Abstract
Objective
To investigate the usefulness of a novel marker for melanocytic proliferations.
Design
Using a novel monoclonal antibody against soluble adenylyl cyclase (sAC), various benign and malignant melanocytic proliferations were immunostained.
Setting
Weill Medical College of Cornell University dermatopathology laboratory.
Main Outcome Measures
The results were qualitative, not quantifiable.
Results
The sAC immunostaining produced distinctive patterns that paralleled melanomagenesis. At one pole of the spectrum were benign nevi, including atypical nevi of special sites and recurrent nevi showing a distinct pattern of dotlike Golgi staining, while at the opposite pole was melanoma, in which many cells demonstrated an intense pannuclear expression pattern, often accompanied by loss of the Golgi expression pattern. Melanomas of lentigo maligna and acral lentiginous subtypes exhibited the most striking pannuclear expression, while nodular melanomas showed the least, although with supervening enhanced diffuse cytoplasmic expression. Loss of the Golgi expression pattern was a feature of malignant melanoma.
Conclusion
The sAC expression pattern is complex but seems discriminatory, with distinctive and variable staining patterns according to the nature of the lesion biopsied.
Melanocytic proliferations are among the most critical diagnostic challenges in the field of dermatopathology. They represent a group of lesions that exhibit striking heterogeneity in terms of morphologic structure and biologic behavior. At one pole of the spectrum are completely benign lesions that are not diagnostically difficult, such as congenital and common acquired nevi. At the opposite pole of the spectrum are overtly malignant melanomas. More problematic is a broad group of pigmented lesions that exhibit variable atypia, including dysplastic nevi, atypical nevi of special sites, Spitz nevi, and melanocytic tumors of undetermined malignant potential (ie, borderline tumors).1
A few immunohistochemical stains, such as HMB45 and Ki67, are routinely used to distinguish benign lesions from melanoma. Neither stain is specific nor especially sensitive, and HMB45 is positive in junctional nevomelanocytes and the superficial aspects of the dermal component, with a progressive decrease in expression toward the dermal base of a benign nevus.2–4 Expression at varying levels throughout the dermal component can be observed in the setting of melanomas other than desmoplastic melanoma, in which this marker is usually negative. Nevertheless, there are benign lesions that express HMB45 in a pandermal fashion, including inverted type A nevus, deep penetrating nevus, blue nevus, and cellular blue nevus. Ki67 is a nuclear proliferation marker that shows low-level expression in nevi, typically with a zonation paralleling that of HMB45, with discernible nuclear staining present within the epidermis and superficial dermis. In borderline melanocytic lesions, the proliferation index is higher, although not typically in the range of that seen in melanoma. In our experience, the proliferation index in borderline tumors can be as high as 10% to 15% of melanocytes, while melanomas often (but not always) express Ki67 in greater than 15% to 20% of nuclei. To our knowledge, there are no other discriminatory immunostains used in routine practice to help distinguish benign from malignant melanocytic proliferations.2–4
Cyclic adenosine monophosphate (cAMP) is a ubiquitous signaling molecule involved in cellular growth and apoptosis that is evolutionarily conserved from bacteria to humans. In mammalian cells, there exist 2 classes of the protein adenylyl cyclase, the enzyme that synthesizes cAMP from adenosine triphosphate. One class, encoded by 9 transmembrane adenylyl cyclase genes, is constitutively localized to the plasma membrane and is sensitive to activation by extracellular signals via activation of G protein–coupled receptors.5 The other class is encoded by the soluble adenylyl cyclase (sAC) gene. Unlike transmembrane adenylyl cyclases, sAC is not permanently localized to the plasma membrane but is present in the cytoplasm, mitochondria, centrioles, and nucleus.6–10 In collaboration with the laboratory that cloned and purified the sAC protein,11 we identified the sAC protein in human epidermal keratinocytes and melanocytes5 and showed that its localization in keratinocytes is not fixed. In particular, in certain hyperproliferative keratinocyte diseases, sAC moves from the cytoplasm into the nucleus.1 Based on these prior studies, we chose to study sAC protein localization in various melanocytic lesions, including benign nevi and melanomas.12 Distinct staining patterns emerged that may have diagnostic implications and perhaps reflect the presumptive biologic progression inherent to melanoma and its precursors. The objective of this study was to investigate the usefulness of sAC as a discriminatory stain between benign nevi vs melanomas. The advantages of sAC immunostaining as a diagnostic adjunct in the assessment of melanocytic proliferations are the same as those for immunohistochemistry in general, namely, cost, speed, and ease of interpretation.
METHODS
The immunohistochemical staining procedure for sAC as previously published5 is described in the subsection that follows. A spectrum of melanocytic lesions was assessed. Among the cases studied were benign nevi,13 atypical nevi of special sites,10 recurrent nevi,10 capsular nevi,4 and malignant melanoma (superficial spreading and lentigo maligna,14 acral lentiginous,3 and nodular subtypes7), as well as metastatic melanoma.7
IMMUNOHISTOCHEMISTRY ON HUMAN TISSUE
Cases were retrospectively identified using archival tissue from the database of the Weill Medical College of Cornell University dermatopathology laboratory. Immunostaining of patient samples was approved under institutional review board protocol 0710009479, Weill Medical College of Cornell University. The study was conducted in accord with the principles of the Declaration of Helsinki. All steps were performed using a commercially available stainer (Bond-Max Autostainer; Leica Microsystems, Bannockburn, Illinois) as previously reported.5 Formalin-fixed paraffin-embedded samples were first dewaxed and then prepared for immunostaining. R21 is a mouse monoclonal antibody directed against amino acids 203 to 216 of human sAC protein.5 All sections were treated as follows for sAC immunostaining5: after the dewaxing procedure, the samples were pretreated (HIER1, Leica Microsystems), followed by HIER1 pretreatment for 30 minutes at 100°C and then HIER1 pretreatment for 12 minutes at ambient temperature. The primary antibody (3 mg/mL) was applied for 25 minutes. The optimal dilution was determined to be 1:1200. Following this step, the sections were treated by a postprimary alkaline phosphatase step for 20 minutes for signal amplification, and the mixed red substrate was applied for 10 minutes, followed by washing and mounting with a coverslip.
