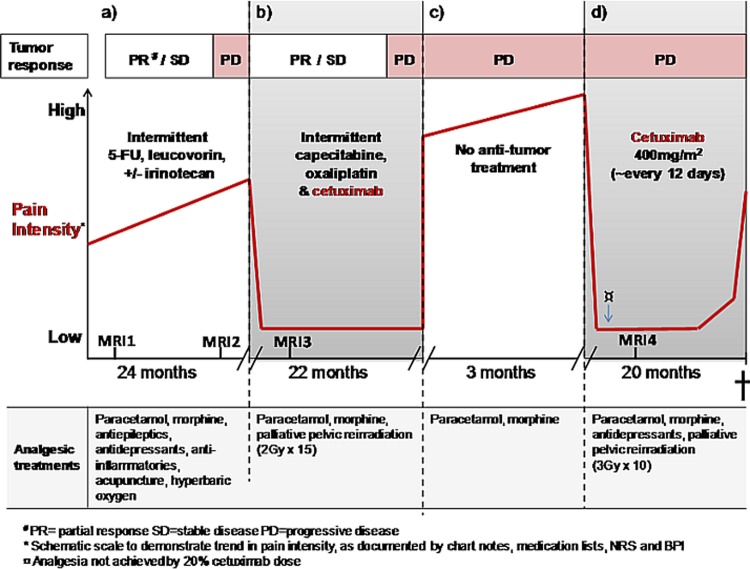
A previously healthy 62-year-old male underwent curative resection of a rectal tumour in April, 2001. The tumour proved to be a Dukes B, moderately differentiated adenocarcinoma with wild-type K-ras status. At a routine follow-up 29 months later, he was found to have an anastomotic recurrence, without distant metastases. He then received preoperative chemoradiotherapy (50 Gy) and the subsequent surgical re-excision was regarded as R0. Over the course of several months following the second operation, the patient developed pelvic pain radiating down his left lower extremity. Nine months postoperatively (corresponds to figure 1a) he was diagnosed with asymptomatic pulmonary metastases, and systemic palliative treatment was initiated with the Nordic FLIRI (irinotecan and 5-fl ourouracil) chemotherapy regimen.
Figure 1.
(a–d) Cetuximab alleviates neuropathic pain despite tumour progression.
Due to progressive pain despite antineoplastic treatment, neurological consultation was obtained. Examination revealed decreased sensibility over the dorsum of his left foot and decreased strength of dorsiflexion. Electromyography and electroneuronography revealed findings consistent with lumbosacral plexopathy. Repeated neurological testing showed worsening neurological pathology after 1 month. MRI (corresponding MRI 1 in figure 1a) confirmed a presacral re-recurrence of rectal cancer, involving the patient’s sacral plexus and left sciatic nerve. His pain was regarded as neuropathic, caused by the cancer recurrence.
The patient’s pain gradually increased to a level that he characterised as ‘unbearable’. Several treatments, including the triplet chemotherapy, paracetamol, morphine (90 mg/24t), antiepileptics, antidepressants, antinflammatories, acupuncture and hyperbaric oxygen, were attempted without satisfactory analgesia (table 1).
Table 1.
Treatments given in an attempt to relieve the patient’s pain
| Type of intervention | Specific treatment |
| Analgesic medication | Paracetamol |
| Morphine | |
| Antiepileptic medication | Gabapeintin |
| Carbamazepine | |
| Pregabalin | |
| Antidepressant medication | Amitriptyline |
| Anti-inflammatory medication | Prednisolone |
| Non-steroidal anti-inflammatories | |
| Chemotherapy | 5-Fluorouracil/leucovorin |
| Capecitabine | |
| Irinotecan | |
| Oxaliplatin | |
| Other | Hyperbaric oxygen |
| Acupuncture | |
| Palliative radiation |
After nearly 2 years of intermittent first line palliative chemotherapy, MRI (corresponding to MRI2 in figures 1a and 2a) revealed progression of the tumour affecting the sciatic nerve, and CT scan showed progression of lung metastases.
Figure 2.
