Abstract
Background
Isolated, non-syndromic cleft lip with or without cleft palate (iCL±P) is a common human congenital malformation with a complex and heterogeneous etiology. Genes coding for fibroblast growth factors and their receptors (FGF/FGFR genes) are excellent candidate genes.
Methods
We tested single nucleotide polymorphic (SNP) markers in 10 FGF/FGFR genes (including FGFBP1, FGF2, FGF10, FGF18, FGFR1, FGFR2, FGF19, FGF4, FGF3, and FGF9) for genotypic effects, interactions with one another, and with common maternal environmental exposures in 221 Asian and 76 Maryland case-parent trios ascertained through a child with iCL±P.
Results
Both FGFR1 and FGF19 yielded evidence of linkage and association in the transmission disequilibrium test, confirming previous evidence. Haplotypes of three SNPs in FGFR1 were nominally significant among Asian trios. Estimated ORs for individual SNPs and haplotypes of multiple markers in FGF19 ranged between1.31-1.87. We also found suggestive evidence of maternal genotypic effects for markers in FGF2 and FGF10 among Asian trios. Tests for gene-environment (GxE) interaction between markers in FGFR2 and maternal smoking or multivitamin supplementation yielded significant evidence of GxE interaction separately. Tests of gene-gene (GxG) interaction using Cordell's method yielded significant evidence between SNPs in FGF9 and FGF18, which was confirmed in an independent sample of trios from an international consortium.
Conclusion
Our results suggest several genes in the FGF/FGFR family may influence risk to iCL±P through distinct biological mechanisms.
Keywords: FGF/FGFR, oral clefts, maternal effects, gene-environment interaction, gene-gene interaction
Isolated, non-syndromic cleft lip with or without cleft palate (iCL±P) represents one of the most common human birth defects (Mossey and Little, 2002) and has a complex and heterogeneous etiology that remains poorly understood (Jugessur and Murray, 2005). There is a strong genetic component to the etiology of this common birth defect. A multifactorial threshold model of inheritance reflecting multiple distinct causal genes is often assumed (Grosen et al., 2010). Genome-wide linkage screens in multiplex families have shown multiple regions of the genome may harbor causal genes with a high degree of linkage heterogeneity (Marazita et al., 2004; 2009). Recently, genome wide association studies (GWAS) have identified a region on chromosome 8q24 as strongly associated with risk to iCL±P; although it is relatively devoid of genes, raising the possibility that non-coding genetic regions are also critical (Birnbaum et al., 2009; Grant et al., 2009; Beaty et al., 2010). Several studies have also shown suggestive evidence for interaction between candidate genes and environmental risk factors, especially maternal smoking (Zeiger et al., 2005; Shi et al., 2007a) and nutrient intake (Shaw et al., 1998) in controlling risk for iCL±P, although the evidence for gene environment (GxE) interaction remains difficult to confirm. Therefore, genes and regulatory elements outside of coding regions, plus their possible interactions with one another (GxG interaction) and with environmental factors, should be considered when searching for potential causal genes for iCL±P.
Genes in the fibroblast growth factor (FGF) signaling pathway are excellent candidate genes for iCL±P (Nie et al., 2006; Riley et al., 2007a; 2007b; 2007c; Menezes et al., 2008). Here we tested markers in 10 FGF and FGF receptor (FGFR) genes for their potential role in controlling risk to iCL±P using 297 case–parent trios from four populations.
MATERIALS AND METHODS
Sample description
As part of an international study, we collected peripheral blood, environmental exposures, and other data on iCL±P case-parent trios recruited through treatment centers in Maryland (MD), (Johns Hopkins and University of Maryland), Chang Gung Memorial Hospital in Taiwan (TW), KK Women's and Children's Hospital in Singapore (SP), and Yonsei Medical Center in South Korea (KR). History of maternal exposure to cigarette smoking, alcohol consumption, and vitamin supplementation was collected from a personal interview of mothers covering the peri-conceptual period (three months before conception through the first trimester). All probands underwent clinical genetic evaluation (including checking for other congenital anomalies or major developmental delays) and were classified as having an isolated, non-syndromic iCL±P. Research protocols were reviewed and approved by institutional review boards (IRBs) at each participating institution.
SNP selection, DNA & genotyping
SNPs were selected in 10 FGF/FGFR genes (including FGFBP1, FGF2, FGF10, FGF18, FGFR1, FGFR2, FGF19, FGF4, FGF3, and FGF9) with a goal of identifying one SNP per 5kb of physical distance. Because Hapmap data were not fully available at the time our SNPs were chosen, we could not identify all possible tagging SNPs. Variants with “SNP scores” > 0.6 (an assessment of design quality of the Illumina assay based on a proprietary algorithm), high validation levels in dbSNP (including validation on multiple platforms), and high heterozygosity levels (particularly in multiple populations) were given priority. SNP markers were genotyped using Illumina's Golden-Gate chemistry at the Genetic Resources Core Facility (GRCF) at Johns Hopkins. Two duplicates and four controls from the Centre d'Etude du Polymorphisme Humain (CEPH) collection were included on each plate to evaluate genotyping consistency within and between plates.
