Abstract
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin growth factor family and is implicated as a modulator of neuronal survival and differentiation, synaptic plasticity, and higher order cognitive functions such as learning and memory. A common single-nucleotide polymorphism (SNP) has been identified in the human BDNF gene (BDNF Val66Met) that leads to decreased BDNF secretion and impairments in specific forms of learning in humans. To better understand the impact of this SNP on biological function, the authors generated a mouse model containing the BDNF Met allele, which they found to replicate the key phenotypes observed in humans and provided further insight into the functional impact of this SNP in vivo. They used a “bottom-up” approach to study the BDNF SNP, which provided external validation in biologically less complex, genetically uniform systems, which minimized the variability inherent in human studies. In this review, the authors discuss the impact of the BDNF SNP on learning and memory while providing arguments for the relevance of a vertically integrated approach to studying human genetic variants.
Keywords: brain-derived neurotrophic factor Val66Met, single-nucleotide polymorphism, learning and memory, animal model, synaptic plasticity
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin growth factor family, which regulates neuronal differentiation, survival, and synaptic plasticity (Poo 2001). It is expressed throughout the developing and mature brain with highest levels in the prefrontal cortex (PFC) and hippocampus (Pezawas and others 2004). BDNF plays a critical role in the development and plasticity of the central nervous system (Huang and Reichardt 2001; Chao 2003) and has been implicated in the biology of psychiatric disorders as well as learning and memory. A common single-nucleotide polymorphism (SNP) has been identified in the human BDNF gene, which results in a single-nucleotide change from G to A at nucleotide position 196 in the protein coding sequence of the gene, as well as subsequent change in amino acid from valine to methionine at position 66 (e.g., Val66Met) in the prodomain of the BDNF protein (BDNF Met). It has been suggested that this SNP leads to decreased availability of BDNF in the brain due to decreased secretion of the variant form of BDNF (Egan and others 2003). The BDNF Val66Met polymorphism has been the focus of a large number of genetic association studies of cognitive function and a host of neuropsychiatric disorders. As is the case for many candidate gene studies of behavior, the ability of independent groups to replicate initial findings has led to inconsistent results. In this review, we propose that the variability in associations derives from the complexity of human behavioral phenotypes and the difficulty in reliably assessing human behavior for genetic studies. We discuss studies on the impact of the BDNF Val66Met SNP on human cognition and, more specifically, on learning and memory. In such studies, findings related to BDNF Val66Met have been generally consistent, likely because cognitive function, unlike psychiatric disorders, relates to basic neural processes that can be reliably and quantitatively assessed. However, some controversies in the literature regarding BDNF Val66Met still remain (Groves 2007). We then discuss a novel approach to understanding the biological effects of the BDNF Val66Met SNP at the molecular, cellular, and behavioral levels through the use of a genetic knock-in mouse model that recapitulates the Val66Met polymorphism and allows for controlled experiments of its phenotypic effects. We then translate these findings to further characterize the BDNF Val66Met polymorphism in a human genetic study with biologically constrained a priori hypotheses rooted in our observations of our knock-in mouse model. We refer to this approach to elucidating the behavioral effects of a human genetic variant as a “bottom-up” approach.
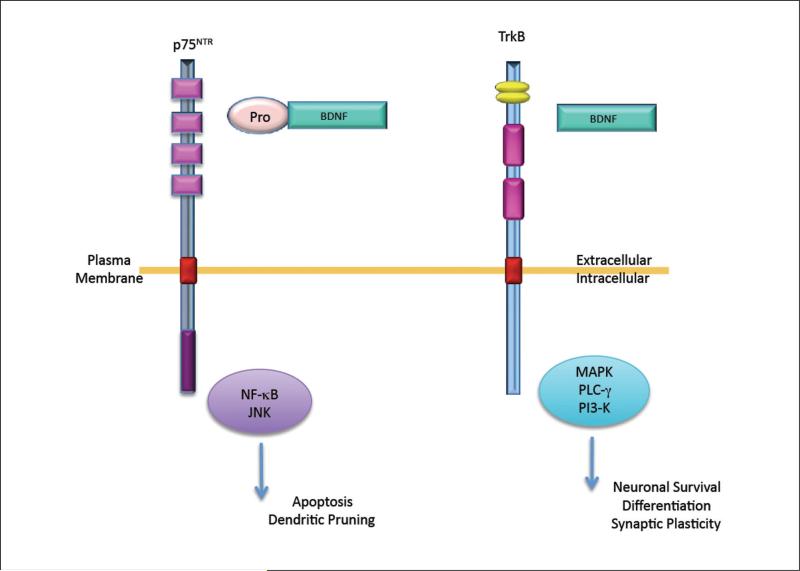
BDNF is initially synthesized in neurons as a precursor protein, pro-BDNF, which is subsequently cleaved to produce mature BDNF (Greenberg and others 2009). The pro and mature forms of BDNF activate distinct signaling pathways, leading to different functional outcomes (Fig. 1). Pro-BDNF preferentially binds to the p75 neurotrophin receptor (Lee and others 2001; Teng and others 2005), which is a member of the tumor necrosis factor receptor super family (Chao 2003). Activation of the p75 receptor elicits apoptosis and pruning of dendritic spines through nuclear factor κB (NF-κB) and c-Jun N-terminal kinase (JNK) activation (Roux and Barker 2002). Mature BDNF binds specifically to the tropomyosin-related kinase B (TrkB) receptor, activating the downstream signaling mediators, including mitogen-activated protein kinase (MAPK), phospholipase C gamma (PLC-γ), and phosphatidylinositol-3-kinase (PI3-K), ultimately leading to neuronal survival, differentiation, and synaptic plasticity (Cowley and others 1994; Mazzucchelli and others 2002; Rosenblum and others 2002; Chao and others 2006; Minichiello 2009).
Figure 1.
Schematic representation of pro and mature brain-derived neurotrophic factor (BDNF) signaling pathways. Pro-BDNF binds preferentially to p75 neurotrophin receptor, activating nuclear factor κB (NF-κB) and c-Jun N-terminal kinase (JNK), leading to apoptosis and dendritic pruning. Mature BDNF binds specifically to tropomyosin-related kinase B (TrkB) receptor, activating mitogen-activated protein kinase (MAPK), phospholipase C gamma (PLC-γ), and phosphatidylinositol-3-kinase (PI3-K), leading to neuronal survival differentiation and synaptic plasticity.