ASSESSMENT OF sAC IMMUNOSTAINING
An estimate about the percentage of cells exhibiting a particular staining pattern was made (0%, 1%–10%, >10%–25%, >25%–50%, >50%–75%, >75%–90%, or >90%). The main staining patterns were cytoplasmic and nuclear. With respect to cytoplasmic staining, the following 3 main patterns were observed: (1) Golgi pattern with a discrete dotlike morphologic structure, (2) Golgi pattern showing a broad granular pattern, and (3) diffuse cytoplasmic pattern that was increased relative to the background staining found within the cytoplasm of nonlesional cells (ie, keratinocytes within the epidermis). For cytoplasmic staining, intensity was graded on a scale from 1 to 3. With regard to nuclear staining, the following 3 main patterns were assessed: (1) nucleolar pattern, which was graded on a scale of 1 to 3 according to intensity; (2) focal granular staining within the nucleus, with intensity graded on a scale of 1 to 3; and (3) pannuclear pattern, where 80% or more of the nucleus diameter showed a homogeneous pattern of staining, granular pattern of staining, or both. Initial observations and analysis were performed by one of us (C.M.M.) in a non-blinded manner to establish the algorithm. Following the establishment of a consensus of analysis and an algorithm for assessing sAC immunostaining by C.M.M., 30 unequivocal benign nevi and melanoma cases stained only with anti-sAC antibody were sent to another of us (A.N.C.), along with the established algorithm to determine reproducibility in a blinded manner. The slides analyzed by 3 of us (C.M.M., A.N.C., and G.D.) constituted our training set. Using these slides, we trained 2 medical students with basic histologic experience and a dermatologist (J.H.Z.) on how to identify pannuclear- and perinuclear dotlike Golgi–stained melanocytes. As a validation set, they were then given 36 unequivocal benign nevi and melanoma cases stained only with R21 (ie, no hematoxylineosin–stained cases were provided). They were asked to score each slide for the presence or absence of pannuclear or perinuclear dotlike Golgi staining relative to a cell percentage cutoff as discussed in the next subsection.
STATISTICAL ANALYSIS
To determine if particular staining patterns were present or absent in a given type of lesion more often than simple chance, we performed a binomial proportion test (with 50% assumption being simple chance) using 2-sided t test. To determine if there was a qualitative difference in staining pattern between diagnoses, we performed a rank order analysis, followed by the χ2 test. To determine if there was a quantitative difference in staining pattern between diagnoses, we performed an NPAIR1WAY using the Mantel-Haenszel χ2 test and confirmed these trends using the Spearman rank correlation coefficient analysis. To determine an appropriate threshold for the frequency of a given staining pattern, a receiver operating characteristic curve analysis with a 4-number ordinal rating scale was performed and was compared with a “clinical” presence or absence of a given diagnosis for each staining pattern frequency. From these data, we established a cutoff of 25% between specific percentages of cells with a given staining pattern and a given diagnosis. However, aside from performing a receiver operating characteristic curve analysis, the patterns clearly separated at 25%. Using these cutoffs, we established the sensitivity and specificity of the sAC immunostaining pattern in the training set (Table 1). Sensitivity was determined by calculating the number of melanoma diagnoses with 25% or less cells having dotlike Golgi expression, more than 25% having pannuclear expression, or a combination of each as indicated in Table 1 divided by the total number of melanomas examined. Specificity was determined by calculating the number of benign nevi diagnoses with 25% or less cells having dot-like Golgi expression, more than 25% having pannuclear expression, or a combination of each as indicated in Table 1 divided by the total number of nevi examined. Following confirmation of the accuracy of these cutoffs, we trained 2 medical students with basic histologic experience and a dermatologist on how to identify pannuclear- and perinuclear dotlike Golgi–stained melanocytes. We then randomly selected 36 unequivocal benign nevi and melanoma cases to be stained only with R21. Using the afore-described cutoff, slides were scored for either staining pattern and were compared with pathologist-rendered diagnoses (C.M.M.). Specificity, sensitivity, positive predictive value, and negative predictive value were then calculated.
Table 1.
Sensitivity and Specificity of Benign Nevi vs Melanomas in the Training Set
| sAC Immunostaining Patterna | Diagnosisb | Sensitivity, % | Specificity, % | P Value | No. of Cases |
|---|---|---|---|---|---|
| Dotlike Golgi and pannuclear | All melanomas | 100 | 50 | <4.1E-7 | 67 |
| Dotlike Golgi or pannuclear | All melanomas | 88 | 100 | <3.9E-6 | 67 |
| Pannuclear | All melanomas | 90 | 57 | <5.2E-7 | 66 |
| Dotlike Golgi | All melanomas | 98 | 50 | <4.5E-6 | 67 |
| Dotlike Golgi and pannuclear | Superficial spreading melanoma | 100 | 61 | <8.6E-5 | 39 |
| Dotlike Golgi or pannuclear | Superficial spreading melanoma | 78 | 100 | <2.2E-4 | 39 |
| Pannuclear | Superficial spreading melanoma | 80 | 100 | <6.1E-5 | 38 |
| Dotlike Golgi | Superficial spreading melanoma | 94 | 62 | <2.6E-4 | 39 |
| Dotlike Golgi and pannuclear | Lentigo maligna melanoma | 100 | 93 | <1.8E-5 | 23 |
| Dotlike Golgi or pannuclear | Lentigo maligna melanoma | 56 | 100 | <.02 | 23 |
| Pannuclear | Lentigo maligna melanoma | 60 | 100 | <.007 | 23 |
| Dotlike Golgi | Lentigo maligna melanoma | 89 | 93 | <1.5E-4 | 23 |
| Dotlike Golgi and pannuclear | Nodular melanoma | 100 | 88 | <.001 | 21 |
| Dotlike Golgi or pannuclear | Nodular melanoma | 50 | 100 | <.047 | 21 |
| Pannuclear | Nodular melanoma | 54 | 100 | <.02 | 21 |
| Dotlike Golgi | Nodular melanoma | 83 | 87 | <.005 | 21 |
| Dotlike Golgi and pannuclear | Metastatic melanoma | 100 | 100 | <8.6E-6 | 21 |
| Dotlike Golgi or pannuclear | Metastatic melanoma | 50 | 100 | <.047 | 21 |
| Pannuclear | Metastatic melanoma | 54 | 100 | <.02 | 21 |
| Dotlike Golgi | Metastatic melanoma | 88 | 100 | <6.8E-5 | 21 |
Abbreviation: sAC, soluble adenylyl cyclase.
Pattern descriptions are as follows: Dotlike Golgi indicates 25% or less of total melanocytes contain dotlike Golgi pattern. Pannuclear indicates more than 25% of total melanocytes contain pannuclear pattern. Dotlike Golgi and pannuclear indicates 25% or less of total melanocytes contain dotlike Golgi pattern and more than 25% of total melanocytes contain pannuclear pattern. Dotlike Golgi or pannuclear indicates 25% or less of total melanocytes contain dotlike Golgi pattern or more than 25% of total melanocytes contain pannuclear pattern.
All melanomas indicates lentigo maligna, acral lentiginous, superficial spreading, and nodular melanoma data combined.
RESULTS
sAC IMMUNOSTAINING ALGORITHM
Results of initial observations and analysis were used to establish the sAC immunostaining algorithm. A summary is given in Table 2.
Table 2.
