Abstract
Alcohol consumption in man, when seen in its extreme form of alcoholism, is a complex and socially disruptive disorder that can result in significant levels of liver injury. Here the rodent “intragastric feeding model” was used together with UHPLC-TOFMS analysis to determine changes in global metabolite profiles for plasma and urine from alcohol treated rats and mice compared to control animals. Multivariate statistical analysis (using principal components analysis, PCA) revealed robust differences between profiles from control and alcohol-treated animals from both species. A large number of metabolites were seen to differ between control and alcohol-treated animals, for both biofluids.
Keywords: Alcohol, Metabolite profiling, Ultra Performance Liquid Chromatography (UPLC), Time of Flight mass spectrometry (TOF-MS), metabonomics, metabolomics
1. Introduction
Alcohol consumption in humans, especially the chronic exposure seen with alcoholism, can result in significant levels of liver injury. In order to gain insights into the biological effects of alcohol disruption much research has been undertaken using animal models, including e.g., the intragastric infusion model [1–4]. Recently the effects of ethanol exposure to rodents on global endogenous metabolite profiles has become an area of increasing interest with the application of metabonomics/metabolomics approaches based on FT-MS [5], LC-MS [6,7] and NMR [8,9] spectroscopy. These studies have highlighted the plethora of changes occurring to the metabolic phenotypes (metabotypes [10]) of organs and biofluids of rats and mice exposed to ethanol. Recently we described the effects of ethanol exposure on the metabolic profiles of liver in rat and mouse following the intragastric feeding of ethanol [7] where profound changes were observed in metabolic profiles, particularly fatty acids, fatty acid ethyl esters, glycerolipids, and phosphatidyl-ethanol homologues. Thus metabolites such as octadecatrienoic acid, eicosapentaenoic acid, ethyl arachidonate, ethyl docosahexaenoic acid, ethyl linoleate, and ethyl oleate as well as phosphatidylethanol (PEth) homologues (including PEth 18:0/18:2 and PEth 16:0/18:2) were up-regulated [7]. Such findings are of considerable interest in developing appropriate animal models for man as e.g., PEth homologues are currently considered as potential biomarkers for harmful, and prolonged, alcohol consumption in humans [11–13]. Whilst the metabolite profiles observed were broadly similar in both rodent species a number of differences were detected including a marked down-regulation of retinol and free cholesterol in the mouse compared to the rat following alcohol administration. Further to our investigation of the effects of intragastric ethanol administration to rodents, we now present in the current manuscript an examination of the metabolic profiles of blood plasma and urine from both rats and mice (the study on mice included animals treated for two different periods). The final goal of the research was to characterise metabolome alterations following alcohol exposure and to gain new insight in the biochemical mechanism of alcohol toxicity in the animal models.
2. Experimental
2.1 Chemicals
All solvents used were of HPLC grade and obtained from Fisher Scientific (Loughborough, Leicestershire, UK). Formic acid of analytical grade was also purchased from Fisher Scientific. Ultra Pure Water (18.2M ) was obtained from a Purelab Ultra system from Elga (Marlow, Bucks, UK). All other chemicals, and metabolites used as standards (including taurine, lysine, arginine, glutamine, 4-hydroxy-L-proline, 2-methylglutamic acid, urinc acid, N-acetylglutamine, N-acetylglycine, nicotinic acid, nicotinamide, 3-hydroxykynurenine, 5-oxoproline (pyroglutamic acid), 5-hydroxy-L-tryptopuhan, leucine, kynurenine, homogentisic acid, 3-hydroxyanthanilic acid, adipic acid, tryptophan, xanthurenic acid, kynurenic acid, 4-hydroxyphenylacetic acid, pimelic acid, 3-phenylactic acid, indole-3-lactic acid, 3- and 4-methylhippuric acids, taurocholic acid, linolenic acid), were obtained from Sigma Aldrich (Poole, Dorset, UK).
2.2 Study design
Male C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and male Wistar rats were purchased from Harlan (Indianapolis, IN, USA). The intragastric ethanol infusion of mice and rats was performed as described previously [1–4,7].
Briefly, at the initial ethanol dose, total caloric intake derived from diet and ethanol for both mice and rats was initially set at 568 cal/kg/day, and the caloric percentages of ethanol, dietary carbohydrate (dextrose), protein (lactalbumin hydrolysate), and fat (corn oil) were 24.3%, 15.7%, 25%, and 35%, respectively. The daily ethanol dose was increased gradually from week 1 to week 4. The highest caloric percentage of ethanol at the end of four weeks accounted for 34.4% of total calories. Adequate vitamin and salt mix were included at the amounts recommended by the Committee on Animal Nutrition of the National Research Council (AIN-76A, 4.42 g/L, and 15.4 g/L, respectively, Dyets Inc, PA). The animals were treated in accordance with the Guide for Care and Use of Laboratory Animals (NIH, Bethesda, MD, Publication 86–23, 1985).
2.3 Samples
Blood plasma and urine samples were collected from two different groups of mice at either two or four weeks after the commencement of ethanol dosing (for each group: n=6, 3 control, 3 ethanol treated at each time point). Plasma and urine samples were also collected from rats (n=6, 3 control and 3 ethanol treated) four weeks after commencing intragastric feeding with ethanol. All samples were stored at −80°C until analysed.
2.4 Sample Preparation
For blood plasma 20 μL aliquots of each sample were protein precipitated by the addition of 60 μl of cold acetonitrile. After centrifugation at 18,000 g for 10 min, 50 μL of the supernatants were diluted with 25 μL water. A plasma quality control (QC) sample was prepared by mixing 10 μL of all plasma samples. From the derived bulk sample an aliquot of 20 μL was prepared for analysis in the same way as the individual samples. Urine samples were firstly centrifuged at 18,000 g for 5 min to remove particulates and then aliquots of 20 μL were diluted with 40 μL of water. Centrifugation of the diluted samples was performed again at 2,400 g for 10 min prior to analysis. A urine QC sample was also prepared by mixing 10 μL from each of the samples and from the final pooled sample an aliquot of 20 μL was subsequently diluted with 40 μL water and centrifuged for analysis.