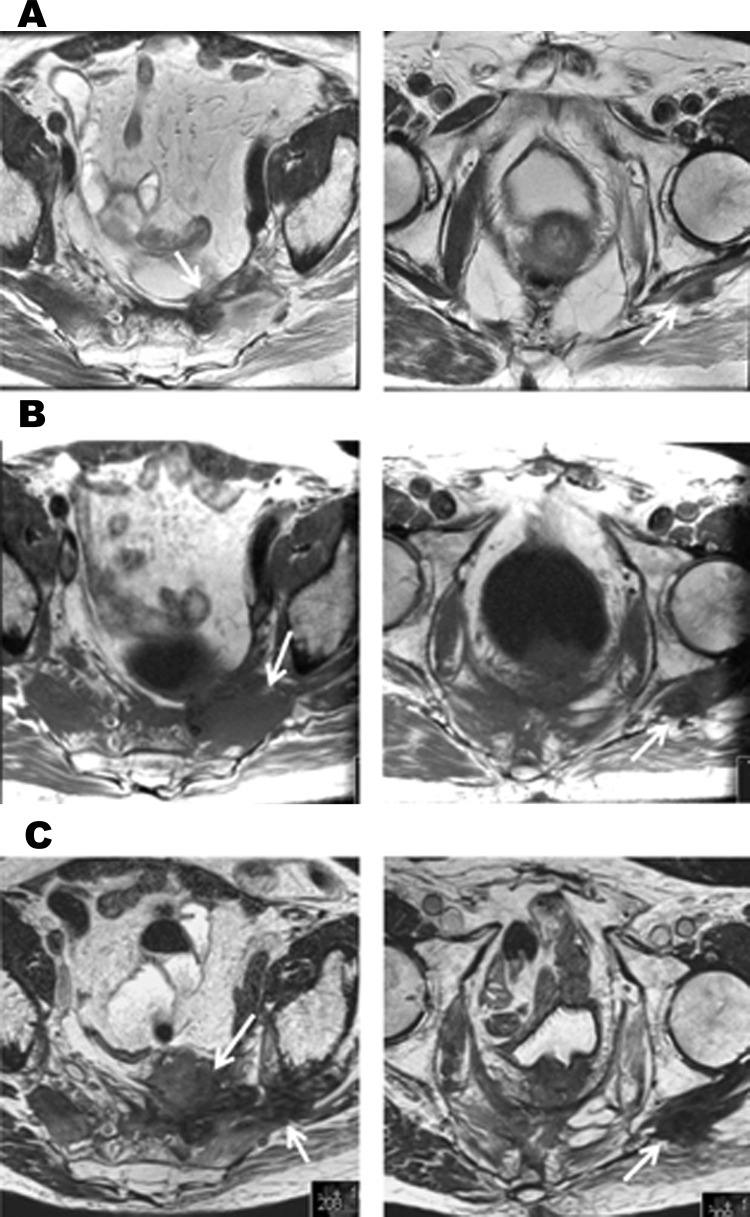
Recurrent and progressive rectal cancer. Arrows indicate tumour changes affecting the left sacral plexus and left sciatic nerve. (a) MRI taken three months prior to starting capecitabine, oxaliplatin and cetuximab (corresponds to MRI 2 in figure 1a). There is a presacral recurrence that extends along the left sciatic nerve. (b)MRI taken 4 months after starting capecitabine, oxaliplatin and cetuximab (corresponds to MRI 3 in figure 1b). Both the presacral recurrence and its extension along the sciatic nerve have increased in size. (c) MRI taken eight months after starting cetuximab monotherapy for analgesia (corresponds to MRI 4 in figure 1d). There is further progression of the recurrence in the presacral area and along the left sciatic nerve.
Due to failure of first line chemotherapy, second line treatment with XELOX (capecitabine and oxaliplatin) chemotherapy and the anti EGFR antibody cetuximab was initiated (figure 1b). At the outset of this treatment the patient required 200 mg morphine/24 h. After nearly 3 years of worsening pain, the patient experienced dramatic relief just hours after an infusion of oxaliplatin and cetuximab. Three weeks after his first course, he reported that the pain had diminished from eight to two on a ten-point numeric rating scale and he no longer required opiates.
During chemotherapy holidays, a common practice in palliative oncologic treatment, the patient’s pain recurred and he required increasing doses of opiates. On two such occasions (figure 1b,d), trials of palliative pelvic radiotherapy, intended to relieve pain, were not effective.
However, re-introduction of the combination of chemotherapy and cetuximab repeatedly led to analgesic response within hours. Although the remarkable analgesic effect continued, there was an approximately 20% increase in size of the sciatic nerve lesion on pelvic MRI (corresponding to MRI3 in figures 1b and 2b) after 4 months of treatment. Due to continued good clinical response and stable disease by response evaluation criteria in solid tumours,15 chemotherapy in combination with cetuximab was continued. After 22 months of this treatment, CT scan revealed progressive lung metastases and as a result, both chemotherapy and antibody were discontinued.
