MEKK1 (MEK kinase 1) is a mammalian serine/threonine kinase in the mitogen-activated protein kinase (MAPK) kinase kinase (MAPKKK) group (1). Being the first mammalian homolog of STE11, a MAPKKK that activates the pheromone responsive MAPK cascade of budding yeast, MEKK1 as its name indicates was thought to be an activator of the MAPK kinase (MAPKK) MEK1/2 and thus an activator of the ERK MAPK cascade. It therefore was rather surprising that titration experiments (2) or analysis of cells engineered to express MEKK1 from an inducible promoter (3) revealed that it is a far more potent activator of the JNK MAPK cascade. These observations made by using either the catalytic domain of MEKK1 (MEKK1Δ) or a 672-aa C-terminal fragment recently were confirmed by using full-length human MEKK1 (Y. Xia, Z. Wu, B. Su, B. Murray, and M.K., unpublished work). Most importantly, when different mammalian MAPKKs were examined in vitro or in vivo for phosphorylation and activation by MEKK1, a MAPKK called JNKK1 (MKK4 or SEK1), whose function is JNK (and p38 MAPK) activation (4) was found to be the preferred MEKK1 substrate (Y. Xia, Z. Wu, B. Su, B. Murray, and M.K., unpublished work). Based on specificity constants, MEKK1 phosphorylates JNKK1 45-fold more efficiently than it phosphorylates MEK1/2 (Y. Xia, Z. Wu, B. Su, B. Murray, and M.K., unpublished work), thus providing a clear biochemical explanation for the marked pro-JNK bias of MEKK1. Targets for JNK include transcription factors c-Jun and ATF2, which are components of the AP-1 dimer that are involved in induction of the c-jun protooncogene (5). JNK-mediated phosphorylation enhances the transcriptional activity of both c-Jun and ATF2 (6, 7). Correspondingly, MEKK1 expression plasmids are potent activators of a chimeric c-Jun-GAL4 transcription factor, in which the c-Jun activation domain is fused to the GAL4 DNA binding domain (8). Overexpression of a catalytically inactive MEKK1(KM) mutant inhibits JNK activation by either epidermal growth factor (EGF) or tumor necrosis factor (TNF) (refs. 8 and 9 and Y. Xia, Z. Wu, B. Su, B. Murray, and M.K., unpublished work). This mutant was used to show that signals generated by occupancy of TNF type I receptor (TNF-RI) diverge downstream to the signaling proteins TRAF2 and RIP, which are recruited to TNF-RI, such that one pathway leads to JNK (and p38 MAPK) activation followed by stimulation of AP-1 activity and the other mediates NF-κB activation (10, 11) (Fig. 1). These experiments also demonstrated that NF-κB activation protects cells against TNF-induced apoptosis, whereas JNK (and p38) activation does not affect programmed cell death either positively or negatively. Similar results were obtained by analysis of mice and cells deficient in the RelA(p65) subunit of NF-κB (12, 13).
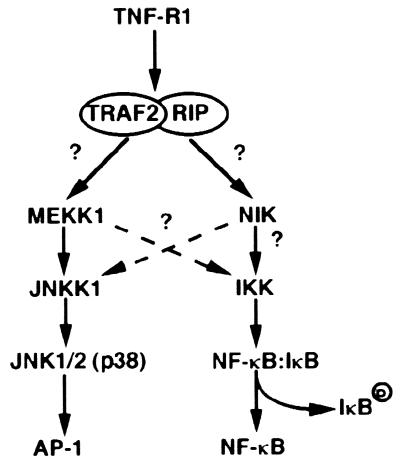
Figure 1.
Signal transduction from TNF receptor type I (TNF-RI) to transcription factors AP-1 and NF-κB. Activation of TNF-RI results in recruitment of several signaling proteins including TRAF2 and RIP. By yet unidentified mechanisms these proteins lead to activation and/or recruitment of MAPKKKs, such as MEKK1 and NIK. MEKK1 is responsible for direct activation of JNKK1, a MAPKK that directly activates JNK1/2 and p38 MAPK, thereby leading to stimulation of transcription factor AP-1. MEKK1 also may be involved in NF-κB activation. NIK or a closely related family member leads to activation of the IKK complex, which leads to phosphorylation of IκBs, thereby triggering their degradation. This results in activation of NF-κB.
