Abstract
TNF-Related Apoptosis Inducing Ligand Receptor 2 (TRAIL-R2 or DR5) is expressed at elevated levels in a broad range of solid tumors to mediate apoptotic signals from TRAIL or agonist antibodies. We tested the hypothesis that DR5 DNA vaccination will induce pro-apoptotic antibody to trigger apoptosis of tumor cells. BALB/c mice were electrovaccinated with DNA encoding wild type human DR5 (phDR5) or its derivatives. Resulting immune serum or purified immune IgG induced apoptosis in triple negative breast cancer (TNBC) cells, which were also TRAIL-sensitive. The pro-apoptotic activity of immune serum at dilutions of 0.5-2% was comparable to that of 1-2 μg/ml of TRAIL. Apoptotic activity of immune serum was enhanced by antibody cross-linking. Apoptotic cell death induced by anti-DR5 antibody was shown by the cleavage of PARP and caspase-3. In contrast, immune serum had no effect on the proliferation of activated human T cells, which expressed low levels of DR5. In vivo, hDR5 reactive immune serum prevented growth of SUM159 TNBC cells in SCID mice. DR5 specific IFN-γ secreting T cells were also induced by DNA vaccination. Furthermore, the feasibility to overcome immune tolerance to self DR5 was shown by the induction of mouse DR5 binding antibody after electrovaccination of BALB/c mice with pmDR5ectm-Td1 encoding a fusion protein of mouse DR5 and an immunogenic fragment of tetanus toxin. These findings support DR5 as a promising vaccine target for controlling TNBC and other DR5 positive cancers.
Keywords: DR5 (TRAIL-R2), DNA vaccine, antibody, apoptosis, triple negative breast cancer
Introduction
The ideal target of a cancer vaccine would be a molecule that is critical to tumor cell survival, is expressed at elevated levels on tumor cell surface and therapeutic benefit should be demonstrable with antibody or T cells to this molecule (1). By these criteria, TRAIL death receptors stand out as excellent vaccine candidates. These receptors, which are elevated in a wide range of solid tumors, mediate apoptosis in tumor cells while sparing normal cells (2), demonstrating both selectivity and therapeutic activity. When considering this family of antigens as vaccine targets, the first challenge is whether vaccine-induced immune sera will trigger apoptotic signals because many receptor binding antibodies block, rather than trigger signals, such as antibodies to HER-2 (3).
Of the five known TRAIL receptors, DR4 (TRAIL-R1) and DR5 (TRAIL-R2) are agonist receptors that transmit death signals (4-9). In humans, DR4 and DR5 are expressed in both solid tumors and hematological malignancies as well as in some normal tissues. In mice, only one agonist TRAIL receptor DR5 has been identified (10). Although the mechanism for preferential TRAIL-induced apoptosis in tumors is not fully understood, there is data supporting the expression of common oncogenes, such as myc and ras which sensitize cancer cells to the extrinsic pathway of apoptosis (11). Coordinate activation of the endogenous death pathway by stress, hypoxia or other stimuli can also enhance signaling by TRAIL. Conversely, expression of decoy receptors, and apoptosis inhibitors, e.g. FLIP, IAP or XIAP, modulates the susceptibility to TRAIL-induced apoptosis (12).
Using modified TRAIL isoforms which bind specifically to DR4 or DR5, signaling through DR5 shows greater apoptotic effect on human solid tumors than signaling through DR4, indicating DR5 as the preferred target for solid tumor treatment (13). Analysis of human DR5/TRAIL crystal structure shows that three DR5 molecules form a complex with TRAIL trimer (14). DR5 has a strong propensity to self-associate in the absence of the ligand (15), but without forming the threefold symmetry. Apoptosis signals are initiated when DR5 transmembrane helices and cytosolic domains are precisely positioned by the binding of TRAIL or agonist monoclonal antibody (mAb) that constrain the receptors into the functional trimers. This property of DR5 may lend itself to apoptosis signals when conjugated with immune serum. We have therefore chosen to test DR5 as a vaccine target for solid tumors (16).
In 15-20% of breast cancer patients, their tumors do not express ER/PR or HER2. Patients with these triple negative breast cancers (TNBC) do not have the option of hormone or molecularly targeted therapy after they receive conventional treatment, but basal type TNBC appear sensitive to extrinsic apoptosis (17). Here we describe the induction of DR5-specific agonist antibody and T cells by DR5 DNA vaccination and inhibition of TNBC growth by hDR5 immune serum both in vitro and in vivo.
Material and Methods
Mice
All animal procedures were conducted in accordance with accredited institution guidelines and the US Public Health Service Policy on Humane Care and Use of Laboratory Animals (http://grants.nih.gov/grants/olaw/olaw.htm#pol). BALB/c and SCID (age 6-8 weeks) female mice were purchased from Charles River Laboratory (Frederick, MD).
Cell lines and reagents
Tissue culture reagents and cell line maintenance were as previously reported (18). Antigen presenting cells (APC) 3T3/hDKB and control 3T3/KB were generated in our lab. Briefly, BALB/c NIH 3T3 fibroblasts were transfected with hDR5, Kd and B7.1 (hDKB). Stable clones were selected and surface expression of hDR5 was confirmed by flow cytometry using either mAb HS201 paired with phycoerythrin (PE) conjugated secondary antibody (Jackson ImmunoResearch, West Grove, PA), or PE-conjugated DJR2-4 anti-human DR5 monoclonal antibody (eBioscience, San Diego, CA). Normal mouse serum or isotype-matched mAb was the negative control. SUM159 and SUM149 cells, originally isolated from primary human breast tumors, were maintained in RPMI plus 5% fetal bovine serum (FBS), 5 μg/mL insulin and 1 μg/mL hydrocortisone (19). MDA-MB231, BT-474 and SKBR3 were obtained from the ATCC (Manassas, VA) and maintained in the recommended culture media. Human PBL were isolated from whole blood by ficoll-paque separation (GE Healthcare) and stimulated by 20 ng/mL OKT3 (mAb to human CD3, ORTHOCLONE by JOM Pharmaceutical Services, Inc., Shepherdsville, KY) together with 100 U/mL human IL-2 (PROLEUKIN by Novartis) for 5 days, by CD3/CD28 magnetic beads (Dynabeads, Invitrogen) for 5 days or 50 ng/mL ionomycin (Sigma) for 6 hours in RPMI media supplemented with 10% FBS. IL-2 was replenished every two days.
Authentification of cell lines by STR profiling was carried out with Promega’s Cell ID System as described by the supplier (performed by the Applied Genetics Technology Center of our institution; data shown in Supplementary Table 1).
