Abstract
Prevalence and phenotype of BRCA mutation can vary by race. The purpose of this study is to evaluate the prevalence of BRCA1/2 mutations in non-familial breast cancer patients with high risks in Korea. A subset of 758 patients was selected for this study from the KOHBRA nationwide multicenter prospective cohort study. Mutations in BRCA1/2 genes were tested using fluorescent-conformation sensitive gel electrophoresis, denaturing high performance liquid chromatography or direct sequencing. Mutation of BRCA1/2 genes were identified in 65 (8.6%) patients among total 758 patients [BRCA1 mutation: 25 (3.3%), BRCA2 mutation: 40 (5.3%)]. According to risk groups, mutation of BRCA1/2 genes were identified in 53 (8.5%) of 625 early onset patients (age ≤40), in 22 (17.7%) of 124 bilateral breast cancer patients, in 3 (50.0%) of 6 breast and ovarian cancer patients, in one (5.9%) of 17 male breast cancer patients, in 5 cases (7.6%) of 66 multiple organ cancer patients. The most common mutation was 509C>A for BRCA1 and 7708C>T for BRCA2. The prevalence of BRCA1/2 mutations by age in early onset patients was significantly different (age <35 vs age ≥35; 10.0 vs 2.9%, p = 0.0007). BRCA1/2 mutations for non-familial Korean breast cancer patients were detected at a high rate, particularly, in patients with early onset of less than 35 years of age, bilateral breast cancer, and breast and ovarian cancer. Individualized genetic counseling should be offered for non-familial breast cancer patients with these risk factors.
Keywords: BRCA1, BRCA2, Hereditary breast cancer, Korean, Non-familial, Prevalence
Introduction
Although hereditary breast cancer is responsible for about 5–10% of all breast cancers, women carrying BRCA1 and BRCA2 gene mutations have especially high risk of breast cancer development. Mutations in BRCA1/2 genes have an estimated lifetime risk of breast cancer between 60 and 85%, and a lifetime risk of ovarian cancer between 26 and 54% for BRCA1 and between 10 and 23% for BRCA2. They are responsible for about 45% of families with multiple cases of breast cancer and up to 90% of families with both breast and ovarian cancer [1–4]. There are specific clinical and pathological features associated with hereditary BRCA1 or BRCA2 mutation associated breast cancers [5–7].
Genetic counseling and genetic testing to identify BRCA1 and BRCA2 gene mutations in high risk (a significant risk ≥10% of mutation) patients is widely available and commonly employed in the US and Europe [1, 8]. Management for breast cancer patients with a BRCA mutation should be established based on the clinicopathologic status as well as the result of genetic testing. Both prophylactic mastectomy and prophylactic oophorectomy are performed in approximately one-third and one half of mutation carriers in Western countries, respectively [9, 10]. However, decision-making for specific management should be done by a physician knowledgeable about the implications and lifetime risks of both breast and ovarian cancer.
There are some limitations to applying the clinical practice guidelines for genetic testing based on data from Western countries due to a lack of BRCA mutation data in breast cancer patients of Korean and other racial backgrounds [1, 11, 12]. First, the clinicopathological features associated with breast cancer may differ between races. For example, the proportion of triple negative breast cancer is higher in African-Americans and Asians compared to Caucasian Americans [13, 14]. Although the incidence of breast cancer in Asian countries, such as Korea, is lower than in Europe and the US, the incidence of Korean breast cancer has increased continuously by about threefold during the last two decades. The most striking difference compared to that of Western countries is that breast cancer among Korean women develops at a younger age [15, 16]. Sixty percent of patients in Korea are premenopausal compared to 30% in the United States. As ER-negative breast cancers are more common in premenopausal women, one may expect differences in frequencies of molecular subtypes of breast cancer between Korean and Caucasian women [13, 17]. There may be differences between countries in terms of genetic–environment interaction for development of breast cancer, ethnic diet, or environmental exposures. Consideration of family structure between in Korea and in Western countries is also needed because smaller family size makes family history less useful for identification of germline genetic risk [18].
Prevalence and phenotype of BRCA mutation varies according to country and race [19–21]. Several reports on BRCA gene mutation in Korean breast cancer patients have been published over a decade since a first report of BRCA1 among Korean pedigrees in 1995 [22–30]. The prevalence of BRCA1 and BRCA2 mutations were reported in 2.5–3.1% for sporadic breast cancers, in 19.4–42.9% for familial breast cancer patients with two or more affected first- and second-degree relatives with breast or ovarian cancers, and in 9.6–18.3% for early onset breast cancers. BRCA1 and BRCA2 mutations were also identified in 15.4% for bilateral breast cancer and in 17.9% for multiple organ cancer, and BRCA2 mutations were identified in 25% for male breast cancer in one study [27]. However, the data in high-risk breast cancer patients without family history of breast or ovarian cancer did not accurately reflect the prevalence of BRCA mutation in Korean breast cancer patients because of the very small sample size. A large scale multicenter study was needed to evaluate the prevalence of BRCA mutation among Korean breast cancer patients nationwide.
The KOHBRA study was designed by the Korean Breast Cancer Society to investigate the prevalence of BRCA1/2 mutations in several groups of subjects seen in high-risk breast cancer clinics in Korea between 2007 and 2010 [31]. These groups included patients with a family history of breast or ovarian cancers, patients with non-familial breast and ovarian cancer but with other risk factors of genetic disease, and family members of breast cancer patients with BRCA1/2 mutations. The KOHBRA study also investigated the prevalence of ovarian cancer in females with BRCA1/2 mutations
The present study evaluated the prevalence of BRCA1 and BRCA2 mutations in the second KOHBRA group, breast cancer patients without family history of breast or ovarian cancer with other risk factors for genetic predisposition.
