Abstract
Understanding the mechanisms of neuronal dysfunction and death represents a major frontier in contemporary medicine, involving the acute cell death in stroke, and the attrition of the major neurodegenerative diseases, including Parkinson's, Alzheimer's, Huntington's and Motoneuron diseases. A growing body of evidence implicates mitochondrial dysfunction as a key step in the pathogenesis of all these diseases, with the promise that mitochondrial processes represent valuable potential therapeutic targets. Each disease is characterised by the loss of a specific vulnerable population of cells—dopaminergic neurons in Parkinson's disease, spinal motoneurons in Motoneuron disease, for example. We discuss the possible roles of cell type-specific calcium signalling mechanisms in defining the pathological phenotype of each of these major diseases and review central mechanisms of calcium-dependent mitochondrial-mediated cell death.
Keywords: Mitochondria, Intracellular calcium, Neurodegenerative disease, Glutamate excitotoxicity
Introduction
The fine spatial and temporal organisation of intracellular calcium signals is fundamental to function in the CNS, perhaps more than in any other tissue. Signals are conveyed throughout the CNS by local changes in calcium concentration ([Ca2+]c) carrying information from neuron to neuron, between neurons and glia and even regulating local blood flow in relation to local activity. Local changes in [Ca2+]c are the very stuff of our thoughts and sensations, our memories and dreams. Neuronal energy supplies are meanwhile entirely predicated on mitochondrial oxidative phosphorylation—neurons are almost exclusively dependent on mitochondrial ATP generation and have almost no capacity to upregulate energy supply through glycolysis when oxidative phosphorylation is compromised [33], making them especially vulnerable to mitochondrial dysfunction. Mitochondrial function and Ca2+ signalling are intimately linked, as the [Ca2+]c signal is used as the major medium of a continuous subtle dialogue between the cytosol and its mitochondrial population that regulates energy homeostasis. It is the [Ca2+]c signal that signals to mitochondria that more energy is required, and the [Ca2+]c signal that mediates the mechanisms that deliver increased energy. [Ca2+]c signals even tell mitochondria where to go in the labyrinthine maze of a complex neuron.
These mechanisms are so fundamental, that even subtle disturbances are likely to have far reaching functional consequences in disease. Defects in mitochondrial function are now implicated in a litany of major diseases throughout the CNS. Development of irreversible mitochondrial injury is almost certainly the critical step in the pathway to irreversible injury and cell death in the penumbra of an evolving stroke. Chronic defects in aspects of mitochondrial biology have been linked with most of the major neurodegenerative diseases. These include a range of genetic diseases—Huntington's disease and Friedreich's ataxia, some of the cerebellar ataxias, and heritable, familial forms of Parkinsons' disease, Motoneuron disease (also known as amyotrophic lateral sclerosis (ALS)) and Alzheimer's disease. These latter three diseases are unusual in this respect, as almost identical disease phenotypes are seen both as sporadic forms for which risk increases progressively with age, and as heritable familial forms, in which mutations of specific genes lead to almost identical disease phenotypes that usually occur in a younger age group of patients. The evidence for a mitochondrial pathophysiology is stronger in some of these diseases than in others, and a major challenge remains to define the processes that are primary mechanisms driving the pathophysiology, those which are contributors on the pathways to cell injury caused by other primary processes, and those that are epiphenomena, and ultimately to identify potentially useful therapeutic targets. These diseases are of huge importance as a major frontier in medical research, a major challenge to the pharmaceutical industry, and an enormous and terrifying threat to us all.
In this short essay, I will explore in particular the role of mitochondrial Ca2+ handling in neurons in the pathophysiology of these horrible and disabling diseases, with a focus on Ca2+-mediated cell death as a most extreme form of mitopathy that seems potentially tractable to therapeutic intervention, and that seems also to represent a ubiquitous form of cell injury that is recapitulated in many different disease contexts.
Mitochondrial calcium handling in the CNS
One of the abiding and critical unanswered questions in all the major neurodegenerative diseases is the basis for the selectivity and specificity of the cellular targets of degeneration: the death of motoneurons in ALS, of Purkinje neurons in the cerebellar ataxias, of dopaminergic neurons of the substantia nigra in Parkinson's disease (PD) and of retinal ganglion cells in optic atrophy and Leber's hereditary optic neuropathy. This is most difficult in those genetic diseases for which mutations of proteins have been identified, as, in all of these, the mutant proteins are ubiquitously expressed while only the target cells are affected. This signals a search for mechanisms or features of the target cells that makes them especially vulnerable to the effects of the mutation, but it has to be said that the questions remain in the most part unsatisfactorily answered if answered at all.
There is growing evidence that the answer, at least in some cases, may be embedded in the cell-specific aspects of mitochondrial Ca2+ signalling—the overall theme of this special issue. This theme is perfectly exemplified in the CNS, where there is a striking diversity in the [Ca2+]c signalling pathways employed by different cell populations. Indeed, it seems very plausible that the differences in expression of specific elements in the [Ca2+]c-signalling toolkit may be, at least in part, responsible for the cell type specificity of injury seen in most of the neurodegenerative diseases.
Calcium signalling in neurons is, in general, dominated by Ca2+ influx through voltage- and ligand-gated ion channels, with smaller modulations by intracellular signalling pathways, while [Ca2+]c signalling in glial cells is dominated by Ca2+ release from ER using IP3 as a second messenger system. This very crude classification leads to further sub-specialisations, depending on the classes of ion channels expressed by the neurons, neuronal architecture, cell size, Ca2+ buffering power and patterns of excitability. Thus, the specific Ca2+ signalling physiology of a cerebellar Purkinje cell is radically different from that of the neighbouring cerebellar granule cell, although both are ‘neurons’, reflecting differences in size and complexity and the requirement for local amplifying signalling mechanisms. Spinal motoneurons differ in many respects from many other cell types in their large volume, their enormously long axons and in their expression of Ca2+ permeant ‘AMPA’ subclass of glutamate receptors [13], combined, apparently with a relatively low Ca2+ buffering power [29, 54]. The dopaminergic neurons of the substantia nigra that selectively degenerate in PD have an unusual physiology in terms of their repetitive rhythmic excitability driven by pacemaking L-type Ca2+ channels [64]. Amongst the glia, astrocyte, microglial and oligodendrocyte Ca2+ signalling are all different, still to be fully explored and beyond the scope of this review.