DNA electrovaccination
pVax1 (Invitrogen) was used to generate all the vaccine constructs in this study, unless otherwise specified. pCEP4 DR5 wild type encoding the full length human DR5 and pCEP4 DR5 truncation mutant (hDR5δ encoding human DR5 with a premature termination signal in the death domain (at a.a. 358, which deletes the C-terminal 40% of the conserved death domain) have been described (20). hDR5 coding sequences were obtained by restriction digest with BamHI and HindIII and subcloned into the equivalent sites in pVax1, giving rise to phDR5 (wt) and phDR5δ. phDR5ectm, encoding the extracellular and transmembrane domains of DR5 (aa. 1-226) w a s derived by PCR (3) from wild-type hDR5 using forward primer 5′-ATATCTACAAGCTTGCGACCATG GAACAACGGGGACAGA-3′, which added a 5′ HindIII site, and reverse primer 5′-CTAGATGGATCCTCAGCCTCCACCTGAGCAGATG-3′, which included a 3′ stop codon followed by a BamHI site. The 702 bp PCR product was directionally cloned into the HindIII and BamHI sites of pVax1. pEGFP-N1 purchased from Clonetech was used as the transfection control for gene expression analysis.
To construct pmDR5ectm-Td1, mouse DR5 cDNA (NM_020275) was cloned into the HindIII/XbaI sites of pVax1 giving pmDR5. The intracellular domain was cut with BamHI and XbaI, and the 490 bp fragment containing the death domain was replaced with in-frame humanized tetanus toxin fragment C domain 1 (Td1) cDNA (21) in a 805 bp BamHI/XbaI fragment. The Td1 cDNA insert has a 27 base 5′ leader encoding an added initiating Met codon preceded by a 6 amino acid bridge (LVQCGG). Surface expression of the recombinant gene product in transfected cells was verified with mAb MD5-1(7)
pEFBos/GM-CSF (pGM-CSF) encoding murine granulocyte macrophage colony stimulating factor (GM-CSF) was provided by Dr. N. Nishisaki at Osaka University, Osaka, Japan. All vaccination was conducted with an admix of pGM-CSF and the designated vaccine or blank vector. Mice were injected in the quadriceps muscle with 50 μg of each plasmid DNA followed immediately by square wave electroporation over the injection site using a BTX830 (BTX Harvard Apparatus, Holliston, MA) as we previously described (22,23).
Purification of IgG from mouse serum
Sera were pooled from 5 mice after 4 vaccinations with phDR5ectm and pGM-CSF and purified with a protein G spin column (Pierce) per manufacturer protocol. Briefly, the spin column was equilibrated with the provided binding buffer before 250 μL serum was incubated with protein G resin at room temperature for 10 min with rocking. Unbound fraction was removed by centrifugation and the column was washed 3 times with binding buffer. Three elutions were performed with the provided elution buffer by centrifugation and neutralization buffer was added to each. Elutions were assayed by absorbance at 280 nm and verified by SDS-PAGE with Coommassie blue staining. Antibody binding was determined by flow cytometry and normalized to the original serum titer.
Measurement of anti-hDR5 antibody by ELISA
Human hDR5-Fc chimeric protein consisting of amino acids 1-182 of the extracellular domain of human DR5 and the Fc portion of human IgG1 (EXBIO Antibodies, Cat No. RL-002-C050; Praha, Czech Republic) was immobilized to Immulon 2HB flat-bottom ELISA plates by capturing with goat anti-human IgG. Control was human HER2 conjugated to the Fc portion of human IgG1 (ACRO Biosystems, Cat No. HE2-H5253). Serum samples from control and phDR5 immunized mice were tested at different dilutions and compared to a standard curve generated using agonist mouse mAb631 (R&D Systems, Minneapolis, MN). After 1 h incubation at RT, bound mouse IgG was detected with goat anti-mouse IgG HRP and developed with TMB Substrate Set (BD Biosciences, San Diego, CA). Reactions were terminated with 1 M phosphoric acid and optical density was read at 450-590 nm. The concentration of hDR5 specific IgG was calculated by linear regression based on the standard curve following background subtraction and corrected for the dilution factor to be expressed as μg/mL. Differences in hDR5 antibody concentrations were analyzed by the Student’s t-test.
Measurement of IFN-γ secreting T cells by ELISPOT assay
A total of 5×105 immune spleen cells were incubated with engineered antigen presenting cells (APC) at a 10:1 ratio of spleen cells to APC. The APCs were 3T3/hDKB expressing hDR5, Kd and B7.1 (CD80). 3T3/KB cells were used as control. IFN-γ ELISPOTs were measured as previously described (23) and the results expressed as the number of cytokine producing cells per 106 splenocytes. Data were analyzed using the Student’s t-test.
Measurement of cell proliferation or survival
Tumor cell proliferation was measured indirectly by mitochondrial metabolic activity using a modified MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrasodium bromide) assay (24). SUM159 cells at 400,000 per mL were treated with indicated dilution of immune serum from vaccinated animals, or with media alone, or graded doses of hDR5 agonist mAb631 (R&D Systems, clone 71903, Minneapolis, MN), or recombinant TRAIL (BIOMOL, San Diego, CA). Approximately 20-24 hours after plating, 0.1 volumes of 5 mg/ml MTT in PBS were added to each well and incubated for 4 h at 37°C before the stop reagent (0.04N HCl in isopropanol) was added and the absorbance measured at 600-650 nm. Survival of pre-activated T cells was assayed over three days using Alamar Blue™ (InVitrogen, Carlsbad, CA) according to the manufacture’s specifications.
Measurement of cell apoptosis
Cells were subcultured in 12- or 6- well plates until 70-80% confluence, at which time the medium was replaced and immune or control serum or purified IgG was added to achieve 0.5-2% final concentration. Media alone or known concentrations of agonist mAb631 or TRAIL were used as controls. After 20-24 h incubation cells were stained with Annexin V-PE and 7-AAD using Annexin V-PE Apoptosis Detection Kit I (cat# 559763; BD Biosciences Pharmingen™). In antibody cross-linking experiments, immune serum or mAb was removed 30 minutes after incubation, washed once and treated with either goat-anti-mouse IgG (10 μg/mL) before the cells were further incubated for 20-24 hrs. Stained samples were evaluated immediately by flow cytometry. Data were analyzed using WinMIDI version 2.8 or FlowJo and density plots of Annexin V-PE versus 7-AAD were generated to show the distribution of the live, non-apoptotic cells (Annexin V-PE negative, 7-AAD negative), live apoptotic cells (Annexin V-PE positive, 7-AAD negative), nonviable cells (7-AAD positive), and nonviable, post-apoptotic cells (Annexin V-PE and 7-AAD double positive).
Detection of cleavage products of caspase-3 and PARP by western blot analysis
SUM159 cells at 90% confluence were incubated for 5 hours with non-immune or hDR5 immune serum (1:50) or purified IgG or 5 μg/mL mAb631. Where indicated, cells were pretreated with 20 μM caspase-8 inhibitor Z-IETD-FMK (BD Pharmingen, San Diego, CA) or diluent (DMSO) for 30 minutes prior to and then throughout the incubation with immune serum/antibodies. Whole cell lysates were extracted using 1X Cell Lysis Buffer (#9803, Cell Signaling Technology, Beverly, MA) as recommended by the manufacture’s protocol. Equal amounts of protein were resolved in 4-20% gels PAGr Duraminew® Gels (Cambrex, Rockland, ME) and electro transferred to Immobilon-P (Millipore, Bedford, MA) PVDF membranes. Blots were probed overnight with primary antibodies and detected with peroxidase-conjugated AffiniPure Goat Anti-Mouse (cat# 115-035-071) or Goat Anti-Rabbit (cat# 111-035-046) secondary antibodies from Jackson ImmunoResearch Laboratories. Blots were developed with enhanced SuperSignal®g West Pico Chemiluminescent Substrate (Pierce Biotechnology, Inc.; Rockford, IL) and imaged with Kodak-MR film. Antibodies used for Western blot detection included: mouse mAb against cleaved PARP (Zymed, Carlsbad, CA), rabbit monoclonal against cleaved caspase-3 (Asp175) (5A1) (#9664, Cell Signaling Technology, Beverly, MA) which only detects cleaved caspase-3, and mouse mAb against B-actin (Sigma, St. Louis, MO).