Methods
Patients
The nationwide prospective cohort “KOHBRA” study accrued 1,967 subjects between May 2007 and May 2010 from 35 hospitals registered in the Korean Breast Cancer Society. The overall protocol is described more in detail in the interim report of the KOHBRA study [31, 32]. Inclusion criteria for this substudy are: patients without family history of breast or ovarian cancer but with at least one of the following risk factors for hereditary predisposition; early onset breast cancer defined as diagnosis at or younger than age 40, bilateral breast cancer, personal history of breast and ovarian cancer, male breast cancer, or cancer of multiple organs that include breast. This study received IRB approval from the individual institutes at the beginning of the study and consent from the study patients at the time of enrollment. Patients who were unable to communication or who did not return the questionnaire were excluded. Seven hundred and fifty-eight patients from the KOHBRA cohort met the criteria for this study.
Among the 758 patients, 741 (97.8%) patients were female and 17 (2.2%) patients were male. The breakdown of the high-risk groups is shown in Table 1. There were 550 (72.6%) patients with early onset breast cancer, 67 (8.8%) patients with bilateral breast cancer, 42 (5.5%) patients with cancer of multiple organs, 15 (2.0%) male breast cancer patients, 5 (0.7%) patients with both breast and ovarian cancer, and 79 (10.4%) patients having two or more of these high genetic risk factors.
Table 1.
Classification of high-risk groups
| High-risk classification | No of patients (%) |
|---|---|
| Early onset breast cancer (age ≤40 years) (E) | 550 (72.6) |
| Bilateral breast cancer (B) | 67 (8.8) |
| Male breast cancer (M) | 15 (2) |
| Breast and ovarian cancer (BO) | 5 (0.7) |
| Multiple organ cancer patients including breast (Mu) | 42 (5.5) |
| Two or more of these high risksa | 79 (10.4) |
| Total | 758 (100.0) |
a E + B:53, E + M:1, E + BO:1, E + Mu:19, B + Mu:3, M + Mu:1, E + B + Mu:1
Collecting clinical data
The family history and epidemiological data was obtained by a baseline questionnaire, the anthropometric data was measured, and the clinical information was collected by physician chart-reviews. Questionnaire included information about basic clinical data, past history, familial cancer history, life style, reproductive factors, social-psychological factors, and history of complementary therapies, etc. Physical information like body weight, height, body circumference, and body composition was acquired via physical examination at the same time of questionnaire. Pedigree of at least three generations was acquired via genetic counseling in all patients. All the data was computerized and regularly reviewed through quality control for missing or error data. Follow-up data of a new cancer, recurrence, and mortality was collected at 1 and 3 years after enrollment.
BRCA1 and BRCA2 genomic mutation analysis
Genomic DNA was extracted from the patients’ peripheral blood. Mutations in BRCA1 and BRCA2 were scanned through the 22 coding exons of BRCA1 and 26 coding exons of BRCA2 using fluorescence-conformation sensitive gel electrophoresis (F-CSGE) or denaturing high performance liquid chromatography (DHPLC). Following identification of a subset of PCR products with aberrant patterns, direct sequencing was performed in these DNA fragments on an ABI3100 or ABI3700 (Applied Biosystems, CA) or a MegaBACE500 (GE Healthcare, UK) genetic analyzer, following the manufacturer’s instructions. Scanning and direct sequencing of BRCA genes was performed based on the institutes’ own protocol and equipment. Some institutes performed direct sequencing through the entire BRCA1 and BRCA2 genes. Among all 758 patients, an F-CSGE performed in 670 cases (88.4%), DHPLC in 14 cases (1.8%), and direct sequencing in 74 cases (9.8%). In this study, the definition of a genetic mutation is confined to the protein-truncating mutation and the missense mutation, which have a confirmed association to disease. Unclassified variants were not considered as a genetic mutation.
Results
Prevalence of BRCA1 and BRCA2 mutations in high-risk breast cancer patients without family history of breast or ovarian cancer
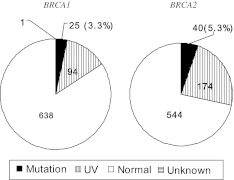
Overall, mutation of BRCA1 or BRCA2 genes was identified in 65 (8.6%) patients among 758 high-risk breast cancer patients without family history of breast or ovarian cancer. BRCA1 mutation was found in 25 (3.3%) patients and BRCA2 mutation was found in 40 (5.3%) patients. One patient had both BRCA1 and BRCA2 mutations (Fig. 1).
Fig. 1.
Prevalence of BRCA1 and BRCA2 mutations in high-risk breast cancer patients without family history of breast or ovarian cancer. Overall BRCA1/2 mutations: 65†/758 (8.6%), †One patient had both BRCA1 and BRCA2 mutations. UV unclassified variant
According to each high-risk group, mutation of BRCA1 and BRCA2 genes were identified in 53 (8.5%) of 625 early onset patients, in 22 (17.7%) of 124 patients with bilateral breast cancer, in 3 (50.0%) of 6 patients with breast and ovarian cancer, in 1 (5.9%) of 17 male breast cancer patients, in 5 (7.6%) of 66 patients with cancer of multiple organs including breast, and in 19 (27.1%) of 79 patients having two or more of these high risks (Table 2). Using non-overlapping risk groups, mutation of BRCA1 or BRCA2 genes were identified in 35 (6.4%) of 550 early onset patients, in 5 (7.5%) of 67 patients with bilateral breast cancer, in 2 (40.0%) of 5 patients with breast and ovarian cancer, in 1 (6.7%) of 15 male breast cancer patients, and in 3 (7.0%) of 42 patients with multiple organ cancers including breast.
Table 2.