Statistical analysis
Genotyping rate, minor allele frequency (MAF), pairwise linkage disequilibrium (LD) and Hardy-Weinberg equilibrium (HWE) were evaluated within each population and in three Asian populations combined. LD was measured as r2 for all SNPs using Haploview (Barrett et al., 2005). SNPs were tested when the following criteria were satisfied: MAF > 1%, compatibility with HWE at p > 0.01 in each group and overall genotyping rate>80%. None of the SNPs between different FGF/FGFR genes on the same chromosome were in LD (data not shown), so the 10 FGF/FGFR genes were analyzed separately.
Statistical significance of transmission distortion from parents to the affected offspring was evaluated using the family based association test (FBAT; http://www.biostat.harvard.edu/~fbat/fbat.htm) for each individual SNP and for haplotypes of multiple SNPs (Laird and Lange, 2006). Numbers of transmitted and non-transmitted alleles for both single SNPs and haplotypes were generated using PLINK (v1.07; http://pngu.mgh.harvard.edu/purcell/plink/, Purcell et al., 2007). The 95% confidence interval (95%CI) for estimated odds ratios (ORs) of over-transmission were calculated by a binomial exact test in STATA (v 10.0).
Analysis of maternally mediated in utero effects was tested using TRIad Multi-Marker (TRIMM) package under an assumption of mating symmetry in the population (Shi et al., 2007b). Paired difference counts (D) of the number of target alleles carried by father and mother is a key component in constructing standardized normal (Z) statistics for the mean D across all markers, and the maximum Z2 served as a test statistic. Empiric significance of maternal genotypic effects was evaluated by permuting these max_Z2 values over random reassignments of “father” and “mother”. To optimize power, max_Z2 and Hotelling's T2 tests were used to generate a combined P-value (sum_logP) from these two tests. If the global max_Z2 test gave a p < 0.1, the apparent risk allele or haplotype was used in the log-linear framework originally proposed by Weinberg et al. (1998) to estimate relative risks. ORs and their significance associated with the mother's carrying one copy (S1) of the risk allele/haplotype was assessed by a likelihood ratio test (LRT) under the log-additive model (where ORs associated with carrying two copies is simply S12) as implemented in the Triad Multi-Marker relative risk Estimation program (TRIMMEST) (www.niehs.nih.gov/research/atniehs/labs/bb/staff/weinberg; Shi et al., 2009). A general model was used to estimate the OR for FGF2, where 4 haplotypes were present, but a more restricted model assuming HWE was used for FGF10 where 11 haplotypes were observed (because the more general model would have required too many parameters).
Family based association tests for individual SNPs or 2-3 SNP sliding window haplotypes incorporating a GxE interaction term with maternal smoking and vitamin supplementation were performed in a combined 2 degree of freedom (df) score test for main effects of genotype (G) and GxE interaction together, followed by a 1 df score test for GxE interaction alone using PBAT (v3.6; http://www.biostat.harvard.edu/~clange/default.htm).
We used Cordell's (2002) LRT for possible GxG interaction assessment among markers in these 10 FGF/FGFR genes. Using a conditional logistic regression model, the observed 2-locus genotype of the case was compared to the 15 possible ‘pseudo-sib’ control genotypes, generating a 4 df test. To address the issue of multiple comparisons, we carried out permutation tests where case vs. pseudo-sib control status was randomly shuffled 1000 times for each trio to generate new sets of data under the null hypothesis. An empirical P-value for the most significant SNP was determined by comparing the observed test statistic to these 1000 replicates.
Although markers in these 10 FGF/FGFR genes were typed as part of a candidate gene study conducted before the international consortium described by Beaty et al. (2010), 157 of these 297 (52.9%) case-parent trios went into that genome wide study. In a confirmatory analysis of GxG interaction, we removed these overlapping trios from the international consortium data set and used all remaining case-parent trios (n=1434) which represents an independent replication sample of iCL±P case-parent trios.
RESULTS
A total of 297 trios were collected from four populations (MD, TW, SP and KR), and Table 1 lists gender and race of all iCL±P probands. Among the 122 SNPs genotyped in these 10 FGF/FGFR genes, nine SNPs were dropped due to low MAF and another 2 SNPs were dropped due to low genotyping call rate. Genotype distributions for the remaining 111 SNPs were all compatible with HWE (data not shown).
TABLE 1.
Gender and racial origin of 297 CL/P probands from 4 populations
| Racial Origin | Maryland | Taiwan | Singapore | Korean | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | |
| European | 41 | 29 | 0 | 0 | 1 | 1 | 0 | 0 | 42 | 30 |
| African | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 |
| Asian | 0 | 0 | 95 | 51 | 23 | 10 | 22 | 18 | 140 | 79 |
| Total | 44 | 32 | 95 | 51 | 24 | 11 | 22 | 18 | 185 | 112 |
When analyzing transmission distortion of individual SNPs among these 10 FGF/FGFR genes, two independent markers in FGF19 showed nominally significant evidence of linkage and association (P<0.05) with iCL±P among Asian trios, and another SNP was significant only among MD trios (Table 2). Estimated ORs for carrying the apparent high-risk allele at each of these three FGF19 SNPs ranged from 1.37 to 1.87.Analysis of sliding window haplotypes using 2-5 SNPs together confirmed the significance of markers in FGF19 among Asian trios, and a 3-SNP haplotype in FGFR1 (rs6987534, rs6474354and rs10958700) gave p = 0.04 (corrected p=0.30) among Asian trios.