Measurement of tumor growth in SCID mice
SUM159 cells were monodispersed in complete growth medium and treated with either 20% final concentration of control or immune serum or control serum spiked with agonist hDR5 mAb631 at a final concentration of 5μg/mL. Cells were incubated at room temperature for 30 minutes with occasional agitation, washed twice with serum free media. Treated cells (3×106 in 50 μL) were injected s.c. into the flanks of SCID mice, with 8 mice per treatment group. Animals were monitored weekly for tumor growth. Tumor volumes were calculated as the product of the XY2/2 (X=long axis, Y=short axis). The statistical significance of differences in percent tumor-free interval was assessed by the proportional hazard model (25). Holm’s step-down procedure was used to adjust obtained p-values for the effects of multiple comparisons. Differences between groups in tumor volume over the 14 wks of observation were assessed using Kruskal-Wallis tests (26). Holm’s procedure was also used to correct for multiple comparisons.
Results
Induction of hDR5 specific pro-apoptotic antibodies and IFN-γ producing T cells by DNA electrovaccination
To evaluate the feasibility of inducing pro-apoptotic antibodies to TRAIL receptor DR5, BALB/c mice were electrovaccinated, i.m., four times at two week intervals with an admix of phDR5 and pGM-CSF (Fig. 1A). Control group received blank vector pVax1 and pGM-CSF. Individual mouse sera were collected 2 weeks after the final vaccination, and hDR5 specific antibody was measured by their binding to mouse NIH 3T3 cells stably transfected to express phDR5, or to triple negative human breast cancer cell line SUM159 cells that express hDR5 (Figure 1B, upper panels, filled histogram). Binding to NIH 3T3 and non-immune serum (open histogram) served as negative controls. The hDR5 specific mAb HS201 (lower panels, filled histogram) was the positive control. hDR5 specific antibodies were further quantified by ELISA using recombinant human DR5-Fc (see Material and Methods): their levels were 149 ± 49 μg/mL (Fig. 1C). Absence of non-specific binding of immune serum was indicated by using recombinant Her2-Fc as antigen.
Figure 1.
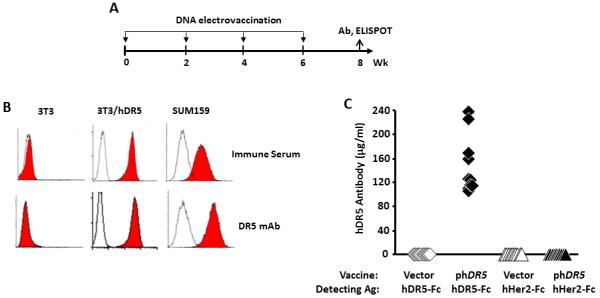
Induction of targeted antibodies by phDR5 DNA electrovaccination. A. BALB/c mice were electrovaccinated four times at two week intervals with an admix of plasmid DNA encoding mouse GM-CSF and human DR5 (50 μg each). Control groups received pGM-CSF and blank vector pVax1. B. Mouse immune sera were tested at 1:20 dilution for specific binding to NIH3T3 cells stably transfected with human DR5 (3T3/hDR5). Flow cytometric histograms showed the binding of hDR5 immune serum (upper panels, shaded histogram) and hDR5 mAb HS201 (lower panels, shaded histogram) to 3T3/hDR5 and SUM159 cells. NIH 3T3 cells (left panels) and non-immune control serum (open histogram) were the negative controls. C. The levels of anti-hDR5 specific IgG in immune sera were quantified by ELISA using recombinant hDR5 (aa 1-182) fused to human IgG Fc (hDR5-Fc). Control wells contained recombinant hHer2 fused to human IgG Fc (hHer2-Fc). (n=10 per group).
To circumvent potentially deleterious signaling from the death domain, DNA vaccines encoding non-functional DR5 variants were tested (Fig. 2A). phDR5δ has a premature stop codon in the death domain (codon 358) resulting from a 2 bp insertion at nucleotide 1065 (codon 338) causing a loss of 57 aa residues at the C-terminus, altered sequence between codons 338-357 and a reduction of ~60-70% of its pro-apoptotic activity (20). phDR5ectm encodes the extracellular and transmembrane domains of hDR5, but lacks all but the first 21 amino acids of the intracellular domain. Both constructs produce stable proteins that are expressed on the cell surface and recognized by an hDR5 specific mAb (Fig. 2B). BALB/c mice were electrovaccinated 4 times and T cell response to hDR5 was analyzed 2 wks post final vaccination by ELISPOT after in vitro stimulation with the engineered antigen presenting cells 3T3/hDKB that expressed human DR5, Kd and B7.1 (CD80). Comparable levels of antigen specific IFN-γ-secreting T-cells were induced by the three DNA constructs, i.e. 593 ± 57 (WT), 508 ± 85 (phDR5δ) and 646 ± 116 (phDR5ectm) spots per 106 spleen cells (Fig. 2C). Thus, DR5 specific immune response was induced by DNA vaccination.
Figure 2.
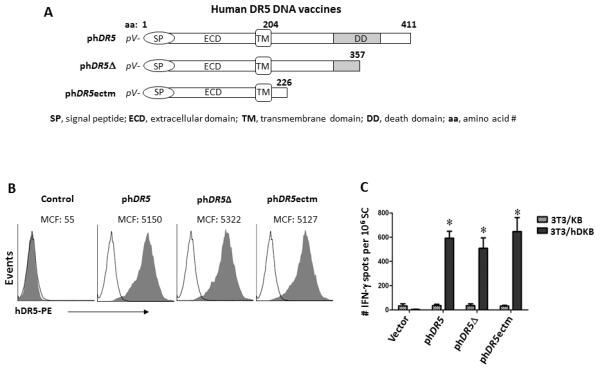
Immunogenicity of death domain deficient hDR5 DNA vaccine constructs. A. Constructs of death domain deficient hDR5 DNA vaccines. B. Expression of recombinant hDR5 derivatives analyzed by flow cytometry after co-transfection of 3T3 cells with the test constructs and pEGFP. Control group was transfected with pEGFP alone. GFP positive cells were gated and cell surface expression of hDR5 was detected with mAb HS201 and PE-conjugated secondary antibody. The majority of GFP-positive transfected cells were also recognized by hDR5 mAb. C. Induction of hDR5-specific T cells. BALB/c mice were electrovaccinated 4 times as described (Material and Methods) with the indicated phDR5 constructs and pGM-CSF. Control group received blank vector (pVax1) and pGM-CSF (n=4-10). At two weeks after the 4th vaccination, T cell response was analyzed by IFN-γ ELISPOT using 3T3/hDKB or control 3T3/KB (n=3-4). *p<0.001 relative to pVax1. No significant differences between hDR5 vaccine groups were observed.