BRCA1 and BRCA2 mutations according to high-risk groups
|
BRCA
mutation |
Early onset ≤40 yrs | Bilateral breast cancer | Male breast cancer | Breast and ovarian cancer | Multiple organ cancer | Two or more high risks |
|---|---|---|---|---|---|---|
| BRCA1/2 | ||||||
|
Mutation no. (%) |
53 (8.5) |
22 (17.7) |
1 (5.9) |
3 (50.0) |
5b (7.6) |
19 (27.1) |
|
No mutation no. (%) |
572 (91.5) |
102 (82.3) |
16 (94.1) |
3 (50.0) |
62 (92.4) |
60 (75.9) |
| BRCA1 c | ||||||
|
Mutation no. (%) |
22 (3.5) |
7 (5.6) |
0 (0.0) |
1 (16.7) |
2 (3.0) |
7 (8.9) |
|
No mutation no. (%) |
523 (83.7) |
97 (78.2) |
17 (100.0) |
5 (83.3) |
58 (87.9) |
62 (78.5) |
|
UVa no. (%) |
79 (12.6) |
19 (15.3) |
0 (0.0) |
0 (0.0) |
6 (9.1) |
9 (11.4) |
| BRCA2 | ||||||
|
Mutation no. (%) |
31 (5.0) |
15 (12.1) |
1 (5.9) |
2 (33.3) |
3 (4.6) |
12 (15.2) |
|
No mutation no. (%) |
456 (73.0) |
85 (68.5) |
12 (70.6) |
3 (50.0) |
42 (63.6) |
53 (67.1) |
|
UVa no. (%) |
138 (22.1) |
24 (19.4) |
4 (23.5) |
1 (16.7) |
21 (31.8) |
14 (17.7) |
| Total | 625 | 124 | 17 | 6 | 66 | 79 |
a UV unverified mutation
bOne patient had both BRCA1 and BRCA2 mutations
cData of BRCA1 test in one patient was not available
BRCA1 and BRCA2 mutation by risk groups
BRCA1 mutations were detected in 22 (3.5%) of 625 early onset patients, in 7 (5.6%) of 124 patients with bilateral breast cancer, in 1 (16.7%) of 6 patients with breast and ovarian cancers, in 2 (3.0%) of 66 patients with multiple organ cancers, and in 7 (8.9%) of 79 patients having two or more of these high-risk factors. There was no BRCA1 mutation detected among 17 male breast cancer patients.
BRCA2 mutations were detected in 31 (5.0%) early onset patients, 15 (12.1%) patients with bilateral breast cancer, 2 (33.3%) patient with breast and ovarian cancer, 3 (4.6%) patients with multiple organ cancers, 1 (5.9%) male breast cancer patient, and 12 (15.2%) patients having two or more of these high risks (Table 2).
BRCA1 and BRCA2 mutations in early onset breast cancer patients
Patients with early onset breast cancer had a variety of BRCA1 and BRCA2 mutations based on the combined risk groups. Of 625 early onset patients, BRCA1 and BRCA2 mutations were detected in 35 (6.4%) of 550 patients without other risks, in 16 (30.2%) of 53 patients with bilateral breast cancer, in one (100%) patient who also had ovarian cancer, and in one (5.3%) of 19 patients with multiple organ cancers (Table 3).
Table 3.
BRCA1 and BRCA2 mutations in patients with early onset breast cancer diagnosed at ≤40 years
| Risk classification | <35 years | ≥35 years | Total | |||
|---|---|---|---|---|---|---|
| Patients no. |
Mutation no. (%) |
Patients no. |
Mutation no. (%) |
Patients no. |
Mutation no. (%) |
|
|
Early onset breast cancer ≤40 yrs only (E)* |
271 | 27 (10) | 279 | 8 (2.9) | 550 | 35 (6.4) |
| E + Bilateral breast cancer | 20 | 7 (35) | 33 | 9 (27.3) | 53 | 16 (30.2) |
| E + Breast and ovarian cancer | 0 | 0 | 1 | 1 (100) | 1 | 1 (100) |
| E + Multiple organ cancer | 5 | 0 | 14 | 1 (7.1) | 19 | 1 (5.3) |
| E + Male breast cancer | 1 | 0 | 0 | 0 | 1 | 0 |
|
E + Bilateral breast cancer + multiple organ cancer |
1 | 0 | 0 | 0 | 1 | 0 |
| Total | 298 | 34 (11.4) | 327 | 19 (5.8) | 625 | 53 (8.5) |
* p = 0.0007
According to age at diagnosis, BRCA1 and BRCA2 mutations were detected in 24.0% of patients 40 years or younger with other risk factors and in 6.4% among early onset patients without other risk factors. However, among the early onset patients without other risk factors, BRCA1 and BRCA2 mutations were detected in 10.0% in patients less than 35 years and in 2.9% in patients 35 years or older (p = 0.0007) (Table 3).
In terms of age group, incidence of BRCA1/2 mutations in patients 35 years or older showed a significant difference depending on whether other risks were combined or not (22.9 vs 2.9%, p = <0.0001). On the other hand, BRCA1/2 mutations in patients less than 35 years showed high incidence rates regardless of the combined risks (25.9 vs 10%, p = 0.01) (Table 4).
Table 4.
BRCA1/2 and BRCA2 mutations by age in patients with early onset breast cancer diagnosed at ≤40 years
| Age | Risk | BRCA1/2 | Total no. (%) |
p value | |
|---|---|---|---|---|---|
| Mutation | No mutation | ||||
| ≥35 yrs | With other risksa | 11 | 37 | 48 | <0.0001 |
| No. (%) | 22.9 | 77.1 | 14.7 | ||
| Without other risks | 8 | 271 | 279 | ||
| No. (%) | 2.9 | 97.1 | 85.3 | ||
| Total | 19 | 308 | 327 | ||
| No. (%) | 5.8 | 94.2 | 100 | ||
| <35 yrs | With other risks | 7 | 20 | 27 | 0.01 |
| No. (%) | 25.9 | 74.1 | 9.1 | ||
| Without other risks | 27 | 243 | 270 | ||
| No. (%) | 10 | 90 | 90.9 | ||
| Total | 34 | 263 | 298 | ||
| No. (%) | 11.4 | 88.6 | 100 | ||
aOther risks included early onset, bilateral breast cancer, male breast cancer, breast and ovarian cancer, and multiple organ cancer
We evaluated each patient’s family history to develop a family structure variable to find whether a limited family structure affects BRCA1/2 mutation prevalence or not. Limited family structure was defined as fewer than two first- or second-degree female relatives surviving past age 45 years in either the maternal or paternal lineage. Otherwise the patient was deemed to have an adequate family structure [18]. BRCA1 and BRCA2 mutations did not show different incidence between limited structure and adequate structure according to family structure in early onset patients (Table 5).