In light of these findings, it was somewhat surprising that under different circumstances overexpression of MEKK1 was found to stimulate NF-κB activity (14, 15). NF-κB is a dimeric transcription factor composed of Rel proteins whose activity is regulated through interaction with specific inhibitors, the IκBs (16–18). In response to cell stimulation the IκBs are rapidly phosphorylated and then undergo ubiquitin-mediated proteolysis, resulting in the release of active NF-κB dimers that translocate to the nucleus. Initially, the demonstration that MEKK1 overexpression leads to NF-κB activation was based solely on the use of an NF-κB transcriptional reporter. As there are ample examples for transcriptional synergy between AP-1 and NF-κB (19, 20), such results should be interpreted with caution. It is expected that a signaling pathway that enhances only AP-1 activity still may stimulate an NF-κB-dependent promoter, even in the absence of overt AP-1 binding sites. Likewise, an AP-1-dependent promoter may respond to NF-κB even in the absence of recognizable NF-κB binding sites. In light of these limitations, a bigger surprise were the results of Lee et al. (21) who reported that addition of recombinant MEKK1Δ to a partially enriched fraction of nonstimulated HeLa cells stimulated a protein kinase activity that phosphorylated IκBα at serines (S) 32 and 36, sites that previously were shown to be phosphorylated in response to cell stimulation with TNF or interleukin 1 (IL-1). Phosphorylation at S32 and S36 results in polyubiquitination and degradation of IκBα (22, 23). Homologous phosphoacceptor sites are essential for the induced degradation of other IκB proteins (23). As activation of an IκB kinase by MEKK1Δ was demonstrated by using a rather crude fraction whose polypeptide composition was not described, the identity of this activity remained a mystery. In the meantime, two other groups working independently have succeeded in purifying an inducible IκB kinase activity from extracts of TNF-stimulated HeLa or Jurkat cells (24, 25). Extensive purification of that activity, named IKK, which elutes from gel filtration columns as a large complex with an apparent molecular mass of 700–900 kDa, revealed the presence of two polypeptides with molecular masses of 85 and 87 kDa that precisely coeluted with IκB kinase activity. Microsequencing and molecular cloning revealed that these polypeptides are closely related protein kinases named IKKα (or IKK1) and IKKβ (or IKK2), respectively (24–26). IKKα and IKKβ also were identified through a different approach, based on yeast two-hybrid screens, as proteins that interact with a MAPKKK called NIK (NF-κB-inducing kinase) (27, 28). NIK originally was identified as a TRAF2-interacting kinase whose overexpression results in potent NF-κB activation (29) without any considerable effect on MAPKs, including JNK (30). Therefore the observed interaction between NIK and the IKKs immediately suggested that NIK may be an upstream activator of IKK (Fig. 1). Although the IKK complex is similar in size to the MEKK1Δ-responsive activity, the relationships between the two remained nebulous, and various attempts to stimulate IKK activity with modest amounts of MEKK1Δ expression vector, that are sufficient for JNK activation, have failed (24) (D. Goeddel, personal communication). In addition, several reports indicating that NF-κB transcriptional reporters are not stimulated by low to modest doses of MEKK1 (which are sufficient for JNK activation), while being highly responsive to cotransfected NIK, have appeared (30, 31). However, in new work published in this issue of the Proceedings, Lee et al. (32) present evidence that the MEKK1Δ-responsive activity they previously identified is none other than the cytokine-responsive IKK. Furthermore, they suggest that MEKK1 may be a direct activator of IKKα and IKKβ.
That IKK activity is regulated through phosphorylation of some of its subunits previously was demonstrated by the use of protein phosphatase 2A (PP2A) catalytic subunit, whose incubation with purified IKK resulted in loss of IκB kinase activity (24). Furthermore, coexpression with NIK stimulates the kinase activity of transiently expressed IKKα, which is also efficiently phosphorylated in vitro by NIK immunoprecipitates (33). Although no specificity constants were determined, IKKβ appears to be a relatively poor NIK substrate (33). Analysis of the IKKα and IKKβ protein sequences reveals several potential phosphoacceptor sites in a region conserved in all protein kinases, the T (or activation) loop, that resemble those that are used by MAPKKKs to activate MAPKKs (25). Indirect evidence that these sites may be used to activate IKKα and IKKβ was provided by site-directed mutagenesis (25, 33), but so far these sites were not shown to be phosphorylated in TNF or IL-1 stimulated cells or be involved in cytokine-mediated IKK activation. Substitution of S176 in IKKα with alanine was found to decrease its phosphorylation and activation by NIK (33), whereas a dual substitution of S177 and S181 of IKKβ with glutamic acid was reported to increase its catalytic activity (25). The current work (32) shows that recombinant MEKK1Δ can phosphorylate a synthetic peptide corresponding to the T loop of IKKβ and that substitution of S177 and S181 with alanines reduces the extent of 32P incorporation (32). It also is shown that incubation of a partially purified preparation with MEKK1Δ results in phosphorylation of two polypeptides whose sizes match those of IKKα and IKKβ (32). However, as these and similar experiments conducted with NIK have not been performed with fully purified proteins the results fall short of a conclusive demonstration that MEKK1 or NIK can directly phosphorylate and activate native IKKα and IKKβ. Nevertheless, the simplest interpretation of past and present results is that either of these MAPKKKs can activate IKK.