Because the pro- or anti-apoptotic activity of vaccine-induced DR5 immune serum could determine whether DR5 is a feasible target for vaccine development, the ability to inhibit tumor cell proliferation by the immune sera (at 2%) was tested with SUM159 cells, using a modified MTT assay. Growth inhibition relative to the normal media control was 44.2 ± 8.3 for mice vaccinated with phDR5 plus pGM-CSF (Fig. 3A), showing the induction of pro-apoptotic antibody by phDR5 vaccine. mAb 631 was the positive control. We further tested the activity of immune sera from mice receiving truncated phDR5δ or phDR5ectm. The mean ± SE for inhibitory activity of individual immune sera was 58 ± 6% (phDR5), 65 ± 5% (phDR5δ) and 72 ± 1% (phDR5ectm) compared to 36, 55 or 65% by 1, 2 or 4 μg/mL mAb631 respectively. Therefore, immunization with phDR5 derived DNA vaccines coding only for the N-terminal half induced comparable levels of growth inhibitory antibodies as phDR5.
Figure 3.
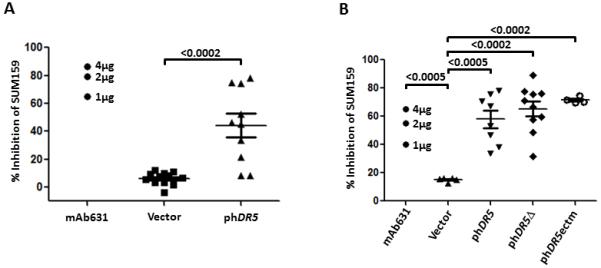
Growth inhibitory activity of immune sera. A. SUM159 cells were treated with 2% hDR5 immune serum in an MTT-based assay as described in Material and Methods. Agonist mAb 631 at 5 μg/ml was the positive control. B. Activity of immune sera induced by hDR5 vaccines encoding wild type or death domain defective constructs measured by MTT assay. Controls were blank pVax1 (Vector) and hDR5 agonist mAb631. Significance was determined using a 2-tailed Student’s t-test. No significant difference was observed between individual hDR5 vaccines.
To further test the mechanism of growth suppression induced by DR5 immune sera, SUM159 cells were treated with hDR5 immune serum, then Annexin V binding was measured by flow cytometry (Fig. 4A). Approximately 70% of immune sera treated cells bound to Annexin V, compared to 80% by mAb 631 (5 μg/ml) and 60% by TRAIL (1 μg/ml). Binding to Annexin V coincided with the classic morphological attributes of apoptosis, such as membrane blebbing, cell shrinkage and nuclear condensation (Fig. 4B). Therefore, the immune sera induced apoptosis in SUM159 cells comparable to that induced by TRAIL or mAb631.
Figure 4.
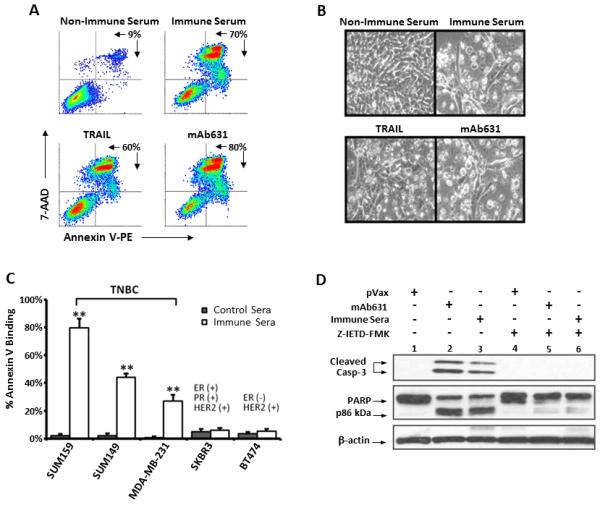

Induction of apoptosis in TNBC cells by hDR5 immune serum. A-B. SUM159 cells were treated for 20 hours with 1% serum from mice vaccinated with pVax1 (non-immune serum) or phDR5 (immune serum) or the hDR5 agonists, mAb631 (5μg/mL) or TRAIL (1 μg/mL). A. Cultured cells were harvested and analyzed by staining with Annexin V and 7-AAD. Percentages represent the number of cells gated as Annexin V/7-AAD-positive cells per total cell count. B. Photomicrographs of representative cell cultures documenting morphological changes consistent with cellular apoptosis. C. The percent Annexin V binding to SUM159 induced by agonist hDR5 antiserum (at 2%) following 20 hr treatment was compared to the indicated TNBC and HER2+/ER+ or HER2+/ER- human breast cancer cell lines (right panel). Statistical analysis was performed using two-tailed Student’s t-tests (**p<0.001; n=4). D. Western blot analysis of SUM159 treated for 5 hours with 2% non-immune (pVax1) or immune (phDR5) serum or 5 μg/mL mAb631 in the absence or presence of 20 μM caspase-8 inhibitor, Z-IETD-FMK as described in Material and Methods. Whole cell lysates were analyzed for cleavage products of caspase-3 (uncleaved caspase-3 not visualized), and PARP; β-actin was used as a loading control. E-F. IgG was purified from immune sera pooled from 5 mice as described in Material and Methods. SUM159 cells were treated for 20 hr with 1% non-immune sera, 1% unpurified pooled immune sera or 1% equivalent purified IgG. Cell apoptosis was measured by staining with Annexin V and 7-AAD (E). Cleavage of PARP was analyzed by Western blot analysis (lower panel) of SUM159 cells treated with the purified IgG, mA631, or non-immune sera. SUM159 growth inhibition was measured by MTT (F) and is displayed as averages of 6 replicates ± SE.
Since DR5 expression and sensitivity to TRAIL are associated with TNBC, in contrast to other breast cancer cells (17), susceptibility to DR5 immune sera was further tested against TNBC cells lines SUM149 and MDA-MB231 as compared to Her-2+ (SKBR3) and Her-2+/ER+ (BT474) cells (Fig. 4C). The experiment was repeated with 4 independent serum samples, with comparable results. Consistent with the reported findings using TRAIL, TNBC, but not SKBR3 or BT474 cells, were sensitive to DR5 immune serum.
Activation of apoptotic signaling pathway was analyzed by measuring the cleavage of caspase-3 and PARP in the presence or absence of a caspase-8 inhibitor (Z-IETD-FMK) (Fig. 4D). Within 5 hours of treatment with mAb631 or immune serum, we observed caspase-3 cleavage to the p17/p19 fragments (lanes 2 and 3) as detected by mAb clone 5A1 specific to cleaved caspase-3. Inhibition of caspase-8 with Z-IETD-FMK blocked caspase-3 cleavage (lane 6). Blockade of caspase-3 cleavage by Z-IETD-FMK was observed with both the immune serum (lanes 3 vs. 6) and mAb631 (lanes 2 vs. 5) indicating a similar role for caspase-8 in the apoptosis induced by immune serum and mAb631. Cleavage of PARP, which is further downstream of caspase-3 was also evident when cells were incubated with immune serum or mAb631 and was similarly blocked by Z-IETD-FMK. Thus, we conclude that hDR5 immune sera initiate the classical extrinsic apoptotic pathway similar to the DR5 agonist mAb631.