Table 5.
BRCA1/2 and BRCA2 mutations according to family structure in patients with early onset breast cancer diagnosed at ≤40 years
| Age | Family structure | p value | ||
|---|---|---|---|---|
| Limited (n = 146) | Adequate (n = 92) | |||
| BRCA1/2 | ||||
| Age <35 yrs | ||||
| Mutation no. (%) |
16 11.0 |
9 9.8 |
0.77 | |
| No mutation no. (%) |
130 89.0 |
83 90.2 |
||
| Age ≥35 yrs | Limited (n = 186) | Adequate (n = 90) | ||
| Mutation no. (%) |
4 2.7 |
1 1.1 |
0.4 | |
| No mutation no. (%) |
142 97.2 |
89 98.9 |
||
| All | Limited (n = 292) | Adequate (n = 182) | ||
| Mutation no. (%) |
20 6.9 |
10 5.5 |
0.56 | |
| No mutation no. (%) |
272 93.1 |
172 94.5 |
||
Possible candidates for founder mutation of BRCA1 and BRCA2 genes
Overall 43 mutations (18 BRCA1 genes and 25 BRCA2 genes) were identified in high-risk breast cancer patients without a family history of breast or ovarian cancer. The most common BRCA1 mutation gene was 509C>A, which was identified separately in four unrelated patients. 1041delAGCinsT, 1630insG, 3746insA, and 5615del11insA were identified separately in two unrelated patients. These five BRCA1 genes accounted for 48% of the BRCA1 mutations identified. The most common BRCA2 mutation gene was 7708C>T, which was identified separately in six unrelated patients. 584delTTAA and 6952delGA was identified in three unrelated patients. 2041dupA, 8542G>T, 1672A>T, 9219T>G, 9481delA, and 999del5 were separately identified in two unrelated patients. These nine BRCA2 genes accounted for 60% of the BRCA2 mutations identified (Table 6).
Table 6.
Frequency of BRCA1 and BRCA2 mutations in high-risk breast cancer patients without family history of breast or ovarian cancer
| BIC nomenclature | Effect on amino acid | n a | % | |
|---|---|---|---|---|
| BRCA2 | 7708C>T | p.Arg2494X hetero | 6 | 9.2 |
| BRCA1 | 509C>A | p.Tyr130X hereto | 4 | 6.2 |
| BRCA2 | 5804delTTAA | p.I1859KfsX3 hetero | 3 | 4.6 |
| BRCA2 | 6952delGAb | p.Asp2242PhefsX2 hetero | 3 | 4.6 |
| BRCA1 | 1041delAGCinsT | p.Ser308X hetero | 2 | 3.1 |
| BRCA1 | 3746insA | p.Glu1210ArgfsX9 hetero | 2 | 3.1 |
| BRCA1 | 5615del11insAc | p.Val1833SerfsX7 hetero | 2 | 3.1 |
| BRCA1 | 1630dupG | p.Lys505X hetero | 2 | 3.1 |
| BRCA2 | 1627A>T | p.Lys467X hetero | 2 | 3.1 |
| BRCA2 | 2041dupA | p.I605NfsX11 hetero | 2 | 3.1 |
| BRCA2 | 8542G>Tb | p.Glu2772X hetero | 2 | 3.1 |
| BRCA2 | 9219T>Gb | p.Tyr2997X hetero | 2 | 3.1 |
| BRCA2 | 9481delA | p.Thr3085GlnfsX19 hetero | 2 | 3.1 |
| BRCA2 | 999del5 | p.Asn257LysfsX17 hetero | 2 | 3.1 |
aFrequency in disease-causing mutation (n = 65)
bNovel
cDetected in only Korean
Discussion
Overall mutation of BRCA1 and BRCA2 genes in high-risk breast cancer patients without family history of breast or ovarian cancer in this study was 8.6% (BRCA1: 3.3%, BRCA2: 5.3%). The prevalence of genetic BRCA1/2 mutation among non-familial patients with high-risk factors in this study was more than three times higher than the 2.6% prevalence observed for BRCA1/2 mutations in sporadic breast cancer patients in Korea [25, 27, 28]. Genetic counseling and testing for BRCA1 and BRCA2 gene mutation has become an integral part of high-risk patient evaluation and management for hereditary breast ovarian cancer [11, 33]. Guideline organizations, including NCCN and ASCO, have advised targeting BRCA1 and BRCA2 testing to probands whose probability of mutation carriage is approximately 10% or greater. However, there might be variation in BRCA1/2 mutation prevalence across race and ethnicity for breast cancer patients with high-risk factors, although the prevalence of BRCA1/2 mutations is comparable among sporadic breast cancer patients of African, Asian, White, and Hispanic descent: approximately 1–4% per gene, with the exception of Ashkenazi Jewish and Icelandic patients [33, 34]. There is a question of whether ethnic differences in genetic, reproductive, or environmental exposures might alter mutation penetrance, which could have significant implications for clinical practice. Therefore, large data collection of BRCA1/2 mutation prevalence and penetrance in individual ethnic groups is necessary for applying individualized guideline for genetic counseling and testing, and management in clinical practice.
This KOHBRA study showed the prevalence of BRCA1/2 mutations in high-risk breast cancer patients without family history of breast or ovarian cancer via a large multicenter nationwide cohort study. Previous reports of BRCA1/2 mutations in Korean breast cancer patients were limited by small sample sizes, and the studies were performed individually at various sites with different screening methods. Overall mutation of BRCA1/2 genes in high-risk breast cancer patients without family history of breast or ovarian cancer in this study was 8.6%. On the other hand, the prevalence of BRCA1/2 mutations among breast cancer patients with family history of breast or ovarian cancers was 24.8% in the KOHBRA study interim report [32]. Notably, BRCA2 mutation was more common than BRCA1 mutation in our study, compared with previous small clinic-based studies [25–27]. This finding is consistent with other studies showing BRCA2 mutations appear as or more commonly than BRCA1 mutations in clinic-based Asians, a difference from other ethnic groups [34].