TABLE 2.
SNPs yielding significant or marginally significant associations from analysis of 111 markers in 10 FGF/FGFR genes from family based association tests for single SNPs and 2-5 SNP haplotypes in analysis of 76 MD trios and 221 Asian trios separately
| Site | Gene | SNP name | Risk allele/haplotype | % | # informative Families | T | NT | OR (95%CI) | P Value |
|---|---|---|---|---|---|---|---|---|---|
| Single SNP Analysis | |||||||||
| Asian | FGF19 | RS3737463 | 1 | 66.6 | 148 | 112 | 78 | 1.44(1.07-1.94) | 0.0136 |
| Asian | FGF19 | RS948992 | 2 | 55.4 | 148 | 114 | 83 | 1.37(1.03-1.85) | 0.0272 |
| MD | FGF19 | RS1789364 | 2 | 38.9 | 33 | 28 | 15 | 1.87(0.96-3.76) | 0.0474 |
| MD | FGF2 | RS308395 | 1 | 18.8 | 18 | 14 | 5 | 2.80(0.95-9.93) | 0.0640 |
| Haplotype Analysis | |||||||||
| Asian | FGFR1 |
RS6987534
RS6474354 RS10958700 |
2,2,1 | 12.4 | 79 | 28 | 55 | 0.51(0.31-0.82) | 0.0411 |
| Asian | FGF19 |
RS3737463
RS948992 |
1,2 | 45.1 | 145 | 117 | 82 | 1.43(1.07-1.92) | 0.0271 |
| Asian | FGF19 |
RS3737463
RS948992 RS1307968 |
1,2,1 | 45.1 | 142 | 119 | 82 | 1.45(1.09-1.95) | 0.0161 |
| Asian | FGF19 |
RS3737463
RS948992 RS1307968 RS1320706 |
1,2,1,1 | 45.3 | 142 | 116 | 80 | 1.45(1.08-1.95) | 0.0272 |
| Asian | FGF19 |
RS3737463
RS948992 RS1307968 RS1320706 RS1789364 |
1,2,1,1,1 | 36.2 | 126 | 101 | 77 | 1.31(0.97-1.79) | 0.0498 |
In testing for possible maternal genotype effects, one 4-SNP haplotype in FGF2 and another 6-SNP haplotype in FGF10 showed significant empiric evidence of an increased risk of iCL±P in offspring that depended solely upon maternal genotype among Asian trios. The corresponding ORs for the child being affected were estimated as 1.72 (χ2 =11.47, empiric p=0.0007) and 1.61 (χ2 =7.29, empiric p=0.0069), if the mother carried one copy of the risk haplotype (Table 3).
TABLE 3.
Maternal risk haplotypes identified by TRIMM from analysis of 28 markers in the FGF2 and FGF10 genes under an additive modelwhere maternal genotype alone (S1) controls risk to CL/P in offspring from TRIMMEST analysis in 221 Asian trios.
| Gene (#Chr) | #SNPs | Sum_logP | SNPs | Risk Allele | Estimated risk for Mat. Hap. S1 | Risk Hap Freq | χ 2 | P | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | ||||||||
| FGF2(4) | 12 | 0.024 | RS3789138 | 1 | 1.72 | 0.22 | 0.52 | 0.26 | 11.47 | 0.0007 |
| RS308388 | 1 | |||||||||
| RS1476214 | 1 | |||||||||
| RS1476217 | 2 | |||||||||
| FGF10(5) | 16 | 0.027 | RS10057630 | 1 | 1.61 | 0.25 | 7.29 | 0.0069 | ||
| RS1448037 | 1 | |||||||||
| RS593307 | 1 | |||||||||
| RS339502 | 2 | |||||||||
| RS1384449 | 2 | |||||||||
| RS2973647 | 1 | |||||||||
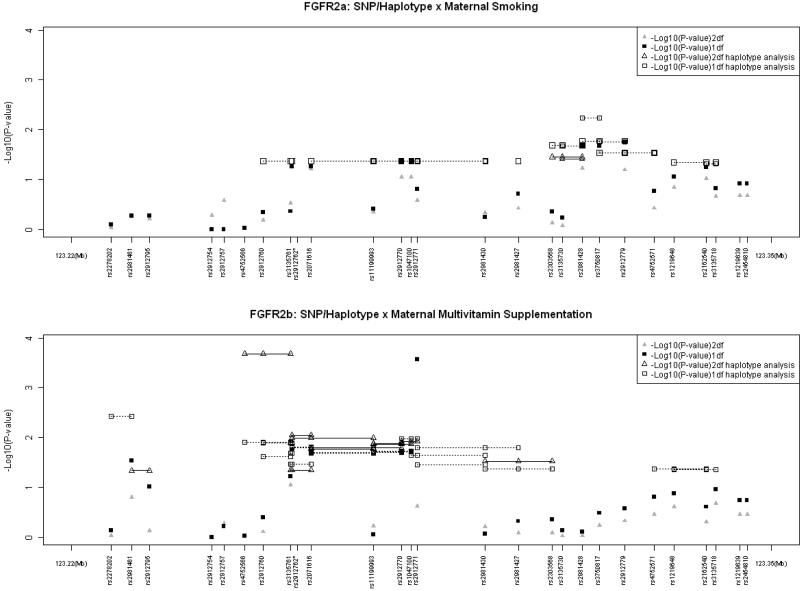
Although the rate of maternal alcohol consumption was too low to permit separate analysis, about 5% and 25% Asian mothers reported smoking and taking vitamin supplements, respectively, during the critical peri-conceptional period (Sull et al., 2009). Significant evidence of GxE interaction was seen among Asian populations for FGFR2 (Figure 1). For GxSmoking analysis, the most significant evidence was seen in a 2-SNP haplotype (rs2981428 and rs3750817) which yielded a p = 0.0058 in a 1 df test (Figure 1) which is not significant after strict Bonferroni correction. For GxVitamin interaction, the strongest evidence in FGFR2was seen both in 1df test for a single SNP (rs2912771; p = 0.00027, corrected p = 0.042) and in the 2 df test for a 3-SNP haplotype (involving rs4752566, rs2912760, and rs3135761; p = 0.00021, corrected p = 0.033). These P-values for GxVitamin interaction remained significant after strict Bonferroni correction for all 156 tests conducted on the 27 markers and their haplotypes in FGFR2. As seen in the lower panel of Figure 1, several other SNPs and haplotypes also showed nominal significance in either the 1 df or the 2 df test for GxVitamin interaction.