To verify that DR5 antibodies induced by DNA vaccination directly induced tumor cell apoptosis, mouse IgG in hDR5 immune sera was isolated by protein G column. Purified immune IgG triggered apoptosis in SUM159 cells as measured by Annexin V/7-AAD staining and PARP cleavage (Fig. 4E). The experiment was performed three times with similar results. Additionally, inhibition of SUM159 growth by purified IgG from hDR5 immunized mice was observed by MTT assay (Fig. 4F). Copurification with IgG of apoptotic signaling in hDR5 immune sera further supports direct death-receptor mediated agonist activity induced by phDR5 vaccines. These results demonstrate direct pro-apoptotic activity of vaccination-induced hDR5 antibody.
A potential adverse effect of inducing pro-apoptotic antibodies is T cell apoptosis because human T cells may also express DR5 when activated (27). Human PBL were stimulated with anti-CD3 and IL-2, PMA/ionomycin, or anti-CD3/anti-CD28 beads. Low-level DR5 expression was detected in cells activated with anti-CD3/IL-2 or PMA/ionomycin, but not with anti-CD3/anti-CD28 (Fig. 5A). When PBL pre-stimulated with anti-CD3 and IL-2 were incubated for 20-24 hrs with immune sera at 1:50 dilution, the proportion of Annexin V binding cells did not increase, indicating resistance to DR5 mediated apoptosis (Fig. 5B). This resistance was verified in cells treated with TRAIL or mAb 631. Survival of PBL stimulated with anti-CD3 and IL-2 in the presence of immune sera, TRAIL or mAb631 was measured by Alamar blue. There was no detectable impact from any of the test agents (Fig. 5C) when compared to control cultures. Under these conditions, normal human PBL demonstrated resistance to hDR5-mediated apoptosis.
Figure 5.
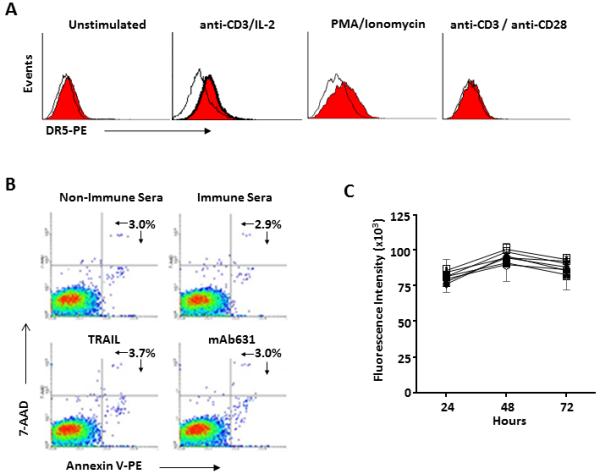
Resistance of DR5+ activated human T cells to DR5 agonists. A. Peripheral blood T cells were activated with anti-CD3 with IL-2, PMA/ionomycin or anti-CD3/anti-CD28 beads as described in Material and Methods and DR5 surface expression was measured by flow cytometry. B-C. T cells activated with anti-CD3 and IL-2 were analyzed for DR5 agonist-induced apoptosis as assessed by 7-AAD and Annexin V binding (B), as well as Alamar Blue cell survival assay (C); ■ control medium; O non-immune sera (2%); ▲ mAb631 (5 ug/ml); ▽ hDR5 immune sera (2%); □ non-immune sera plus TRAIL (1 ug/ml); ◆ mAb631 plus TRAIL; + hDR5 immune sera (2%) plus TRAIL.
Apoptosis signaling induced by hDR5 antiserum is amplified by receptor cross-linking
Crosslinking of the DR5 binding mAb by secondary antibody or FcγR bearing cells has been shown to result in receptor clustering and amplified apoptotic signal and may enhance tumor cell apoptosis in vivo (28). To test if cross-linking of DR5 antibodies enhanced tumor cell apoptosis, SUM159 cells coated for 30 min with immune serum were further incubated with anti-mouse IgG. Annexin V binding apoptotic cells increased from 20% to >75% (Fig. 6A), showing that receptor cross linking amplifies apoptotic signaling. These results may suggest that even weak agonist antibody may render strong anti-tumor activity when cross-linked in vivo which may be mediated by FcγR bearing cells.
Figure 6.

Inhibition of tumor growth by hDR5 reactive immune sera. A. Apoptosis induced by immune sera can be further amplified by IgG-mediated crosslinking. SUM159 cells were treated with 1% immune or non-immune sera for 30 min at 37°C and washed to remove unbound antibody, followed by incubation with goat anti-mouse IgG (α-IgG, 10 μg/mL) for 20h before Annexin V and 7-AAD staining. Relative to non-immune sera group, **p<.005. B. SUM159 cells were incubated with sera from vaccinated mice, or mAb631 (as described in Material and Methods), washed, and 3×106 cells were injected s.c. into SCID mice. Animals were monitored weekly for tumor growth. The median time to palpable tumor was monitored. Tumor incidence in the hDR5 immune sera treated group (n=7) was significantly different from the control (n=8, p=0.02) or the mAb631 treated (n=8, p=0.03). C. Tumor growth rate was documented by weekly assessment of tumor volume as calculated by the XY2/2, X=long axis and Y=short axis. Statistical differences between the three groups are indicated.
Inhibition of TNBC SUM159 growth in vivo by hDR5 immune serum
To test if hDR5-specific immune serum controls tumor growth in vivo, SUM159 cells were incubated with either non-immune serum, serum from hDR5-immunized mice, or agonist mAb631 and monitored for growth after s.c. injection into SCID mice. Immune serum from hDR5 vaccinated mice protected over 85% (6/7) of mice from tumor growth, a significant increase when compared with non-immune serum (12.5%, 1/8). Treatment with the control agonist mAb631 delayed tumor onset, but all eight mice eventually developed tumors (Fig 6B). Significant difference between immune sera and mAb631 was further illustrated by the difference in tumor volume as analyzed by Kruskal-Wallis tests (Fig. 6C). Therefore, immune serum was significantly more effective (p=0.0003) at preventing tumor growth than the mAb631, suggesting that polyclonal immune serum may be superior in inhibiting tumor growth in vivo.
Induction of antibodies to autologous DR5 in mice by DNA electrovaccination
To test if immune response to self DR5 can be induced, a mouse DR5 vaccine was generated and tested in BALB/c mice. pmDR5ectm was constructed to encode mouse DR5 ECD and TM domains, eliminating the intracellular death domain (Fig. 7A). The immunogenic tetanus toxin fragment C (TetC) domain 1 (Td1) was inserted after the TM region to generate pmDR5ectm-Td1.
Figure 7.