According to high-risk groups in this study, mutation of BRCA1/2 genes were identified in 8.5% among early onset patients, in 17.7% among bilateral breast cancer, in 50.0% among patients with both breast and ovarian cancer, in 5.9% among male breast cancer patients, in 7.6% among patients with cancer of multiple organs, and in 27.1% among patients having two or more of these high-risk factors. There are few previous smaller studies of Korean patients which report BRCA mutations by individual risk category and several of these include patients with family history [24, 27]. BRCA1/2 mutations in Korean patients with early onset regardless of family history were detected in 10.4–18.3% in the previous studies (Table 7) [23, 26, 32]. The wide variation in reported prevalence of BRCA1/2 mutation in patients with early onset could be related to variation in number with family history of breast cancer in these studies. When the group of early onset patients under 35 years was considered, we found prevalence of 10% for BRCA1/2 mutations with no other risk factor. The mutation rate increased up to 25.9% for the early onset patients with other risk factor. Therefore, additional risk factors increase the likelihood of BRCA1/2 mutation among young patients.
Table 7.
Prevalence of BRCA1 and BRCA2 mutations among early onset breast cancer patients with or without familial history of breast or ovarian cancers in Korea
| Ahn et al. [26] | Kang et al. [23] | KOHBRA study-interim report [32] | |
|---|---|---|---|
| No. of cases tested (Age) | 183 (Age <35) | 60 (Age <40) | 513 (Age ≤40) |
| History of familial breast or ovarian cancers (%) | 24 (13.1) | 8 (22.0) | 181 (32.0) |
| BRCA1 mutation (%) | 13 (7.1) | 6 (10.0) | 48 (9.4) |
| BRCA2 mutation (%) | 6 (3.3) | 5 (8.3) | 43 (8.4) |
| BRCA1/2 mutations (%) | 19 (10.4) | 11 (18.3) | 91a (17.7) |
aTwo cases of both BRCA1 and BRCA2 mutations
Two studies reviewed the prevalence of BRCA1/2 mutations in breast cancer patients in terms of ethnic difference, finding that those diagnosed at a younger age (mostly under 35 to 45-year old) had prevalence of 5–10% among various races [24, 34]. On the other hand, the prevalence of BRCA1 mutation was particularly high in African-American patients diagnosed before age 35 years (16.7%), compared with young Hispanics (8.9%), non-white Hispanics without Ashkenazi Jewish ancestry (7.2%), and Asian-Americans (2.4%) [35].
Family structure can affect BRCA gene mutation rate and the accuracy of mutation probability models. BRCA1/2 mutations were detected in 13.7% of the limited family structure and in 5.2% of the adequate family structure. Family structure was a significant predictor of mutation status [18]. However, there was no significant difference of BRCA gene mutations between family structures in our study.
The prevalence of BRCA1/2 mutations in bilateral breast cancer patients in Korea was 17.7% in our study and was higher than 15.4% prevalence in a previous small study [27]. BRCA1/2 mutations of bilateral breast cancer in other ethnic countries showed both 29.6% in high-risk Jewish families and in Polish patients with having 46.9–82.4% positive familial history [36, 37].
There is a lack of studies about the prevalence of BRCA1/2 mutations in male breast cancer in Korea. Two (25%) BRCA2 mutations and no BRCA1 mutation were reported among eight male breast cancer patients in a small study [27]. However, the patients with mutation also had familial breast cancer history. Our study had a small number of male breast cancer patients and BRCA2 mutation was detected in only one (5.9%) patients. The BRCA mutations in male breast cancer without familial breast cancer history are usually undetected and if detected, it is almost always in BRCA2 gene.
The prevalence of BRCA2 mutation in male breast cancer varies from 4 to 40% according to racial differences. This variance might be related to the different rates of familial breast cancer history [38–43]. On the other hand, a recently published study accounted for 16% BRCA2 mutation of 115 male breast cancer cases from the United States, including 40% for breast cancer families and 13% for non-familial breast cancer. The study suggested that family history is not a strong predictor of carrying a mutation in males and males who develop breast cancer should be screened for mutations in BRCA2 [44].
The prevalence of BRCA1/2 in multiple organ cancer cases in our study (7.6%) was lower than previously reported (9.1%) for cases without familial history in a previous small study, which also showed 50% with BRCA1/2 mutations in cases with familial breast cancer history [27]. In our study, the other primary cancer sites in breast cancer patients were 46 thyroid cancers, 6 uterine cancers, 5 kidney cancers, 2 stomach cancers, 2 rectal cancers, 2 tongue cancers, 1 liver cancer, 1 pancreatic cancer, and 1 bone cancer. Five BRCA1/2 mutations were detected in 2 thyroid cancers, 2 kidney cancers, and 1 bone cancer, respectively (Table 8). It would seem that genetic testing should be considered whether or not a specific primary cancer is genetically related to breast cancer. For example, the International Consensus Conference on Breast Cancer Risk, Genetics, and Risk Management suggested as candidates for genetic counseling and testing for breast cancer, any patient who has had a family history of prostate cancer, thyroid cancer, sarcoma, endometrial cancer, adrenocortical cancer, brain cancer, or pancreatic cancer—although these involve less strict criteria [1].
Table 8.