Figure 1.
Testing for main effects (G) of individual SNPs (haplotypes) of FGFR2 and gene–environment interaction (GxE) for two common maternal exposures in 221CL/P case–parent trios from Asian populations. Triangles represent the 2 df test of G and GxE interaction, squares represent the 1 df test of G x E only. Haplotypes of 2- and 3-SNPs are connected by solid lines for 2dftest and dotted lines for 1 df test (only nominally significant haplotypes are shown here). rs2912762*: The distance between rs3135761 and rs2912762 were drawn for clarity (true distance between these two SNPs is 199 base pairs).
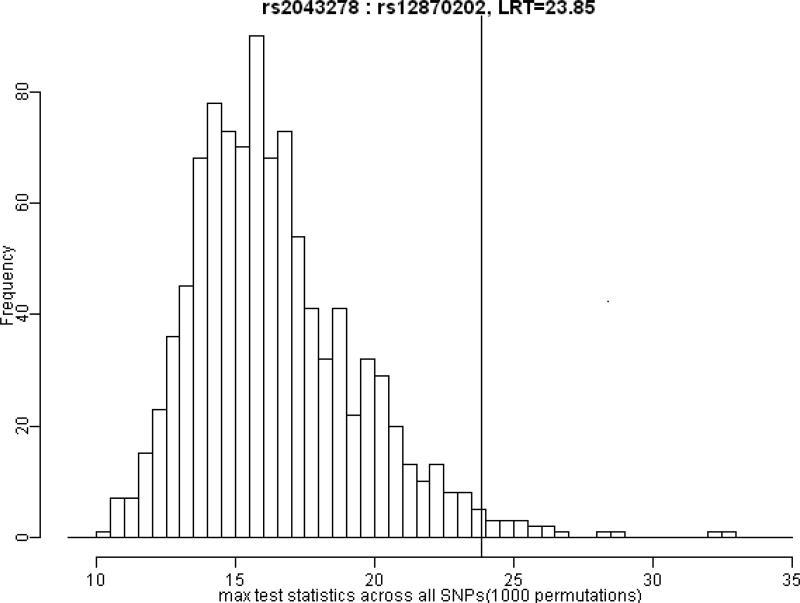
A total of 16 pairs of SNPs in different FGF genes attained nominal significance in tests of GxG interaction (Table 4). The most significant LRT was generated by rs2043278 in FGF18 and rs12870202 in FGF9 (p = 0.0001), which remained significant (p = 0.019) after correcting for multiple comparisons via permutation tests. Only 1.9% of replicates (across the 338 separate tests for GxG interactions) generated under the null hypothesis exceeded this observed test statistic (Table 4 and Figure 2).
TABLE 4.
Significant tests for G×G interaction among 111 markers in 10 different FGF/FGFR genes in 297 CL/P case-parent trios from 4 populations based on 4df LRT testing for 2-way interaction
| First gene | Second gene | Marker 1 | Marker 2 | Test statistic | P-value | ||
|---|---|---|---|---|---|---|---|
| Gene snpl | Chr snpl | Gene snp2 | Chr snp2 | SNP1 | SNP2 | ||
| FGF18 | 5 | FGF10 | 5 | RS4559013 | RS11750845 | 14.255 | 0.0065 |
| FGF18 | 5 | FGF10 | 5 | RS4076077 | RS11750845 | 14.300 | 0.0064 |
| FGF18 | 5 | FGF10 | 5 | RS4076077 | RS1482679 | 14.156 | 0.0068 |
| FGF18 | 5 | FGF10 | 5 | RS3934591 | RS1482679 | 17.743 | 0.0014 |
| FGF18 | 5 | FGFR1 | 8 | RS6887323 | RS2978073 | 13.604 | 0.0087 |
| FGF18 | 5 | FGF3 | 11 | RS6887323 | RS1893047 | 15.135 | 0.0044 |
| FGF18 | 5 | FGF3 | 11 | RS2043278 | RS11263592 | 14.199 | 0.0067 |
| FGF18 | 5 | FGF3 | 11 | RS2043278 | RS1893047 | 13.958 | 0.0074 |
| FGF18 | 5 | FGF9 | 13 | RS2043278 | RS12870202 | 23.854 | 0.0001* |
| FGFR2 | 10 | FGF10 | 5 | RS2981430 | RS593307 | 13.556 | 0.0089 |
| FGFR2 | 10 | FGF10 | 5 | RS2981430 | RS339502 | 13.815 | 0.0079 |
| FGFR2 | 10 | FGF10 | 5 | RS2981430 | RS1384449 | 14.338 | 0.0063 |
| FGFR2 | 10 | FGFR1 | 8 | RS2981427 | RS4733930 | 15.973 | 0.0031 |
| FGF3 | 11 | FGFBP1 | 4 | RS11263592 | RS732245 | 13.590 | 0.0087 |
| FGF3 | 11 | FGFBP1 | 4 | RS11263587 | RS732245 | 13.563 | 0.0088 |
| FGF9 | 13 | FGFR1 | 8 | RS6490667 | RS4733930 | 14.010 | 0.0073 |
Only this 2–SNP interaction remained significant after 1,000 permutations with P value of 0.019.