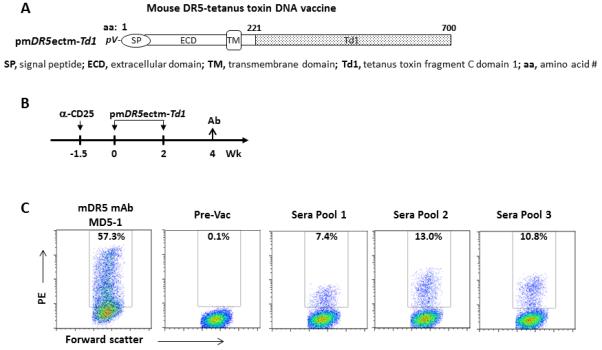
Induction of antibodies to autologous DR5 in mice by DNA electrovaccination. A.. Mouse DR5 DNA vaccine. pmDR5ectm-Td1 encodes mouse DR5 ECD and TM domains fused to tetanus toxin fragment C domain 1 (Td1). B. Vaccination Scheme. A total of 15 BALB/c mice were treated i.p. with 0.5 mg anti-CD25 mAb, PC61, to deplete Treg 10 days before the first electrovaccination with pmDR5ectm-Td1. Vaccination was repeated once. Sera were collected two weeks after the second vaccination and pooled into 3 samples, each from 5 mice. Pre-vaccination (Pre-Vac) sera were used as the negative control. C. Binding of immune sera to mouse DR5. 3T3 cells were transiently transfected with pmDR5ectm and expression was confirmed by staining with mDR5 mAb MD5-1 (left panel). Pre-vaccination and post-vaccination mouse sera (1:20) were incubated with transfected 3T3 cells followed by detection with PE-conjugated secondary antibody.
Normal BALB/c mice were electrovaccinated twice with pmDR5ectm-Td1 and pGM-CSF after Treg were depleted with CD25 mAb PC61 (Fig. 7B) as previously described (18). A total of 15 mice were vaccinated with pmDR5ectm-Td1. Immune sera were pooled into 3 groups with 5 samples in each group. To measure antibody response to autologous mDR5, 3T3 cells were transiently transfected with mDR5ectm. After 2-3 days, transfected cells appeared to undergo apoptosis even though mDR5ectm is free of the intracellular domain. Before transfected cells underwent apoptosis, they expressed mDR5 as detected by mAb MD5-1 (Fig. 7C). Serum samples were incubated with 3T3/mDR5ectm. mDR5 positive cells were detected with PE-conjugated secondary antibody. Induction of mDR5 binding antibody was detected in all 3 serum pools. These results support the feasibility of overcoming DR5 immune tolerance with DNA vaccination.
Discussion
Intramuscular electrovaccination with human DR5 DNA elicits DR5 specific antibody and T cells. Resulting anti-DR5 antibody induces apoptosis via the surface receptor-mediated caspase cascade to inhibit the proliferation of TNBC cells in vitro and block tumor growth in SCID mice. The pro-apoptotic activity of immune serum can be further augmented by IgG cross linking. Purified IgG from hDR5 immunized mice demonstrate direct pro-apoptotic activity and induce PARP cleavage, showing activation of receptor mediated apoptosis comparable to agonist mAb631. This is the first description of DR5 DNA vaccine that elicits agonist antibodies capable of direct binding to DR5 on human TNBC cells and inducing apoptosis. Furthermore, electrovaccination of mice with pmDR5ectm-Td1 that encodes self DR5 fused to Td1 induced anti-mouse DR5 antibodies to support the feasibility of overcoming immune tolerance to autologous DR5.
We previously reported that vaccine-induced Her-2/neu antibodies interfere with growth factor signaling (3). Here we describe a new DR5 DNA vaccine that induces an agonist, rather than antagonist antibody to initiate tumor cell apoptosis. The mechanism contributing to the induction of agonist, rather than antagonist antibody is not known, but may be related to the pre-existing DR5 complex which may be readily oriented into functional trimer when engaged with antibody (15).
The induction of pro-apoptotic antibody may be advantageous in patients with triple negative breast cancers, for whom a lack of targeting molecules renders a paucity of treatment options. It has been demonstrated that TNBCs have high expression of DR5, and that these cell lines are highly sensitive to DR5 agonist therapy (17). By inducing DR5 agonist antibodies, this vaccine may be used to prevent or treat TNBC as well as other TRAIL-sensitive tumors such as lung, colon, prostate, pancreatic and ovarian cancers
Ongoing and completed Phase I and II clinical trials targeting death receptors with recombinant TRAIL and/or death receptor mAb are showing disease stabilization without dose limiting toxicities. (http://clinicaltrials.gov/ct2/results?term=TRAIL+AND+cancer) (11,29-31). Asymptomatic elevation of liver enzyme activity was the primary indicator of toxicity, but only at very high doses and without clinical symptoms. These results have validated TRAIL death receptors as important clinical targets for cancer therapy. However, these therapeutic agents are limited in that they require repeated administration over prolonged periods and can only be given therapeutically to patients with active disease. On the other hand, DR5 vaccination induces agonist antibodies that are produced by the host continuously to provide long-term surveillance against disease occurrence or recurrence. Additionally, DR5-targeted vaccination may potentially be given prophylactically to focus the process of tumor immunosurveillance and prevent the occurrence of tumors that are typically undetectable at early stages. Finally, because TRAIL occurs naturally in the human body, vaccination to induce agonist DR5 antibodies reinforces the existing anti-tumor defense.
There is concern that such a potent anti-DR5 immune response may have deleterious effects on immune effectors such as activated lymphocytes which are known to express DR5. We analyzed the effects of immune serum, agonist antibodies and TRAIL on activated T cells and showed they are resistant to apoptosis when treated in vitro (Fig. 5B, C). Others have documented that upon activation, as cell surface DR5 expression increases, T and NK cells upregulate FLIP and XIAP, potent inhibitors of death receptor mediated apoptosis (27). Furthermore, clinical trials using death receptor agonist therapies including the various formulations of TRAIL and agonist antibodies specific for DR4 (HGS-ETR1, mapatumumab) and DR5 (HGS-ETR2, Lexatumumab, ApoMab and CS-1008 (Tra-8)) have reported no specific toxicity to lymphocytes. It is still possible that DR5-specific T-cells may cause damage to certain normal cells, and this potential should be closely monitored.
In our studies, coating SUM159 TNBC cells with immune serum prevented tumor growth in SCID mice, whereas a defined hDR5 agonist mAb was only capable of delaying tumor onset. Antibody-induced death receptor activation and ADCC are likely responsible for the observed greater efficacy. Since cross-linking of antibody-bound receptors on tumor cells with anti-IgG greatly amplified the induction of apoptosis in vitro, interactions with FcγR-bearing immune cells potentially mimicked this effect in vivo to provide an additional level of tumor destruction.
Since active vaccination also elicited hDR5 specific T cell response, DR5 expressing tumor cells could additionally be the targets of T cell mediated destruction. Numerous mechanisms confer intrinsic TRAIL-resistance in cancer, including upregulation of apoptosis inhibitors such as cFLIP or XIAP (32), defects in O-linked glycosylation of DR5 (33), and down-regulation of cell surface death receptor expression via endocytosis or impaired trafficking (34). Even in these instances, DR5 epitopes can still be presented by the major histocompatibility complex (MHC) to cytotoxic CD8 T cells to mediate tumor cell destruction. Thus, a broad range of tumors may be potential targets of DR5 vaccine.