Recent studies have shed light on molecular interactions that regulate sorting of the BDNF in the biosynthetic pathway. The BDNF polypeptide interacts with the sorting receptor carboxypeptidase E (CPE) through a specific sorting motif (I16E18I105D106) in the mature domain, which has been shown to play a role in sorting BDNF to the regulated secretory pathway. Site-directed mutagenesis of the putative sorting motif at the two acidic residues in BDNF that are suggested to interact with two basic residues in CPE abolishes activity-dependent secretion of BDNF from AtT 20 cells and leads to a small increase in basal BDNF secretion. In addition, there was no activity-dependent secretion of BDNF in cortical neurons of Cpe−/− mice and higher basal BDNF release compared to neurons from wild-type mice. These results suggest that an interaction of the sorting motif with CPE is necessary for BDNF sorting into the regulated secretory pathway, and the absence of such interaction may result in aberrant sorting of BDNF (Lou and others 2005). BDNF polypeptide sorting into the regulated secretory pathway has also been shown to be regulated by the VPS10 domain protein, sortilin, which binds BDNF specifically in the prodomain encompassing amino acids 44 to 102, wherein the Val66Met substitution occurs (Chen and others 2005). In this context, heterologous expression of the BDNF Met peptide in primary cultured neurons leads to three trafficking defects relative to wild-type BDNF (BDNF Val): 1) decreased variant BDNF Met distribution into neuronal dendrites, 2) decreased variant BDNF Met targeting to secretory granules, and 3) subsequent impairment in regulated secretion (Egan and others 2003; Chen and others 2004; Chen and others 2005). In addition, when expressed together in the same cell, BDNF Met alters the trafficking of BDNF Val through the formation of heterodimers that are less efficiently sorted into the regulated secretory pathway (Chen and others 2004). These initial findings are consistent with previous studies indicating that the prodomain of neurotrophins plays an important role in regulating their intracellular trafficking to secretory pathways (Suter and others 1991). Together, these in vitro overexpression studies with BDNF Met point to the presence of a specific trafficking signal in the BDNF prodomain region encompassing the Met substitution that is required for efficient BDNF sorting. In particular, the single substitution of Val to Met at position 66 leads to decreased interaction of BDNF with sortilin and suggests that decreased protein-protein interaction between BDNF and the trafficking machinery is a plausible molecular model for the secretion defect observed with the variant BDNF. This variant BDNF provides an example of how appropriate trafficking of BDNF may have a significant impact on the physiological responses to neurotrophins.
In addition, recently, an RNA binding protein, translin, was also shown to bind BDNF mRNA in this same coding region. All BDNF transcripts contain a constitutively active dendritic targeting signal with which translin interacts and mediates mRNA translocation to dendrites. However, this signal is suppressed in transcripts containing exon 1 or 4, restricting their localization to the cell soma and proximal dendrites. Thus, translin binding facilitates selective dendritic targeting of alternatively spliced BDNF transcripts. Interestingly, the same G to A switch at position 196 of the BDNF gene that leads to the Val66Met substitution also disrupts translin binding to BDNF mRNA and impairs dendritic targeting of transcripts (Chiaruttini and others 2009). This suggests that some of the biological consequences of this SNP may be due to altered sub-cellular BDNF mRNA sorting as well as altered BDNF protein secretion.
BDNF has been shown to be released from neuronal terminals primarily in an activity-dependent manner, and in many brain regions, including the hippocampus, it has been shown to regulate synaptic plasticity (Poo 2001; Lu B 2003a, 2003b). In particular, BDNF has been shown to modulate latephase hippocampal LTP (Lu Y and others 2008; Bramham and Messaoudi 2005). Thus, alteration in BDNF availability and signaling related to the G196A (Val66Met) polymorphism would be predicted to have profound effects on cognitive processes such as learning and memory. This hypothesis has been recently tested in a number of human genetic association studies using batteries of neuropsychological tests to characterize and quantify human cognition. In general, these studies have been consistent in identifying decreased cognitive performance in individuals with the BDNF Met allele.
Impact of BDNF Val66Met Polymorphism on Human Cognitive Processes
Because of the critical role BDNF plays in synaptic plasticity, the first studies of the impact of the BDNF Val66Met polymorphism were focused on hippocampal learning and memory (Egan and others 2003). The Val66Met amino acid switch was associated with impaired episodic memory as measured by delayed recall on the revised version of the Wechsler Memory Scale (WMS-R). There was also an altered hippocampal activation pattern observed on functional magnetic resonance imaging (fMRI) in individuals performing the N-back working memory task, which typically results in suppression of the hippocampus (Egan and others 2003). Furthermore, healthy BDNF Met allele carriers displayed reduced hippocampal activity on fMRI during the memory-related processes of encoding, determining if an image was an “indoor” or “outdoor” scene, and retrieval, determining if a scene was “new” or “old” (Hariri and others 2003). After these initial studies, subsequent groups have attempted to further characterize the effect of the Val66Met polymorphism on human cognition with varying degrees of success (Table 1). For brevity, not all cognition studies are included in the main text, but a summary of additional studies can be found in Table 3. The BDNF Met allele was once again associated with a lower score on the WMS-R, which measures episodic memory, but the effect did not reach statistical significance (Dempster and others 2005). Structural MRI studies in healthy individuals supported previous findings showing that BDNF Met carriers displayed smaller hippocampal volumes compared to Val/Val homozygotes (Pezawas and others 2004; Szeszko and others 2005). In addition, there was volume reduction of the gray matter in the dorsolateral prefrontal cortex (PFC), which along with the hippocampus is implicated in learning in memory (Pezawas and others 2004). Supporting this structural alteration in a population of bipolar patients, BDNF Met carriers performed significantly worse than their Val/Val counterparts on the Wisconsin Card Sorting Test (WCST), which measures PFC function (Rybakowski 2003). BDNF Val/Val schizophrenia patients had a higher percentage of correct reactions in the N-back test, which scores visual working memory, than BDNF Met allele carrier patients (Rybakowski, Borkowska, Skibinska, Szczepankiewicz, and others 2006). BDNF Val/Val homozygote bipolar patients scored significantly better on three of five domains of the WCST. There was no association found between BDNF genotype and performance on the N-back test in this patient group (Rybakowski, Borkowska, Skibinska, Szczepankiewicz, and others 2006; Rybakowski, Borkowska, Skibinska, and Hauser 2006). In a Japanese population, an fMRI study of hippocampal activity during an episodic memory task showed a decrease in encoding-related brain activity in the bilateral hippocampi and right parahippocampal gyrus in BDNF Met carriers. There was no genotype effect observed on brain activity during retrieval or on percent correct answers on the memory task itself (Hashimoto and others 2008). Thus, the study only partially replicated in an Asian population the results observed in primarily white population samples using the WMS-R episodic memory task (Egan and others 2003; Hariri and others 2003). This result might suggest that ethnic stratification of the BDNF Val66Met polymorphism may influence behavioral phenotype.