Calcium regulates local mitochondrial metabolism
While much of the early literature demonstrating pathways for physiological mitochondrial Ca2+ accumulation and extrusion arose from studies of isolated mitochondria from liver or heart, it turns out that many of the earliest demonstrations of physiological mitochondrial Ca2+ uptake came from neuronal systems. A recurrent theme throughout this special issue is the activation of mitochondrial metabolism through the stimulation of the tricarboxylic acid (TCA) cycle by a rise in the matrix Ca2+ concentration [48]. One of the earliest demonstrations that a rise in Ca2+ concentration can increase mitochondrial oxidative phosphorylation was made in the photoreceptor, albeit from that strangest of creatures, the horseshoe crab [24] nearly a quarter of a century ago. Around the same time, Thayer and colleagues showed that during the time period immediately following (but not before) generation of Ca2+ signals through physiological pathways (although admittedly not to stimuli that could be called very physiological) in sensory neurons, the collapse of mitochondrial membrane potential by an uncoupler caused a large Ca2+ signal, presumably due to the release of Ca2+ from mitochondria, inferring that the mitochondria had been Ca2+ loaded by the prior Ca2+ increase, i.e. the mitochondria were accumulating Ca2+ during a physiological stimulus, a controversial issue at that time [66]. This was followed by our own work, also in sensory neurons, showing changes in mitochondrial function—a brief transient mitochondrial depolarization associated with mitochondrial Ca2+ uptake across the inner membrane, followed by prolonged, Ca2+-dependent increase in NADH—following depolarization-induced Ca2+ signals also in sensory neurons, again with the inference that mitochondria must be taking up Ca2+ under these physiological conditions. The interpretation was that mitochondria were taking up Ca2+, which activated the TCA cycle, increasing NADH and driving increased oxidative phosphorylation [22]. Even in complex preparations of neuronal networks, such as the hippocampal slice preparation, stimulation of synaptic pathways led to Ca2+-dependent changes in NADH fluorescence suggesting direct modulation of metabolism by Ca2+ signals [38, 60]. Thus, the Ca2+ signals that are essential for synaptic transmission and therefore for transmission of information throughout the CNS are transmitted to the mitochondria where it is assumed that Ca2+ modulates mitochondrial metabolism as described elsewhere—with upregulation of the TCA cycle, of the ATP synthase, of the aspartate carrier [56] and presumably with a resultant increase in the supply of ATP. I am intentionally cautious here, as a formal direct demonstration of increased ATP generation in neurons in response to synaptic stimulation is lacking as yet, although this has been demonstrated in other cell types [37].
If mitochondria take up Ca2+, it follows that they have a potential to act as local Ca2+ buffers. The effective impact is a quantitative matter—how densely packed are the mitochondria and what is their capacity to accumulate Ca2+? There are good data to show that mitochondrial Ca2+ uptake has a sufficient impact on local Ca2+ signalling to help shape synaptic transmission, both at the neuromuscular junction [18, 19] and at the giant synapse of the calyx of Held [8]. We also showed that mitochondrial Ca2+ uptake has sufficient buffering power to determine the rate of propagation of Ca2+ signals as waves through astrocytes [9]. As the subtle shaping and the spatial and temporal characteristics of Ca2+ signals are essential in shaping neuronal activity, it is very tempting to speculate that this subtle impact will be altered in disease states in which mitochondrial function is disturbed, having a disproportionate impact on CNS function. This remains hard to study directly and remains largely speculative.
Calcium directs the mitochondrial traffic
Calcium signals also seem to play a critical role in directing the mitochondrial traffic in complex neuronal networks. Mitochondria must be continually refreshed with new mitochondrial protein (the vast bulk of which is encoded by nuclear DNA), and must then be transported sometimes very long distances along axonal routes to reach the synaptic terminals where they are most needed. Travelling mitochondria have to find their way through complex dendritic trees and up and down long axons in a cell that may be effectively a metre long. Trafficking in general is beyond the scope of this review, but in principle involves the interaction between proteins on the outer mitochondrial membrane, including mitofusin 2 (MFN2) and the Ca2+-dependent Miro1. The local positioning of mitochondria near points of high energy demand seems functionally important and appears to be largely orchestrated by Ca2+. Thus, a local rise in [Ca2+]c interacts with the outer mitochondrial protein Miro, inhibiting trafficking through the kinesin motor KIF5, so that mitochondria stop where they are, and remain localized at areas where [Ca2+]c is high—presumably the places where energy demand will be highest and where local Ca2+ buffering is also likely to be important [44]. Defects in axonal mitochondrial transport lead to long tract dysfunction, such as the Charcot Marie Tooth peripheral sensory neuropathy (type 2A) that involves a defect in the protein MFN2 which interacts with Miro1 and 2 [50]. Defects in mitochondrial trafficking proteins have also been associated with Parkinson's disease. The kinase PTEN induced kinase 1 (PINK1) is one of an increasingly large family of proteins for which mutations are associated with familial forms of PD (see also below). PINK1 expression on the outer mitochondrial membrane increases in depolarized mitochondria, and recruits the ubiquitin ligase Parkin to mitochondria, promoting mitochondrial autophagy (mitophagy). It turns out that PINK1 phosphorylates Miro, activating its proteosomal degradation through the PINK1-dependent recruitment of Parkin. It is proposed that degradation of Miro limits mitochondrial movement, and might serve to ‘quarantine damaged mitochondria’, prior to their removal by autophagy with the suggestion that mutations in these proteins will impair the processes that segregate and remove dysfunctional mitochondria, allowing their accumulation and so leading to progressive neurodegeneration [50]. Defective axonal transport of mitochondria has also been associated with Motoneuron disease (discussed briefly below).
Roles of mitochondria in calcium-dependent neurotoxicity
A decisive element in the neuronal signalling machinery is the expression of the glutamate receptor families. Of these, two are highly Ca2+ permeable—the NMDA receptor and the Ca2+-permeant AMPA receptor. These play a crucial role in regulating Ca2+ influx related to synaptic activity and modulation, but they also create a precarious balancing act for neuronal Ca2+ homeostasis. It has been apparent for many years that prolonged exposure of neurons in culture to high concentrations of glutamate leads to Ca2+-dependent cell death—a phenomenon described as excitotoxicity [16]. Not only was it clear that glutamate caused Ca2+-dependent toxicity but it also seemed that not all Ca2+ is the same—the toxicity of Ca2+ depends on the route of entry. Michael Tymianski coined the term ‘the source specificity’ of Ca2+-dependent toxicity, showing that Ca2+ influx into hippocampal neurons via L-type voltage-gated Ca2+ channels was innocuous, while a similar global Ca2+ load (measured using radiolabelled calcium to avoid artefacts due to dye saturation) arriving in the cell through NMDA receptors was lethal [67]. His group later went on to show in a most elegant series of experiments that the source specificity arises from the colocalisation of NMDA receptors with nNOS, coupled through the post synaptic density protein, psd-95 [58]. The Ca2+-dependent nNOS was thus exposed to local microdomains of very high [Ca2+]c as the ion enters through the Ca2+-permeant NMDA channels, generating NO which in combination with the [Ca2+]c signal is toxic to the cells. Importantly, cells could be rescued by nNOS inhibition or by decoupling the nNOS from the NMDA receptor.
This process is probably not an experimental oddity of neurons in tissue culture but is thought to occur in the brain under pathological conditions. The clearest role is in ischaemic disease in the CNS—in stroke for example—where glutamate can rapidly accumulate in the extracellular space to toxic concentrations of hundreds of micromolar. It is believed that this mechanism is likely responsible for the progression of a stroke over the first few days after an ischaemic episode, extending the areas of brain injury beyond the core ischaemic zone into a penumbra. What completed the circle very nicely was the demonstration by Tymianski and colleagues that decoupling nNOS from the NMDA receptors using small peptides rendered membrane permeant with the HIV TAT sequence rescued CNS tissue in an in vivo stroke model [1].