2.5 Liquid Chromatography
An Acquity UPLC™ chromatographic system (Waters, Millford, MA) was used with an Acquity BEH C18 column (2.1 x 150 mm), 1.7 μm particle size (Waters Corporation, Milford, USA) maintained at 60°C throughout the study. The mobile phase consisted of A: water 0.1% formic acid and B: acetonitrile 0.1% formic acid and was delivered at 300 μl . min−1. For plasma samples the gradient elution program started with a 1 min isocratic step at 5% B, then a 1–3 min linear increase to 55% B followed by a linear increase to 100 % B from 3–9.5 min. A 1.5 min isocratic step at 100% B followed. The gradient applied for urine samples was as follows: 0 min 5% B, then a 0–5 min linear increase to 20% B, a 5–7.5 min linear increase to 45% B and finally 7.5–9.8 min linear increase to 100% B. The solvent composition was then held isocratically at 100% B for a further 1.2 min, before returning to the initial conditions (5% B). Re-equilibration occurred for 3 min. Before each injection of 2 μL of sample the injection valves and syringes were washed consecutively with a weak solvent (98% water-2% methanol) and a strong solvent (80% methanol-20% water).
2.6 Mass spectrometry
The UPLC system was coupled to a Q-TOF Micro quadrupole Time of Flight Mass Spectrometer (Waters, Milford, USA) operating in positive or negative ion electrospray mode (+/−ESI). ESI source working parameters were: capillary voltage ±3.5 kV; cone voltage ±28 V; source temperature 120°C; desolvation temperature 300°C; cone gas flow-rate 10 l/ h and desolvation gas flow-rate 700 l/ h. The scan time was 0.3 sec monitoring m/z values ranging from 100 to 800 with interscan time of 0.1 sec.
Leucine enkephalin at m/z 556.277 in +ESI and at m/z 554.261 amu in -ESI was used as lock mass for exact mass measurement correction. MarkerLynx v 4.0 software (Waters) was used for system controlling and data acquisition.
2.7 Sample analysis
Positive and negative ionisation data were acquired in two separate analyses. All samples were analysed in a random sequence to obtain metabolite profiles. Three QC samples were run at the beginning of the sample sequence to condition the system. Afterwards QC injections were made every eight samples for mice, or four samples for rat, in order to control the analytical variability of the system [14]. Two replicate injections of each test sample were performed in a fully randomised order.
2.8 Data Processing and Analysis
The raw spectrometric data acquired were processed by MarkerLynx V4.1 (Waters) for data analysis. The applied parameters for peak picking and alignment were set as follows. Mass tolerance: 0.1 Da, intensity threshold: 15 counts, mass window: 0.05 Da, retention time window: 0.5 min and noise elimination level: 6. Peak width at 5% height and peak-to-peak baseline noise were calculated automatically whereas C13 isotope signals were also excluded. Peak list data were obtained in the form of peak tables, where a peak is designated as a feature (a pair of retention time and m/z). MS data was normalised to the total signal. Peak tables were further processed by Simca P version 11 from Umetrics (Windsor, UK) for multivariate data analysis. Applications such as Principal Components Analysis (PCA), Orthogonal Partial Least Squares (OPLS) and other multivariate statistical tools () were applied. Pareto scaling (division of measurement by the square root of the standard deviation) was used prior to PCA and OPLS-DA. Peak tables were also imported into spreadsheet programs (Microsoft Excel) for univariate statistical analysis and data scrutiny.
The ions that were found significant from the statistical analysis were investigated in order to find out if they provided potential biomarkers of ethanol toxicity. These ions were tentatively identified based on their m/z value and mass spectrum using in house data. More specifically web based search engines were also used to provide potential identities for these ions as follows: the m/z values from the peak table were inserted in MassTrix, [15] (www.masstrix.org) used for metabolites and pathways annotation (using a setting for 5 ppm mass accuracy). In addition the Human Metabolome Database [16] (www.HMDB.ca) and the lipid specific lipidmaps database [17] (www.lipidmaps.org) were also used. Whenever possible solutions of reference standards, and also appropriately spiked biofluid samples, were analysed in the same UHPLC-TOF-MS system to verify tentative metabolite assignments, and eliminate false identifications, by matching mass and retention time data..
3. Results
3.1 Data Quality
As a first step the validity of the data were investigated by examination of the replicate QC profiles in the data sets as a means of evaluating both retention time and signal intensity repeatability during the analysis (by monitoring a number of peaks along the chromatogram)[14]. Further, the data for the two technical replicates of each test sample were compared in a similar way. Results obtained from both QC and test sample replicates showed high consistency of the profiles. In addition examination of the data by PCA showed that in all datasets the technical replicates for each sample were plotted together (see PCA plots given later in Figures and in supplementary material). QC replicates were also plotted in tight clusters in PCA scores plots indicating highly repeatable UPLC-TOF-MS profiles. Based on these evaluations further examination of the data was undertaken as described below.
3.2 Plasma
As indicated in the experimental section, plasma samples from both rats and mice were analysed by UHLPC using both +ve and −ve ESI. UHLPC-ToF-MS profiles in +ve ESI gave some 672 features (pairs of m/z and retention time data) after processing for mouse plasma extracts whilst −ve ESI gave 130 features. The equivalent numbers were 474 and 107 for +ve and −ve ESI respectively for the profiles of rat plasma extracts.
3.2.1 Mouse plasma samples
In this study mice received ethanol intragastrically for either the two or four week time periods. These two groups of mice were first examined separately. Plasma profiles (+ve ESI) from mice after two weeks of treatment with ethanol, when examined by PCA, gave a significant differentiation between the controls and treated animals (Figure S1a). This differentiation was mainly described by the 1st component, which explained almost 35% of the observed variation. Similarly, the plasma profiles (+ve ESI) obtained after four weeks of treatment showed a clear separation of ethanol administrated mice from controls (see Figures S1b for PCA and S1c for the UHLPC-TOF-MS profiles). These statistical models were built based on the data obtained from both technical replicates of each sample. However, even using the data obtained from only a single replicate for each sample provided statistical models that showed very good separation, with classification of groups in PC1 with R1>37%.