Figure 2.
Distribution of maximum LRT values over 1000 replicates. Histograms represent the frequency of maximum test statistics generated under the null hypothesis of no GxG interaction. The vertical line shows the position of observed test statistic of GxG interaction between rs12870202 and rs2043278. About 1.9% of permutated test statistics exceeded this observed value, giving an empiric p-value corrected for multiple testing (p=0.019).
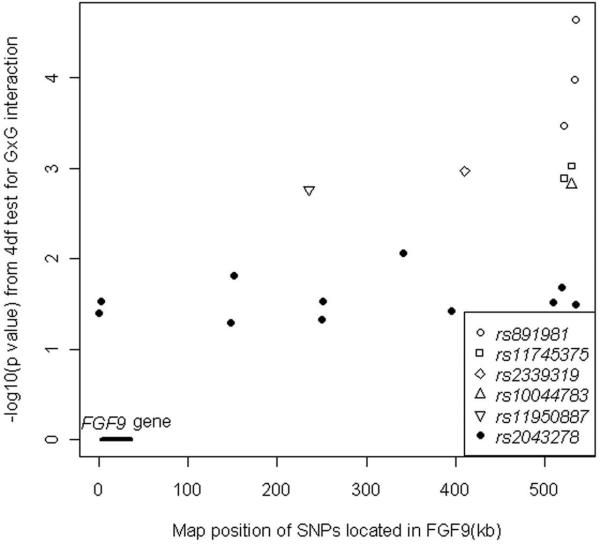
To follow-up on the intriguing evidence for GxG interaction between FGF9 and FGF18 seen here, an independent replication sample of 1,434 case-parent trios was examined in a confirmatory test of GxG interaction using markers in these two genes. Only rs2043278 in FGF18 was included in the genome wide marker panel, but 11 different SNPs in or near FGF9 gave a nominally significant evidence of GxG interaction with this one SNP (Table 5). When all 38 SNPs in FGF18 were examined with the 140 SNPs mapping to FGF9, several additional pairs of SNPs in these two genes showed further evidence of possible GxG interaction (see Figure 3). The most significant pairs involved SNPs in the intergenic region 3' of the gene, some are as far as 500kb away.
TABLE 5.
Tests for G×G interaction between RS2043278 in FGF18 and 11 SNPs in FGF9 that achieved nominal significance in 1434 case-parent trios from the International Cleft Consortium
| SNP_pair | Position in FGF9 | Statistic | Nominal P |
|---|---|---|---|
| RS2043278 : RS829209 | 21133584 | 10.05 | 0.0396 |
| RS2043278 : RS9634328 | 21136546 | 10.71 | 0.0300 |
| RS2043278 : RS672905 | 21281253 | 9.42 | 0.0514 |
| RS2043278 : RS7999069 | 21284490 | 12.27 | 0.0154 |
| RS2043278 : RS17073403 | 21383899 | 9.61 | 0.0475 |
| RS2043278 : RS7338014 | 21384015 | 10.77 | 0.0293 |
| RS2043278 : RS9580272 | 21474452 | 13.59 | 0.0087 |
| RS2043278 : RS12853883 | 21529175 | 10.16 | 0.0378 |
| RS2043278 : RS725600 | 21643202 | 10.70 | 0.0301 |
| RS2043278 : RS9552612 | 21652578 | 11.60 | 0.0205 |
| RS2043278 : RS17326684 | 21667539 | 10.52 | 0.0325 |
Figure 3.
Significance [as –log10(p)] from 4df LRT for Cordell's test of GxG interaction between markers in FGF18 and FGF9 plotted against the physical position in FGF9. SNP (rs2043278) in FGF18 is an intronic marker located at 170,815,212 (Build 36) and showed moderately significant p-values for 11 different SNPs in FGF9 (solid circles), most of which were located in the 3'UTR (some as far as 500 Kb away from the coding region of FGF9). Five additional SNPs in FGF18 also yielded strong evidence of GxG interaction with SNPs in the intergenic region 3' of the FGF9 coding region (open symbols).