The source specificity of glutamate-induced neuronal damage has been refined more recently to suggest that excessive activation of extrasynaptic receptors may induce toxic Ca2+ loads and mitochondrial Ca2+-dependent injury, whilst activation of synaptic receptors tends to cause smaller Ca2+ elevations that play a prosurvival role [61], suggesting that the balance between these two pathways may be critical in some disease models, in which glutamate ‘overspill’ from synaptic release, glutamate release from glial cells by reversal of the glutamate transporter, or impaired glutamate clearance from synaptic clefts might lead to overactivation of extrasynaptic receptors, causing cell injury.
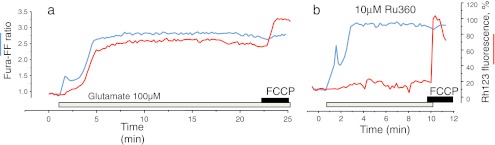
So, where do the mitochondria come in? One of the major features of [Ca2+]c-dependent toxicity is the ‘delayed deregulation of [Ca2+]c homeostasis’ (delayed [Ca2+]c deregulation, or DCD)—a secondary rise in [Ca2+]c that can be delayed by anything from 1 or 2 to 10 min after the initial exposure to glutamate (Fig. 1a). Simultaneous measurements of mitochondrial membrane potential and [Ca2+]c showed that the secondary [Ca2+]c deregulation occurs in synchrony with a [Ca2+]c-dependent collapse of mitochondrial membrane potential (Δψm) [69] (Fig. 1a). The loss of Δψm was preventable using nNOS inhibitors as was the DCD, and indeed, mitochondria were sensitised to otherwise innocuous [Ca2+]c loads by NO donors [39], suggesting a direct link between the events at the plasma membrane and routes to mitochondrial toxicity.
Fig. 1.
Simultaneous measurements of [Ca2+]c (blue trace) and of Δψm (red trace) from single hippocampal neurons in response to toxic glutamate exposure. Δψm was measured using the dye rhodamine 123 which was used in the so-called ‘dequench’ mode, in which mitochondrial depolarization causes an increase in fluorescence signal. [Ca2+]c was measured using fura-FF, a low affinity ratiometric [Ca2+]c indicator. a The response to glutamate shows a stereotypical pattern: the [Ca2+]c increases transiently followed by variable degree of recovery, followed by a progressive increase—referred to as delayed [Ca2+]c deregulation. Δψm changes only slightly during the initial phase, but then the mitochondria show a progressive and complete depolarization (addition of uncoupler which depolarises completely has no further effect) that is exactly synchronous with DCD. b Following preincubation with Ru360, inhibitor of the mitochondrial uniporter, the [Ca2+]c signal was essentially unchanged, but mitochondrial depolarization was suppressed, suggesting that mitochondrial [Ca2+]c uptake is an essential step on the pathway to [Ca2+]c-mediated cell death [2]
Key questions then are what are the mechanisms of DCD and of loss of mitochondrial membrane potential? Once mitochondrial potential is lost in a neuron, that cell is destined to die as neurons have almost no capacity to increase glycolytic ATP once oxidative phosphorylation is lost [33], and so neurons with depolarized mitochondria will undergo bioenergetic collapse and die. It has been generally assumed that the loss of mitochondrial membrane potential is triggered by mitochondrial Ca2+ accumulation (‘overload’), a state where influx into the mitochondria overwhelms matrix Ca2+ buffering power and the capacity of the sodium–calcium exchange efflux pathway. Direct measurements of increased intramitochondrial free Ca2+ during this process have proven remarkably difficult—transfection of neurons with genetically encoded Ca2+ reporters is not easy, and large intracellular pH shifts associated with the massive movement of Na+ and Ca2+ across the membrane introduce major artefacts into most measurements. Using small molecule sensors such as rhod family dyes have also failed in most people's hands, as dye loading invariably is not restricted to the mitochondria and cytosolic [Ca2+]c rises so high that the cytosolic component overwhelms the mitochondrial specific signal, which is probably saturated anyway. It has been shown that brief exposure to uncoupler around the time of glutamate application can be protective [62]. The logic was that the uncoupler, by collapsing the potential at the crucial time, prevents mitochondrial Ca2+ uptake. Even though the change in [Ca2+]c was if anything enhanced, cell viability was improved, arguing that mitochondrial Ca2+ uptake plays a critical role in driving cell death. This is a tricky experiment as uncouplers can only too easily kill the cells, and so the timing is critical.
We showed relatively recently that inhibition of the Ca2+ uniporter using Ru360 prevented the loss of mitochondrial membrane potential and decoupled DCD and mitochondrial depolarization, as DCD proceeded even though mitochondrial potential was preserved [2] (Fig. 1b). This is perhaps the closest so far to a direct demonstration of a role for mitochondrial matrix Ca2+ overload as a determinant of mitochondrial dysfunction (see also [21]). It would be ideal to know whether this is protective against cell death, but Ru360 is highly unstable and tricky to use—it is oxidised rapidly after exposure to room air, and only ‘works’ for about 20–30 min after addition to the cells, making this an impossible experiment, and as yet, the appropriate tools to address this question directly are not available.
The mitochondrial permeability transition pore
Demonstrations of mitochondrial depolarization following a rise in [Ca2+]m and dependent on nitrosative stress immediately flags involvement of the mitochondrial permeability transition pore (mPTP) for any mitochondrial biologist. Therefore it is timely to introduce the permeability transition pore into this text. This is an extraordinary phenomenon, which seems to underlie Ca2+-dependent mitochondrial cell death in most tissues [31]. First described in a series of remarkable papers by Haworth and Hunter [32, 34, 35], this sudden loss of the mitochondrial permeability barrier following additions of Ca2+ or prooxidants was treated for a long while as an artefact of isolated mitochondrial preparations. Much later, it was shown that the permeability increase is due to the opening of a large conductance pore in the inner mitochondrial membrane large enough to admit deoxyglucose into the matrix and is not simply a disruption of the membrane [30]. It has been suggested that the pore is generated by a transformation of membrane proteins with other physiological roles into a pore-forming configuration—favoured candidates included the adenine nucleotide translocase (ANT), a protein which can switch to a pore-forming conformation in the presence of high Ca2+ [57] and pore opening is modulated by drugs which bind to the ANT, and the voltage-dependent anion channel (VDAC) in the outer mitochondrial membrane. However, recent experiments on tissues from an ‘ANT and VDAC knockout mice’ have thrown question marks over this model [6, 42] leaving considerable uncertainty about the molecular identity of the mPTP. What is clear and unambiguous is that the pore opening is regulated by the matrix peptidyl prolyl cis–trans isomerase, the protein cyclophilin D [5, 65]. This is particularly important, as it binds to cyclosporine A (CsA), which prevents pore opening. CsA has become the benchmark for mPTP opening and is now being used in clinical trials for mPTP involvement (along with other mPTP inhibitors) in various pathologies [28, 49].