Data analysis using supervised multivariate analysis via OPLS distinguished the two groups in both cases even more clearly in the 1st component and the examination of the resulting S-plot (pc vs pc(corr)) revealed a number of ions which were responsible for the separation of the groups. The importance of these differentiating features was subsequently verified further by studying their contribution plots, VIP plots (variable importance in the projection) and position in loadings plots. To represent good potential biomarkers ions should show acceptable signal variability in the QC samples (CV <30%) and have non-zero values in 4 out of 6 injections in one of the groups. Further to these examinations, extracted ion chromatograms for each of these differentiating features were generated and compared between samples from treated and control animals. A T-test was also applied between the groups (as described in the following paragraphs). The most significant of these ions are listed in Table 1.
Table 1.
Detected masses-rt pairs contributing significantly to the differentiation of ethanol treated mouse blood plasma samples from the corresponding controls (+ESI dataset). The trend is referred to ethanol treated animals. (metabolites in italic = by comparison with databases).
| Period of treatment (week) | m/z | Rt (min) | Fold Change | p-value | Putative ID (Lipidmaps annotation in the footnote) |
|---|---|---|---|---|---|
| 2 | 383.1167 | 1.10 | 0.53 | 0.0001 | |
| 2 | 132.0839 | 1.11 | 1.7 | 0.009 | |
| 4 | 160.1357 | 1.20 | 0.4 | <10e-4 | |
| 2, 4 | 245.0647 | 1.38 | NA | <10e-3 | |
| 2, 4 | 240.0776 | 2.90 | 0.47 | 0.03 | |
| 4 | 480.2748 | 3.77 | NA | <10e-4 | |
| 2, 4 | 516.3035 | 3.70 | 18.7 | <10e-4 | |
| 4 | 462.2669 | 4.06 | 673 | <10e-4 | |
| 4 | 498.2885 | 4.06 | 141 | <10e-4 | |
| 4 | 533.3278 | 4.06 | 461 | <10e-4 | |
| 2 | 207.0572 | 4.45 | 0 | <10e-3 | |
| 4 | 500.3057 | 4.46 | 233 | <10e-4 | |
| 4 | 517.3123 | 4.46 | |||
| 2 | 494.3211 | 6.32 | 0.32 | <10e-3 | |
| 516.3053 | 0.34 | <10e-3 | |||
| 2 | 478.3015 | 6.64 | 2.4 | 0.006 | |
| 521.3476 | 2.4 | <10e-4 | |||
| 2, 4 | 544.3353 | 6.65 | 0.53 | 0.05 | Glycerophosphocholine(C28H50NO7P)1 |
| 4 | 519.7202 | 6.70 | 0.22 | 0.05 | |
| 2, 4 | 570.3554 | 7.18 | 0.08 | <10e-4 | Glycerophosphoserine (C28H44NO9P)2 |
| 2, 4 | 592.3481 | 7.2 | 0.01 | 0.001 | Glycerophosphocholine(C30H58NO8P)3 |
| 2 | 522.9712 | 7.45 | 0.58 | 0.005 | |
| 2 | 525.3708 | 8.31 | 6.3 | 0.05 |
1-(5Z,8Z,11Z,14Z-eicosatetraenoyl)-sn-glycero-3-phosphocholine or 2-(5Z,8Z,11Z,14Z-eicosatetra-enoyl)-sn-glycero-3-phosphocholine
1-(4Z,7Z,10Z,13Z,16Z,19Z-docosahexaenoyl)-glycero-3-phosphoserine
1-(9Z-octadecenoyl)-2-butyryl-sn-glycero-3-phosphocholine,
Fold change denotes the ratio between the average signal from samples from treated animals versus the average signal from samples from control animals. Values >1 denote increase in treated animals. Values <1 denote decrease in treated animals. NA signifies that the ion was not detected in the control samples. Zero value signifies that the ion was not detected in treated samples
As might be expected, some of these features were the same for the two periods of treatment showing an increasing trend in ethanol exposed animals (e.g., the ion eluting at 1.38 min, m/z 245.06 that shows the same level of differentiation between treated and controls for both short, 2 week, and longer, 4 week, treatment periods). However, ions that started to be significantly higher in ethanol-exposed animals only after four-weeks of treatment are also included in the list given in Table 1. e.g., the cluster of ions eluting at 4.06 min (see trend plot Figure S2). Finally there were also ions that were found to be relatively lower in intensity in ethanol-treated vs control animals, at both time points.
The (+ve ESI) plasma data were finally examined as a combined data set for both two and four weeks of treatment and showed similar PCA patterns with clear-cut separation of ethanol treated from control animals. The resulting PCA plot is given in Figure 1 in 3D mode showing that the groups were in addition separated according to their duration of treatment. Thus there were clear metabolic changes during the exposure to ethanol from the 2nd to the 4th week along with normal changes in metabolite profiles that are seen as animals age (more evident in the t2 component). In the next step t-tests were performed on all data sets to find differences between the treated and control groups. The samples obtained from mice after four weeks of intragastric ethanol infusion generated a higher differentiation than the two week samples and gave also a larger number of significant ions (cut-off p<0.05). To further explore these findings the UHLPC-MS traces were examined individually and the most significant ions are also included in Table 1.
Fig 1.
3D PCA scores plot of mouse plasma samples (+ve ESI) after 2 and 4 weeks of ethanol treatment. Bigger symbols correspond to 4 weeks samples and smaller to 2 weeks samples (both replicates are plotted).
The ions listed in Table 1, were scrutinised by searches in the MassTrix search engine [15] (www.masstrix.org) and the lipid maps database [17] (www.lipidmaps.org). A number these ions appeared to correspond to glycerophosphocholines or glycerolphosphoserines based on their mass.
The −ve ESI UHLPC-MS data for plasma samples were also examined, but did not provide enough information to enable the different groups to be separated by PCA. Examination of these data using PLS-DA models did show a separation of the groups but, following the exclusion of the ion for ethyl glucuronide (m/z 221.06, RT 1.33 min) few ions remained that could be considered significant differentiators. Almost all of these showed a decreasing trend in samples from ethanol treated mice compared to controls and exhibited masses greater than m/z 500. These high mass molecules eluted late in the gradient, with high percentages of acetonitrile in the mobile phase, suggesting that they were lipids. Among the few significant peaks detected by −ve ESI the ion at m/z 407.23, that eluted at 4.96 min, was possibly derived from an isomer of cholic acid.