Over the next 3 months, the patient’s pain increased profoundly (figure 1c). Standard treatments for NP were inadequate or their side-effects prohibitive. His morphine requirement rose to 320 mg/24 h, without satisfactory effect.
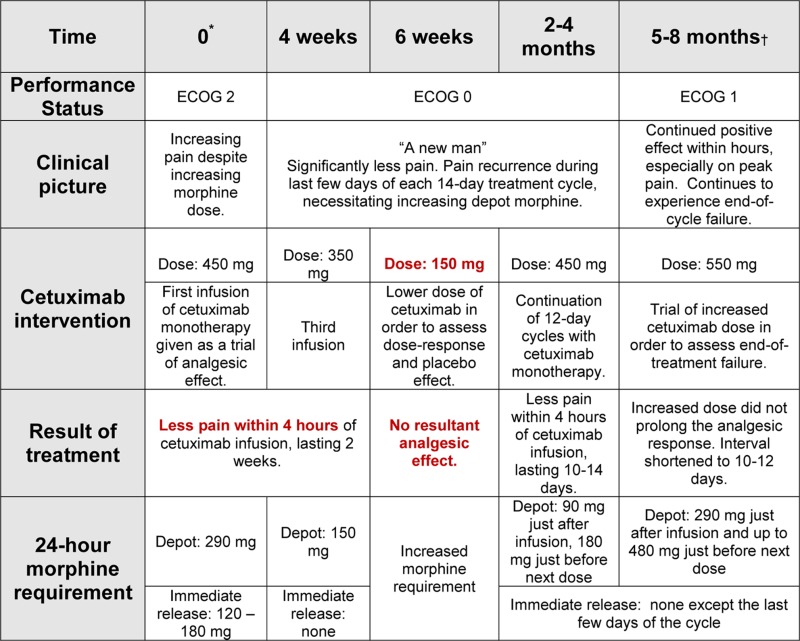
Based on its previously observed rapid analgesic effect, cetuximab monotherapy was attempted (figure 1d). Within 3–4 h after the first infusion, the patient experienced significant pain relief, analogous to that seen after earlier infusions of the XELOX and cetuximab combination (figure 1b). Once again, the patient was able to reduce his dose of depot opiates (from 290 mg/day to 150 mg/day) and he no longer required immediate release morphine. He received cetuximab monotherapy infusions every 10–14 days for 20 months (figure 1d). During this period, he consistently responded with effective analgesia within hours, lasting nearly 2 weeks each time.
Increasing opiate requirements that were observed during the last days before treatment suggest an end of dose effect. Observed side effects of cetuximab consisted of mild and transient acne. The most prominent burden of treatment was the need for repeated intravenous infusions.
In order to test for placebo effect, the patient received (unknown to him) 20% of his usual cetuximab dose on one occasion and experienced no resultant pain relief (table 2). A trial of dose escalation from 450 mg to 550 mg did not prolong the analgesic effect. Thus, the treatment interval was shortened to 10–12 days.
Table 2.
The effect of cetuximab monotherapy during progression of rectal cancer recurrence causing neuropathic pain and a detailed description of the patient’s clinical course during the first months of treatment (as depicted in Figure 1d)
 |
Eight months after starting cetuximab monotherapy, MRI again demonstrated growth of the offending lesion (figure 1d, MRI4 and figure 2c). Despite this growth, cetuximab consistently produced a remarkable analgesic response for a total of 12 additional months.
Investigations
See figure 2.