The role of the mPTP in cell death during ischaemia and reperfusion in the heart is clear and unambiguous. In the CNS, the storey has been less straightforward. CsA proved to be protective in stroke models [26] and in culture models of glutamate toxicity. The problem is that CsA binds to all cyclophilins, and these include cytosolic proteins that regulate calcineurin, which in turn mediates the Ca2+-dependent activation of nNOS [20]. Given the role of nNOS described above, this undermines the interpretation of experiments that demonstrate any protective action of CsA and, in the absence of other more specific pharmacological agents, has made it difficult to assign an unambiguous role for mPTP in glutamate toxicity or stroke. After generation of the CypD knockout mouse, it was soon shown that infarct size was reduced in the CypD knockout, arguing for a role for the PTP in stroke and cell death in the CNS [5]. To our surprise, however, the loss of mitochondrial potential in response to glutamate was not notably altered in cell models from the CypD knockout mouse [2], arguing that the initial loss of potential cannot be a simple consequence of mPTP opening. What we did find was that the loss of potential that was normally irreversible became reversible in the CypD knockout, suggesting that during a prolonged mitochondrial depolarization there was a delayed transition from depolarized mitochondria to mPTP opening [2].
A role for PARP?
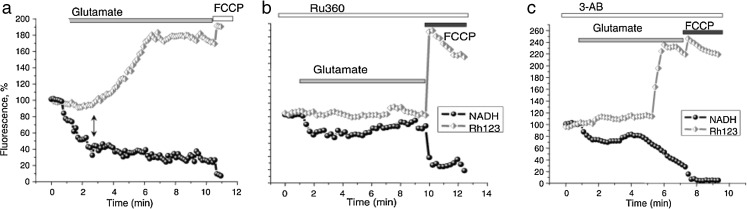
This leaves the question of the cause of the initial mitochondrial depolarization caused by glutamate excitotoxicity. In the late 1990s, evidence from several labs suggested that glutamate toxicity engaged the enzyme PARP-1 [23, 43, 46]. Poly (ADP ribose) polymerase-1 is a DNA repair enzyme that is activated by single strand DNA breaks, usually the result of oxidative stress. Activation of PARP uses NAD+ to generate polymers—poly (ADP ribose), or PAR polymers—which are relatively shortlived as these are rapidly degraded by the enzyme PAR Glycohydrolase [63]. It appears that PAR polymers can release apoptosis inducing factor (AIF) from mitochondria, suggesting that cell death may be mediated by AIF translocation to the nucleus and a ‘caspase-independent apoptosis’—a form of cell death that has been termed ‘parthanatos’ [70]. This is a fascinating model, but remains somewhat controversial. Several groups have shown that PARP-1 is hyperactivated by glutamate toxicity, but details of the pathways to cell death are less clear. Thus, it has been proposed that activation of NMDA receptors can activate the enzyme NADPH oxidase, generating oxidative stress that then activates PARP [10]. The activation of PARP consumes NAD+, prevents cell metabolism and the cells die. AIF may be released but seems immaterial, as the cells will die through bioenergetic collapse [3, 4]. In this model, it is hard to identify any specific role for mitochondrial Ca2+ uptake or to see why preventing mitochondrial Ca2+ uptake should be protective. Another group have suggested that the oxidative stress that activates PARP-1 is the result of mitochondrial Ca2+ uptake—i.e. mitochondria take up Ca2+, the matrix Ca2+ overload generates excessive free radical species, this causes activation of PARP-1 which then depletes NAD+ and the cells die [21]. Our own data suggest that mitochondrial Ca2+ uptake is indeed critical for PARP-1 activation, and suggest that PARP-1 activation causes the loss of mitochondrial membrane potential by inhibition of glycolysis and so depletion of mitochondrial substrate supply [2]. Thus, we found that application of glutamate to neurons causes a remarkably rapid decrease in the autofluorescence of NADH that precedes the loss of potential (Fig. 2a). Importantly, loss of NADH and mitochondrial depolarization were prevented by Ru360, (Fig. 2b) arguing that mitochondrial Ca2+ uptake is an essential step on the pathway to PARP activation and to cell death [2]. Pharmacological inhibition of PARP delayed both the NADH oxidation and the loss of potential (Fig. 2c). Remarkably, the mitochondrial membrane potential could be restored using substrates that bypass glycolysis—pyruvate or methyl succinate for example, suggesting that mitochondrial depolarization occurs as a failure of substrate supply as NAD+ is consumed and inhibits glycolytic supply of pyruvate. Again, it was hard to see an essential role for AIF, as the loss of potential condemns these cells to die. So the precise role for AIF remains a little unclear. What again brings the data full circle is the recent demonstration that small molecule PARP inhibitors are protective in a stroke model, putting the mechanism into a bigger context of brain injury and stroke [51]. The proposed full pathways of excitotoxicity are illustrated schematically in Fig. 3.
Fig. 2.
Simultaneous measurements of Δψm and of NADH autofluorescence from single hippocampal neurons. a Glutamate exposure caused a rapid oxidation of NADH causing a decrease in signal (as NAD+ is not fluorescent). Once the NADH oxidation reaches a nadir, Δψm started to fall (an increase in fluorescence signal). Final addition of uncoupler, FCCP, which causes maximal oxidation of mitochondrial NADH had no effect, suggesting that the NADH pool was already mostly oxidised. b Both the changes in Δψm and in NADH were largely inhibited following preincubation with Ru360 and were profoundly delayed by the PARP inhibitor 3-AB (c). Modified from [2]
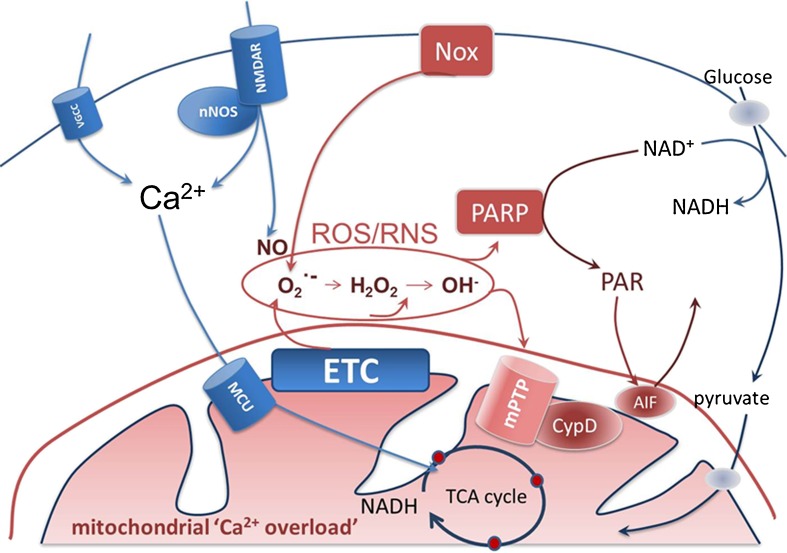
Fig. 3.