Comparison of these data for mouse plasma with our previous results for liver extracts [7] showed no common markers amongst the ions detected as being significant for group differentiation in either +ve or −ve ESI.
3.2.2 Rat plasma samples
When the data obtained via UHLPC-MS in +ve ESI for plasma samples from control rats and rats treated with ethanol for four weeks were examined by PCA there was no clear separation of the two groups since one of the ethanol treated rats was plotted within the control group (see Figure S3a). OPLS could separate the two groups but the model was not reliable based on permutation tests results and thus this dataset was not pursued any further.
The same result was observed for the −ve ESI plasma dataset which provided insufficient information for the differentiation of the two groups of rats. In Figure S3b the PCA plot is given, where marginal separation in PC2 is seen.
3.3. Urine samples
UHLPC-ToF-MS profiling of the mouse urinary metabolites after processing gave some 1195 features in +ve ESI and 181 in −ve ESI. These numbers were 579 and 105 respectively for the +ve and −ve ESI profiles of rat urine samples.
3.3.1 Mouse urine samples
As for plasma, mouse urine samples analysed in +ve ESI mode were initially examined separately according to the duration of ethanol treatment. The PCA results obtained for both two and four weeks of treatment were similar, as illustrated in Figure S4 a and b, and showed, in both cases, a high level of separation of the controls vs the treated mice. These PCA models explained almost 60% of the variation in the data in the t1 and t2 components. Furthermore, when the UHLPC-MS data from all of the urine samples were analysed together as a single data-set by PCA there was, as seen for plasma, also a clear separation according to the different duration of the treatment (see Figure 2a).
Figure 2.
(a) 3D PCA scores plot of mouse urine samples (+ve ESI) after 2 and 4 weeks of ethanol treatment. Bigger symbols correspond to 4 weeks samples and smaller to 2 weeks samples (both replicates are plotted). (b) 3D PCA scores plot of mouse urine samples (−ve ESI) after 2 and 4 weeks of ethanol treatment after exclusion of variables from ethanol metabolites. Bigger symbols correspond to 4 weeks samples and smaller to 2 weeks samples (both replicates are plotted).
Compared to the plasma-generated results, urine datasets were found to be more information-rich: thus, for urine a much larger number of ions were found to be significant for the separation of the treated vs control group compared to plasma. The S-plot derived from the OPLS model was used to find features providing a high contribution to the differentiation of the two groups (see Table 2). From this analysis it was observed that most of the significant ions were the same in both the two and four weeks treatment groups, although some of these potential markers were observed to increase in intensity at four compared to two weeks of ethanol administration. In Figure 3 the intensity profile of such a case (the ion exhibiting m/z 147.05 (+veESI) and Rt:1.83 min) is shown, with an increasing trend along the duration of the treatment. An interesting finding is that the majority of the differentiating features from urine exhibited relatively short retention times. In particular, all of the ions that were found to be decreased in the ethanol treated group were eluted within 1 min or less, suggesting that they corresponded to highly polar metabolites. Similarly, most of the ions which were increased in the ethanol-dosed mice were eluted in the first part of the chromatogram.
Table 2.
Detected masses-rt pairs contributing significantly to the differentiation of ethanol treated mouse urine samples from the corresponding controls (+ESI dataset). The trend is referred to ethanol treated animals. (Metabolites in Bold = comparison with authentic standards, metabolites in italic = by comparison with databases).
| Period of treatment (week) | m/z | Rt (min) | Fold Change | p-value | Putative ID |
|---|---|---|---|---|---|
| 2, 4 | 144.0006 | 0.98 | 0.16 | <10e-4 | |
| 2, 4 2,4 |
167.0142 | 0.98 0.98 |
0.28 | 0.001 | Taurine adduct [+ ACN+H]+ |
| 125.9952 | 0.25 | <10e-4 | Taurine | ||
| 2,4 | 214.9176 | 0.97 | 0.16 | <10e-4 | |
| 273.9545 | 0.14 | <10e-4 | |||
| 296.9717 | 0.14 | <10e-4 | |||
| 344.8783 | 0.06 | <10e-4 | |||
| 2 | 158.0181 | 1.00 | 0.21 | 0.001 | |
| 2,4 | 175.0647 | 1.1 | 675 | <10e-4 | Arginine |
| 4 | 132.0833 | 1.13 | 17 | 0.001 | Hydroxyproline |
| 4 | 130.0689 | 1.31 | 3.0 | 0.0001 | 5-oxoproline (pyroglutamic acid) |
| 4 | 189.0891 | 1.24 | 32.4 | <10e-4 | N-Acetylglutamine |
| 2 | 221.0603 | 1.33 | 2 | 0.001 | 5-Hydroxy-Tryptophan |
| 2, 4 | 196.0811 | 1.43 | 312 | <10e-4 | 2-Amino-2-deoxy-D-gluconate |
| 4 | 223.0392 | 1.47 | 41 | 0.0003 | |
| 2 | 483.1201 | 1.54 | NA | <10e-4 | |
| 4 | 200.0527 | 1.56 | 384 | <10e-4 | |
| 2 | 501.0751 | 1.57 | NA | <10e-4 | |
| 2, 4 | 205.0712 | 1.59 | 490 | <10e-4 | |
| 2, 4 | 141.0245 | 1.60 | NA | <10e-4 | |
| 159.0329 | <10e-4 | ||||
| 2, 4 | 147.0511 | 1.83 | 35.8 | <10e-4 | |
| 2, 4 | 257.1131 | 2.12 | NA | 0.001 | |
| 2, 4 | 453.2012 | 2.46 | 7.1 | 0.001 | |
| 2 | 493.0725 | 2.50 | 0 | <10e-4 | |
| 2 | 206.0443 | 2.70 | 4.0 | 0.0001 | Xanthurenic acid |
| 2 | 172.1012 | 5.12 | 93 | <10e-4 |
Fold change denotes the ratio between the average signal from samples from treated animals versus the average signal from samples from control animals. Values >1 denote increase in treated animals. Values <1 denote decrease in treated animals. NA signifies that the ion was not detected in the control samples. Zero value signifies that the ion was not detected in treated samples
Figure 3.