Treatment
Outcome and follow-up
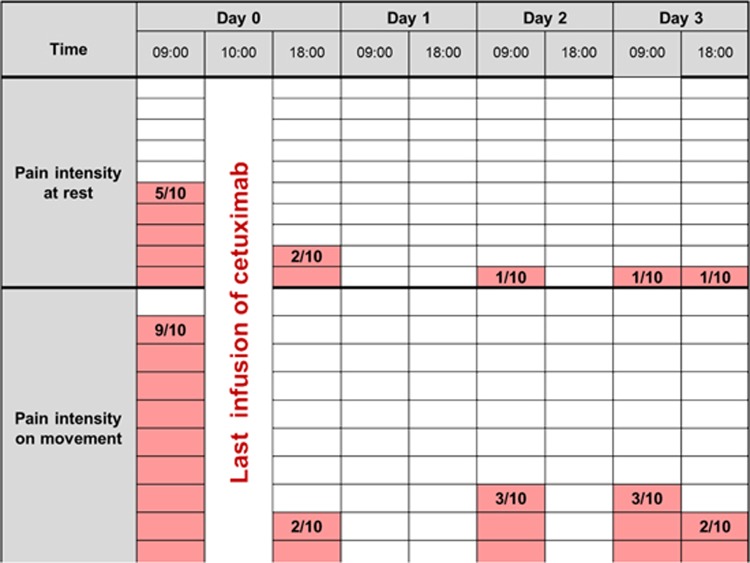
Toward the end of his life, the patient required increasing doses of depot morphine and tended to experience shorter time intervals without ‘unbearable’ peak pain. On the day before his very last cetuximab infusion, which was administered after 20 months of monotherapy, the patient was admitted to hospital with intense pain. Just hours after the cetuximab infusion, the patient reported that pain at rest had been reduced from five to two and pain on movement had been reduced from nine to two on a ten-point numeric rating scale (figure 3), without increase in analgesic medications or any other interventions. The patient died 1 month later.
Figure 3.
The patient reported pain immediately prior and subsequent to his last infusion of cetuximab (10-point rating scale).
Discussion
To our knowledge, there exist no reports describing an analgesic effect of cetuximab without tumour response. Until now, pain relief in response to cetuximab has been correlated with tumour shrinkage.16
Cetuximab was developed to inhibit EGFR1-activation and MAPK-signalling by EGF in cancers.17 Side effects such as allergic reactions, skin changes and diarrhea are usually mild, manageable and transient.18 By blocking EGFR1, cetuximab also has the potential to inhibit other EGFR1-binding ligands,19 either directly or by inhibition of human epidermal growth factor receptor (HER) family heterodimerisation.20 21 Our patient experienced dramatic analgesic effect just hours after treatment with cetuximab, although his tumour was in progression. The pain relief is well documented and importantly, did not respond to dose reduction. The timing of our patient’s pain relief correlates with the pharmacokinetics of cetuximab.22
After nerve injury, neurons upregulate members of the HER-family of receptors,7 23 24 thereby potentially increasing their activation of the MAPK signalling10 cascade. This may lead to further interaction between cells in the NP triad. Additionally, it has been shown that EGF has the potential to act in a rapid, but short-lasting manner on nociceptive neurons, which is consistent with our clinical observation.25 We therefore hypothesise a direct inhibition of MAPK-signalling by cetuximab in neuronal or glial cells.
Data from two published clinical trials may support the protective role of cetuximab on the development of oxaliplatin-induced neuropathy. Both the Nordic VII26 and COIN27 studies analysed the potential survival benefit of adding cetuximab to oxaliplatin-containing 5FU-based chemotherapy in patients with metastatic colorectal cancer. In both studies, no survival benefit was shown and patients treated with and without cetuximab received similar doses of the neuropathy-inducing chemotherapy, oxaliplatin. Interestingly, in both studies, the incidence of peripheral neuropathy was 30% less in the arms that also received cetuximab. This finding was statistically significant in the COIN study (p=0.0053).
At this time, we can only speculate about the analgesic mechanism in our patient, but the rapid onset indicates a direct effect on neuronal cells or on the communication among the NP triad.7 If confirmed, these findings may have important implications for the treatment of NP. Further preclinical and clinical studies of EGFR-inhibition, including the use of oral agents are warranted.
Learning points.
Many treatments of NP are characterised by a narrow therapeutic index. Inhibition of MAPK-signalling is proposed to be a promising target.
We present a case of repeated and dramatic relief of NP within hours after infusion of the EGFR-inhibitor cetuximab.
The duration of analgesia is consistent with the pharmacokinetics of cetuximab and the patient recognised blinded dose reduction.
EGFR-inhibition in NP warrants further study.
Footnotes
Competing interests: We, the authors, have filed a US provisional patent application for the use of EGFR as a clinical target for treatment of neurological disorders. There are no other potential conflicts of interest.
Patient consent: Obtained.