Table 1.
Summary of Human Studies Showing Association of Met Allele and Memory
| Author | Brain Area | Task | Met Carriers Compared to VV | Memory/Cognitive Function | Total Number of Subjects |
|---|---|---|---|---|---|
| Egan and others (2003) | Hippocampus/medial temporal lobe | WMS-R; N-back; fMRI | Lower score; abnormal activation pattern | Episodic memory | 641 |
| Hariri and others (2003) | Hippocampus | Scene recognition fMRI | Reduced activity | Episodic/declarative memory | 64 |
| Pezawas and others (2004) | Hippocampus | Structural MRI | Smaller volume | — | 214 |
| Szeszko and others (2005) | Hippocampus | Structural MRI | Smaller volume | — | 19 schizophrenic 125 healthy volunteers |
| Pezawas and others (2004) | Gray matter in the dorsolateral prefrontal cortex | Structural MRI | Smaller volume | — | 214 |
| Rybakowski and others (2003) | Prefrontal cortex | WCST | Lower score | Prefrontal cognition | 54 |
| Rybakowski, Borkowska, Skibinska, Szczepankiewicz, and others (2006) | Prefrontal cortex | N-back | Lower score schizophrenic patients | Visual working memory | 111 bipolar 129 schizophrenic |
| Rybakowski, Borkowska, Skibinska, and Hauser (2006) | Prefrontal lobe | WCST | Lower score bipolar patients | Working memory | 111 bipolar 129 schizophrenic |
| Miyajima and others (2008) | Hippocampus | Delayed recall; alphabet-coding task | Reduced performance | Memory/cognitive ability | 722 |
| Goldberg and others (2008) | Medial temporal lobe | Depth of encoding and delay of recall | Lower % correct hits | Verbal recognition memory | 48 |
| Schofield and others (2009) | Fronto-hippocampal systems | IntegNeuro | Higher number of errors | Verbal learning and memory | 475 |
| Hippocampal gray matter | |||||
| Hippocampus | |||||
| Dorsolateral PFC | Structural MRI | Smaller volume | |||
| fMRI oddball task | Increased activity | ||||
| fMRI oddball task | Decreased activity | ||||
| Hashimoto and others (2008) | Bilateral hippocampi and right parahippocampal gyrus | WMS-R; fMRI | Decreased activation | Encoding of episodic memory | 58 |
| Beste and others (2011) | — | Partial report | Decreased time of stability | Visual-sensory (iconic) memory | 211 |
| Ho and others (2006) | Medial temporal lobe | Rey Auditory Verbal Learning Test; WMS-R; Rey-Osterrieth Complex Figure Test; WAIS-R; Benton Judgment of Line Orientation | Decreased performance | Verbal memory; visuospatial abilities | 114 healthy volunteers 293 schizophrenic |
WCST = Wisconsin Card Sorting Test; WMS-R = Wechsler Memory Scale–Revised; WAIS-R = Wechsler Adult Intelligence Scale–Revised; PFC = prefrontal cortex; VV = Val/Val; — = not applicable.
Table 3.
Additional Cognition Studies
| Author | Task | Findings for Met Carriers Compared to VV | Memory/Cognitive Function | Total Number of Subjects |
|---|---|---|---|---|
| Tsai (2004) | Wechsler Adult Intelligence Scale–Revised | Lower score | Performance IQ | 114 |
| Gong (2009) | Recall of three-digit numbers | Decrease | Digital working memory | 711 |
| Localization, mental rotation, touching blocks, Gestalt picture | Decrease | Spatial localization ability | ||
| Foltynie (2005) | Tower of London | Met allele associated with better performance | Planning ability | 291 |
| Guerini (2009) | Mini-Mental State Examination | Cognitive impairment | Cognition | 527 |
VV = Val/Val.
In a community of elderly white individuals, BDNF Val/Val homozygotes performed significantly better compared to both Val/Met heterozygous individuals and Met/Met homozygous individuals on a delayed recall task and an alphabet-coding task (ACT), a measure of processing speed. The authors also noted a decrease in hippocampal volume in Met allele carries, although it did not reach significance. However, taken together with the results on the cognitive tasks, this study supports the hypothesis of altered hippocampal function in individuals with the BDNF Met allele (Miyajima and others 2008). In addition, BDNF Met carriers performed worse than the control subjects on a task for delay of recall, and performance worsened when the time for recall was increased. Words deeply encoded by a decision such as if they were living or nonliving were recognized more accurately than words encoded at a shallow level, such as if they contained the letter A (Goldberg and others 2008).
BDNF Met/Met homozygous healthy individuals had higher verbal recall errors than both BDNF Val/Val and BDNF Val/Met counterparts on the “IntegNeuro” neuro-psychological battery of tests assessing cognitive performance, including learning and memory. Structural MRI studies showed a decrease in gray matter volume of the hippocampus in BDNF Met allele carriers compared to BDNF Val/Val individuals. fMRI showed reduction of dorsolateral PFC activation in Met allele carriers performing the auditory oddball task, which elicits hippocampal and frontoparietal activation. Interestingly, there was a significant increase in hippocampal activity in BDNF Met carriers compared to BDNF Val/Val homozygotes during the same auditory oddball task. This finding was not in direct accordance with previously published reports of decreased hippocampal activity in BDNF Met allele carries, but the authors argue that BDNF Met carriers might require greater hippocampal activation than their BDNF Val/Val counterparts to process stimuli presented during the task, inadvertently depleting resources for prefrontal processing of the stimuli, and that similar circuit dysregulation has been observed previously (Schofield and others 2009).