Soluble Adenylyl Cyclase (sAC) Immunostaining Algorithm
| Diagnosis | sAC Immunostaining Pattern, %
|
||||
|---|---|---|---|---|---|
| Dotlike Golgi | Broad Golgi | Nuclear or Granular Nuclear | Pannuclear | Diffuse Cytoplasm | |
| Benign nevi | |||||
| Compound, dermal, congenital | >50 | NA | 50 | <10 | NA |
| Capsular nevus | >50 | NA | 50 | <10 | NA |
| Atypical nevus of special site | >50 | NA | NA | >10a | NA |
| Melanomas | |||||
| Lentigo maligna | NA | NA | NA | >90 | NA |
| Acral lentiginous | Broad Golgib | NA | NA | >90 | NA |
| Superficial spreading | <25c | >50 | >25 | >50 | Variable |
| Nodular | NA | NA | 50 | ≥25 | 50 |
| Metastatic | NA | NA | NA | >50 | 50 |
Abbreviation: NA, pattern not seen for the given diagnosis.
Never in dermis.
A rare finding.
Sometimes exceeds 50%.
BENIGN NEVI
Congenital and Common Acquired Nevi
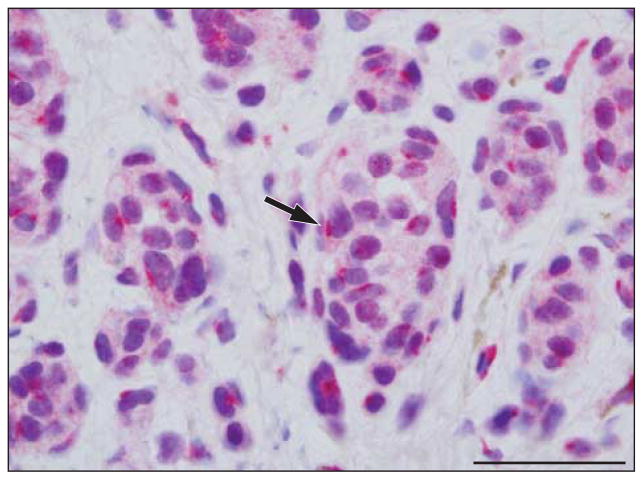
In 14 cases of benign congenital and common acquired nevi without any morphologic features indicative of dysplasia, the sAC antibody profile was characterized by a discrete perinuclear dotlike Golgi staining pattern (arrow in Figure 1) in most melanocytes within the epidermis and dermis (P=.001). Nucleolar staining could be seen and was usually weak (P=.008). Focal weak granular staining in the nucleus was seen in a few cases (P=.59). Most cases did not exhibit any pannuclear staining; however, rare cells with this particular staining pattern could be observed (P=.71).
Figure 1.
Benign dermal nevus with soluble adenylyl cyclase (sAC) immunostain and hematoxylineosin costain. The sAC immunostaining pattern (red) is characterized by small dotlike Golgi positivity (arrow) around the nucleus; pannuclear staining is not observed. Weak incomplete granular nuclear staining is sometimes present. Scale bar, 25 μm.
Recurrent Nevi
We examined 10 cases of recurrent nevi (eFigure 1 [http://www.archdermatol.com]) in which there was enhanced intraepidermal and superficial dermal atypia localized to the zone of the cicatrix. The residual lesion or the previously biopsied melanocytic lesion was in the context of a benign nevus. Despite the enhanced atypia, the staining pattern essentially mirrored that encountered in the original lesion, defined by a predominant perinuclear dotlike staining pattern. A few melanocytes exhibited a pannuclear staining pattern.
Atypical Nevi of Special Sites
Among 10 cases of atypical acral nevi examined, the pattern was similar to that noted with benign common acquired nevi,3 namely, a prominent perinuclear dotlike Golgi pattern (arrow in eFigure 2) (P=.045), with variable weak nucleolar staining (P=.045). In cases exhibiting mild atypia, pannuclear staining was typically seen in less than 10% of intraepidermal melanocytes and was often accompanied by weak nucleolar staining. In nevi exhibiting a higher grade of atypia, more intraepidermal melanocytes showed pannuclear staining, while other melanocytes showed an intense incomplete granular staining pattern. There was preservation of the dotlike Golgi pattern in dermal melanocytes, where pannuclear expression was not observed.
MELANOMAS
Melanomas With a Radial Growth Phase (Acral Lentiginous, Lentigo Maligna, and Superficial Spreading)
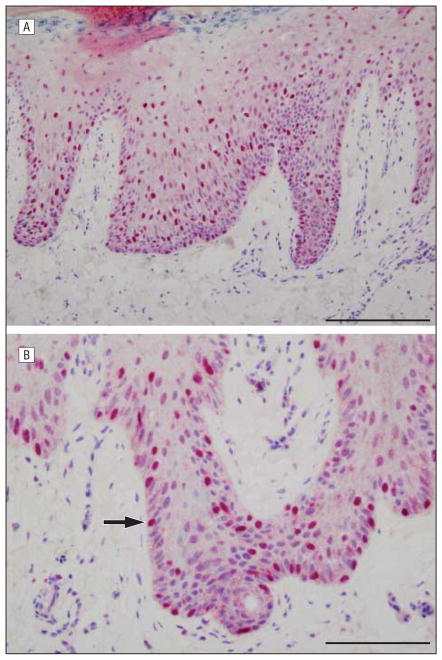
In acral lentiginous melanomas, neoplastic melanocytes demonstrated a distinctive pattern of lentiginous dysplasia characterized by enlarged cells with darkly staining angulated nuclei, representing 90% or more of intraepidermal melanocytes in most cases (Figure 2). These cells exhibited an intense broad Golgi decoration pattern, with most cells demonstrating pannuclear sAC immunostaining.
Figure 2.
Acral lentiginous melanoma with soluble adenylyl cyclase (sAC) immunostain and hematoxylineosin costain. The sAC immunostaining pattern (red) demonstrates extensive pannuclear staining of melanocytes (arrow) within the epidermis, highlighting the atypical melanocytes in a lentiginous and pagetoid array. Discernible Golgi staining is not apparent. Scale bar, 100 μm (A) and 75 μm (B).