An important question, however, that is yet to be answered is which MAPKKKs are physiologically involved in IKK and NF-κB activation and whether different NF-κB-activating stimuli use the same MAPKKKs. It is also to be resolved whether MEKK1 acts exclusively on the JNK (and p38) to AP-1 pathway or whether it also is involved in IKK and NF-κB activation. In this respect, it would be useful to compare whether the specificity constants for IKKα or IKKβ phosphorylation by MEKK1 match the one for JNKK1, the most efficient and relevant MEKK1 substrate identified so far. Most groups who cotransfected varying amounts of truncated MEKK1 expression vectors with either a JNK reporter plasmid or an NF-κB transcriptional reporter find that JNK activity is potently stimulated at low input levels whereas NF-κB transcriptional activity is stimulated only by very high doses of MEKK1 (10, 30–32). High doses of MEKK1 are known to have nonspecific effects (2). Activation of a GAL4 transcriptional reporter by the c-Jun-GAL4 chimera and cotransfected MEKK1 parallels the stimulation of JNK activity (8, 34), but a different AP-1 reporter containing multiple c-Jun:ATF2 binding sites is stimulated only by very high doses of MEKK1, similar to those required for stimulation of the NF-κB reporter (32). Although all groups seem to agree that cotransfection of a NIK expression vector has no effect on JNK activity, some find that it nevertheless can enhance AP-1 activity, albeit less efficiently than MEKK1 (31). Currently it is hard to reconcile all of these results even if one invokes transcriptional synergy between AP-1 and NF-κB. More puzzling differences are found when the abilities of MEKK1Δ and NIK to activate NF-κB and IKK are compared. All groups agree that MEKK1 is a much poorer activator of the NF-κB transcriptional reporter than NIK is (30–32). However, some find that MEKK1 and NIK expression plasmids have similar effects on the activity of transiently expressed IKKα (32), and others find that NIK is a much more potent activator of IKKα than MEKK is, whereas IKKβ is slightly more responsive to MEKK1 than to NIK (35). A major difference between measuring the response of an NF-κB transcriptional reporter to MEKK1 vs. activation of a transiently expressed epitope tagged IKKα or IKKβ is that in the former case NF-κB activation depends on stimulation of endogenous (physiological) IKK activity, whereas in the latter case the transiently overexpressed IKK subunit probably is not incorporated into the physiological IKK complex. In fact, protein purification and immunoprecipitation experiments strongly suggest that most of the IKK complexes are IKKα:IKKβ heterodimers plus additional subunits and that very little IKKα or IKKβ homodimeric complexes exist (E. Zandi, D. Rothwarf, and M.K., unpublished results). It is important to express only small amounts of exogenous IKKα or IKKβ to ensure their incorporation into the physiological 900-kDa IKK complex (26). It is therefore safer to compare the abilities of NIK and MEKK1 to activate the endogenous IKK complex rather than the artificial IKKα or IKKβ homodimers generated by transient overexpression. When such a comparison is performed, transient transfection of a NIK vector into 293 cells results in preferential activation of endogenous IKK whereas transfection of a full-length MEKK1 vector results in preferential JNK activation (Fig. 2A). However overexpression of NIK can lead to JNK activation whereas overexpression of MEKK1 can lead to IKK activation (Fig. 2B). These results are in complete agreement with all published comparisons of the effect of these MAPKKKs on NF-κB and AP-1 transcriptional reporters. Nevertheless, it should be realized that these results do not rule out the possibility that, although weak, MEKK1 may contribute to IKK activation nor do they prove that, although potent, NIK is a physiological NF-κB activator. In addition these experiments highlight the potential pitfalls associated with overexpression of signaling proteins.
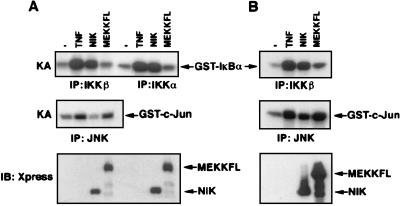
Figure 2.