BRCA1 and BRCA2 mutations in patients with multiple organ cancer
| Number of total subjects | BRCA1 mutation no. | BRCA2 mutation no. | Overall prevalence (%) | |
|---|---|---|---|---|
| Breast and other cancers | 66 | 2 | 3 | 7.6 |
| Thyroid cancer | 46 | 1 | 1 | 4.3 |
| Uterine cancer | 6 | 0 | 0 | 0.0 |
| Kidney cancer | 5 | 1a | 1a | 40.0 |
| Stomach cancer | 2 | 0 | 0 | 0.0 |
| Rectal cancer | 2 | 0 | 0 | 0.0 |
| Tongue cancer | 2 | 0 | 0 | 0.0 |
| Liver cancer | 1 | 0 | 0 | 0.0 |
| Pancreas cancer | 1 | 0 | 0 | 0.0 |
| Bone cancer | 1 | 0 | 1 | 100.0 |
aOne patient with kidney cancer had both BRCA1 and BRCA2 mutations
A study reported that the germline mutation rate in BRCA1 was 2.7% in 37 Korean sporadic ovarian cancer patients unselected for family history, which was slightly lower than rates obtained in other countries [45]. The prevalence of BRCA1/2 mutations in patients with both breast and ovarian cancer in our study was 50% and higher than that in Myriad data (20%), although the number of cases were small [46]. This high prevalence supports current practice guidelines of genetic testing for all patients with breast and ovarian cancers, regardless of age or family cancer history [11].
Although germline mutations were scattered through the BRCA1 and BRCA2 genes, the BRCA1, 509C>A gene which was identified separately in four unrelated patients, and the BRCA2, 7708C>T gene which was identified separately in six unrelated patients, are the most frequent mutation in either genes and accounted for 16 and 15% of mutations detected in BRCA1 and BRCA2 respectively in this study of Korean women. 509C>A gene was also detected in Asian population and 5615del11insA were detected in only Korean patients in the BIC database. The BRCA2, 7708C>T gene was detected in Asian and West European population and detected frequently in Korean patients in the previous studies [23–25, 27–29]. These genes would be possible candidates for founder mutations of BRCA1/2 in high-risk Korean breast cancer patients. However, defining founder genes in Korean breast cancer patients should be approached carefully, because there is no data of mutation frequency in the Korean general population and additional data about BRCA mutations is required even though the KOHBRA study was a nationwide multicenter study.
The selection of genetic testing criteria would be clinically important because the mutation result might affect the decision-making for operation method and follow-up method. Although widely accepted clinical criteria for referral are usually suggested, genetic testing criteria may differ between countries based on mutation prevalence because the prevalence of BRCA1/2 mutations varies considerably among ethnic groups and geographical areas [12]. As the NCCN guideline currently recommends genetic counseling and genetic testing for any patient with high risk 10% or more of mutation, the prevalence of BRCA1/2 mutations in high-risk breast cancer patients without family history of breast or ovarian cancer—noted in this study—may play a key role for genetic testing criteria in Korean breast cancer patients with high risk of mutation. BRCAPRO and Myriad II, two of the most commonly used computer predictive models on clinical expertise, show accuracy of approximately 80% for whites with strong personal and/or familial history of breast, ovarian, and/or male breast cancer. However since these models show underestimation of Asian mutation carriers because of their differences in performance by race/ethnicity, using these models to predict the BRCA1/2 mutation probability among Korean patients may result in inaccuracies [34]. Therefore, large nationwide trials like the KOHBRA study are needed to continue developing the appropriate guidelines for genetic counseling, genetic testing, and management as well as the suitable predictive model for BRCA1/2 mutation patients or carriers in Korea. It is important to evaluate whether the difference of BRCA1/2 mutation prevalence exists between Koreans and other races. Identifying racial differences in genetic or lifestyle factors, which may modify the cancer risk of BRCA1/2 mutations, is also a priority for future research. So far, the management for BRCA1/2 mutation patients or carriers in Korea is recommending that application is individually based on the prevalence data of BRCA1/2 mutation of the KOHBRA study, as well as the worldwide guidelines such as the NCCN guideline or the International Consensus Conference guideline.
Our study has some limitations despite being a large multicenter nationwide study. Three different screening methods for testing BRCA1/2 mutations were performed although most patients were tested with the F-CSGE method. This heterogeneous screening method might affect the prevalence of BRCA mutation. Also, the number of patients varied according to risk groups. For instance, the prevalence of BRCA1/2 mutations in male breast cancer patients as well as in patients with breast and ovarian cancer in this study must be interpreted with caution due to the very small number of subjects. Another limitation involves the various additional primary organ sites for patients with multiple organ cancers. Additional primary cancer sites in breast cancer patients in our study were thyroid, uterus, kidney, stomach, tongue, rectum, liver, bone, and pancreas. Whether cancers from these organs are genetically related to breast cancer or BRCA1/2 mutation requires confirmation. Besides the differences in number of cases with familial breast cancer history, the different sites of multiple-organ cancers might affect the many discrepancies in the prevalence of BRCA1/2 in multiple organ cancers cases between our study and the previous smaller study [27].
In conclusion, BRCA1 and BRCA2 mutations were detected at higher frequency than reported for Korean sporadic breast cancer patients in those patients with no family history of breast or ovarian cancer but with other risk factors of genetic disease. In particular, the patients with early onset of less than 35 years of age, bilateral breast cancer, and personal history of breast and ovarian cancer had greater than 10% prevalence of BRCA1/2 mutations. Genetic testing may be indicated for these high-risk patient groups.
Acknowledgments
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea [0720450]; This study was registered at clinicaltrials.gov (NCT0059534). We thank all participants and investigators of the KOHBRA study: Beom Seok Kwak, Byeong-Woo Park, Byung Ho Son, Byung-In Moon, Cha Kyong Yom, Chan Heun Park, Chan Seok Yoon, Chang Hyun Lee, Dae Sung Yoon, Dong-Young Noh, Doo Ho Choi, Eundeok Chang, Eun-Kyu Kim, Eunyoung Kang, Hae Kyung Lee, Hai-Lin Park, Hyde Lee, Hyeong-Gon Moon, Hyun-Ah Kim, Il-Kyun Lee, Jeong Eon Lee, Jong Won Lee, Jong-Han Yu, Joon Jeong, Jung-Hyun Yang, Keumhee Kwak, Ki-Tae Hwang, Ku Sang Kim, Lee Su Kim, Min Hee Hur, Min Hyuk Lee, Myung Chul Chang, Nam Sun Paik, Sang Ah Han, Sang Seol Jung, Sang Uk Woo, Se Jeong Oh, Sehwan Han, Sei Joong Kim, Sei-Hyun Ahn, Seok-Jin Nam, Seung Sang Ko, Sung Hoo Jung, Sung Soo Kang, Sung Yong Kim, Sung-Won Kim, Tae Hyun Kim, Tae Woo Kang, Wonshik Han, Woo-Chul Noh, Yong Lai Park, Yongsik Jung, Young Jin Suh, Young Tae Bae, Young Up Cho, Young-Ik Hong, Sue K. Park, Yoon Joo Jung, Su Yun Choi, Young Bum Yoo, Soo-Jung Lee.