Finally, there are numerous preclinical reports demonstrating that the anti-tumor effects of DR5 agonists can be greatly enhanced with chemotherapeutic agents (35-37) that induce TRAIL or DR5 expression (38), suggesting potential synergy between DR5 vaccine and conventional therapy. This may broaden the scope of tumors that can be treated to include tumors with lower levels of intrinsic sensitivity to DR5 agonists or possibly reducing the necessary dose of chemotherapeutic agents. The pro-apoptotic activity of DR5 immune serum warrants a full investigation of DR5 DNA as a cancer vaccine.
Supplementary Material
NOVELTY AND IMPACT STATEMENT.
This study is the first demonstration that pro-apoptotic, agonist antibodies are induced by electrovaccination with DNA encoding human TRAIL death receptor DR5 or its derivatives. Purified immune IgG binds to triple negative breast cancer (TNBC) cells to induce apoptosis. In vivo tumor growth is inhibited by immune sera. DR5 is a new vaccine target with the potential to trigger functional humoral and cellular immunity against TNBC.
Acknowledgement
The authors wish to thank Jonathan Ringler and Jessica Back for their input, and David Shim, Joyce Reyes and Andi Cani for their excellent technical support. We acknowledge the Microscopy, Imaging and Cytometry core and the Genomics core of the Karmanos Cancer Institute for their support of this study.
This study was supported by DOD W81XWH-10-1-0466 and NIH CA 76340, CA125680 (WZW)
Nonstandard Abbreviations
- ADCC
Antibody Dependent Cell-mediated Cytotoxicity
- APC
Antigen presenting cells
- DR4
Death Receptor 4
- TRAIL-R1
TNF-Related Apoptosis Inducing Ligand Receptor 1
- DR5
Death Receptor 5
- TRAIL-R2
TNF-Related Apoptosis Inducing Ligand Receptor 2
- ER
Estrogen Receptor
- GM-CSF
Granulocyte Macrophage Colony Stimulating Factor
- Her-2
Human Epidermal Growth Factor Receptor -2
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrasodium bromide
- PE
phycoerythrin
- PR
Progesterone Receptor
- SCID
Severe Combined Immune Deficient
- TNBC
Triple Negative Breast Cancers
- TRAIL
TNF-Related Apoptosis Inducing Ligand
References
- 1.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 3.Whittington PJ, Piechocki MP, Heng HH, Jacob JB, Jones RF, Back JB, Wei W-Z. DNA vaccination controls Her-2+ tumors that are refractory to targeted therapies. Cancer Research. 2008;68:7502–7511. doi: 10.1158/0008-5472.CAN-08-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hampton T. Novel targeted cancer drugs highlighted. JAMA. 2006;296:270. doi: 10.1001/jama.296.3.270. [DOI] [PubMed] [Google Scholar]
- 5.Cretney E, Takeda K, Smyth MJ. Cancer: Novel therapeutic strategies that exploit the TNF-related apoptosis-inducing ligand (TRAIL)/TRAIL receptor pathway. Int J Biochem Cell Biol. 2007;39:280–286. doi: 10.1016/j.biocel.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Rowinsky EK. Targeted induction of apoptosis in cancer management: the emerging role of tumor necrosis factor-related apoptosis-inducing ligand receptor activating agents. J Clin Oncol. 2005;23:9394–9407. doi: 10.1200/JCO.2005.02.2889. [DOI] [PubMed] [Google Scholar]
- 7.Takeda K, Yamaguchi N, Akiba H, Kojima Y, Hayakawa Y, Tanner JE, Sayers TJ, Seki N, Okumura K, Yagita H, Smyth MJ. Induction of Tumor-specific T Cell Immunity by Anti-DR5 Antibody Therapy. J Exp Med. 2004;199:437–448. doi: 10.1084/jem.20031457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi J, Zheng D, Liu Y, Sham MH, Tam P, Farzaneh F, Xu R. Overexpression of soluble TRAIL induces apoptosis in human lung adenocarcinoma and inhibits growth of tumor xenografts in nude mice. Cancer Res. 2005;65:1687–1692. doi: 10.1158/0008-5472.CAN-04-2749. [DOI] [PubMed] [Google Scholar]
- 9.Clancy L, Mruk K, Archer K, Woelfel M, Mongkolsapaya J, Screaton G, Lenardo MJ, Chan FK. Preligand assembly domain-mediated ligand-independent association between TRAIL receptor 4 (TR4) and TR2 regulates TRAIL-induced apoptosis. Proc Natl Acad Sci U S A. 2005;102:18099–18104. doi: 10.1073/pnas.0507329102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu GS, Burns TF, Zhan Y, Alnemri ES, El-Deiry WS. Molecular cloning and functional analysis of the mouse homologue of the KILLER/DR5 tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor. Cancer Res. 1999;59:2770–2775. [PubMed] [Google Scholar]
- 11.Ashkenazi A, Holland P, Eckhardt SG. Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/Tumor necrosis factor-related apoptosis-inducing ligand (rhApo2L/TRAIL) J Clin Oncol. 2008;26:3621–3630. doi: 10.1200/JCO.2007.15.7198. [DOI] [PubMed] [Google Scholar]
- 12.Duiker EW, Mom CH, de Jong S, Willemse PH, Gietema JA, van der Zee AG, de Vries EG. The clinical trail of TRAIL. Eur J Cancer. 2006;42:2233–2240. doi: 10.1016/j.ejca.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 13.Kelley RF, Totpal K, Lindstrom SH, Mathieu M, Billeci K, Deforge L, Pai R, Hymowitz SG, Ashkenazi A. Receptor-selective mutants of apoptosis-inducing ligand 2/tumor necrosis factor-related apoptosis-inducing ligand reveal a greater contribution of death receptor (DR) 5 than DR4 to apoptosis signaling. J Biol Chem. 2005;280:2205–2212. doi: 10.1074/jbc.M410660200. [DOI] [PubMed] [Google Scholar]
- 14.Hymowitz SG, Christinger HW, Fuh G, Ultsch M, O’Connell M, Kelley RF, Ashkenazi A, de Vos AM. Triggering cell death: the crystal structure of Apo2L/TRAIL in a complex with death receptor 5. Mol Cell. 1999;4:563–571. doi: 10.1016/s1097-2765(00)80207-5. [DOI] [PubMed] [Google Scholar]
- 15.Wassenaar TA, Quax WJ, Mark AE. The conformation of the extracellular binding domain of Death Receptor 5 in the presence and absence of the activating ligand TRAIL: a molecular dynamics study. Proteins. 2008;70:333–343. doi: 10.1002/prot.21541. [DOI] [PubMed] [Google Scholar]
- 16.Natoni A, MacFarlane M, Inoue S, Walewska R, Majid A, Knee D, Stover DR, Dyer MJ, Cohen GM. TRAIL signals to apoptosis in chronic lymphocytic leukaemia cells primarily through TRAIL-R1 whereas cross-linked agonistic TRAIL-R2 antibodies facilitate signalling via TRAIL-R2. Br J Haematol. 2007;139:568–577. doi: 10.1111/j.1365-2141.2007.06852.x. [DOI] [PubMed] [Google Scholar]