Scheme of pathways involved in glutamate-induced excitotoxicity. Calcium influx through voltage-gated or NMDAR-gated channels is followed by mitochondrial Ca2+ influx through the mitochondrial calcium uniporter (MCU). While the physiological consequence of raised intramitochondrial [Ca2+] is an increased activity of the three rate limiting enzymes of the TCA cycle, pathological and prolonged Ca2+ influx leads to mitochondrial Ca2+overload. NMDAR-mediated Ca2+ influx is closely coupled to the generation of NO by nNOS; raised Ca2+ may activate the NADPH oxidase (Nox), while mitochondrial Ca2+ overload may also increase generation of superoxide by the electron transport chain (ETC). Nitrosative or oxidative stress arising either from the ETC or from Nox activation may cause overactivation of PARP. PARP consumes NAD + to form PAR polymers, causing depletion of NAD+, failure of glycolysis and so failure of mitochondrial substrate supply. This culminates in the loss of Δψm, ATP depletion and cell death. The PAR polymers generated by PARP may also cause release of AIF which amplifies cell death following its translocation to the nucleus
Mitochondrial calcium handling and Parkinson's disease
Similar mechanisms of Ca2+-induced cell injury have been implicated in the pathogenesis of PD. The dopaminergic cells of the substantia nigra that degenerate in PD have an unusual electrical feature as pacemaking neurons, in which electrical activity is driven largely by L-type Ca2+ channels [64]. It has been argued that this Ca2+-mediated activity sensitises the pacemaking dopaminergic cells to injury, to the extent that inhibition of L-type Ca2+ channels (a condition in which electrical activity is taken over by Na+-mediated activity) can protect the cells against injury by toxins such as 6-(OH)DA [15]. The specific role of downstream pathways involving mitochondrial Ca2+ handling are perhaps less clear. The Ca2+ load appears to increase mitochondrial free radical generation, which in normal mice induces a protective mechanism through a mild uncoupling mechanism mediated by the uncoupling proteins UCP3 and 4, with the suggestion that this might reduce mitochondria free radical generation and so serve a protective role. Thus, a modest mitochondrial depolarization was inhibited by the UCP antagonist genipin. Of greatest interest perhaps was the loss of this protective mechanism by knockout of the protein DJ-1 and protection by overexpression of DJ-1. DJ-1 (also known as PARK7) is a protein in which mutations are associated with early onset familial form of PD. The data presented by the Surmeier group suggested that PTP and CypD were not involved, but the mechanism of cell death remains unclear [15]. Interestingly, other data also point to a role for PARP in PD but again the specific link between mitochondrial dysfunction, mitochondrial Ca2+ uptake, Ca2+ signalling and PARP does not seem to have been made [36, 45].
Amongst the neurodegenerative diseases, the case for a role of mitochondrial dysfunction in PD is probably the strongest. Several models involving toxic damage to the CNS, using the mitochondrial complex I inhibitor, rotenone, the drug derived compound MPTP, which also blocks complex I, and 6(OH)-DA, all cause mitochondrial and selective damage to dopaminergic neurons. Coupled with findings of Complex I dysfunction in SN from post mortem material from PD patients, it seems likely that this points to a major role for mitochondrial bioenergetic dysfunction in PD [59]. Huge strides have been made in the genetics of familial PD over recent years, with the identification of a string of proteins in which mutations cause early onset PD (for a review see: [25]). Remarkably, the picture that is emerging from these studies again points towards a pivotal role for mitochondrial dysfunction, as almost all of these proteins seem either to associate with mitochondria or to lie on mitochondrial associated pathways. Detailed discussion of this literature and the proteins involved is outside the scope of this essay, and I refer the reader to excellent recent reviews for further detail [11, 12]. However, the notion that the underlying defect may involve the failure of appropriate removal of dysfunctional mitochondria allowing the slowly progressive accumulation of mitochondrial defects as the basis for a neurodegenerative disease [53] seems quite appealing, even though these proteins are ubiquitous and so leaves us once again with the question why the dopaminergic SN neurons should be especially vulnerable.
In our own work, we addressed the functional bioenergetic consequences of a mutation of the protein PINK1 and its consequences for the neuronal vulnerability to calcium-mediated injury [27]. In PINK1 deficient cells, mitochondrial potential was reduced and was largely maintained by the ATPase running in reverse mode. This state was reversed by the provision of substrates for complex I and II (pyruvate or methyl succinate), respectively, suggesting a defect in substrate supply as a key mechanism underlying the bioenergetic defect. We also found that mitochondrial Ca2+ efflux was profoundly delayed in these cells, suggesting impaired Na+/Ca2+ exchange in mitochondrial Ca2+ homeostasis. The functional consequence of this defect was to make mitochondria far more vulnerable to Ca2+ overload and to dramatically reduce the effective threshold for mPTP opening, leading to Ca2+-dependent cell death. At present, it is not clear how to match these findings with our understanding of a role for PINK1 as a regulator of mitochondrial autophagy, unless we propose that the bioenergetic deficiencies are a consequence of failure of autophagic removal of damaged mitochondria. However, the observations provide a link between the Ca2+ models of PD dysfunction and the emerging literature pointing towards mitochondrial dysfunction as a pathophysiological mechanism in PD.
In Huntington's disease, there are also data suggesting that the pathology involves increased mitochondrial sensitivity to Ca2+ leading to a decreased threshold for mPTP opening. Huntington's disease is caused by an expansion of exonic CAG triplet repeats in the gene encoding the Huntingtin protein (Htt) that appear to be toxic. Mitochondria isolated from the brains of transgenic mice expressing mutant Htt showed depolarisation at lower Ca2+ loads than controls. Remarkably, this observation could be replicated in normal mitochondria exposed to long polyglutamine repeats, suggesting some kind of direct sensitising effect of the mutant protein [55].
Motoneuron disease
The other major neurodegenerative disease that I must touch on is Motoneuron disease, or ALS. This is an appalling rapidly progressive and fatal disease involving the selective degeneration of spinal motoneurons. As in PD, there are rare familial forms of the disease which have been associated with mutations of superoxide dismutase (mSOD-1) and more recently with mutations of the protein vesicle associated membrane protein associated protein B (VAPB) [52]. The transgenic mutant mouse expressing the G93A mSOD-1 mutation develops selective degeneration of spinal motoneurons, and so represents a very appealing model to study ALS. There is always a major issue with using the mutations associated with any of the familial forms of major neurodegenerative diseases in an attempt to understand the sporadic disease which affects the majority of ALS patients. Familial ALS (fALS) associated with the SOD-1 mutation accounts for only a fraction of the total number of patients suffering this horrible disease: the incidence of familial ALS is usually estimated at 5–10 % of all patients with ALS, of which only one fifth express the SOD-1 mutation—i.e. this mutation accounts for about 2 % of all ALS patients, and yet it is the major model that is studied, if only for want of an adequate model of the sporadic disease. It is hard to know how much these studies will really tell us about the sporadic disease, but there is no doubt that it does tell us a great deal about general principles governing pathophysiological mechanism.