Visual, sensory, and spatial tasks, which are cortical dependent, are also affected by the BDNF Val66Met polymorphism. A healthy German population was studied for the effects of the BDNF Val66Met polymorphism on visual sensory memory performance, referred to as iconic memory on a partial report task, involving the identification of previously presented letters. BDNF Met allele carries displayed a decrease in stability of information stored in iconic memory compared to BDNF Val/Val homozygotes, suggesting that BDNF Val/Val individuals retain the information in iconic memory for a longer period than BDNF Met allele carriers (Beste and others 2011). Schizophrenia-spectrum disorder patients but not healthy controls carrying the BDNF Met allele displayed visuospatial defects. This effect was underscored by smaller cortical gray matter volume in BDNF Met carriers. There was a verbal memory impairment associated with the BDNF Met allele in both schizophrenia patients and healthy controls, as well as a decreased volume of the temporal and occipital lobar gray matter (Ho and others 2006).
The Val66Met polymorphism has also been suggested to affect the sensorimotor cortex activity as well as motor learning (Table 2). A short period of motor training improved corticostriatal output and increased motor map area in BDNF Val/Val individuals but not in individuals with at least one BDNF Met allele, as measured by transcranial magnetic stimulation to the cortical area corresponding to the first dorsal interosseous muscle of the right hand (Kleim and others 2006). Interestingly, this deficiency in short-term cortical plasticity can be overcome by intensive motor training (McHughen and others 2011). Using fMRI regional activation of the sensorimotor network showed a greater reduction of the volume of activation in BDNF Met carriers after 25 minutes of right index finger training, whereas controls displayed an expansion in activation volume. In addition, BDNF Met allele carriers committed more errors during short-term learning and had poorer retention compared to controls on a driving-based motor learning task, which involved a steering wheel connected to a monitor simulating the road to be followed (McHughen and others 2010).
Table 2.
Val66Met Polymorphism on Motor Learning
| Author | Brain Area | Task | Findings for Met Carriers Compared to VV | Total Number of Subjects |
|---|---|---|---|---|
| Kleim and others (2006) | Motor cortex | Transcranial magnetic stimulation; finger-tapping rate, nine-hole pegboard, pinch-grip strength | No motor training improvement in corticostriatal output; no increase in motor map area | 78 |
| McHughen and others (2010) | Sensorimotor network | fMRI; index finger training; driving-based motor learning | Greater reduction of activation after index finger training; less short-term learning and poorer retention on driving-based learning | 25 experiment 129 experiment 2 |
| McHughen and others (2011) | Motor cortex | Transcranial magnetic stimulation; marble navigation task | No cortical motor map plasticity; training overcomes deficiency | 24 |
| Fritsch (2010) | — | Sequential visual isometric pinch force for humans; mouse rotarod | Reduced motor skill acquisition Mice showed a decreased latency to fall off | 36 |
VV = Val/Val.
To date, a number of studies have suggested that the BDNF Val66Met polymorphism leads to impairments in hippocampal and cortical function, especially that related to learning and memory processes. However, not all studies come to those same conclusions. In young healthy individuals, working memory performance of BDNF Met carriers was decreased compared to BDNF Val/Val homozygotes, but there was no association observed between BDNF genotype and cognitive performance or hippocampal volume (Richter-Schmidinger and others 2011). A study of young adults with a history of childhood-onset mood disorder did not find an association of Val66Met polymorphism and declarative memory function on sub-tests of the WMS (Strauss and others 2004). The BDNF Met allele was found not to be associated with alterations in hippocampal structure and performance on four memory tasks in healthy individuals (Karnik and others 2010), and BDNF Met carriers exhibited increased medial temporal lobe activation in memory tests during encoding and retrieval compared to controls (Dennis and others 2010). The discrepancies between findings could be due to a number of variables. These include differences in study design, including smaller sample size, differences in subject age at the time of testing, differential ethnic representations in the sample, and differences in cognitive task administration. However, the general consensus from the numerous studies has been that in healthy white populations, when challenged with various cognitive or motor learning behavioral tasks, humans with one or more copies of the BDNF Met allele have altered performance suggestive of a decrease in plasticity.
Impact of BDNF Val66Met Polymorphism on Neuropsychiatric Disease
Because many psychiatric disorders such as depression and anxiety disorders involve a dysregulation in emotional responses and because emotional behaviors involve adaptive responses that require synaptic plasticity, it has also been hypothesized that the BDNF Met allele could present a genetic risk factor for these disorders. Human genetic studies testing for associations between the BDNF SNP and a range of mood and anxiety disorders have yielded tantalizing evidence that the BDNF Val66Met polymorphism contributes to risk for these disorders. However, in comparison to the studies of BDNF Val66Met and cognition, these associations have been less consistently replicated, especially when compared to those that assessed cognition in healthy individuals.
The lack of consistency in genetic association studies of the BDNF Val66Met polymorphism limits their ability to guide understanding of emotional variability and/or the diagnosis and treatment of mood and anxiety disorders. Although there are a number of reasons that these studies have yielded such variable results, much of the difficulty likely lies in the complexity of human behavior and the difficulty of defining behavior precisely for genetic studies. Genetic variants are expressed phenotypically when they alter the function of a specific biological process. Unfortunately, little is known about the biological basis of human emotional behavior, and thus most genetic studies rely on assessments of behavioral phenomena such as mood, feelings, appetite, and activity levels. These phenomena are most commonly assessed through self-report and therefore inevitably involve subjective recollections of complex feelings and events that are imprecisely recalled and understood differently by individuals. Moreover, personality traits and emotional disorders often result from a complex interaction between the genetic and biological background of an individual and his or her unique experiences. In such a complex context, it can be very difficult to precisely and reliably identify the effects of any particular genetic variant.