Intensity profile of ion m/z 147.05 eluted at 1.83 min found as significant differentiator of control vs ethanol treated mice in urine samples (+ve esi data) showing increasing intensity with the duration of the treatment.
In order to attempt to identify the ions highlighted in Table 2, searches in in-house and web-based metabolite databases (www.HMDB.ca, www.masstrix.org) were performed based on the ions m/z value and a number of tentative identities were reached. To verify these findings UHLPC-MS analysis of available reference standards and spiked samples was performed (for a list of the reference compounds examined see section 2.1). From these a number of potential markers were verified, based on m/z, mass spectrum comparison and retention time data as shown in Table 2, as corresponding to metabolites such as arginine (m/z 175.06), hydroxyproline (m/z 132.08), taurine (m/z 125.99), 5-oxoproline (pyroglutamic acid m/z 130.07), N-acetyl-glutamine (m/z 189.09) 5-Hydroxy-Tryptophan (m/z196.08) and xanthurenic acid (m/z 206.04) (tryptophan metabolism) etc. Others such as e.g., 2-Amino-2-deoxy-D-gluconate, were only tentatively identified based on database searches. Interestingly, a number of metabolites remained unaffected by exposure to ethanol such as e.g. hippuric acid (detected both in +ve and −ve ESI) eluting at 4.4 min.
Urine profiles acquired in −ve ESI mode showed an even higher differentiation of samples in treated and control animals by PCA than those seen for +ve ESI. In Figure 4 the combined (mean average of peak intensities) chromatogram (−ve ESI) for urine from mice treated for four weeks and the corresponding averaged chromatogram for the control mice are illustrated. The effect of treatment on the urinary metabolite profile of the animals can easily be seen. The peaks of major ethanol metabolites are predominant in the chromatographic trace. From this it can be seen that ethyl glucuronide (EtG) and ethyl sulphate (EtS), which elute at 1.62 and 1.34 min respectively, represent the major peaks in urine samples from ethanol-treated animals. For this reason all features emanating from the EtG and EtS peak, with their most abundant masses at m/z 221.06 and m/z 124.99 respectively (spectra are given in Figure S5), were excluded from the variables list by excluding the region of 1.31–1.42 and 1.56–1.79 min. Despite this PCA still showed a clear separation of the groups in the first component (t1: 40%). In Figure S6a and b the PCA scores plots are given for the two and the four weeks of treatment separately. In Figure 2b a three dimensional PCA scores plot examining both time points as a combined dataset is shown, indicating that the samples from the treated animals are in addition separated according to the period of treatment. The most highly differentiating ions are listed in Table 3. From that list it is clear that, in addition to the early eluting components in this −ve ESI dataset, significant peaks are detected eluting in the latter part of the chromatogram. These late eluting peaks, with m/z values above 200, could be fatty acids that do not efficiently ionise in +ve ESI. The majority of the ions were the same for urine samples from both periods of treatment.
Figure 4.
Combined (−) ESI TIC chromatograms (mean average of peak intensities) of mice urine treated for four weeks (right) and the corresponding control (left).
Table 3.
Detected masses-rt pairs contributing significantly to the differentiation of ethanol treated mouse urine samples from the corresponding controls (-ESI dataset). The trend is referred to ethanol treated animals. (Metabolites in Bold = comparison with authentic standards, metabolites in italic = by comparison with databases).
| Week of treatment | m/z | Rt (min) | Fold Change | p-value | Putative ID |
|---|---|---|---|---|---|
| 4 | 288.9323 | 0.98 | 0.17 | <10e-4 | |
| 2, 4 | 304.9162 | 0.98 | 0.05 | <10e-4 | |
| 4 | 434.8781 | 0.98 | 0.02 | 0.0001 | |
| 4 | 174.9547 | 0.99 | 0.08 | 0.0001 | |
| 2, 4 | 209.0292 | 1.12 | 5.25 | 0.001 | |
| 4 | 166.0171 | 1.17 | 5.2 | <10e-4 | |
| 4 | 263.9836 | 1.17 | 4,7 | 0.001 | |
| 4 | 216.9078 | 1.18 | 0.02 | <10e-4 | |
| 4 | 199.0394 | 1.21 | NA | 0.0009 | |
| 2, 4 | 254.9792 | 1.22 | 0.37 | 0.001 | |
| 4 | 188.0557 | 1.50 | 3.0 | <10e-4 | |
| 4 | 142.0485 | 2.42 | 15 | 0.0005 | |
| 4 | 435.1399 | 2.61 | 0.13 | 0.001 | |
| 2 | 230.9948 | 2.69 | 19 | 0.01 | 2 / 4-hydroxyphenylacetic sulphate |
| 4 | 186.0752 | 2.75 | NA | <10e-4 | |
| 2 | 233.1026 | 2.85 | NA | 0.001 | |
| 4 | 156.0692 | 3.68 | 2.0 | 0.05 | |
| 2, 4 | 212.0007 | 3.71 | 0.4 | 0.001 | |
| 2 | 387.1359 | 3.82 | 0.07 | 0.02 | |
| 2 | 249.0972 | 4.22 | NA | <10e-4 | |
| 4 | 401.1577 | 5.13 | 0.19 | <10e-4 | |
| 2, 4 | 192.0665 | 5.22 | 0.08 | <10e-4 | 4-Hydroxyphenylacetylglycine /Phenylacetylglycine |
| 4 | 187.0059 | 5.24 | 0.26 | 0.0002 | |
| 2 | 435.1447 | 5.85 | 0.17 | 0.008 | |
| 4 | 172.0991 | 5.87 | NA | 0.0006 | |
| 4 | 283.0809 | 5.92 | 0.24 | 0.01 | |
| 4 | 263.1137 | 6.15 | 153 | 0.001 | |
| 4 | 321.1553 | 7.27 | 0.09 | <10e-4 | |
| 2 | 277.1312 | 7.41 | 0.14 | 0.005 | |
| 4 | 319.1421 | 8.34 | 15 | 0.0009 | |
| 2 | 319.1391 | 8.34 | 0.29 | 0.08 | |
| 2 | 305.1573 | 8.74 | 18 | 0.01 | Eicosatrienoic acid |
| 2 | 359.1935 | 8.90 | 1.2 | 0.08 | |
| 2 | 317.1647 | 9.01 | 1.6 | 0.08 | |
| 2 | 453.1782 | 9.70 | NA | 0.0008 | Urso /Chenodeoxycholic acid sulphate |
| 4 | 521.1604 | 9.70 | 0.2 | 0.005 |