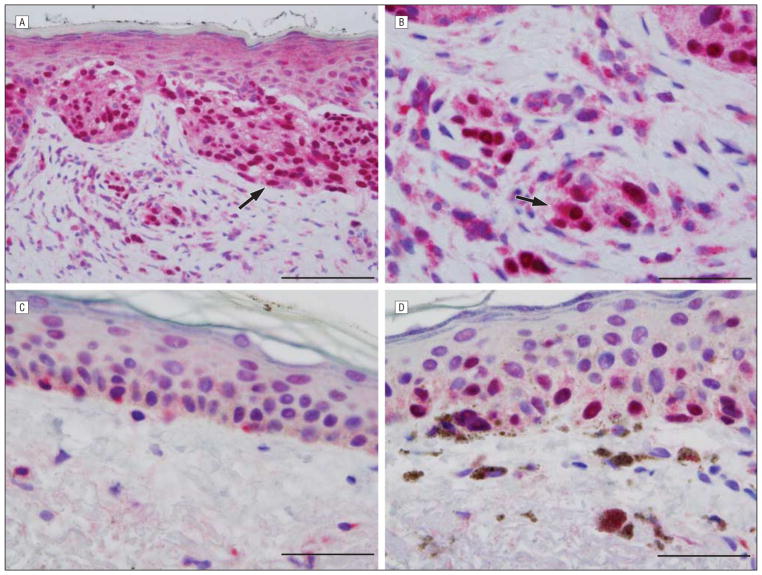
In lentigo maligna melanomas, most cases demonstrated prominent pannuclear homogeneous or granular expression (arrow in Figure 3A) (P=.003). Of the cases studied, all but 1 showed pannuclear staining involving most neoplastic melanocytes. Most cases had no discernible Golgi expression, but where Golgi decoration was discernible, it was typically of the broad pattern (arrow in Figure 3B). In addition, the pannuclear staining allowed easy discrimination between lentigo maligna and background changes of chronic photoactivation shown in Figure 3C (negative margin) and Figure 3D (positive margin).
Figure 3.
Lentigo maligna melanoma. A and B, Central tumor with soluble adenylyl cyclase (sAC) immunostain and hematoxylineosin costain. The sAC immunostaining pattern (red) demonstrates extensive pannuclear staining of melanocytes (arrow in A) within the epidermis and dermis. A broad Golgi pattern is seen (arrow in B). C and D, Negative (C) and positive (D) margins with sAC immunostain (red) and hematoxylineosin costain. C, Benign photoactivated melanocytes exhibiting a perinuclear dotlike Golgi pattern are noted at the negative lateral margin of resection. D, In contrast, striking pannuclear staining of malignant melanocytes is present at a positive inked margin of resection. Scale bar, 75 μm (A), 50 μm (B), 25 μm (C), and 25 μm (D).
Superficial spreading melanomas displayed pannuclear expression in epidermal and dermal melanocytes (Figure 4, eFigure 3, and eFigure 4) (P < .001). In all cases, 25% or more of intraepidermal and dermal melanocytes showed pannuclear expression. In 50% of cases, 50% or more of intraepidermal and dermal melanocytes exhibited pannuclear expression (Figure 4), while in 30% of cases more than 75% of melanocytes showed pannuclear expression. Most cases had a broad Golgi expression pattern (P=.03), while a predominant dotlike Golgi pattern was seen in less than 10% of cases, with no discernible Golgi expression in 3 cases. Many neoplastic melanocytes demonstrated combined incomplete nuclear and macronucleolar staining (arrow C in Figure 4C) (P < .001) in addition to pannuclear staining (arrow A in Figure 4C). In some melanomas, cells were devoid of all staining (arrow B in Figure 4C). In melanomas arising in preexisting nevi, the sAC expression pattern could be used to discriminate invasive melanoma cells from residual nevus cells (eFigure 3), with the latter having classic perinuclear Golgi expression (arrow A in eFigure 3) without pannuclear expression vs the invasive melanoma component demonstrating a pannuclear pattern (arrow B in eFigure 3). This discriminatory pattern helped determine the depth of invasion. In eFigure 4A (arrow), pannuclear sAC expression clearly defines the in situ and superficially invasive components of the melanoma, while in routine stains nuclear atypia of the precursor component prohibited this distinction. There was a clear difference between the pannuclear staining of melanoma cells above vs the dotlike Golgi expression in the subjacent benign melanocytes (arrow in eFigure 4B). The sAC expression pattern in superficial spreading melanoma was constant regardless of anatomic location. Acral melanomas of superficial spreading subtype demonstrated mostly pannuclear melanocytes, a few with dotlike Golgi, and diffuse cytoplasmic staining and bore no resemblance to acral lentiginous melanomas, which showed intense pannuclear but little cytoplasmic expression (Figure 2).
Figure 4.
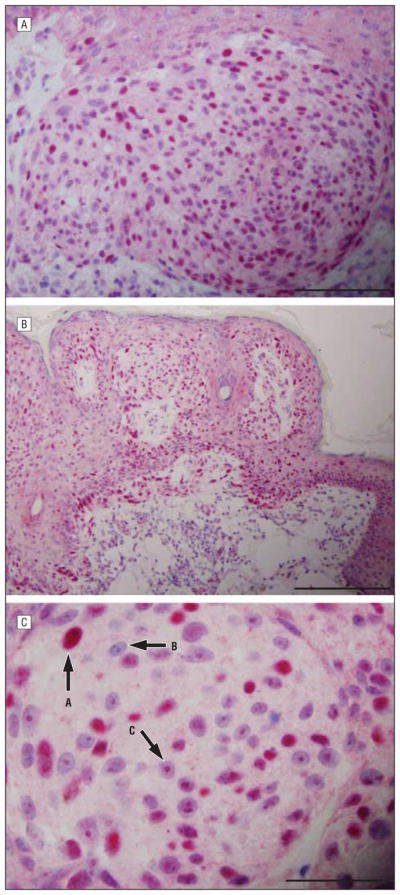
Malignant melanoma of superficial spreading subtype. A, B, and C, Soluble adenylyl cyclase (sAC) immunostain and hematoxylineosin costain show different morphologic structures of melanoma. The sAC immunostaining pattern (red) demonstrates fairly extensive pannuclear staining within the epidermis. C, There is focal pannuclear staining amid neoplastic cells in the dermis (arrow A). Some nuclei are devoid of staining (arrow B). There is conspicuous nucleolar staining (arrow C). A noticeable lack of Golgi staining is apparent. Scale bar, 75 μm (A), 100 μm (B), and 50 μm (C).
Nodular Melanomas
Seven cases of nodular melanomas showed pannuclear expression (P=.008) but in fewer cells compared with the other melanoma subtypes. Pandermal melanocyte nuclear expression was observed. Many cells also had a prominent nucleolar staining pattern (P=.03). The Golgi expression pattern was typically lost, and cytoplasmic expression was primarily a diffuse and intense pattern (P=.01). The classic sAC expression profile of nodular melanoma comprised pannuclear expression in nuclei at all levels, cells exhibiting a clustered pattern with nucleolar expression, and diffuse cytoplasmic expression, typically unaccompanied by discrete Golgi expression. Diffuse cytoplasmic expression could be seen in the other melanoma subtypes but was quantitatively and qualitatively less compared with that in nodular melanomas. The neoplastic cells that did not exhibit a pannuclear staining pattern showed a prominent nucleolar and incomplete granular nuclear staining pattern (Figure 5).
Figure 5.