DISCUSSION
In our analysis of 111 markers in 10 FGF/FGFR genes using 297 case−parent trios collected from an international study, SNPs in seven of these genes gave some evidence of linkage and association with unobserved causal variants for iCL±P. Genes in the FGF/FGFR pathway are considered good candidates for iCL±P because they play important roles in craniofacial development (Kurose et al., 2004; Rice et al., 2004; Jugessur et al., 2009) and several of them (FGFR1, FGFR2, and FGF10) control Mendelian malformation syndromes which can include oral clefts as a hallmark feature (Slaney et al., 1996; Dodéet al., 2003; Entesarian et al., 2005). Genes can contribute to the etiology of non−syndromic forms of oral clefts as well as Mendelian malformation syndromes, e.g. interferon regulatory factor 6 (Kondo et al., 2002; Zucchero et al., 2004).
Fine mapping linkage scans in the 8p11−23 chromosomal region (Riley et al., 2007c), association and sequencing studies (Riley et al., 2007a; 2007b) offered further support for FGFR1 as a good candidate for iCL±P. Our analysis of markers in FGFR1 gene confirmed these previous reports. However, neither results from our or Riley's studies would retain statistical significance if strict Bonferroni correction for multiple testing were used. Although there is no previous evidence that FGF19 influences risk to iCL±P, our results combined with evidence from animal models (Kurose et al., 2004) suggest further investigation of this gene may be warranted.
Mutations in non−coding regions of FGF2 and FGF10 can result in impaired transcription (Riley et al., 2007b). Our analysis showed intriguing evidence for maternal genotypic effects controlling the offspring's risk of iCL±P for markers in these two genes among Asians, though fetal effects identified in two previous studies for SNPs in FGF10 (Riley et al., 2007b; Menezes et al., 2008) were not confirmed here. Maternal genes control the in utero environment, so potential maternal genotype effects of markers in FGF2 and FGF10 genes seen among our Asian trios may be important (Boyles et al., 2009).
Sequence analysis identified several rare mutations in coding regions of FGFR2, which may be causal (Riley et al., 2007a; 2007b). In our study, SNPs in FGFR2 showed suggestive evidence of GxE interaction with maternal smoking and significant evidence of interaction with vitamin supplementation (even after Bonferroni correction). Evidence from association studies with markers inFGFR2 across studies has been inconsistent (Riley et al., 2007a; Menezes et al., 2008), but our suggestion of GxE interaction deserves further investigation given the potential for public health intervention with modifiable environmental risk factors.
Potentially important mutations in non-coding regions of FGF9 were identified in a separate sequencing study (Riley et al., 2007a; 2007b). Our analysis of 297 trios showed intriguing evidence for possible GxG interaction between FGF18 and FGF9 that may be important for iCL±P. Using independent trios from the International Cleft Consortium described by Beaty et al. (2010), we confirmed the significance of GxG interaction between markers in FGF9 and FGF18 identified in these 297 trios, although the strongest evidence was seen for markers distal to the 3' end of FGF9 (well away from coding regions). The combination of statistical evidence from these 297 trios and from the 1434 independent trios from the GWAS makes it more likely these findings are biologically meaningful. Very few GWAS signals are actually found in coding sequences (Hindorff et al., 2009) and the strongest association signal for iCL±P in Europeans lies in an apparent “gene desert” on chromosome 8q24 (Birnbaum et al., 2009; Grant et al., 2009, Beaty et al., 2010). Similar regulators may underlie these associations, and one can imagine a shared enhancer sequence could explain the suggested interaction between these two FGF genes.
Our association results confirmed some previous findings from published linkage, association and sequencing analysis for various FGF/FGFR genes, and provided new clues about how these different genes may act through potential maternal genotypic effects, GxE and GxG interactions to control risk of iCL±P. Although some of the statistical evidence presented here did not retain significance after strict Bonferroni correction, mechanisms of GxG interaction in particular require further investigation.
ACKNOWLEDGEMENTS
We thank all participants who donated samples for this multi−center study of oral clefts, as well as the staff at each participating site and institution.
This research was supported by R21−DE−013707 and R01−DE−014581 from the National Institute of Dental & Craniofacial Research, and D43-TW006176 from Fogarty Institution.
Footnotes
The authors declare that they have no conflict of interest.
Contributor Information
Hong Wang, Department of Epidemiology and Biostatistics, School of Public Health, Peking University, Beijing, China.
Tianxiao Zhang, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Tao Wu, Department of Epidemiology and Biostatistics, School of Public Health, Peking University, Beijing, China.
Jacqueline B. Hetmanski, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Ingo Ruczinski, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Holger Schwender, Faculty of Statistics, TU Dortmund University, Dortmund, Germany.
Kung Yee Liang, Department of Life Sciences and Institute of Genome sciences, National Yang-Ming University, Taipei, Taiwan.
Tanda Murray, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
M. Daniele Fallin, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Richard J. Redett, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Gerald V. Raymond, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Sheng-Chih Jin, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Yah-Huei Wu Chou, Chang Gung Memorial Hospital, Taipei, Taiwan.
Philip Kuo-Ting Chen, Chang Gung Memorial Hospital, Taipei, Taiwan.
Vincent Yeow, Department of Plastic Surgery, K K Womens and Childrens Hospital, Singapore.
Samuel S. Chong, National University of Singapore, Singapore.
Felicia S. H. Cheah, National University of Singapore, Singapore.