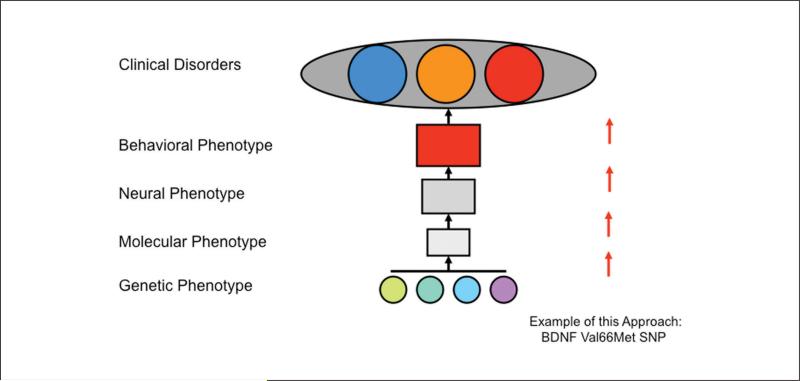
To circumvent the complexities of human genetic association studies of behavioral disorders, we propose a “bottom-up” approach to identifying the behavioral effects of the BDNF Val66Met polymorphism. This bottom-up approach may provide instructive guidance for behavioral studies of other polymorphisms (Figure 2). This approach is focused on the effects of polymorphisms such as BDNF Val66Met at low levels of biological complexity such as the molecular and cellular levels and then relating those biological differences stepwise to ever more complex behavioral processes that are known to relate to the biology of the basic processes in which the polymorphism is implicated. The major benefit of this approach is that it allows for controlled scientific studies in which all variables other than the polymorphism are kept constant.
Figure 2.
The “bottom-up” approach to behavioral genetics begins in biologically less complex, genetically uniform systems, minimizing variability and providing external validation to gene association studies in the human population.
The bottom-up approach as we have applied it to the BDNF Val66Met polymorphism involved the initial identification of a sortilin-binding deficit of the BDNF Met allele, leading to a trafficking and secretion deficit of the BDNF peptide using molecular and cellular expression systems. To further the bottom-up approach, we used genetic knock-in technology to introduce the BDNF Met allele into the genome of inbred mice. This advance allows us to study the impact of the Val66Met allele on brain structure and function in inbred animals that are littermates and are therefore genetic clones except for the BDNF polymorphic locus and have shared environmental experiences from the time their in utero development began. In such a setting, any differences identified between wild-type (Val allele) mice and variant Met allele mice can reliably be ascribed to the effects of the polymorphism. Moreover, use of mice allows precise manipulations and invasive analyses to be performed.
BDNF Val66Met Knock-in Mouse Model
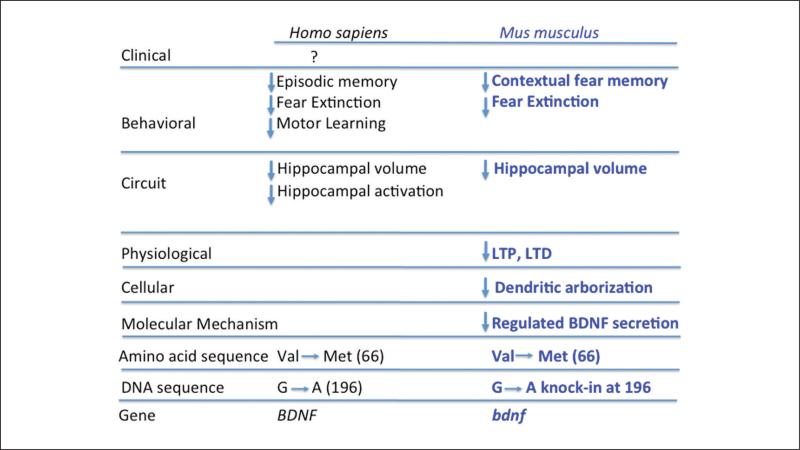
The human ancestral BDNF Val allele is highly conserved evolutionarily, meaning that the genomes of all mouse strains contain only the BDNF Val form of the BDNF protein. The BDNF Met mouse was generated using genetic knock-in technology that allows targeted manipulation of the mouse genome (Chen and others 2006). In this knock-in strain, we introduced DNA that coded for a methionine residue in position 66 instead of the wild-type valine residue. Because only the single amino acid is altered, the expression of BDNF Met in this mouse model is regulated by the physiologic endogenous BDNF promoter elements. This knock-in mouse has provided an invaluable tool for assessment of the in vivo consequences of the BDNF polymorphism in biochemical, anatomical, and behavioral studies (Chen and others 2008). Initially, we repeated the BDNF secretion experiments described above using cultured neurons from the BDNF Met mice and wild-type littermate controls. These experiments confirmed the results from the BDNF Met overexpression studies. There was a ~30% decrease of activity-dependent BDNF release from homozygous knock-in BDNF Met/Met mice, which represents a large drop in BDNF availability at synapses because in neurons, the majority of BDNF is released through the regulated secretory pathway. Having confirmed the molecular phenotype of the Val66Met polymorphism in the knock-in strain, we next addressed effects of the secretion deficit at the next level of biological complexity: cellular and anatomical alterations related to the BDNF secretion deficit. These studies revealed a significant decrease in hippocampal volume in both BDNF Val/Met heterozygous and BDNF Met/Met homozygous mice to the level of BDNF+/− heterozygous knock-out mice. Furthermore, morphological analyses of Golgi-stained neurons suggested a decrease in dendritic arbor complexity of dentate gyrus neurons in the hippocampus of BDNF Met/Met mice compared to littermate controls at eight weeks of age. These findings provided a potential mechanistic basis to explain reports in human brain imaging studies, which reliably identified a reduction in hippocampal volume in BDNF Met allele carriers. In combination, these findings validated the BDNF Met knock-in mouse as a model, which replicated the core phenotypes observed in human carriers of the Val66Met polymorphism.
Having demonstrated that the BDNF Val66Met knock-in mouse recapitulated molecular phenotypic properties observed in vitro and the anatomical phenotypes observed in humans, we were interested in identifying effects of the BDNF Met allele on behavioral outcomes. To do this, we used the BDNF Val66Met knock-in mice and assessed behavioral parameters related to cognitive and emotional processing found to be altered in human carriers. As an initial focus, we used standard tests of anxiety-like behaviors in mice (the open field and elevated plus maze tests) to investigate the impact of this SNP on behavioral response to being placed in an anxiety-provoking context. In both of these behavioral assays, mice must resolve the conflict of their initial anxiety associated with being placed in a new context with unknown potential danger and their inherent propensity to explore new places. Mice that explore potentially dangerous areas (center of the arena or open arms of the plus maze) more are those with lower levels of anxiety-like behavior. We found that the BDNF Met/Met mice preferred safe environments compared to wild-type BDNF Val/Val mice, suggesting the polymorphism does in fact alter emotional behaviors, providing some support for the positive human association findings related to depression and anxiety disorders (Verhagen and others 2010). Because anxiety in humans is an emotional state and in mice we can only infer an emotional state from observation of behavior, our ability to translate these results across species is limited.