Stimulation of endogenous IKK activity by NIK and MEKK1. 293 cells were transiently transfected with either Xpress-tagged NIK or Xpress-tagged MEKK1 full-length expression vectors (1 μg DNA in A or 2 μg DNA in B/60-mm plate). After 24 hr, cells were treated or not with TNF (20 ng/ml for 10 min) and then lysed. Cell lysates were immunoprecipitated (IP) with either anti-IKKα or anti-IKKβ or anti-JNK antibodies. The IKK activity (KA) was determined by using glutathione S-transferase (GST)-IκBα (1–54) as a substrate. The JNK activity (KA) was determined by using GST-cJun (1–79) as a substrate.
In addition to proinflammatory cytokines IKK activity is potently stimulated by the Tax transactivator protein of human T cell leukemia virus (HTLV) (36, 37). This response leading to NF-κB activation is likely to play a major role in the leukemogenic function of this virus. Like proinflammatory cytokines Tax was proposed to act either via MEKK1 (37) or NIK (36). Although the strongest evidence in favor of MEKK1 as a target is based on its ability to physically interact with Tax (37), the pro-NIK evidence is based on genetic arguments (36). Uhlik et al. (36) isolated variants of the Jurkat T cell line that fail to activate NF-κB in response to Tax. Although the basis for this defect currently is unknown, it can be complemented by transient expression of NIK but not by MEKK1 overexpression (36).
A major problem in sorting out the exact physiological functions of these and other MAPKKKs is the difficulty in detecting considerable changes in their enzymatic activity in response to cell stimulation by using conventional immunoprecipitation experiments. This deficiency can be overcome by genetic experiments similar to those that established the function of the yeast MAPKKK STE11 in three distinct MAPK cascades (38, 39). Although we will have to await the results of gene knockout experiments in which the activities of NIK and MEKK1 are selectively abolished, similar experiments conducted with components of the TNF-RI response pathway clearly support the earlier conclusion (10) that the pathways leading from this receptor to either JNK and AP-1 or IKK and NF-κB diverge at the level of TRAF2 and RIP (Fig. 1). Cells established from TRAF2 knockout embryos are defective in JNK activation in response to TNF, while exhibiting only a slightly retarded NF-κB activation response (40). In contrast, cells derived from RIP knockout embryos are defective in NF-κB activation, while exhibiting a normal JNK activation response (41). As MAPKKKs, like MEKK1 and NIK, are thought to act downstream to TRAF2 and RIP rather than upstream to them, it is unlikely that they play equal roles in transducing signals generated by TNF-RI activation to transcription factors. It is also possible that neither MEKK1 nor NIK are involved in TNF signaling. After all, the MAPKKK family contains many other members in addition to these two usual suspects.
Acknowledgments
We thank Dr. D. Goeddel for his comments. Research in our laboratory is supported by the National Institutes of Health (R01 AI43477). M.D. was supported by a postdoctoral fellowship from the D. Collen Research Foundation vzw.
Footnotes
The companion to this commentary is published on pages 9319–9324.
References
- 1.Lange-Carter C A, Pleiman C, Gardner A, Blumer K, Johnson G. Science. 1993;260:315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- 2.Minden A, Lin A, McMahon M, Lange-Carter C, Dérijard B, Davis R J, Johnson G L, Karin M. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 3.Yan M, Dai T, Deak J C, Kyriakis J M, Zon L I, Woodgett J R, Templeton D J. Nature (London) 1994;372:798–800. doi: 10.1038/372798a0. [DOI] [PubMed] [Google Scholar]
- 4.Lin A, Minden A, Martinetto H, Claret F X, Lange-Carter C, Mercurio F, Johnson G L, Karin M. Science. 1995;268:286–290. doi: 10.1126/science.7716521. [DOI] [PubMed] [Google Scholar]
- 5.Karin M. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 6.Smeal T, Binétruy B, Mercola D, Birrer M, Karin M. Nature (London) 1991;354:494–496. doi: 10.1038/354494a0. [DOI] [PubMed] [Google Scholar]