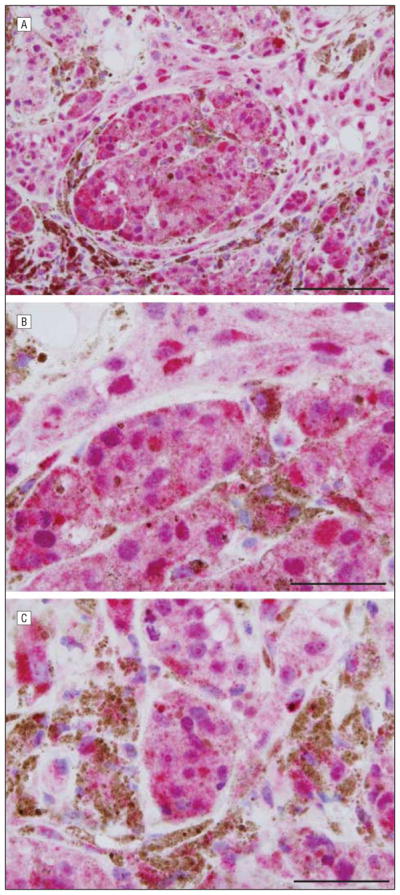
Nodular melanoma showing soluble adenylyl cyclase immunostain and hematoxylineosin costain. A, B, and C, All 3 panels show striking pannuclear staining. Golgi staining is present in a few cells. There is diffuse intense cytoplasmic staining. Scale bar, 75 μm (A), 50 μm (B), and 50 μm (C).
Table 2 summarizes the dotlike Golgi and pannuclear staining patterns in benign nevi compared with those in melanomas in our training set. We reasoned that loss of dotlike Golgi expression with gain of pannuclear expression was indicative of melanoma. Based on clinical correlation and statistical analysis, we established that melanoma was consistent with loss of dotlike Golgi expression representing 25% or less of total melanocytes having this staining pattern (>50% to 75% for benign nevi; 0%–25% for melanomas) and with gain of pannuclear expression representing greater than 25% of total melanocytes having this staining pattern (0%–25% for benign nevi; >50% to 90% for melanomas). It was rare to find a benign nevus with dotlike Golgi staining below 50% or pannuclear staining exceeding 10%, and it was unusual to find an unequivocal melanoma with dotlike Golgi staining exceeding 25% or pannuclear staining below 50%, making these cutoffs fairly discriminatory. The absence of dotlike Golgi expression combined with the presence of pannuclear expression proved to be very sensitive and specific for the diagnosis of melanoma (Table 2). We wanted to test the ability of this stain when used without hematoxylineosin by non–pathology-trained personnel to distinguish between benign nevi and melanomas. Therefore, we trained 2 medical students with basic histologic experience and a dermatologist on how to identify pannuclear- and dotlike Golgi–stained melanocytes. They then examined 36 unequivocal benign nevi and melanomas and determined if dotlike Golgi or pannuclear staining represented more than 25% or 25% or less of total melanocytes. This analysis was performed blinded from each other and blinded to the diagnosis rendered by the pathologist. This method proved reproducible in that the 3 observers disagreed on only 3 of 72 determinations (36 pannuclear and 36 dotlike Golgi). For pannuclear staining, the sensitivity was 75%, the specificity was 94%, the positive predictive value was 92%, and the negative predictive value was 81% for the diagnosis of melanoma (P < .001 for all). For dotlike Golgi staining, the sensitivity was 63%, the specificity was 67%, the positive predictive value was 63%, and the negative predictive value was 67% for the diagnosis of melanoma (P < .001 for all).
EXTRACUTANEOUS MELANOCYTIC PROLIFERATIONS
Benign Capsular Nevus
Four cases of benign capsular nevi were studied. In all 4 cases, the staining pattern was a weak cytoplasmic one without any nuclear staining or was a dotlike Golgi pattern (P=.03). In no case were the findings comparable to those observed in melanomas or other high-grade melanocytic proliferations (Figure 6).
Figure 6.
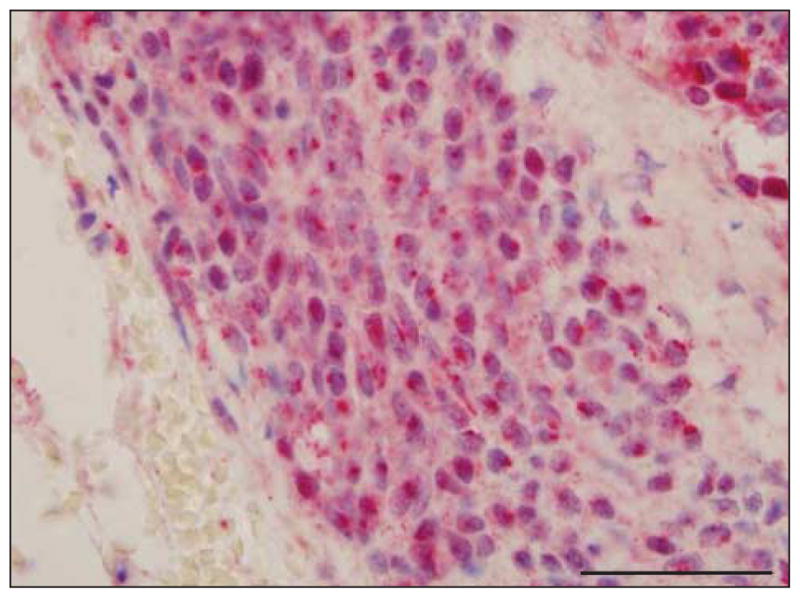
Capsular nevus showing soluble adenylyl cyclase (sAC) immunostain and hematoxylineosin costain. The sAC Golgi staining pattern (red) is noted around the nucleus, and there is occasional incomplete granular nuclear staining. Pannuclear staining is not identified. Scale bar, 50 μm.
Metastatic Melanoma
Seven cases of metastatic melanoma were studied, comprising 5 cases of disease metastasized to the lung and 2 cases of disease involving regional lymph nodes. In melanomas with a spindled cytomorphologic structure, most cells exhibited a prominent pannuclear staining pattern (Figure 7) (P=.008). In metastatic lesions exhibiting a high-grade epithelioid appearance, pannuclear staining was noted but was present in a minor cell population (data not shown). There was a complete absence of Golgi staining in 5 of 7 cases. In 2 cases in which Golgi staining was observed, few cells stained with sAC antibody, and the pattern was broad as opposed to the sharp dotlike staining pattern of a benign or dysplastic nevus. The intense diffuse pattern of cytoplasmic staining was observed in all but 1 case (P=.03).
Figure 7.