There is a substantial literature suggesting that motoneuron death in the mSOD-1 G93A genetic model of fALS is associated with mitochondrial dysfunction and some evidence linking changes in Ca2+ and mitochondrial Ca2+ overload. Thus, motoneurons express Ca2+-permeable AMPA receptors and also appear to have a relatively low Ca2+ buffering capacity. Exposure of motoneurons to AMPA caused increased ROS generation, loss of mitochondrial potential and cell death [14]. Furthermore, motoneurons expressing the G93A SOD-1 mutation were protected by overexpression of the Ca2+-buffering protein, parvalbumin [68], and also by knockdown of cyclophilin D [47], together pointing towards mPTP-related mechanism of glutamate-induced injury, mediated by AMPA receptors rather than the NMDA receptors that drive glutamate toxicity in cortical and hippocampal neurons. Interestingly, it seems that neurodegeneration in the fALS model is initiated at nerve terminals and tracks back along the axons. Gathering evidence suggests that Ca2+-mediated mitochondrial toxicity may be associated with repetitive stimulation in peripheral motoneuron axons [7], perhaps operating exactly the same mechanisms discussed above. Indeed, there is some evidence for a role of PARP in ALS as well, suggesting that elements of this Ca2+-dependent mitochondrial cell death pathway are recapitulated in different diseases, perhaps shaped by the specific physiology of each cell type [17, 40, 41].
There is also substantial evidence pointing to a disturbance in mitochondrial axonal transport in fALS. Thus, the VAPB mutation also seems to target Ca2+ and mitochondria but relating to mitochondrial transport in axons. A general defect in retrograde axonal transport has been described in the axons of G93A mutant mice, preceding the onset of symptoms, while a selective defect of anterograde axonal mitochondrial transport and a decrease in axonal mitochondrial content has also been described in the G93A mutant [52]. The VAPB mutation (VAPB P56S) also causes a selective defect in anterograde transport of mitochondria in axons associated with a rise in local [Ca2+]c, probably related to ER disruption (VAPB is an ER protein). The local elevation of the [Ca2+]c signal regulates Miro1-mediated axonal mitochondrial transport (see above) leading to impaired mitochondrial trafficking and distribution. It is not clear why this defect should selectively affect motoneurons, nor why it should lead ultimately to motoneuron degeneration.
Conclusions
Mitochondrial dysfunction is clearly implicated in the pathogenesis of the major neurodegenerative diseases and plays a critical role as a determinant of irreversible cell injury following stroke. Increasing evidence suggests that specialisations of Ca2+ signalling pathways in different cell types may play a major role in shaping the patterns of cell loss in each of the major neurodegenerative diseases. Understanding these pathways brings the promise of defining new potential therapeutic targets for these dreadful diseases.
Acknowledgments
I would like to thank Dr Andrey Abramov, whose industry was responsible for much of the work described here that originated from my lab. Work in the Duchen lab is largely funded by the Wellcome Trust and the Medical Research Council.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Footnotes
This article is published as part of the special issue on “Cell-specific roles of mitochondrial Ca2+ handling.”
References
- 1.Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, Wang YT, Salter MW, Tymianski M. Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science. 2002;298:846–850. doi: 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- 2.Abramov AY, Duchen MR. Mechanisms underlying the loss of mitochondrial membrane potential in glutamate excitotoxicity. Biochim Biophys Acta. 2008;1777:953–964. doi: 10.1016/j.bbabio.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 3.Alano CC, Ying W, Swanson RA. Poly(ADP-ribose) polymerase-1-mediated cell death in astrocytes requires NAD + depletion and mitochondrial permeability transition. J Biol Chem. 2004;279:18895–18902. doi: 10.1074/jbc.M313329200. [DOI] [PubMed] [Google Scholar]
- 4.Alano CC, Garnier P, Ying W, Higashi Y, Kauppinen TM, Swanson RA. NAD + depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J Neurosci. 2010;30:2967–2978. doi: 10.1523/JNEUROSCI.5552-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 6.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrett EF, Barrett JN, David G. Mitochondria in motor nerve terminals: function in health and in mutant superoxide dismutase 1 mouse models of familial ALS. J Bioenerg Biomembr. 2011;43:581–586. doi: 10.1007/s10863-011-9392-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Billups B, Forsythe ID. Presynaptic mitochondrial calcium sequestration influences transmission at mammalian central synapses. J Neurosci. 2002;22:5840–5847. doi: 10.1523/JNEUROSCI.22-14-05840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boitier E, Rea R, Duchen MR. Mitochondria exert a negative feedback on the propagation of intracellular Ca2+ waves in rat cortical astrocytes. J Cell Biol. 1999;145:795–808. doi: 10.1083/jcb.145.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brennan AM, Suh SW, Won SJ, Narasimhan P, Kauppinen TM, Lee H, Edling Y, Chan PH, Swanson RA. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci. 2009;12:857–863. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burchell VS, Gandhi S, Deas E, Wood NW, Abramov AY, Plun-Favreau H. Targeting mitochondrial dysfunction in neurodegenerative disease: Part I. Expert Opin Ther Targets. 2010;14:369–385. doi: 10.1517/14728221003652489. [DOI] [PubMed] [Google Scholar]
- 12.Burchell VS, Gandhi S, Deas E, Wood NW, Abramov AY, Plun-Favreau H. Targeting mitochondrial dysfunction in neurodegenerative disease: Part II. Expert Opin Ther Targets. 2010;14:497–511. doi: 10.1517/14728221003730434. [DOI] [PubMed] [Google Scholar]
- 13.Carriedo SG, Yin HZ, Weiss JH. Motor neurons are selectively vulnerable to AMPA/kainate receptor-mediated injury in vitro. J Neurosci. 1996;16:4069–4079. doi: 10.1523/JNEUROSCI.16-13-04069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carriedo SG, Yin HZ, Sensi SL, Weiss JH. Rapid Ca2+ entry through Ca2 + -permeable AMPA/Kainate channels triggers marked intracellular Ca2+ rises and consequent oxygen radical production. J Neurosci. 1998;18:7727–7738. doi: 10.1523/JNEUROSCI.18-19-07727.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan CS, Gertler TS, Surmeier DJ. Calcium homeostasis, selective vulnerability and Parkinson's disease. Trends Neurosci. 2009;32:249–256. doi: 10.1016/j.tins.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi DW. Glutamate neurotoxicity in cortical cell culture is calcium dependent. Neurosci Lett. 1985;58:293–297. doi: 10.1016/0304-3940(85)90069-2. [DOI] [PubMed] [Google Scholar]