To refine and improve the translational applicability of the anxiety-like phenotype of the BDNF Met mice, and in keeping with the biologically based bottom-up approach, we used a conditioned fear-learning paradigm to assess emotional learning, hippocampal-dependent memory, and the ability of these mice to suppress a learned fear response. Fear learning is a variant of Pavlovian classical conditioning in which a neutral stimulus such as an acoustic tone is paired with an intrinsically aversive stimulus such as an electric shock. After a small number of pairings of the aversive shock (unconditioned stimulus) with the neutral cue (conditioned stimulus), the previously neutral stimulus takes on the aversive properties of the unconditioned stimulus, eliciting a stereotyped autonomic fear response such as dilated pupils or increased activity of sweat glands in humans or freezing behavior in mice. In all cases, the fear response is a reflexive response of the sympathetic nervous system to danger that can be objectively and reliably measured. Once the conditioned stimulus has been associated with the unconditioned stimulus, a process referred to as acquisition, the fear association can be modified through extinction learning, in which the conditioned stimulus is presented alone. Over repeated exposures to the conditioned stimulus alone, the organism learns that it no longer predicts danger, and the fear response is reduced. Extinction is an active adaptive learning process in which the organism learns that environmental conditions have changed and the conditioned stimulus no longer predicts danger. Fear learning is an excellent behavioral target phenotype for the bottom-up approach as applied to BDNF Val66Met for several reasons. First, fear learning is a primitive and highly conserved response to environmental threats, and the neural circuitry involved in forming and modulating fear associations has been shown to be very similar in mice and humans. As a result, the translational validity of fear learning is much greater than anxiety-related pheno-types. Moreover, the ability of humans to extinguish learned fear associations has been shown to be inversely correlated with anxious personality traits and anxiety disorders, ensuring that this basic form of adaptive learning is relevant to more complex and subjective emotional states. Finally, adaptive fear responses are learned behaviors that require synaptic and morphologic plasticity of neurons in the fear-learning circuitry, meaning they should be and in fact have been demonstrated to be dependent on intact BDNF signaling (Frielingsdorf and others 2010; Mahan and Ressler 2011)—an instance of matching the biology of the polymorphism's molecular properties with the known biology of a higher order behavioral process.
We trained wild-type and BDNF Met mice in a standard fear-learning paradigm. To do this, we presented the mice with multiple 30-second tones (conditioned stimulus [CS]) that were immediately followed by the administration of a mild shock (unconditioned stimulus [US]) through an electrified grid as the floor. All mice rapidly acquired the association between the tone (CS) and the shock (US). However, during extinction training, when the CS was repeatedly presented without a shock, the BDNF Met mice failed to extinguish the learned fear response compared with wild-type mice.
Having established that BDNF Met mice have a deficit in extinction learning, we tested human participants in a standard human fear-learning paradigm. Humans were exposed to a colored square as the conditioned stimulus and a loud burst of white noise as the unconditioned stimulus. Fear responses were quantified using the galvanic skin response, a measure of skin conductance related to the production of sweat as part of the sympathetic nervous system's response to threat. Individuals were genotyped for the BDNF Val66Met polymorphism using genomic DNA extracted from saliva. Because of our earlier finding in mice, this study was grounded in the directional a priori hypothesis that the BDNF Val66Met polymorphism would be selectively associated with deficits in extinction learning and that the BDNF Met allele would be associated with impaired (as opposed to improved) extinction learning. We confirmed that hypothesis and identified a statistically significant effect of the BDNF Met allele in reducing the efficiency of extinction learning (Soliman and others 2010).
Future Directions
In this review, we have described a novel approach to understanding the role of genetic variation in interindividual differences in behavior as applied to the BDNF Val66Met polymorphism. Although this approach is labor intensive and not easily applied to all potentially important polymorphisms, such as polymorphisms that do not alter well-conserved amino acids, we suggest that this bottom-up approach is critical to progress in behavioral genetics. The major methodologies for genetic studies that are based in purely statistical genotypephenotype associations have not proved successful. Candidate gene approaches have suffered from an unacceptable level of nonreplication that has led to controversy in the literature about the effects of the most commonly studied polymorphisms. The major alternative approach, the genome-wide association study (GWAS), which is essentially hypothesis free, has proven inadequate to identify polymorphisms of even moderate effect size, and many disease-related GWAS studies have failed to identify any significantly associated polymorphisms when correction for the 500,000 or more genetic tests inherent in the GWAS methodology is taken into account. The lack of success of these traditional association methods is related in large part to the factors we have described in this review: the complexity of human behavior and the imprecision and nonbiologic grounding of behavioral disorders as defined for genetic studies.
In this article, we have described the bottom-up analysis of the BDNF Val66Met polymorphism from the molecular level to the cellular and anatomic levels to the level of basic learning processes in vivo. The next critical step is to refine the definitions of clinical disorders in a manner consistent with the biological properties we have characterized for the BDNF Val66Met polymorphism. One example of this approach would be to characterize individuals with mood or anxiety disorders according to their performance in fear-learning paradigms. Anxious individuals who have problems with extinction learning would be a reasonable subgroup to test for the overrepresentation of the BDNF Met allele relative to controls or anxiety-disordered individuals with intact extinction learning. Another direction to apply the bottom-up approach is in terms of therapeutic response. Although effective behavioral and pharmacologic treatments have been developed for anxiety disorders, there is great heterogeneity in terms of how well individuals respond to a particular treatment. Growing evidence suggests that both the major drug therapies for anxiety disorder, the serotonin-selective reuptake inhibitors (SSRIs) and behavioral treatments based in exposure therapies, act through improving extinction of aversive experiences. One would predict, therefore, that the BDNF Met allele would be associated with poor response to these treatments. We have demonstrated that SSRIs are ineffective in reducing anxiety in BDNF Met mice, predicting that nonresponsive humans may form a biologically meaningful subcategory of anxiety-disordered individuals that may relate to the BDNF Val66Met polymorphism (Chen and others 2006). Alternative treatments such as the cognitive enhancer d-cycloserine, which we have found improves extinction learning in BDNF Met as well as wild-type mice (Yu and others 2009), may be a more effective treatment for anxious individuals with the BDNF Met allele than SSRIs or may serve as a necessary adjunct to extinction-based, exposure-based therapies for anxiety disorders.