Fold change denotes the ratio between the average signal from samples from treated animals versus the average signal from samples from control animals. Values >1 denote increase in treated animals. Values <1 denote decrease in treated animals. NA signifies that the ion was not detected in the control samples. Zero value signifies that the ion was not detected in treated samples
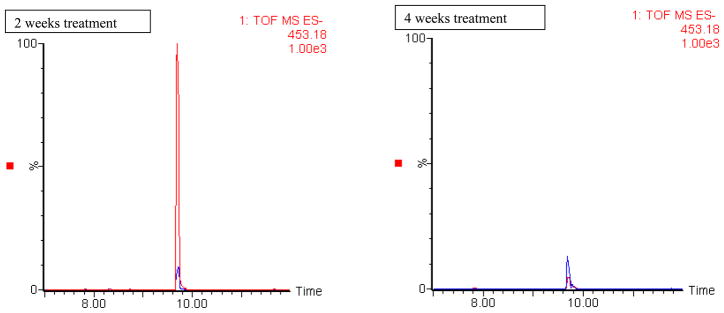
Based on MS database (www.HMDB.ca, www.masstrix.org) and the analysis of reference standards and spiked samples the ion m/z 305.16 (also detected as significant in liver extracts from the same control and treated animals [7]) was tentatively identified as eicosatrienoic acid. An interesting finding is that in some cases the increase or decrease of signal intensity in the ethanol exposed animals was higher in the early time point of 2 weeks than at 4 weeks. Thus, e.g. the peak area of the putative eicosatrienoic acid (m/z 305.16) was 30 times higher in the treated animals (compared to controls) at the 2 week time point reducing to only 6 times higher in samples from animals treated for 4 weeks. In Figure 5 the extracted ion chromatogram of m/z 453.18 which could correspond to either urso- or chenodeoxycholic acid sulphate is given for mouse urine samples (−ve ESI) for both 2 and 4 weeks of treatment showing also this type of trend. It should be mentioned that t-tests were additionally performed in the two datasets and ions with a p-value of <0.0001 were examined manually in the chromatographic data. These ions are also included in Tables 2 and 3 when this examination confirmed the t-test results.
Figure 5.
Ion 453.18 at 9.7 in mice urinary profiles (−ve ESI) probably urso/chenodeoxycholic showing higher increase in the earlier time point. Blue trace: control, red trace: treated
3.3.2 Rat urine samples
Examination of the PCA scores plots obtained from the +ve ESI profiles of rat urine showed the control group tightly clustered away from the ethanol treated rats (Figure 6a). Interestingly examination of the ions contributing to the differentiation of the ethanol-treated vs control rats showed that these ions were not the same as those found as differentiators for the mouse urine samples. In Table 4 the differentiating ions found from S-plot examination are given. A t-test was also performed between the two groups which confirmed the statistical significance of these ions.
Figure 6.
PCA scores plot of rat urine samples in a) +ve esi and b) −ve esi. Both replicates are included
Table 4.
Detected masses-rt pairs contributing to the separation of the ethanol treated rat (4 weeks) urine samples from the corresponding controls (+ESI dataset). The trend is referred to ethanol treated animals. (Metabolites in Bold = comparison with authentic standards, metabolites in italic = by comparison with databases).
| m/z | Rt (min) | Fold Change | p-value | Putative ID |
|---|---|---|---|---|
| 271.9967 | 0.98 | 21 | 0.0005 | |
| 148.0124 | 1.10 | 21 | 0.001 | |
| 201.0507 | 1.22 | 28 | 0.001 | |
| 240.0916 | 1.67 | NA | 0.001 | |
| 501.0758 | 0.0001 | |||
| 141.0234 | 1.60 | NA | 0.006 | |
| 159.0321 | 0.006 | |||
| 723.1499 | 1.69 | NA | 0.0007 | |
| 209.1288 | 1.81 | 82 | 0.0009 | |
| 199.1077 | 2.28 | NA | <10e-4 | |
| 453.1954 | 2.56 | 25 | 0.05 | |
| 206.0445 | 2.70 | 1.9 | 0.02 | Xanthurenic acid |
| 483.3342 | 10.65 | 0.5 | 0.002 | |
| 397.2957 | 11.01 | 0.04 | 0.0002 | |
| 227.1265 | 11.02 | 0.43 | 0.0002 | |
| 419.2775 | 0.43 | 0.0002 | ||
| 441.3204 | 0.44 | 0.0002 | ||
| 485.3463 | 0.42 | 0.0002 | ||
| 502.3760 | 0.43 | 0.0002 |
Fold change denotes the ratio between the average signal from samples from treated animals versus the average signal from samples from control animals. Values >1 denote increase in treated animals. Values <1 denote decrease in treated animals. NA signifies that the ion was not detected in the control samples. Zero value signifies that the ion was not detected in treated samples
Examination of the −ve ESI rat urine data allowed ready differentiation of control and treated groups to be made merely by visual examination of the ion chromatograms. As seen for the mouse urine samples in the urine of the alcohol treated rats peaks corresponding to EtS and EtG showed high signal intensities. In general the treated animals did not show a homogeneous MS profile, with some peaks being higher in one of the samples compared to the others, e.g. it seems that the metabolic profile of animal 6 was less affected by treatment (in Figure S7 the ethanol metabolites peaks are shown as being of different intensity). These differences noted between individual animals, based on examination of the UHPLC-MS profiles, were also observed in PCA, where the sample from this animal clusters closer to the control group than those of the other alcohol-treated rats as also observed from its plasma profile (See section 3.2.2 and Figure S3a). It should be noted that the differentiation of the control group from the ethanol-treated animals is clear in the first PCA component (t1 accounting for 34% of the variation) even after exclusion of the ethanol metabolites (See Figure 6b). The differentiating ions found from the S-plot of the OPLS model are given in Table 5 and an example of an ion with an increasing trend in the treated animals is given in Figure S8. In general it is observed that different ions are appearing as significant in the urinary profiles of the rat compared to the mouse.