- 17.Chung YH, Joo KM, Lee YJ, Shin DH, Cha CI. Reactive astrocytes express PARP in the central nervous system of SOD(G93A) transgenic mice. Brain Res. 2004;1003:199–204. doi: 10.1016/j.brainres.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 18.David G, Barrett EF. Stimulation-evoked increases in cytosolic [Ca(2+)] in mouse motor nerve terminals are limited by mitochondrial uptake and are temperature-dependent. J Neurosci. 2000;20:7290–7296. doi: 10.1523/JNEUROSCI.20-19-07290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.David G, Barrett JN, Barrett EF. Evidence that mitochondria buffer physiological Ca2+ loads in lizard motor nerve terminals. J Physiol. 1998;509(Pt 1):59–65. doi: 10.1111/j.1469-7793.1998.059bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diaz-Ruiz A, Vergara P, Perez-Severiano F, Segovia J, Guizar-Sahagun G, Ibarra A, Rios C. Cyclosporin-A inhibits constitutive nitric oxide synthase activity and neuronal and endothelial nitric oxide synthase expressions after spinal cord injury in rats. Neurochem Res. 2005;30:245–251. doi: 10.1007/s11064-005-2447-0. [DOI] [PubMed] [Google Scholar]
- 21.Duan Y, Gross RA, Sheu SS. Ca2 + -dependent generation of mitochondrial reactive oxygen species serves as a signal for poly(ADP-ribose) polymerase-1 activation during glutamate excitotoxicity. J Physiol. 2007;585:741–758. doi: 10.1113/jphysiol.2007.145409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duchen MR. Ca(2+)-dependent changes in the mitochondrial energetics in single dissociated mouse sensory neurons. Biochem J. 1992;283(Pt 1):41–50. doi: 10.1042/bj2830041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eliasson MJ, Sampei K, Mandir AS, Hurn PD, Traystman RJ, Bao J, Pieper A, Wang ZQ, Dawson TM, Snyder SH, Dawson VL. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med. 1997;3:1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- 24.Fein A, Tsacopoulos M. Activation of mitochondrial oxidative metabolism by calcium ions in Limulus ventral photoreceptor. Nature. 1988;331:437–440. doi: 10.1038/331437a0. [DOI] [PubMed] [Google Scholar]
- 25.Fitzgerald JC, Plun-Favreau H. Emerging pathways in genetic Parkinson's disease: autosomal-recessive genes in Parkinson's disease—a common pathway? FEBS J. 2008;275:5758–5766. doi: 10.1111/j.1742-4658.2008.06708.x. [DOI] [PubMed] [Google Scholar]
- 26.Friberg H, Ferrand-Drake M, Bengtsson F, Halestrap AP, Wieloch T. Cyclosporin A, but not FK 506, protects mitochondria and neurons against hypoglycemic damage and implicates the mitochondrial permeability transition in cell death. J Neurosci. 1998;18:5151–5159. doi: 10.1523/JNEUROSCI.18-14-05151.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gandhi S, Wood-Kaczmar A, Yao Z, Plun-Favreau H, Deas E, Klupsch K, Downward J, Latchman DS, Tabrizi SJ, Wood NW, Duchen MR, Abramov AY. PINK1-associated Parkinson's disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell. 2009;33:627–638. doi: 10.1016/j.molcel.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomez L, Li B, Mewton N, Sanchez I, Piot C, Elbaz M, Ovize M. Inhibition of mitochondrial permeability transition pore opening: translation to patients. Cardiovasc Res. 2009;83:226–233. doi: 10.1093/cvr/cvp063. [DOI] [PubMed] [Google Scholar]
- 29.Grosskreutz J, Van Den Bosch L, Keller BU. Calcium dysregulation in amyotrophic lateral sclerosis. Cell Calcium. 2010;47:165–174. doi: 10.1016/j.ceca.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Halestrap AP. The mitochondrial permeability transition: its molecular mechanism and role in reperfusion injury. Biochem Soc Symp. 1999;66:181–203. doi: 10.1042/bss0660181. [DOI] [PubMed] [Google Scholar]
- 31.Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol. 2009;46:821–831. doi: 10.1016/j.yjmcc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 32.Haworth RA, Hunter DR. The Ca2 + -induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch Biochem Biophys. 1979;195:460–467. doi: 10.1016/0003-9861(79)90372-2. [DOI] [PubMed] [Google Scholar]
- 33.Herrero-Mendez A, Almeida A, Fernandez E, Maestre C, Moncada S, Bolanos JP. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat Cell Biol. 2009;11:747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- 34.Hunter DR, Haworth RA. The Ca2 + -induced membrane transition in mitochondria. I. The protective mechanisms. Arch Biochem Biophys. 1979;195:453–459. doi: 10.1016/0003-9861(79)90371-0. [DOI] [PubMed] [Google Scholar]
- 35.Hunter DR, Haworth RA. The Ca2 + -induced membrane transition in mitochondria. III. Transitional Ca2+ release. Arch Biochem Biophys. 1979;195:468–477. doi: 10.1016/0003-9861(79)90373-4. [DOI] [PubMed] [Google Scholar]
- 36.Infante J, Sanchez-Juan P, Mateo I, Rodriguez-Rodriguez E, Sanchez-Quintana C, Llorca J, Fontalba A, Terrazas J, Oterino A, Berciano J, Combarros O. Poly (ADP-ribose) polymerase-1 (PARP-1) genetic variants are protective against Parkinson's disease. J Neurol Sci. 2007;256:68–70. doi: 10.1016/j.jns.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci U S A. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kann O, Schuchmann S, Buchheim K, Heinemann U. Coupling of neuronal activity and mitochondrial metabolism as revealed by NAD(P)H fluorescence signals in organotypic hippocampal slice cultures of the rat. Neuroscience. 2003;119:87–100. doi: 10.1016/S0306-4522(03)00026-5. [DOI] [PubMed] [Google Scholar]
- 39.Keelan J, Vergun O, Duchen MR. Excitotoxic mitochondrial depolarisation requires both calcium and nitric oxide in rat hippocampal neurons. J Physiol. 1999;520(Pt 3):797–813. doi: 10.1111/j.1469-7793.1999.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim SH, Henkel JS, Beers DR, Sengun IS, Simpson EP, Goodman JC, Engelhardt JI, Siklos L, Appel SH. PARP expression is increased in astrocytes but decreased in motor neurons in the spinal cord of sporadic ALS patients. J Neuropathol Exp Neurol. 2003;62:88–103. doi: 10.1093/jnen/62.1.88. [DOI] [PubMed] [Google Scholar]
- 41.Kim SH, Engelhardt JI, Henkel JS, Siklos L, Soos J, Goodman C, Appel SH. Widespread increased expression of the DNA repair enzyme PARP in brain in ALS. Neurology. 2004;62:319–322. doi: 10.1212/01.WNL.0000103291.04985.DC. [DOI] [PubMed] [Google Scholar]
- 42.Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lo EH, Bosque-Hamilton P, Meng W. Inhibition of poly(ADP-ribose) polymerase: reduction of ischemic injury and attenuation of N-methyl-d-aspartate-induced neurotransmitter dysregulation. Stroke. 1998;29:830–836. doi: 10.1161/01.STR.29.4.830. [DOI] [PubMed] [Google Scholar]