References
- 1.Jensen TS, Baron R, Haanpää M, et al. A new definition of neuropathic pain. Pain 2011;152:2204–5. [DOI] [PubMed] [Google Scholar]
- 2.Boureau F, Doubrère JF, Luu M. Study of verbal description in neuropathic pain. Pain 1990;42:145–52. [DOI] [PubMed] [Google Scholar]
- 3.Dworkin RH. An overview of neuropathic pain: syndromes, symptoms, signs, and several mechanisms. Clin J Pain 2002;18:343–9. [DOI] [PubMed] [Google Scholar]
- 4.Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain 2010;150:573–81. [DOI] [PubMed] [Google Scholar]
- 5.Jensen MP, Chodroff MJ, Dworkin RH. The impact of neuropathic pain on health-related quality of life: review and implications. Neurology 2007;68:1178–82. [DOI] [PubMed] [Google Scholar]
- 6.Dieleman JP, Kerklaan J, Huygen FJ, et al. Incidence rates and treatment of neuropathic pain conditions in the general population. Pain 2008;137:681–8. [DOI] [PubMed] [Google Scholar]
- 7.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 2007;10:1361–8. [DOI] [PubMed] [Google Scholar]
- 8.Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 2010;9:807–19. [DOI] [PubMed] [Google Scholar]
- 9.Ji RR. Mitogen-activated protein kinases as potential targets for pain killers. Curr Opin Investig Drugs 2004;5:71–5. [PubMed] [Google Scholar]
- 10.Ji RR, Gereau RW, Malcangio M, et al. MAP kinase and pain. Brain Res Rev 2009;60:135–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yasuda S, Sugiura H, Tanaka H, et al. p38 MAP kinase inhibitors as potential therapeutic drugs for neural diseases. Cent Nerv Syst Agents Med Chem 2011;11:45–59. [DOI] [PubMed] [Google Scholar]
- 12.Calvo M, Zhu N, Grist J, et al. Following nerve injury neuregulin-1 drives microglial proliferation and neuropathic pain via the MEK/ERK pathway. Glia 2011;59:554–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anand P, Shenoy R, Palmer JE, et al. Clinical trial of the p38 MAP kinase inhibitor dilmapimod in neuropathic pain following nerve injury. Eur J Pain 2011;15:1040–8. [DOI] [PubMed] [Google Scholar]
- 14.Martin YB, Herradón G, Ezquerra L. Uncovering new pharmacological targets to treat neuropathic pain by understanding how the organism reacts to nerve injury. Curr Pharm Des 2011;17:434–48. [DOI] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 16.Mesía R, Palmero R, Cos M, et al. Rapid palliation of symptoms with platinum-based chemotherapy plus cetuximab in recurrent oral cancer: a case report. Head Neck Oncol 2010;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vincenzi B, Zoccoli A, Pantano F, et al. Cetuximab: from bench to bedside. Curr Cancer Drug Targets 2010;10:80–95. [DOI] [PubMed] [Google Scholar]
- 18.Holt K. Common side effects and interactions of colorectal cancer therapeutic agents. J Pract Nurs 2011;61:7–20. [PubMed] [Google Scholar]
- 19.Wheeler DL, Dunn EF, Harari PM. Understanding resistance to EGFR inhibitors-impact on future treatment strategies. Nat Rev Clin Oncol 2010;7:493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nautiyal J, Rishi AK, Majumdar AP. Emerging therapies in gastrointestinal cancers. World J Gastroenterol 2006;12:7440–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schamel WW, Dick TP. Signal transduction: specificity of growth factors explained by parallel distributed processing. Med Hypotheses 1996;47:249–55. [DOI] [PubMed] [Google Scholar]
- 22.Ramanathan RK. Alternative dosing schedules for cetuximab: a role for biweekly administration? Clin Colorectal Cancer 2008;7:364–8. [DOI] [PubMed] [Google Scholar]
- 23.Liu B, Neufeld AH. Activation of epidermal growth factor receptors in astrocytes: from development to neural injury. J Neurosci Res 2007;85:3523–9. [DOI] [PubMed] [Google Scholar]
- 24.Carroll SL, Miller ML, Frohnert PW, et al. Expression of neuregulins and their putative receptors, ErbB2 and ErbB3, is induced during Wallerian degeneration. J Neurosci 1997;17:1642–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andres C, Meyer S, Dina OA, et al. Quantitative automated microscopy (QuAM) elucidates growth factor specific signalling in pain sensitization. Mol Pain 2010;6:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tveit K, Guren T, Glimelius B, et al. Randomized phase III study of 5-fluouracil/folinate/oxaliplatin given continously or intermittently with or witout cetuximab: the NORDIC VII study (NCT00145314). Ann Oncol. 2010;21(Suppl 8):viii1–2. [Google Scholar]
- 27.Maughan TS, Adams RA, Smith CG, et al. ; MRC COIN Trial Investigators. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet 2011;377:2103–14. [DOI] [PMC free article] [PubMed] [Google Scholar]