Conflict of interest
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Schwartz GF, Hughes KS, Lynch HT, et al. Proceedings of the international consensus conference on breast cancer risk, genetics, & risk management, April, 2007. Cancer. 2008;113:2627–2637. doi: 10.1002/cncr.23903. [DOI] [PubMed] [Google Scholar]
- 2.Brose MS, Rebbeck TR, Calzone KA, et al. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst. 2002;94:1365–1372. doi: 10.1093/jnci/94.18.1365. [DOI] [PubMed] [Google Scholar]
- 3.Easton DF, Bishop DT, Ford D, et al. Genetic linkage analysis in familial breast and ovarian cancer: result from 214 families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1993;52:678–701. [PMC free article] [PubMed] [Google Scholar]
- 4.King MC, Marks JH, Mandel JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 5.Honrado E, Osorio A, Palacios J, et al. Pathology and gene expression of hereditary breast tumors associated with BRCA1, BRCA2 and CHEK2 gene mutations. Oncogene. 2006;25:5837–5845. doi: 10.1038/sj.onc.1209875. [DOI] [PubMed] [Google Scholar]
- 6.Foulkes WD, Stefansson IM, Chappuis PO, et al. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst. 2003;95:1482–1485. doi: 10.1093/jnci/djg050. [DOI] [PubMed] [Google Scholar]
- 7.Liede A, Karlan BY, Narod SA. Cancer risks for male carriers of germline mutations in BRCA1 or BRCA2: a review of the literature. J Clin Oncol. 2004;22:735–742. doi: 10.1200/JCO.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 8.Robson ME. Treatment of hereditary breast cancer. Semin Oncol. 2007;34:384–391. doi: 10.1053/j.seminoncol.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Wainberg S, Husted J. Utilization of screening and preventive surgery among unaffected carriers of a BRCA1 or BRCA2 gene mutation. Cancer Epidemiol Biomarkers Prev. 2004;13:1989–1995. [PubMed] [Google Scholar]
- 10.Narod SA, Offit K. Prevention and management of hereditary breast cancer. J Clin Oncol. 2005;23:1656–1663. doi: 10.1200/JCO.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 11.Clinical Practice Guidelines in Oncology (2010) Genetic/familial high risk assessment: breast and ovarian cancer. 2010. National Comprehensive Cancer Network. http://www.nccn.org/professionals/physician_gls/PDF/genetics_screening.pdf. Accessed 20 Dec 2010
- 12.Balmaña J, Diez O, Rubio I, et al. BRCA in breast cancer: ESMO Clinical Practice Guidelines. Ann Oncol. 2010;21(supple):v20–v22. doi: 10.1093/annonc/mdq161. [DOI] [PubMed] [Google Scholar]
- 13.Lee JA, Kim KI, Bae JW, et al. Triple negative breast cancer in Korea-distinct biology with different impact of prognostic factors on survival. Breast Cancer Res Treat. 2010;123:177–187. doi: 10.1007/s10549-010-0998-5. [DOI] [PubMed] [Google Scholar]
- 14.Chlebowski RT, Chen Z, Anderson GL, et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst. 2005;97:439–448. doi: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 15.Son BH, Kwak BS, Kim JK, et al. Changing patterns in the clinical characteristics of Korean patients with breast cancer during the last 15 years. Arch Surg. 2006;141:155–160. doi: 10.1001/archsurg.141.2.155. [DOI] [PubMed] [Google Scholar]
- 16.Ahn SH, Yoo KY, The Korean Breast Cancer Society Chronological changes of clinical characteristics in 31,115 new breast cancer patients among Koreans during 1996–2004. Breast Cancer Res Treat. 2006;99:209–214. doi: 10.1007/s10549-006-9188-x. [DOI] [PubMed] [Google Scholar]
- 17.American Cancer Society . Breast cancer facts & figures 2009–2010. Atlanta: American Cancer Society, Inc.; 2009. [Google Scholar]
- 18.Weitzel JN, Lagos VI, Gullinane CA, et al. Limited family structure and BRCA gene mutation status in single cases of breast cancer. JAMA. 2007;297:2587–2595. doi: 10.1001/jama.297.23.2587. [DOI] [PubMed] [Google Scholar]
- 19.Nagy R, Sweet K, Eng C. Highly penetrant hereditary cancer syndromes. Oncogene. 2004;23:6445–6470. doi: 10.1038/sj.onc.1207714. [DOI] [PubMed] [Google Scholar]
- 20.Liede A, Narod SA. Hereditary breast and ovarian cancer in Asia: genetic epidemiology of BRCA1 and BRCA2. Hum Mutat. 2002;20:413–424. doi: 10.1002/humu.10154. [DOI] [PubMed] [Google Scholar]
- 21.Neuhausen SL. Founder populations and their uses for breast cancer genetics. Breast Cancer Res. 2000;2:77–81. doi: 10.1186/bcr36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh JH, Noh DY, Choe KJ, et al. Germline mutation of BRCA1 gene in Korean breast and ovarian cancer patients. J Korean Cancer Assoc. 1995;27:1061–1069. [Google Scholar]
- 23.Kang HC, Kim IJ, Park JH, et al. Germline mutations of BRCA1 and BRCA2 in Korean breast and/or ovarian cancer families. Hum Mutat. 2002;20:235–239. doi: 10.1002/humu.9059. [DOI] [PubMed] [Google Scholar]
- 24.Choi DH, Lee MH, Bale AE, et al. Incidence of BRCA1 and BRCA2 mutations in young Korean breast cancer patients. J Clin Oncol. 2004;22:1638–1645. doi: 10.1200/JCO.2004.04.179. [DOI] [PubMed] [Google Scholar]