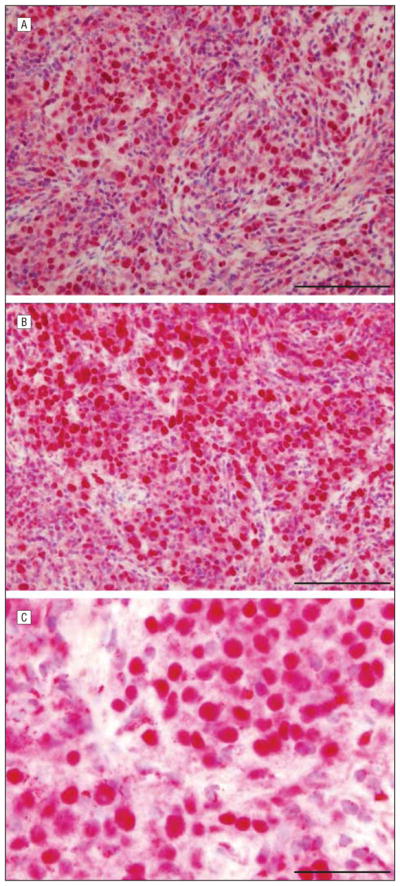
Metastatic melanoma in the lung showing soluble adenylyl cyclase (sAC) immunostain and hematoxylineosin costain. Images A, B, and C increase in magnification. The sAC immunostaining pattern exhibited extensive pannuclear staining without cytoplasmic staining. Scale bar, 100 μm (A), 100 μm (B), and 50 μm (C).
CONSENSUS REVIEW
Based on these results, the sAC immunostaining algorithm was confirmed (Table 2). One of us (A.N.C.) rendered diagnoses based on the sAC algorithm and in a blinded analysis accurately identified the main subtype of melanocytic lesion based on the sAC pattern in 90% of 30 cases reviewed.
COMMENT
We examined the immunolocalization of the sAC protein in benign and malignant melanocytic lesions and found that characteristic patterns emerge with this antibody depending on the nature of the melanocytic lesion. The most extreme variants of sAC immunostaining were typified (1) by unequivocally benign dermal nevi, which demonstrated a monotypic pattern of discrete perinuclear dotlike staining; and (2) by melanomas, in which, especially when associated with a prominent lentiginous pattern, greater than 50% of lesional melanocytes exhibited pannuclear staining without conspicuous Golgi staining or, if there was discernible Golgi staining, the pattern was broad, a point that will be elaborated herein.
In melanomas, the Golgi expression pattern was altered compared with that in most banal nevi: a discrete dotlike Golgi pattern was very uncommon unless it was amid residual nevus. It could be absent, especially in those melanomas with intense pannuclear staining or in nodular melanoma. If there was a discernible Golgi expression pattern, it was often broad, perhaps best typified by the melanomas of superficial spreading subtype. This pattern was heterogeneous not only between cases but among cells in an individual case; because of the quantitative differences between staining patterns, the sAC immunostain seemed sensitive and specific when distinguishing between benign nevi and melanomas in the training set (Table 1) and in the validation set. Because of the ease of detection and the robust nature of pannuclear staining, this proved to be a more consistent marker for melanoma in both the training and validation sets. Dotlike Golgi staining can be challenging to detect, especially when some background keratinocyte staining is present, and this pattern is not universally “negative” in melanoma. For these reasons, this staining pattern is less robust in distinguishing benign nevi from melanomas.
The staining pattern also varied according to the subtype of melanoma. In melanomas of superficial spreading subtype, especially in those cases arising in precursor dysplastic nevi, preservation of the Golgi pattern of staining could be found. However, the discriminating feature found in superficial spreading melanoma was the presence of supervening pannuclear staining, affecting at least 25% of the cell populace in the epidermis and in the dermis, with dermal pannuclear staining present in cases in which the tumor had assumed an invasive growth pattern. In fact, a significant degree of pannuclear staining amid cells in the dermis (as opposed to intraepidermal staining) was an important finding signifying invasive melanoma. In acral lentiginous melanoma and lentigo maligna melanoma, the extent of pannuclear staining was greatest compared with that in nodular melanoma or superficial spreading melanoma. Due to the consistent nature of this extensive pannuclear pattern amid lesional melanocytes, we find the sAC immunostain to be very useful in assessing lentigo maligna margins (Figure 3C and D) or in delineating residual disease from cicatricial reactive lentiginous melanocytic hyperplasia (data not shown). In cicatricial lentiginous melanocytic hyperplasia and in chronic photoactivation, a characteristic pattern of pannuclear staining is not observed. Recurrent nevi can exhibit severe atypia, which in concert with inflammation and fibrosis can exactly recapitulate partially regressed malignant melanoma. The sAC immunostain mirrored the pattern of a benign nevus despite enhanced cytologic atypia in the zone of recurrence (eFigure 1). In nodular melanoma, the enhanced diffuse cytoplasmic staining without Golgi localization defined the dominant pattern; pannuclear staining was found in randomly disposed neoplastic cells. Notably, the nodular melanoma pattern was reminiscent of the sAC immunostaining pattern found in extracutaneous metastatic melanoma and was dissimilar to other subtypes of cutaneous melanoma, indicating that the biologic activity of this cutaneous tumor (from a sAC protein “point of view”) may be more similar to metastatic disease than to other cutaneous subtypes of melanoma (ie, superficial spreading melanoma, lentigo maligna melanoma, and others). Because of the difference in aggressiveness of nodular melanoma compared with that of other cutaneous melanomas, sAC signaling may provide information about key steps in the melanomagenesis and progression of cutaneous melanomas to metastatic disease.
Metastatic melanoma exhibited absent Golgi and nucleolar expression. In metastatic deposits associated with an epithelioid appearance, there was diffuse cytoplasmic and focal pannuclear expression recapitulating the pattern seen in nodular melanoma. Metastatic spindle cell melanomas had a pannuclear pattern reminiscent of the patterns seen in acral lentiginous melanoma and lentigo maligna melanoma, subtypes that are characteristically associated with a spindle cell vertical growth–phase component. When comparing the expression pattern with that of benign capsular nevus cells, the differences were obvious. The metastatic melanoma deposits had an abnormal pattern typical of melanoma with prominent pannuclear staining or no staining, while capsular nevus cells showed a reproducible dotlike Golgi expression pattern. The biologic basis for loss of Golgi, nucleolar, or cytoplasmic sAC expression in metastatic melanoma is unclear but nonetheless seems to be a hallmark.