As noted above, the bottom-up approach is not easily applied to all categories of functional polymorphisms, but as transgenic techniques for manipulating the genomes of mice advance, it will become feasible to introduce large portions of the human genome that contain selected polymorphisms into mice that would also carry with them all of the related regulatory sequence that would allow accurate recapitulation of the phenotypic effects of these variants in mice. We suggest that although the bottom-up approach requires substantial effort above and beyond the major statistically based genetic methods in behavior, it is one critical response to the limitations that are now clear in those methodologies.
Figure 3.
Comparison of brain-derived neurotrophic factor (BDNF) Val66Met in humans and mice.
Acknowledgments
We thank Kevin Bath, Catia C. Proenca, Minseok Song, and Matthew Malter Cohen for helpful discussions and feedback on the manuscript.
Funding The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the DeWitt-Wallace Fund of the New York Community Trust (F.S.L.), Irma T. Hirschl/Monique Weill-Caulier Trust (I.D., F.S.L.), International Mental Health Research Organization (F.S.L.), Burroughs Wellcome Foundation (F.S.L.), Pritzker Consortium (F.S.L.), NARSAD (F.S.L.), and National Institutes of Health grants NS052819 (F.S.L.) and MH088814 (F.S.L.).
Footnotes
Declaration of Conflicting Interests The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Beste C, Schneider D, Epplen JT, Arning L. The functional BDNF Val66Met polymorphism affects functions of pre-attentive visual sensory memory processes. Neuropharmacology. 2011;60:467–471. doi: 10.1016/j.neuropharm.2010.10.028. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Chao MV, Rajagopal R, Lee FS. Neurotrophin signalling in health and disease. Clin Sci (Lond) 2006;110:167–73. doi: 10.1042/CS20050163. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Bath K, McEwen B, Hempstead B, Lee F. Impact of genetic variant BDNF (Val66Met) on brain structure and function. Novartis Found Symp. 2008;289:180–8. doi: 10.1002/9780470751251.ch14. discussion 188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Ieraci A, Teng H, Dall H, Meng CX, Herrera DG, et al. Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J Neurosci. 2005;25:6156–66. doi: 10.1523/JNEUROSCI.1017-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–3. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, et al. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci. 2004;24:4401–11. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaruttini C, Vicario A, Li Z, Baj G, Braiuca P, Wu Y, et al. Dendritic trafficking of BDNF mRNA is mediated by translin and blocked by the G196A (Val66Met) mutation. Proc Natl Acad Sci U S A. 2009;106:16481–6. doi: 10.1073/pnas.0902833106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–52. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Dempster E, Toulopoulou T, McDonald C, Bramon E, Walshe M, Filbey F, et al. Association between BDNF val66 met genotype and episodic memory. Am J Med Genet B Neuropsychiatr Genet. 2005;134B:73–5. doi: 10.1002/ajmg.b.30150. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Cabeza R, Need AC, Waters-Metenier S, Goldstein DB, Labar KS. Brain-derived neurotrophic factor val-66met polymorphism and hippocampal activation during episodic encoding and retrieval tasks. Hippocampus. 2010 May 20; doi: 10.1002/hipo.20809. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–69. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Foltynie T, Lewis SG, Goldberg TE, Blackwell AD, Kolachana BS, Weinberger DR, et al. The BDNF Val66Met polymorphism has a gender specific influence on planning ability in Parkinson's disease. J Neurol. 2005;252:833–8. doi: 10.1007/s00415-005-0756-5. [DOI] [PubMed] [Google Scholar]
- Frielingsdorf H, Bath KG, Soliman F, Difede J, Casey BJ, Lee FS. Variant brain-derived neurotrophic factor Val-66Met endophenotypes: implications for posttraumatic stress disorder. Ann N Y Acad Sci. 2010;1208:150–7. doi: 10.1111/j.1749-6632.2010.05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 2010;66:198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Iudicello J, Russo C, Elvevag B, Straub R, Egan MF, et al. BDNF Val66Met polymorphism significantly affects d′ in verbal recognition memory at short and long delays. Biol Psychol. 2008;77:20–4. doi: 10.1016/j.biopsycho.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Gong P, Zheng A, Chen D, Ge W, Lv C, Zhang K, et al. Effect of BDNF Val66Met polymorphism on digital working memory and spatial localization in a healthy Chinese Han population. J Mol Neurosci. 2009;38:250–6. doi: 10.1007/s12031-009-9205-8. [DOI] [PubMed] [Google Scholar]
- Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: implications in CNS function. J Neurosci. 2009;29:12764–7. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves JO. Is it time to reassess the BDNF hypothesis of depression? Mol Psychiatry. 2007;12:1079–88. doi: 10.1038/sj.mp.4002075. [DOI] [PubMed] [Google Scholar]
- Guerini FR, Beghi E, Riboldazzi G, Zangaglia R, Pianezzola C, Bono G, et al. BDNF Val66Met polymorphism is associated with cognitive impairment in Italian patients with Parkinson's disease. Eur J Neurol. 2009;16:1240–5. doi: 10.1111/j.1468-1331.2009.02706.x. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23:6690–94. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R, Moriguchi Y, Yamashita F, Mori T, Nemoto K, Okada T, et al. Dose-dependent effect of the Val66Met polymorphism of the brain-derived neurotrophic factor gene on memory-related hippocampal activity. Neurosci Res. 2008;61:360–7. doi: 10.1016/j.neures.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Ho BC, Milev P, O'Leary DS, Librant A, Andreasen NC, Wassink TH. Cognitive and magnetic resonance imaging brain morphometric correlates of brain-derived neurotrophic factor Val66Met gene polymorphism in patients with schizophrenia and healthy volunteers. Arch Gen Psychiatry. 2006;63:731–40. doi: 10.1001/archpsyc.63.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnik MS, Wang L, Barch DM, Morris JC, Csernansky JG. BDNF polymorphism rs6265 and hippocampal structure and memory performance in healthy control subjects. Psychiatry Res. 2010;178:425–9. doi: 10.1016/j.psychres.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Chan S, Pringle E, Schallert K, Procaccio V, Jimenez R, et al. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci. 2006;9:735–7. doi: 10.1038/nn1699. [DOI] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–8. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Lou H, Kim SK, Zaitsev E, Snell CR, Lu B, Loh YP. Sorting and activity-dependent secretion of BDNF require interaction of a specific motif with the sorting receptor carboxypeptidase E. Neuron. 2005;45:245–55. doi: 10.1016/j.neuron.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003a;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B. Pro-region of neurotrophins: role in synaptic modulation. Neuron. 2003b;39:735–8. doi: 10.1016/s0896-6273(03)00538-5. [DOI] [PubMed] [Google Scholar]