- 7.Gupta S, Campbell D, Dérijard B, Davis R J. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 8.Minden A, Lin A, Claret F X, Abo A, Karin M. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 9.Coso O A, Chiariello M, Yu J C, Teramoto H, Crespo P, Xu N, Miki T, Gutkind J S. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 10.Liu Z-G, Hu H, Goeddel D V, Karin M. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 11.Natoli G, Costanzo A, Ianni A, Templeton D J, Woodgett J R, Balsano C, Levrero M. Science. 1997;275:200–203. doi: 10.1126/science.275.5297.200. [DOI] [PubMed] [Google Scholar]
- 12.Beg A A, Baltimore D. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 13.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 14.Hirano M, Osada S, Aoki T, Hirai S, Hosaka M, Inoue J, Ohno S. J Biol Chem. 1996;271:13234–13248. doi: 10.1074/jbc.271.22.13234. [DOI] [PubMed] [Google Scholar]
- 15.Meyer C F, Wang X, Chang C, Templeton D, Tan T H. J Biol Chem. 1996;271:8971–8976. doi: 10.1074/jbc.271.15.8971. [DOI] [PubMed] [Google Scholar]
- 16.Baeuerle P A, Baltimore D. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 17.Baldwin A S. Annu Rev Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 18.Verma I M, Stevenson J K, Schwarz E M, Van Antwerp D, Miyamoto S. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 19.Stein B, Baldwin A S, Jr, Ballard D W, Green W C, Angel P, Herrlich P. EMBO J. 1993;12:3879–3891. doi: 10.1002/j.1460-2075.1993.tb06066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yasumoto K, Okamoto S, Mukaida N, Murakami S, Mai M, Matsushima K. J Biol Chem. 1992;267:22506–22511. [PubMed] [Google Scholar]
- 21.Lee F S, Hagler J, Chen Z J, Maniatis T. Cell. 1997;88:213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- 22.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Science. 1995;267:1485–1491. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 23.DiDonato J A, Mercurio F, Rosette C, Wu-li J, Suyang H, Ghosh S, Karin M. Mol Cell Biol. 1996;16:1295–1304. doi: 10.1128/mcb.16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. Nature (London) 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 25.Mercurio F, Zhu H, Murray B W, Shevchenko A, Bennett B L, Li J, Young D B, Barbosa M, Mann M, Manning A, Rao A. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 26.Zandi E, Rothwarf D, Delhase M, Hayakawa M, Karin M. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 27.Régnier C H, Yeong Song H, Gao X, Goeddel D V, Cao Z, Rothe M. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 28.Woronicz J D, Gao X, Cao Z, Rothe M, Goeddel D V. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 29.Malinin N L, Boldin M P, Kovalenko A V, Wallach D. Nature (London) 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 30.Song H Y, Régnier C H, Kirschning C J, Ayres T M, Goeddel D V, Rothe M. Proc Natl Acad Sci USA. 1997;94:9792–9796. doi: 10.1073/pnas.94.18.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Natoli G, Costanzo A, Moretti F, Fuleo M, Balsano C, Levrero M. J Biol Chem. 1997;272:26079–26082. doi: 10.1074/jbc.272.42.26079. [DOI] [PubMed] [Google Scholar]
- 32.Lee F S, Peters R T, Dang L C, Maniatis T. Proc Natl Acad Sci USA. 1998;95:9319–9324. doi: 10.1073/pnas.95.16.9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ling L, Cao Z, Goeddel D V. Proc Natl Acad Sci USA. 1998;95:2791–2797. doi: 10.1073/pnas.95.7.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cavigelli M, Dolfi F, Claret F X, Karin M. EMBO J. 1995;14:5957–5964. doi: 10.1002/j.1460-2075.1995.tb00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakano H, Shindo M, Sakon S, Nishinaka S, Mihara M, Yagita H, Okumura K. Proc Natl Acad Sci USA. 1998;95:3537–3542. doi: 10.1073/pnas.95.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uhlik, M., Good, L., Xiao, G., Harhaj, E. W., Zandi, E., Karin, M. & Sun, S.-C. (1998) J. Biol. Chem., in press. [DOI] [PubMed]
- 37.Yin M J, Christerson L B, Yamamoto Y, Kwak Y-T, Xu S, Mercurio F, Barbosa M, Cobb M H, Gaynor R B. Cell. 1998;93:875–884. doi: 10.1016/s0092-8674(00)81447-6. [DOI] [PubMed] [Google Scholar]
- 38.Herskowitz I. Cell. 1995;80:187–197. doi: 10.1016/0092-8674(95)90402-6. [DOI] [PubMed] [Google Scholar]
- 39.Posas F, Saito H. Science. 1997;276:1702–1705. doi: 10.1126/science.276.5319.1702. [DOI] [PubMed] [Google Scholar]
- 40.Yeh W C, Shahinian A, Speiser D, Kraunus J, Billia F, Wakeham A, de la Pompa J L, Ferrick D, Hum B, Iscove N, et al. Immunity. 1997;7:715–725. doi: 10.1016/s1074-7613(00)80391-x. [DOI] [PubMed] [Google Scholar]
- 41.Kelliher M A, Grimm S, Ishida Y, Kuo F, Stanger B Z, Leder P. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]