Sun Ha Jee, Institute for Health Promotion, Yonsei University, Seoul, Korea.
Ethylin W. Jabs, Mount Sinai School of Medicine, New York City, New York USA.
Alan F. Scott, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Terri H. Beaty, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
REFERENCES
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Beaty TH, Murray JC, Marazita ML, Munger RG, Ruczinski I, Hetmanski JB, Liang KY, Wu T, Murray T, Fallin MD, et al. A genome-wide association study of cleft lip with and without cleft palate identifies risk variants near MAFB and ABCA4. Nat Genet. 2010;42:525–529. doi: 10.1038/ng.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum S, Ludwig KU, Reutter H, Herms S, Steffens M, Rubini M, Baluardo C, Ferrian M, Almeida de Assis N, Alblas MA, et al. Key susceptibility locus for nonsyndrominc cleft lip with or without cleft palate on chromosome 8q24. Nat Genet. 2009;41:473–477. doi: 10.1038/ng.333. [DOI] [PubMed] [Google Scholar]
- Boyles AL, Wilcox AJ, Taylor JA, Shi M, Weinberg CR, Meyer K, Fredriksen A, Ueland PM, Johansen AM, Drevon CA, et al. Oral facial clefts and gene polymorphisms in metabolism of folate/one-carbon and vitamin A: a pathway-wide association study. Genet Epidemiol. 2009;33(3):247–255. doi: 10.1002/gepi.20376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell HJ. Epistasis: what it means, what it doesn't mean, and statistical methods to detect it in humans. Hum Mol Genet. 2002;11:2463–2468. doi: 10.1093/hmg/11.20.2463. [DOI] [PubMed] [Google Scholar]
- Dodé C, Levilliers J, Dupont JM, De Paepe A, Le Dû N, Soussi-Yanicostas N, Coimbra RS, Delmaghani S, Compain-Nouaille S, Baverel F, et al. Loss-of-function mutations in FGFR1 cause autosomal dominant Kallmann syndrome. Nat Genet. 2003;33:463–465. doi: 10.1038/ng1122. [DOI] [PubMed] [Google Scholar]
- Entesarian M, Matsson H, Klar J, Bergendal B, Olson L, Arakaki R, Hayashi Y, Ohuchi H, Falahat B, Bolstad AI, et al. Mutations in the gene encoding fibroblast growth factor 10 are associated with aplasia of lacrimal and salivary glands. Nat Genet. 2005;37:125–127. doi: 10.1038/ng1507. [DOI] [PubMed] [Google Scholar]
- Grant SF, Wang K, Zhang H, Glaberson W, Annaiah K, Kim CE, Bradfield JP, Glessner JT, Thomas KA, Garris M, et al. A genome-wide association study identifies a locus for nonsyndromic cleft lip with or without cleft palate on 8q24. J. Pediatr. 2009;155(6):909–913. doi: 10.1016/j.jpeds.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Grosen D, Chevrier C, Skytthe A, Bille C, Mølsted K, Sivertsen A, Murray JC, Christensen K. A cohort study of recurrence patterns among more than 54,000 relatives of oral cleft cases in Denmark: support for the multifactorial threshold model of inheritance. J. Med Genet. 2010;47:162–168. doi: 10.1136/jmg.2009.069385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindorff LA, Sethupathy P, Junkins HA, Ramos EA, Mehta JP, Collins FS, Manolio TA. Potential etiologic and functional implications of genome wide association loci for human diseases and traits. Proc Natl Acad Sci USA. 2009;106:9362–9367. doi: 10.1073/pnas.0903103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jugessur A, Murray JC. Orofacial clefting: recent insights into a complex trait. Curr Opinion Genet Dev. 2005;15:270–278. doi: 10.1016/j.gde.2005.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jugessur A, Farlie PG, Kilpatrick N. The genetics of isolated orofacial clefts: from genotypes to subphenotypes. Oral Dis. 2009;15(7):437–453. doi: 10.1111/j.1601-0825.2009.01577.x. [DOI] [PubMed] [Google Scholar]
- Kondo S, Schutte BC, Richardson RJ, Bjork BC, Knight AS, Watanabe Y, Howard E, de Lima RL, Daack-Hirsch S, Sander A, et al. Mutations in IRF6 cause Van der Woude and popliteal pterygium syndromes. Nat Genet. 2002;32:285–289. doi: 10.1038/ng985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurose H, Bito T, Adachi T, Shimizu M, Noji S, Ohuchi H. Expression of Fibroblast growth factor 19 (Fgf19) during chicken embryogenesis and eye development, compared with Fgf15 expression in the mouse. Gene Expression Patterns. 2004;4:687–693. doi: 10.1016/j.modgep.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Laird NM, Lange C. Family-based designs in the age of large-scale gene-association studies. Nat Rev Genet. 2006;7:385–394. doi: 10.1038/nrg1839. [DOI] [PubMed] [Google Scholar]