- 25.Seo JH, Cho DY, Ahn SH, et al. BRCA1 and BRCA2 germline mutations in Korean patients with sporadic breast cancer. Hum Mutat. 2004;24:350. doi: 10.1002/humu.9275. [DOI] [PubMed] [Google Scholar]
- 26.Ahn SH, Hwang UK, Kwak BS, et al. Prevalence of BRCA1 and BRCA2 Mutations in Korean Breast Cancer Patients. J Korean Med Sci. 2004;19:269–274. doi: 10.3346/jkms.2004.19.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahn SH, Son BH, Yoon KS, et al. BRCA1 and BRCA2 germline mutations in Korean breast cancer patients at high risk of carrying mutations. Cancer Lett. 2007;8(245):90–95. doi: 10.1016/j.canlet.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 28.Han SH, Lee KR, Lee DG, et al. Mutation analysis of BRCA1 and BRCA2 from 793 Korean patients with sporadic breast cancer. Clin Genet. 2006;70:496–501. doi: 10.1111/j.1399-0004.2006.00717.x. [DOI] [PubMed] [Google Scholar]
- 29.Kim BY, Lee DG, Lee KR, et al. Identification of BRCA1 and BRCA2 mutations from Korean breast cancer patients using denaturing HPLC. Biochem Biophys Res Commun. 2006;349:604–610. doi: 10.1016/j.bbrc.2006.08.086. [DOI] [PubMed] [Google Scholar]
- 30.Kim TJ, Lee KM, Choi CH, et al. Germline mutations of BRCA1 in two Korean hereditary breast/ovarian cancer families. Oncol Rep. 2006;15:565–569. [PubMed] [Google Scholar]
- 31.Kim EK, Kim KS, Park SK, et al. The Korean Hereditary Breast Cancer (KOHBRA) Study: protocol review. J Breast Cancer. 2007;10:241–247. doi: 10.4048/jbc.2007.10.4.241. [DOI] [Google Scholar]
- 32.Han SA, Park SK, Ahn SH, et al. The Korean Hereditary Breast Cancer (KOHBRA) Study: protocols and report. Clin Oncol (R Coll Radiol) 2011;23(7):434–441. doi: 10.1016/j.clon.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 33.U.S. Preventive Services Task Force Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility: recommendation statement. Ann Intern Med. 2005;5:355–361. doi: 10.7326/0003-4819-143-5-200509060-00011. [DOI] [PubMed] [Google Scholar]
- 34.Kurian AW. BRCA1 and BRCA2 mutations across race and ethnicity: distribution and clinical implications. Curr Opin Obstet Gynecol. 2010;22:72–78. doi: 10.1097/GCO.0b013e328332dca3. [DOI] [PubMed] [Google Scholar]
- 35.John EM, Miron A, Gong G, et al. Prevalence of pathogenic BRCA1 mutation carriers in 5 US racial/ethnic groups. JAMA. 2007;298:2869–2876. doi: 10.1001/jama.298.24.2869. [DOI] [PubMed] [Google Scholar]
- 36.Gershoni-Baruch R, Dagan E, Fried G, et al. BRCA1 and BRCA2 founder mutations in patients with bilateral breast cancer. Eur J Hum Genet. 1999;7:833–836. doi: 10.1038/sj.ejhg.5200371. [DOI] [PubMed] [Google Scholar]
- 37.Rogozińska-Szczepka J, Utracka-Hutka B, Grzybowska E, et al. BRCA1 and BRCA2 mutations as prognostic factors in bilateral breast cancer patients. Ann Oncol. 2004;15:1373–1376. doi: 10.1093/annonc/mdh352. [DOI] [PubMed] [Google Scholar]
- 38.Basham VM, Lipscombe JM, Ward JM, et al. BRCA1 and BRCA2 mutations in a population based study of male breast cancer. Breast Cancer Res. 2002;4:1–5. doi: 10.1186/bcr419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Couch FJ, Farid LM, Deshano ML, et al. BRCA2 germline mutations in male breast cancer cases and breast cancer families. Nat Genet. 1996;13:123–125. doi: 10.1038/ng0596-123. [DOI] [PubMed] [Google Scholar]
- 40.Friedman LS, Gayther SA, Kurosaki T, et al. Mutation analysis of BRCA1 and BRCA2 in a male breast cancer population. Am J Hum Genet. 1997;60:313–319. [PMC free article] [PubMed] [Google Scholar]
- 41.Mavraki E, Gray IC, Bishop T, et al. Germline BRCA2 mutations in men with breast cancer. Br J Cancer. 1997;76:1428–1431. doi: 10.1038/bjc.1997.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haraldsson K, Loman N, Zhang OX, et al. BRCA2 germline mutations are frequent in male breast cancer patients without a family history of the disease. Cancer Res. 1998;58:1367–1371. [PubMed] [Google Scholar]
- 43.Csokay B, Udvarhelyi N, Sulyok Z, et al. High frequency of germline BRCA2 mutations among Hungarian male breast cancer patients without a family history. Cancer Res. 1999;59:995–998. [PubMed] [Google Scholar]
- 44.Ding YC, Steele L, Kuan CJ, et al. Mutations in BRCA2 and PALB2 in male breast cancer cases from the United States. Breast Cancer Res Treat. 2011;126:771–778. doi: 10.1007/s10549-010-1195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim YT, Nam EJ, Yoon BS, et al. Germline mutations of BRCA1 and BRCA2 in Korean sporadic ovarian carcinoma. Gynecol Oncol. 2005;99:585–590. doi: 10.1016/j.ygyno.2005.06.058. [DOI] [PubMed] [Google Scholar]
- 46.Frank TS, Deffenbaugh AM, Reid JE, et al. Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol. 2002;20:1480–1490. doi: 10.1200/JCO.20.6.1480. [DOI] [PubMed] [Google Scholar]