Table 5.
Detected masses-rt pairs contributing to group separation of the ethanol treated rat (4 weeks) urine samples from the corresponding controls (-ESI dataset). The trend is referred to ethanol treated animals. (Metabolites in Bold = comparison with authentic standards)
| m/z | Rt (min) | Fold Change | p-value | Putative ID |
|---|---|---|---|---|
| 166.0169 | 1.17 | 4 | <10e-4 | |
| 267.0709 | 1.6 | NA | 0.0009 | |
| 283.0841 | 5. 89 | 9.1 | 0.01 | |
| 297.0968 | 6.23 | NA | 0.006 | |
| 277.1292 | 7.54 | NA | 0.002 | |
| 157.0380 | 1.12 | 11 | <10e-4 | |
| 167.0203 | 1.21 | 1 | <10e-4 | |
| 175.0224 | 1.22 | 0.39 | <10e-4 | |
| 254.9795 | 0.35 | <10e-4 | ||
| 145.0521 | 11.64 | 4 | 0.0003 |
Fold change denotes the ratio between the average signal from samples from treated animals versus the average signal from samples from control animals. Values >1 denote increase in treated animals. Values <1 denote decrease in treated animals. NA signifies that the ion was not detected in the control samples. Zero value signifies that the ion was not detected in treated samples
4. Discussion
Not unexpectedly, especially when our previous results on the metabolite composition of liver extracts are considered [7], there were large changes in the plasma and urinary metabolomes of mice fed intragastrically with ethanol for periods of 2 and 4 weeks. In addition the alcohol fed mice showed progression between the two dosing periods. Such progressive changes have also been noted in the urinary metabolomes in studies on the Ppara-null mouse [6]. Similarly there were detectable changes in the plasma metabolome of rats intra-gastrically fed with ethanol for four weeks, though these were not as marked as seen with mice. Large effects were also noted on the urinary metabolome of rats administered ethanol for 4 weeks. These differences remained even after the removal of ethanol metabolites such as ethyl glucuronide and ethyl sulphate from consideration. These data enabled the detection of a large number of ions via PCA analysis of both +ve and −ve ESI data, for both rats and mice, that contributed to the separation of control and intra-gastrically infused animals.
In plasma the effects noted on both mouse and rat metabolomes covered two categories of molecules exhibiting either relatively short retention time and low molecular mass (indicative of polar metabolites) or longer retention time and higher molecular mass (indicative of non-polar species, presumably lipids). Such results are consistent with changes in the lipid profile of plasma seen in other metabolic profiling studies in rodents. For example, a recent lipidomic study [9] in male Fischer 334 rats, fed with 5% alcohol (in a Leiber-DeCarli diet) for 1 month, which analysed solvent extracts of plasma (prepared using methyl tert butyl ether) using 1H and 31P NMR spectroscopy, demonstrated significant changes in phospholipid composition compared to control animals. These changes involved decreases in the signals observed for methylated fatty acids and phosphatidlyethanolamine/lysophosphatidyl choline in the plasma extracts. In the present study we have tentatively identified decreases in the relative amounts of glycerophosphocholines and glycerolphosphoserines that also indicate effects of ethanol administration on the plasma metabolome.
For urine, again for both species, the range of metabolites seen to change in response to ethanol exposure also covered both polar and relatively non-polar species. In general those ions noted as significant for the separation of treatment and control animals generally appeared to be different from those detected and identified in liver extracts from the same animals in our previous study [7] (although eicosatrienoic acid appeared to be present in the urine of rats having also been found to be elevated in liver extracts [7]).
We also noted changes in the concentrations of a number of ions identified as the tryptophan metabolites 5-hydroxytryptophan (m/z 221.06) and xanthurenic acid (m/z 206.04). Such changes are interesting given the well documented association of effects on liver tryptophan pyrrolase and tryptophan metabolism in rodents and man in response to chronic ethanol exposure [18, 19], reviewed in [20]. Alterations in tryptophan metabolism and disposition have effects on brain serotonin synthesis with obvious potential for behavioural effects [16]. Whilst such results are therefore perhaps not unexpected it is still reassuring that a non-targeted metabolic profiling approach nevertheless did indeed detect changes in tryptophan metabolites. In addition other metabolites such as arginine (m/z 175.06), hydroxyproline (m/z 132.08) and taurine (m/z 125.995) were also observed to change on alcohol administration. Whilst it is tempting to try to assign biological meaning to these observations it is also the case that, in these preliminary studies, many differentiating ions still remain to be fully characterised and identified. It is more than likely that some of these will prove to be much more important as mechanistic biomarkers of the effects of alcohol exposure and toxicity. It is also noteworthy in this context that recent studies examining the effects of ad libitum dosing of ethanol (in a Leiber-DeCarli diet) to wild type and Ppara-null mice [6] also showed robust changes in metabolome composition over time, with indole-3-lactic acid (m/z 204.07) seen as a unique marker for ethanol exposure in the Ppara-null mice [6]. Other identified markers of ethanol exposure in this study were pimelic and adipic acids (m/z 159.032 and 145.05 respectively), 2- and 4-hydroxyphenylacetic acids and 4-hydroxyphenylacetic acid sulphate (m/z 151.04 and 230.99 respectively) and, as with our investigation, a significant number of features remained unidentified. However, comparison of the data from the present intragastric feeding study did not identify the masses found in the investigation using the Ppara-null mice as biomarkers. Such differences of course may result from the use of different experimental models, and different analytical protocols, and indeed similar, but different instrumentation. For example we have recently shown that metabolite profiling with different mass spectrometers connected simultaneously to the same UHLPC separation can highlight different sets of molecules as potential biomarkers [21].
In addition, given the polar nature of some of the potential ethanol-exposure markers the use of other types of LC-separation such as e.g., HILIC may be of benefit for metabolic profiling [22–23]. Clearly therefore, whilst preliminary, the data from this, and other rodent-based studies, confirm that metabonomics may provide a promising method for the study of these model systems in elucidating the biochemical effects of ethanol exposure. In order to translate these studies to humans, further work is required to fully characterise the changes and extract biological meaning from them. However, the present study, and the study conducted in the Ppara-null mouse [6], show the potential of UHPLC-based metabolite profiling of urine and blood plasma in the elucidation of the mechanisms of alcohol toxicity in rodents. Further work in these models could well result in the discovery of new insights into the biochemical effects of prolonged ethanol exposure and biomarkers for monitoring the progression of alcoholism in man.