The question arises as to the biologic significance of sAC expression profiles in melanocytic lesions. Melanocytes are very sensitive to changes in cAMP levels; primary melanocytes cannot be grown without providing high levels of cAMP. These levels are most commonly induced in cells by hormones such as melanocyte-stimulating hormone and corticotropin, products of Pro-opiomelanocortin (POMC) protein. Pro-opiomelanocortin protein is expressed in the pituitary gland and locally in the skin. Local production of POMC protein is thought to be the result of a skin-based stress response. The main receptor leading to increased levels of cAMP in melanocytes is the melanocortin receptor, a canonical G protein–coupled receptor that activates a heterotrimeric G protein, leading to cAMP synthesis via transmembrane adenylyl cyclases.13,15,16 It should be noted that sAC does not respond to G proteins but instead is directly stimulated by calcium and bicarbonateions. The primary outcome of cAMP elevation in melanocytes is increased growth and melanogenesis17; in addition, cAMP is implicated in melanocyte migration,18 vascular mimicry,19 and immune regulation.20,21 Lester et al14 and Sheppard et al22,23 found that in vivo cAMP level and adenylyl cyclase activity are upregulated in murine melanoma and that metastatic capability of B16 melanoma clones correlated with cAMP accumulation.
Many of these processes also involve key signaling cascades, such as the Ras/Raf/extracellular signal–regulated kinase (ERK) and PI3K/AKT pathways.24–27 cAMP is known to activate Ras via a poorly characterized melanocyte exchange protein25,28,29 and Rap via an exchange protein directly activated by cAMP (EPAC).18 Ras and Rap normally signal via B-Raf to activate ERK18,30 because under normal conditions, cAMP activation of PKA phosphorylates key inhibitory sites on C-Raf, preventing it from activation. PKA was found to be essential for B-Raf and ERK activation and for melanoma proliferation.31 The regulatory subunit 1A of PKA (PKAR1A), the protein component that binds cAMP, is upregulated in human melanoma compared with normal melanocytes, and genetic downregulation of PKAR1A decreases cell growth and induces apoptosis.32 These observations are intriguing because Zippin et al10 observed that sAC colocalizes with PKAR1A in the nucleolus of cancer cell lines. It is clear that cAMP has numerous roles in melanocyte biologic function, suggesting that perturbation of normal cAMP signaling could have serious repercussions on cells, such as allowing or facilitating transformation.13,15,16
Zippin et al5,10 demonstrated that nuclear sAC is capable of activating a nuclear cAMP microdomain, leading to activation of the transcription factor CREB (cAMP response element binding protein). In addition, Zippin et al10 previously demonstrated that nuclear sAC is associated with activated CREB protein in keratinocyte malignant neoplasms, such as squamous cell carcinoma. The accumulation of sAC in the nucleus may represent a necessary step in malignant transformation or may occur in tandem with transformation and serve a supportive role. Previous work highlighting the ability of nuclear sAC to regulate CREB purports the likely role of nuclear sAC in the regulation of gene expression.10 However, several studies5,6,10,11 demonstrate the existence of distinct sAC-modulated cAMP signaling microdomains localized throughout the cell. It is intriguing to note that Raf and EPAC (2 cAMP-regulated signaling proteins) are enriched in the perinuclear Golgi area and shuttle between this location and the plasma membrane on activation.18 Therefore, one could infer from sAC immunostaining patterns that it may not be the appearance of sAC in the nucleus that induces a malignant phenotype but instead its absence from the perinuclear Golgi (ie, the dotlike Golgi pattern) cAMP microdomain. Future work will be focused on determining the role of sAC in melanomagenesis at each of these subcellular locations.
The sAC expression profile seems to provide a confirmatory test to distinguish benign from malignant lesions. Soluble adenylyl cyclase is ubiquitously expressed and is present in other cell types of the skin, a drawback that proves problematic in the assessment of low-density single-cell lentiginous melanocytic proliferations. However, neither the cytologic characteristics nor the architecture is obscured by the sAC immunostain: melanin pigment is preserved, and a nested epidermal and dermal growth pattern characteristic of melanocytic lesions is easily discernible. These features aid considerably in the distinction from non-melanocytic skin components. The sAC antibody seems to have practical application to the assessment of capsular nevomelanocytic deposits, proliferative nodules in congenital nevi, and lentigo maligna margin evaluation in the delineation of invasive melanoma from residual nevus cells and may prove useful in the evaluation of prognostically indeterminate melanocytic proliferations. The sAC profile in problematic pigmented lesions is being investigated.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported in part by the Dermatology Foundation, La Roche-Posay North American Foundation, Woman’s Dermatological Society, and American Skin Association (all awarded to Dr Zippin). All the technical staffing and slide preparations were paid for by dematopathology research funds from the Department of Dermatology (Dr Magro).
Footnotes
Author Contributions: All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Magro and Zippin. Acquisition of data: Magro, Crowson, and Desman. Analysis and interpretation of data: Magro, Crowson, Desman, and Zippin. Drafting of the manuscript: Magro and Zippin. Critical revision of the manuscript for important intellectual content: Crowson, Magro, and Zippin. Obtained funding: Zippin. Administrative, technical, and material support: Magro. Study supervision: Magro and Zippin.
Financial Disclosure: None reported.
Additional Contributions: Lonny R. Levin, PhD, and Jochen Buck, MD, PhD, provided the R21 antibody and critically read the manuscript, Adam Widman, BA, and Matt Sagrawsky, BS, scored the sAC immunostaining, and Mary Chang performed the statistical analysis.
Online-Only Material: The eFigures are available at http://www.archdermatol.com.