- 17.Rahman M, Davis SR, Pumphrey JG, Bao J, Nau MM, Meltzer PS, Lipkowitz S. TRAIL induces apoptosis in triple-negative breast cancer cells with a mesenchymal phenotype. Breast Cancer Res Treat. 2008;113:217–230. doi: 10.1007/s10549-008-9924-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei WZ, Jacob JB, Zielinski JF, Flynn JC, Shim KD, Alsharabi G, Giraldo AA, Kong YM. Concurrent induction of antitumor immunity and autoimmune thyroiditis in CD4+ CD25+ regulatory T cell-depleted mice. Cancer Research. 2005;65:8471–8478. doi: 10.1158/0008-5472.CAN-05-0934. [DOI] [PubMed] [Google Scholar]
- 19.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, Speed T, Spellman PT, DeVries S, Lapuk A, Wang NJ, Kuo WL, Stilwell JL, Pinkel D, Albertson DG, Waldman FM, McCormick F, Dickson RB, Johnson MD, Lippman M, Ethier S, Gazdar A, Gray JW. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pai SI, Wu GS, Ozoren N, Wu L, Jen J, Sidransky D, El-Deiry WS. Rare loss-of-function mutation of a death receptor gene in head and neck cancer. Cancer Res. 1998;58:3513–3518. [PubMed] [Google Scholar]
- 21.Radkevich-Brown O, Piechocki MP, Back JB, Weise AM, Pilon-Thomas S, Wei WZ. Intratumoral DNA electroporation induces anti-tumor immunity and tumor regression. Cancer Immunol Immunother. 2010;59:409–417. doi: 10.1007/s00262-009-0760-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei WZ, Shi WP, Galy A, Lichlyter D, Hernandez S, Groner B, Heilbrun L, Jones RF. Protection against mammary tumor growth by vaccination with full-length, modified human ErbB-2 DNA. Int J Cancer. 1999;81:748–754. doi: 10.1002/(sici)1097-0215(19990531)81:5<748::aid-ijc14>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 23.Jacob J, Radkevich O, Forni G, Zielinski J, Shim D, Jones RF, Wei W. Activity of DNA vaccines encoding self or heterologous Her-2/neu in Her-2 or neu transgenic mice. Cellular Immunology. 2006;240:96–106. doi: 10.1016/j.cellimm.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 25.Cox DR. Regression Models and Life Tables (with Discussion) 1972:187–220. [Google Scholar]
- 26.Conover WJ. Practical Nonparametric Statistics. Wiley; New York: 1999. pp. 223–236. [Google Scholar]
- 27.Mirandola P, Ponti C, Gobbi G, Sponzilli I, Vaccarezza M, Cocco L, Zauli G, Secchiero P, Manzoli FA, Vitale M. Activated human NK and CD8+ T cells express both TNF-related apoptosis-inducing ligand (TRAIL) and TRAIL receptors but are resistant to TRAIL-mediated cytotoxicity. Blood. 2004;104:2418–2424. doi: 10.1182/blood-2004-04-1294. [DOI] [PubMed] [Google Scholar]
- 28.Adams C, Totpal K, Lawrence D, Marsters S, Pitti R, Yee S, Ross S, Deforge L, Koeppen H, Sagolla M, Compaan D, Lowman H, Hymowitz S, Ashkenazi A. Structural and functional analysis of the interaction between the agonistic monoclonal antibody Apomab and the proapoptotic receptor DR5. Cell Death Differ. 2008;15:751–761. doi: 10.1038/sj.cdd.4402306. [DOI] [PubMed] [Google Scholar]
- 29.Ichikawa K, Liu W, Zhao L, Wang Z, Liu D, Ohtsuka T, Zhang H, Mountz JD, Koopman WJ, Kimberly RP, Zhou T. Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nature Medicine. 2001;7:954–960. doi: 10.1038/91000. [DOI] [PubMed] [Google Scholar]
- 30.Hotte SJ, Hirte HW, Chen EX, Siu LL, Le LH, Corey A, Iacobucci A, MacLean M, Lo L, Fox NL, Oza AM. A phase 1 study of mapatumumab (fully human monoclonal antibody to TRAIL-R1) in patients with advanced solid malignancies. Clin Cancer Res. 2008;14:3450–3455. doi: 10.1158/1078-0432.CCR-07-1416. [DOI] [PubMed] [Google Scholar]
- 31.Plummer R, Attard G, Pacey S, Li L, Razak A, Perrett R, Barrett M, Judson I, Kaye S, Fox NL, Halpern W, Corey A, Calvert H, de BJ. Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin Cancer Res. 2007;13:6187–6194. doi: 10.1158/1078-0432.CCR-07-0950. [DOI] [PubMed] [Google Scholar]
- 32.Thorburn A, Behbakht K, Ford H. TRAIL receptor-targeted therapeutics: resistance mechanisms and strategies to avoid them. Drug Resist Updat. 2008;11:17–24. doi: 10.1016/j.drup.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner KW, Punnoose EA, Januario T, Lawrence DA, Pitti RM, Lancaster K, Lee D, von GM, Yee SF, Totpal K, Huw L, Katta V, Cavet G, Hymowitz SG, Amler L, Ashkenazi A. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med. 2007;13:1070–1077. doi: 10.1038/nm1627. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Zhang B. TRAIL resistance of breast cancer cells is associated with constitutive endocytosis of death receptors 4 and 5. Mol Cancer Res. 2008;6:1861–1871. doi: 10.1158/1541-7786.MCR-08-0313. [DOI] [PubMed] [Google Scholar]
- 35.Ohtsuka T, Buchsbaum D, Oliver P, Makhija S, Kimberly R, Zhou T. Synergistic induction of tumor cell apoptosis by death receptor antibody and chemotherapy agent through JNK/p38 and mitochondrial death pathway. Oncogene. 2003;22:2034–2044. doi: 10.1038/sj.onc.1206290. [DOI] [PubMed] [Google Scholar]
- 36.Jin H, Yang R, Ross J, Fong S, Carano R, Totpal K, Lawrence D, Zheng Z, Koeppen H, Stern H, Schwall R, Ashkenazi A. Cooperation of the agonistic DR5 antibody apomab with chemotherapy to inhibit orthotopic lung tumor growth and improve survival. Clin Cancer Res. 2008;14:7733–7740. doi: 10.1158/1078-0432.CCR-08-0670. [DOI] [PubMed] [Google Scholar]
- 37.Luster TA, Carrell JA, McCormick K, Sun D, Humphreys R. Mapatumumab and lexatumumab induce apoptosis in TRAIL-R1 and TRAIL-R2 antibody-resistant NSCLC cell lines when treated in combination with bortezomib. Mol Cancer Ther. 2009;8:292–302. doi: 10.1158/1535-7163.MCT-08-0918. [DOI] [PubMed] [Google Scholar]
- 38.Ding Z, Zhou JY, Wei WZ, Baker VV, Wu GS. Induction of apoptosis by the new anticancer drug XK469 in human ovarian cancer cell lines. Oncogene. 2002;21:4530–4538. doi: 10.1038/sj.onc.1205545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