5. Conclusions
The results from this preliminary examination of blood plasma and urine from mice treated with alcohol provided LC-TOF-MS profiles that differed greatly from those obtained from control animals, with urine enabling the best differentiation between the groups. Temporal effects were also noted in mice, with ethanol administration for four weeks with ethanol providing stronger effects on the metabolome compared to two weeks treatment. For rats plasma was less useful for studying the effects of exposure to ethanol but differentiation was readily achieved based on urine profiles.
Supplementary Material
Highlights.
Urine and Plasma from Rodent “intragastric alcohol feeding model” metabolically profiled with UPLC-TOF-MS.
Scope was to determine changes in the metabolite profiles due to alcohol treatment.
PCA revealed robust differences between profiles from control and alcohol-treated animals from both species.
A large number of metabolites were seen to differ between control and alcohol-treated animals, for both biofluids.
Acknowledgments
The authors wish to thank the EU committee for funding through a Transfer of Knowledge Industry-Academia partnership grant (TOK-IAP 29640). H.G.Gika is grateful to the EU Committee for a re-integration grant (EIRG 202132) funding her work at the Aristotle University Thessaloniki. Cheng Ji is supported by the US NIH/NIAAA grants R01AA018846-3 and R01AA018612-2 This research has been co-financed by the European Union (European Social Fund – ESF) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF) - Research Funding Program: Heracleitus II, Investing in knowledge society through the European Social Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tsukamoto H, Reidelberger RD, French SW, Largman C. Am J Physiol. 1984;247:R595. doi: 10.1152/ajpregu.1984.247.3.R595. [DOI] [PubMed] [Google Scholar]
- 2.Tsukamoto H, Gaal K, French SW. Hepatology. 1990;12:599. doi: 10.1002/hep.1840120325. [DOI] [PubMed] [Google Scholar]
- 3.Tsukamoto H, Mkrtchyan H, Dynnyk A. Methods Mol Biol. 2008;447:33. doi: 10.1007/978-1-59745-242-7_3. [DOI] [PubMed] [Google Scholar]
- 4.Shinohara M, Ji C, Kaplowitz N. Hepatology. 2010;51:796. doi: 10.1002/hep.23391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradford BU, O’Connell TM, Han J, Koysk O, Shymonyak S, Ross PK, Winnike J, Kono H, Rusyn I. Toxicol Appl Pharmacol. 2008;232:236. doi: 10.1016/j.taap.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manna SK, Patterson AD, Yang Q, Krausz KW, Li H, Idle HJ, Jr, Fornace AJ, Gonzalez FJ. J Proteome Res. 2010;9:4176. doi: 10.1021/pr100452b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loftus N, Barnes A, Ashton S, Michopoulos F, Theodoridis G, Wilson ID, Ji C, Kaplowitz N. J Proteome Res. 2011;10:705. doi: 10.1021/pr100885w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicholas PC, Kim D, Crews TT, Macdonald JM. Chem Res Toxicol. 2008;21:408. doi: 10.1021/tx700324t. [DOI] [PubMed] [Google Scholar]
- 9.Fernando H, Kondraganti S, Bhopale KK, Volk DE, Neerathilingham M, Kaphalia BS, Luxon BA, Boor PJ, Ansari GAS. Alcohol Clin Exp Res. 2010;34:1937. doi: 10.1111/j.1530-0277.2010.01283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gavaghan CL, Holmes E, Lenz E, Wilson ID, Nicholson JK. FEBS Lett. 2000;484:169. doi: 10.1016/s0014-5793(00)02147-5. [DOI] [PubMed] [Google Scholar]
- 11.Laposata M. Addiction Biol. 1998;3:5. doi: 10.1080/13556219872308. [DOI] [PubMed] [Google Scholar]
- 12.Gustavsson L. Alcohol Alcoholism. 1995;30:391. [PubMed] [Google Scholar]
- 13.Helander A, Zheng Y. Clin Chem. 2009;55:1395. doi: 10.1373/clinchem.2008.120923. [DOI] [PubMed] [Google Scholar]
- 14.Gika H, Macpherson E, Theodoridis G, Wilson ID. J Chromatogr B. 2008;871:299. doi: 10.1016/j.jchromb.2008.05.048. [DOI] [PubMed] [Google Scholar]
- 15.Suhre K, Schmitt-Kopplin P. Nucleic Acids Res. 2008;36(Web Server issue):W481. doi: 10.1093/nar/gkn194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wishart DS, Tzur D, Knox C, et al. 2007;35(Database issue):D521. doi: 10.1093/nar/gkl923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fahy E, Subramaniam S, Murphy RC, Nishijima M, Raetz CRH, Shimizu T, Spener F, vanMeer G, Wakelam MJO, Dennis EA. J Lipid Res. 2009;50:9. doi: 10.1194/jlr.R800095-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badawy AB, Evans M. Adv Exp Med Biol. 1973;35:105. doi: 10.1007/978-1-4757-0632-1_15. [DOI] [PubMed] [Google Scholar]
- 19.Badawy AB, Evans M. Adv Exp Med Biol. 1975;59:229. doi: 10.1007/978-1-4757-0632-1_15. [DOI] [PubMed] [Google Scholar]
- 20.Badawy AB. Nutrition Res Rev. 2002;15:123. doi: 10.1079/NRR200133. [DOI] [PubMed] [Google Scholar]
- 21.Gika HG, Theodoridis GA, Earll M, Snyder RW, Sumner SJ, Wilson ID. Anal Chem. 2010;82:8226. doi: 10.1021/ac1016612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spagou K, Wilson ID, Masson P, Theodoridis G, Raikos N, Coen M, Holmes E, Lindon JC, Plumb RS, Nicholson JK, Want EJ. Anal Chem. 2011;83:382. doi: 10.1021/ac102523q. [DOI] [PubMed] [Google Scholar]
- 23.Tolstikov VV, Fiehn O. Anal Biochem. 2002;301:298. doi: 10.1006/abio.2001.5513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.