References
- 1.Crowson AN, Magro CM, Mihm MC. The Melanocytic Proliferations: a Comprehensive Textbook of Pigmented Lesions. New York, NY: Wiley-Liss; 2001. [Google Scholar]
- 2.Lebe B, Pabuççuoğlu U, Ozer E. The significance of Ki-67 proliferative index and cyclin D1 expression of dysplastic nevi in the biologic spectrum of melanocytic lesions. Appl Immunohistochem Mol Morphol. 2007;15(2):160–164. doi: 10.1097/01.pai.0000209868.58699.64. [DOI] [PubMed] [Google Scholar]
- 3.Li LX, Crotty KA, McCarthy SW, Palmer AA, Kril JJ. A zonal comparison of MIB1-Ki67 immunoreactivity in benign and malignant melanocytic lesions. Am J Dermatopathol. 2000;22(6):489–495. doi: 10.1097/00000372-200012000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Rothberg BE, Moeder CB, Kluger H, et al. Nuclear to non-nuclear Pmel17/gp100 expression (HMB45 staining) as a discriminator between benign and malignant melanocytic lesions. Mod Pathol. 2008;21(9):1121–1129. doi: 10.1038/modpathol.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zippin JH, Chadwick PA, Levin LR, Buck J, Magro CM. Soluble adenylyl cyclase defines a nuclear cAMP microdomain in keratinocyte hyperproliferative skin diseases. J Invest Dermatol. 2010;130(5):1279–1287. doi: 10.1038/jid.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Acin-Perez R, Salazar E, Kamenetsky M, Buck J, Levin LR, Manfredi G. Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab. 2009;9(3):265–276. doi: 10.1016/j.cmet.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng Q, Zhang Y, Li Y, Liu Z, Zuo J, Fang F. Two domains are critical for the nuclear localization of soluble adenylyl cyclase. Biochimie. 2006;88(3–4):319–328. doi: 10.1016/j.biochi.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Feng QP, Zuo J, Meng Y, Fang FD. Nuclear localization region in soluble adenylyl cyclase [in Chinese] Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2005;27(3):280–284. [PubMed] [Google Scholar]
- 9.Zippin JH, Chen Y, Nahirney P, et al. Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. FASEB J. 2003;17(1):82–84. doi: 10.1096/fj.02-0598fje. [DOI] [PubMed] [Google Scholar]
- 10.Zippin JH, Farrell J, Huron D, et al. Bicarbonate-responsive “soluble” adenylyl cyclase defines a nuclear cAMP microdomain. J Cell Biol. 2004;164(4):527–534. doi: 10.1083/jcb.200311119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buck J, Sinclair ML, Schapal L, Cann MJ, Levin LR. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc Natl Acad Sci U S A. 1999;96(1):79–84. doi: 10.1073/pnas.96.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magro CM, Crowson AN, Mihm MC, Jr, Gupta K, Walker MJ, Solomon G. The dermal-based borderline melanocytic tumor: a categorical approach. J Am Acad Dermatol. 2010;62(3):469–479. doi: 10.1016/j.jaad.2009.06.042. [DOI] [PubMed] [Google Scholar]
- 13.Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21 (5):457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- 14.Lester BR, Greig RG, Buscarino C, Sheppard JR, Corwin SP, Poste G. cAMP metabolism in B16 melanoma clones during the formation of experimental and spontaneous metastases. Int J Cancer. 1986;38(3):405–411. doi: 10.1002/ijc.2910380316. [DOI] [PubMed] [Google Scholar]
- 15.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84(4):1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 16.Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80(3):979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 17.Jean D, Bar-Eli M. Regulation of tumor growth and metastasis of human melanoma by the CREB transcription factor family. Mol Cell Biochem. 2000;212(1–2):19–28. [PubMed] [Google Scholar]
- 18.Gao L, Feng Y, Bowers R, et al. Ras-associated protein-1 regulates extracellular signal-regulated kinase activation and migration in melanoma cells: two processes important to melanoma tumorigenesis and metastasis. Cancer Res. 2006;66(16):7880–7888. doi: 10.1158/0008-5472.CAN-06-0254. [DOI] [PubMed] [Google Scholar]
- 19.Lissitzky JC, Parriaux D, Ristorcelli E, Vérine A, Lombardo D, Verrando P. Cyclic AMP signaling as a mediator of vasculogenic mimicry in aggressive human melanoma cells in vitro. Cancer Res. 2009;69(3):802–809. doi: 10.1158/0008-5472.CAN-08-2391. [DOI] [PubMed] [Google Scholar]
- 20.Böhm M, Eickelmann M, Li Z, et al. Detection of functionally active melanocortin receptors and evidence for an immunoregulatory activity of α-melanocyte-stimulating hormone in human dermal papilla cells. Endocrinology. 2005;146 (11):4635–4646. doi: 10.1210/en.2005-0665. [DOI] [PubMed] [Google Scholar]
- 21.Eves P, Haycock J, Layton C, et al. Anti-inflammatory and anti-invasive effects of α-melanocyte-stimulating hormone in human melanoma cells. Br J Cancer. 2003;89(10):2004–2015. doi: 10.1038/sj.bjc.6601349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheppard JR, Koestler TP, Corwin SP, et al. Experimental metastasis correlates with cyclic AMP accumulation in B16 melanoma clones. Nature. 1984;308 (5959):544–547. doi: 10.1038/308544a0. [DOI] [PubMed] [Google Scholar]
- 23.Sheppard JR, Lester B, Doll J, et al. Biochemical regulation of adenylate cyclase in murine melanoma clones with different metastatic properties. Int J Cancer. 1986;37(5):713–722. doi: 10.1002/ijc.2910370512. [DOI] [PubMed] [Google Scholar]
- 24.Brennesvik EO, Ktori C, Ruzzin J, Jebens E, Shepherd PR, Jensen J. Adrenaline potentiates insulin-stimulated PKB activation via cAMP and Epac: implications for cross talk between insulin and adrenaline. Cell Signal. 2005;17(12):1551–1559. doi: 10.1016/j.cellsig.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Buscà R, Abbe P, Mantoux F, et al. Ras mediates the cAMP-dependent activation of extracellular signal-regulated kinases (ERKs) in melanocytes. EMBO J. 2000;19(12):2900–2910. doi: 10.1093/emboj/19.12.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filippa N, Sable CL, Filloux C, Hemmings B, Van Obberghen E. Mechanism of protein kinase B activation by cyclic AMP-dependent protein kinase. Mol Cell Biol. 1999;19(7):4989–5000. doi: 10.1128/mcb.19.7.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sable CL, Filippa N, Hemmings B, Van Obberghen E. cAMP stimulates protein kinase B in a Wortmannin-insensitive manner. FEBS Lett. 1997;409(2):253–257. doi: 10.1016/s0014-5793(97)00518-8. [DOI] [PubMed] [Google Scholar]
- 28.Obara Y, Horgan AM, Stork PJ. The requirement of Ras and Rap1 for the activation of ERKs by cAMP, PACAP, and KCl in cerebellar granule cells. J Neurochem. 2007;101(2):470–482. doi: 10.1111/j.1471-4159.2006.04390.x. [DOI] [PubMed] [Google Scholar]
- 29.Khaled M, Larribere L, Bille K, et al. Glycogen synthase kinase 3β is activated by cAMP and plays an active role in the regulation of melanogenesis. J Biol Chem. 2002;277(37):33690–33697. doi: 10.1074/jbc.M202939200. [DOI] [PubMed] [Google Scholar]
- 30.Dumaz N, Hayward R, Martin J, et al. In melanoma, RAS mutations are accompanied by switching signaling from BRAF to CRAF and disrupted cyclic AMP signaling. Cancer Res. 2006;66(19):9483–9491. doi: 10.1158/0008-5472.CAN-05-4227. [DOI] [PubMed] [Google Scholar]
- 31.Calipel A, Lefevre G, Pouponnot C, Mouriaux F, Eychène A, Mascarelli F. Mutation of B-Raf in human choroidal melanoma cells mediates cell proliferation and transformation through the MEK/ERK pathway. J Biol Chem. 2003;278(43):42409–42418. doi: 10.1074/jbc.M308709200. [DOI] [PubMed] [Google Scholar]
- 32.Mantovani G, Bondioni S, Lania AG, et al. High expression of PKA regulatory subunit 1A protein is related to proliferation of human melanoma cells. Oncogene. 2008;27(13):1834–1843. doi: 10.1038/sj.onc.1210831. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.