- Lu Y, Christian K, Lu B. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem. 2008;89:312–23. doi: 10.1016/j.nlm.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 2011 Jul 26; doi: 10.1016/j.tins.2011.06.007. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzucchelli C, Vantaggiato C, Ciamei A, Fasano S, Pakhotin P, Krezel W, et al. Knockout of ERK1 MAP kinase enhances synaptic plasticity in the striatum and facilitates striatal-mediated learning and memory. Neuron. 2002;34:807–20. doi: 10.1016/s0896-6273(02)00716-x. [DOI] [PubMed] [Google Scholar]
- McHughen SA, Pearson-Fuhrhop K, Ngo VK, Cramer SC. Intense training overcomes effects of the val(66)met BDNF polymorphism on short-term plasticity. Exp Brain Res. 2011;213:415–22. doi: 10.1007/s00221-011-2791-z. [DOI] [PubMed] [Google Scholar]
- McHughen SA, Rodriguez PF, Kleim JA, Kleim ED, Marchal Crespo L, Procaccio V, et al. BDNF val66met polymorphism influences motor system function in the human brain. Cereb Cortex. 2010;20:1254–62. doi: 10.1093/cercor/bhp189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minichiello L. TrkB signalling pathways in LTP and learning. Nat Rev Neurosci. 2009;10:850–60. doi: 10.1038/nrn2738. [DOI] [PubMed] [Google Scholar]
- Miyajima F, Ollier W, Mayes A, Jackson A, Thacker N, Rabbitt P, et al. Brain-derived neurotrophic factor polymorphism Val66Met influences cognitive abilities in the elderly. Genes Brain Behav. 2008;7:411–7. doi: 10.1111/j.1601-183X.2007.00363.x. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, et al. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24:10099–102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Richter-Schmidinger T, Alexopoulos P, Horn M, Maus S, Reichel M, Rhein C, et al. Influence of brain-derived neurotrophic-factor and apolipoprotein E genetic variants on hippocampal volume and memory performance in healthy young adults. J Neural Transm. 2011;118:249–57. doi: 10.1007/s00702-010-0539-8. [DOI] [PubMed] [Google Scholar]
- Rosenblum K, Futter M, Voss K, Erent M, Skehel PA, French P, et al. The role of extracellular regulated kinases I/II in late-phase long-term potentiation. J Neurosci. 2002;22:5432–41. doi: 10.1523/JNEUROSCI.22-13-05432.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol. 2002;67:203–33. doi: 10.1016/s0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Borkowska A, Czerski PM, Skibinska M, Hauser J. Polymorphism of the brain-derived neurotrophic factor gene and performance on a cognitive prefrontal test in bipolar patients. Bipolar Disord. 2003;5:468–72. doi: 10.1046/j.1399-5618.2003.00071.x. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Borkowska A, Skibinska M, Hauser J. Illness-specific association of val66met BDNF polymorphism with performance on Wisconsin Card Sorting Test in bipolar mood disorder. Mol Psychiatry. 2006;11:122–4. doi: 10.1038/sj.mp.4001765. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Borkowska A, Skibinska M, Szczepankiewicz A, Kapelski P, Leszczynska-Rodziewicz A, et al. Prefrontal cognition in schizophrenia and bipolar illness in relation to Val66Met polymorphism of the brain-derived neurotrophic factor gene. Psychiatry Clin Neurosci. 2006;60:70–6. doi: 10.1111/j.1440-1819.2006.01462.x. [DOI] [PubMed] [Google Scholar]
- Schofield PR, Williams LM, Paul RH, Gatt JM, Brown K, Luty A, et al. Disturbances in selective information processing associated with the BDNF Val66Met polymorphism: evidence from cognition, the P300 and fronto-hippocampal systems. Biol Psychol. 2009;80:176–88. doi: 10.1016/j.biopsycho.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–6. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss J, Barr CL, George CJ, Ryan CM, King N, Shaikh S, et al. BDNF and COMT polymorphisms: relation to memory phenotypes in young adults with childhood-onset mood disorder. Neuromol Med. 2004;5:181–92. doi: 10.1385/NMM:5:3:181. [DOI] [PubMed] [Google Scholar]
- Suter U, Heymach J, Shooter E. Two conserved domains in the NGF polypeptide are necessary and sufficient for the biosynthesis of correctly processed and biologically active NGF. EMBO J. 1991;10:2394–400. doi: 10.1002/j.1460-2075.1991.tb07778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeszko PR, Lipsky R, Mentschel C, Robinson D, Gunduz-Bruce H, Sevy S, et al. Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Mol Psychiatry. 2005;10:631–6. doi: 10.1038/sj.mp.4001656. [DOI] [PubMed] [Google Scholar]
- Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25:5455–63. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SJ, Hong CJ, Yu YW, Chen TJ. Association study of a brain-derived neurotrophic factor (BDNF) Val66Met polymorphism and personality trait and intelligence in healthy young females. Neuropsychobiology. 2004;49:13–6. doi: 10.1159/000075333. [DOI] [PubMed] [Google Scholar]
- Verhagen M, van der Meij A, van Deurzen PA, Janzing JG, Arias-Vasquez A, Buitelaar JK, et al. Meta-analysis of the BDNF Val66Met polymorphism in major depressive disorder: effects of gender and ethnicity. Mol Psychiatry. 2010;15:260–71. doi: 10.1038/mp.2008.109. [DOI] [PubMed] [Google Scholar]
- Yu H, Wang Y, Pattwell S, Jing D, Liu T, Zhang Y, et al. Variant BDNF Val66Met polymorphism affects extinction of conditioned aversive memory. J Neurosci. 2009;29:4056–64. doi: 10.1523/JNEUROSCI.5539-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]