- 44.Macaskill AF, Rinholm JE, Twelvetrees AE, Arancibia-Carcamo IL, Muir J, Fransson A, Aspenstrom P, Attwell D, Kittler JT. Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron. 2009;61:541–555. doi: 10.1016/j.neuron.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mandir AS, Przedborski S, Jackson-Lewis V, Wang ZQ, Simbulan-Rosenthal CM, Smulson ME, Hoffman BE, Guastella DB, Dawson VL, Dawson TM. Poly(ADP-ribose) polymerase activation mediates 1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism. Proc Natl Acad Sci U S A. 1999;96:5774–5779. doi: 10.1073/pnas.96.10.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mandir AS, Poitras MF, Berliner AR, Herring WJ, Guastella DB, Feldman A, Poirier GG, Wang ZQ, Dawson TM, Dawson VL. NMDA but not non-NMDA excitotoxicity is mediated by Poly(ADP-ribose) polymerase. J Neurosci. 2000;20:8005–8011. doi: 10.1523/JNEUROSCI.20-21-08005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin LJ, Gertz B, Pan Y, Price AC, Molkentin JD, Chang Q. The mitochondrial permeability transition pore in motor neurons: involvement in the pathobiology of ALS mice. Exp Neurol. 2009;218:333–346. doi: 10.1016/j.expneurol.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- 49.Merlini L, Bernardi P. Therapy of collagen VI-related myopathies (Bethlem and Ullrich) Neurotherapeutics. 2008;5:613–618. doi: 10.1016/j.nurt.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Misko A, Jiang S, Wegorzewska I, Milbrandt J, Baloh RH. Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. J Neurosci. 2010;30:4232–4240. doi: 10.1523/JNEUROSCI.6248-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moroni F, Cozzi A, Chiarugi A, Formentini L, Camaioni E, Pellegrini-Giampietro D, Chen Y, Liang S, Zaleska M, Gonzales C, Wood A, Pellicciari R. Long-lasting neuroprotection and neurological improvement in stroke models with new, potent and brain permeable inhibitors of poly(ADP-ribose) polymerase. Br J Pharmacol. 2012;165:1487–1500. doi: 10.1111/j.1476-5381.2011.01666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morotz GM, De Vos KJ, Vagnoni A, Ackerley S, Shaw CE, Miller CC (2012) Amyotrophic lateral sclerosis-associated mutant VAPBP56S perturbs calcium homeostasis to disrupt axonal transport of mitochondria. Hum Mol Genet [DOI] [PMC free article] [PubMed]
- 53.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin-induced mitophagy in the pathogenesis of Parkinson disease. Autophagy. 2009;5:706–708. doi: 10.4161/auto.5.5.8505. [DOI] [PubMed] [Google Scholar]
- 54.Palecek J, Lips MB, Keller BU. Calcium dynamics and buffering in motoneurones of the mouse spinal cord. J Physiol. 1999;520(Pt 2):485–502. doi: 10.1111/j.1469-7793.1999.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Panov AV, Gutekunst CA, Leavitt BR, Hayden MR, Burke JR, Strittmatter WJ, Greenamyre JT. Early mitochondrial calcium defects in Huntington's disease are a direct effect of polyglutamines. Nat Neurosci. 2002;5:731–736. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- 56.Pardo B, Contreras L, Serrano A, Ramos M, Kobayashi K, Iijima M, Saheki T, Satrustegui J. Essential role of aralar in the transduction of small Ca2+ signals to neuronal mitochondria. J Biol Chem. 2006;281:1039–1047. doi: 10.1074/jbc.M507270200. [DOI] [PubMed] [Google Scholar]
- 57.Ruck A, Dolder M, Wallimann T, Brdiczka D. Reconstituted adenine nucleotide translocase forms a channel for small molecules comparable to the mitochondrial permeability transition pore. FEBS Lett. 1998;426:97–101. doi: 10.1016/S0014-5793(98)00317-2. [DOI] [PubMed] [Google Scholar]
- 58.Sattler R, Xiong Z, Lu WY, Hafner M, MacDonald JF, Tymianski M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science. 1999;284:1845–1848. doi: 10.1126/science.284.5421.1845. [DOI] [PubMed] [Google Scholar]
- 59.Schapira AH. Mitochondrial complex I deficiency in Parkinson's disease. Adv Neurol. 1993;60:288–291. [PubMed] [Google Scholar]
- 60.Shuttleworth CW, Brennan AM, Connor JA. NAD(P)H fluorescence imaging of postsynaptic neuronal activation in murine hippocampal slices. J Neurosci. 2003;23:3196–3208. doi: 10.1523/JNEUROSCI.23-08-03196.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stanika RI, Pivovarova NB, Brantner CA, Watts CA, Winters CA, Andrews SB. Coupling diverse routes of calcium entry to mitochondrial dysfunction and glutamate excitotoxicity. Proc Natl Acad Sci U S A. 2009;106:9854–9859. doi: 10.1073/pnas.0903546106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stout AK, Raphael HM, Kanterewicz BI, Klann E, Reynolds IJ. Glutamate-induced neuron death requires mitochondrial calcium uptake. Nat Neurosci. 1998;1:366–373. doi: 10.1038/1577. [DOI] [PubMed] [Google Scholar]
- 63.Strosznajder RP, Czubowicz K, Jesko H, Strosznajder JB. Poly(ADP-ribose) metabolism in brain and its role in ischemia pathology. Mol Neurobiol. 2010;41:187–196. doi: 10.1007/s12035-010-8124-6. [DOI] [PubMed] [Google Scholar]
- 64.Surmeier DJ. Calcium, ageing, and neuronal vulnerability in Parkinson's disease. Lancet Neurol. 2007;6:933–938. doi: 10.1016/S1474-4422(07)70246-6. [DOI] [PubMed] [Google Scholar]
- 65.Tanveer A, Virji S, Andreeva L, Totty NF, Hsuan JJ, Ward JM, Crompton M. Involvement of cyclophilin D in the activation of a mitochondrial pore by Ca2+ and oxidant stress. Eur J Biochem. 1996;238:166–172. doi: 10.1111/j.1432-1033.1996.0166q.x. [DOI] [PubMed] [Google Scholar]
- 66.Thayer SA, Miller RJ. Regulation of the intracellular free calcium concentration in single rat dorsal root ganglion neurones in vitro. J Physiol. 1990;425:85–115. doi: 10.1113/jphysiol.1990.sp018094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tymianski M, Charlton MP, Carlen PL, Tator CH. Source specificity of early calcium neurotoxicity in cultured embryonic spinal neurons. J Neurosci. 1993;13:2085–2104. doi: 10.1523/JNEUROSCI.13-05-02085.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Den Bosch L, Schwaller B, Vleminckx V, Meijers B, Stork S, Ruehlicke T, Van Houtte E, Klaassen H, Celio MR, Missiaen L, Robberecht W, Berchtold MW. Protective effect of parvalbumin on excitotoxic motor neuron death. Exp Neurol. 2002;174:150–161. doi: 10.1006/exnr.2001.7858. [DOI] [PubMed] [Google Scholar]
- 69.Vergun O, Keelan J, Khodorov BI, Duchen MR. Glutamate-induced mitochondrial depolarisation and perturbation of calcium homeostasis in cultured rat hippocampal neurones. J Physiol. 1999;519(Pt 2):451–466. doi: 10.1111/j.1469-7793.1999.0451m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y, Dawson VL, Dawson TM. Poly(ADP-ribose) signals to mitochondrial AIF: a key event in parthanatos. Exp Neurol. 2009;218:193–202. doi: 10.1016/j.expneurol.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]