- Marazita ML, Murray JC, Lidral AC, Arcos-Burgos M, Cooper ME, Goldstein T, Maher BS, Daack-Hirsch S, Schultz R, Mansilla MA, et al. Meta-analysis of 13 genome scans reveals multiple cleft lip/palate genes with novel loci on 9q21 and 2q32-35. Am J Hum Genet. 2004;75:161–173. doi: 10.1086/422475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazita ML, Lidral AC, Murray JC, Field LL, Maher BS, Goldstein McHenry T, Cooper ME, Govil M, Daack-Hirsch S, Riley B, et al. Genome scan, fine-mapping, and candidate gene analysis of non-syndromic cleft lip with or without cleft palate reveals phenotype-specific differences in linkage and association results. Hum Hered. 2009;68(3):151–170. doi: 10.1159/000224636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes R, Letra A, Ruff J, Granjeiro JM, Vieira AR. Studies of genes in the FGF signaling pathway and oral clefts with or without dental anomalies. Am J Med Genet A. 2008;146A:1614–1617. doi: 10.1002/ajmg.a.32341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossey PA, Little J. Epidemiology of oral clefts: an international perspective. In: Wyszynski DF, editor. Cleft Lip and Palate. Oxford University Press; New York: 2002. pp. 127–158. [Google Scholar]
- Nie X, Luukko K, Kettunen P. FGF signalling in craniofacial development and developmental disorders. Oral Dis. 2006;12:102–111. doi: 10.1111/j.1601-0825.2005.01176.x. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, et al. PLINK: a toolset for whole-genome association and population-based linkage analysis. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice R, Spencer-Dene B, Connor EC, Gritli-Linde A, McMahon AP, Dickson C, Thesleff I, Rice DP. Disruption of Fgf10/Fgfr2b-coordinated epithelial-mesenchymal interactions causes cleft palate. J Clin Invest. 2004;113:1692–1700. doi: 10.1172/JCI20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley BM, Mansilla MA, Ma J, Daack-Hirsch S, Maher BS, Raffensperger LM, Russo ET, Vieira AR, Dodé C, Mohammadi M, et al. Impaired FGF signaling contributes to cleft lip and palate. Proc Natl Acad Sci. 2007a;104:4512–4517. doi: 10.1073/pnas.0607956104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley BM, Murray JC. Sequence Evaluation of FGF and FGFR Gene Conserved Non-Coding Elements in Non-Syndromic Cleft Lip and Palate Cases. Am J Med Genet A. 2007b;143A:3228–3234. doi: 10.1002/ajmg.a.31965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley BM, Schultz RE, Cooper ME, Goldstein-McHenry T, Daack-Hirsch S, Lee KT, Dragan E, Vieira AR, Lidral AC, Marazita ML, et al. A genome-wide linkage scan for cleft lip and cleft palate identifies a novel locus on 8p11-23. Am J Med Genet A. 2007c;143A:846–852. doi: 10.1002/ajmg.a.31673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw GM, Wasserman CR, Murray JC, Lammer EJ. Infant TGF-alpha genotype, orofacial clefts, and maternal periconceptional multivitamin use. Cleft Palate Craniofac J. 1998;35:366–370. doi: 10.1597/1545-1569_1998_035_0366_itagoc_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Shi M, Christensen K, Weinberg CR, Romitti P, Bathum L, Lozada A, Morris RW, Lovett M, Murray JC. Orofacial cleft risk is increased with maternal smoking and specific detoxification gene variants. Am J Hum Genet. 2007a;80:76–90. doi: 10.1086/510518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Umbach DM, Weinberg CR. Identification of risk-related haplotypes with the use of multiple SNPs from nuclear families. Am J Hum Genet. 2007b;81:53–66. doi: 10.1086/518670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Umbach DM, Weinberg CR. Using case-parent triads to estimate relative risks associated with a candidate haplotype. Ann Hum Genet. 2009;73(3):346–359. doi: 10.1111/j.1469-1809.2009.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaney SF, Oldridge M, Hurst JA, Moriss-Kay GM, Hall CM, Poole MD, Wilkie AO. Differential effects of FGFR2 mutations on syndactyly and cleft palate in Apert syndrome. Am J Hum Genet. 1996;58:923–932. [PMC free article] [PubMed] [Google Scholar]
- Sull JW, Liang KY, Hetmanski JB, Wu T, Fallin MD, Ingersoll RG, Park JW, Wu-Chou YH, Chen PK, Chong SS, et al. Evidence that TGFA influences risk to cleft lip with/without cleft palate through unconventional genetic mechanisms. Hum Genet. 2009;126:385–394. doi: 10.1007/s00439-009-0680-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg CR, Wilcox AJ, Lie RT. A log-linear approach to case-parent-triad data: assessing effects of disease genes that act either directly or through maternal effects and that may be subject to parental imprinting. Am J Hum Genet. 1998;62:969–978. doi: 10.1086/301802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger JS, Beaty TH, Liang KY. Oral clefts, maternal smoking, and TGFA: a meta-analysis of gene-environment interaction. Cleft Palate Craniofac J. 2005;42(1):58–63. doi: 10.1597/02-128.1. [DOI] [PubMed] [Google Scholar]
- Zucchero TM, Cooper ME, Maher BS, Daack-Hirsch S, Nepomuceno B, Ribeiro L, Caprau D, Christensen K, Suzuki Y, Machida J, et al. Interferon regulatory factor 6 (IRF6) gene variants and the risk of isolated cleft lip or palate. N Engl J Med. 2004;351:769–780. doi: 10.1056/NEJMoa032909. [DOI] [PubMed] [Google Scholar]