Abstract
The heart has both the greatest caloric needs and the most robust oxidation of fatty acids. Under pathological conditions such as obesity and type 2 diabetes, cardiac uptake and oxidation are not balanced and hearts accumulate lipid potentially leading to cardiac lipotoxicity. We will first review the pathways utilized by the heart to acquire fatty acids from the circulation and to store triglyceride intracellularly. Then we will describe mouse models in which excess lipid accumulation causes heart dysfunction and experiments performed to alleviate this toxicity. Finally, the known relationships between heart lipid metabolism and dysfunction in humans will be summarized.
Introduction
Although the heart is far and away the most energy-requiring organ of the body, studies of cardiac lipid metabolism, especially in vivo, are relatively scarce compared with investigations in adipose tissue or liver. In adult fasting mammals, 60–80% of cardiac energy metabolism relies on the oxidation of fatty acids (FAs) with glucose, lactate, and ketones providing substrates for the remainder (Neely et al., 1972). The adult heart, however, has the ability to switch to different substrates for ATP generation depending on feeding, hormonal status and overall nutritional supply as characterized by the Randle cycle (Hue and Taegtmeyer, 2009). Of note, there are major species differences with mice relying more on glucose, lactate and ketone bodies, and much less on fatty acids (30–40% from fat) (Stanley et al., 2005; Stowe et al., 2006). The fetal heart operates under low oxygen pressure and primarily depends on glucose and lactate for ATP generation, whereas the adult heart utilizes FAs but conserves the ability to switch other substrates. Older animals and humans use relatively less FAs and more glucose.
The heart avidly acquires lipids both from circulating non-esterified (free) fatty acids (FFAs) and esterified FAs bound to lipoproteins (Figure 1A). Observations made studying arterial venous differences in substrate concentrations showed that esterified FAs were a major source of lipids for the human heart (Ballard et al., 1960). More recent methods to study heart lipid metabolism have relied on tracers of FFAs in, for example, isolated perfused hearts. These studies quantify conversion of FFAs to CO2 and TCA cycle intermediates under a variety of experimental conditions. In vivo studies can assess the uptake and loss of tracers from the heart. Although the heart can synthesize lipoproteins as it expresses both apoB and microsomal triglyceride transfer protein (Bartels et al., 2009; Nielsen et al., 1998), under most circumstances, the heart probably does not re-secrete appreciable amounts of glucose or lipids, and the uptake should indicate oxidation plus a relatively small amount of substrate that is stored and a small amount of substrate used for structural requirements of the cell.
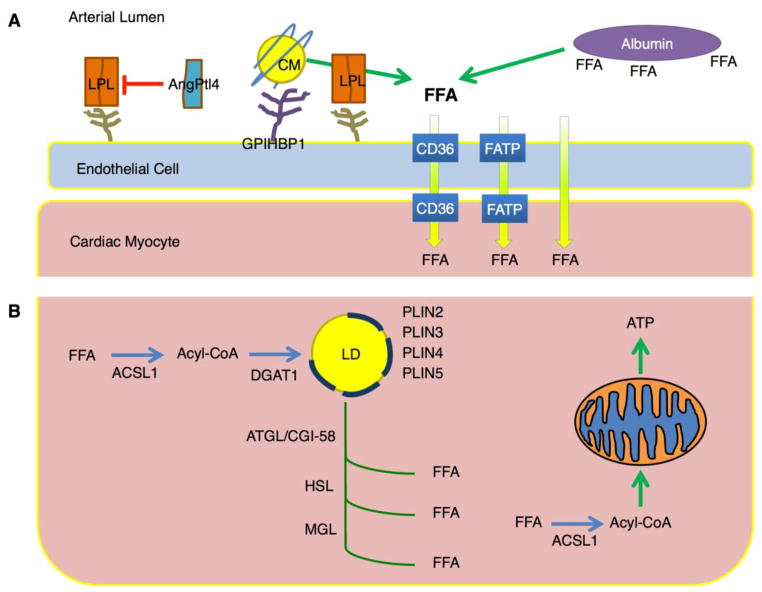
FIGURE 1. Regulation of cardiomyocyte lipid storage.
A. Fatty acids esterified as triacylglycerol (TG) within lipoproteins require hydrolysis by lipoprotein lipase (LpL) associated with proteoglycans and GPIHBP1 on the luminal surface of endothelial cells. Angiopoietin like protein 4 (ANGPTL4) is an LpL inhibitor. Non-esterified fatty acids (FFA) associated with albumin likely are internalized by membrane transporters such as CD36. These lipids must cross the endothelial barrier; how this occurs is unclear. B. Within the cardiomyocytes the fatty acids are esterified to CoA and either stored in the lipid droplet (LD) or used for energy. At least 4 lipid droplet proteins (perilipins – PLINs) are expressed in the heart. The lipid droplet supplies some oxidized fatty acids via the actions of adipose triglyceride lipase (ATGL)/desnutrin and hormone sensitive lipase (HSL). CGI58 is the ATGL co-activator. ATGL and LpL actions both provide ligands for PPAR activation.
In some situations the heart adjusts to maintain lipid homeostasis. Increases in work load (Goodwin et al., 1998) and myocardial ischemia (Lopaschuk et al., 2010) cause a rapid switch from fat to glucose utilization for ATP generation. This finding has led to several animal studies showing that administration of compounds that reduce FA oxidation protect the heart from the consequences of ischemia and ischemia-reperfusion injury (Goodwin et al., 1998; Lopaschuk et al., 2010). This is presumed to be due to reduced oxygen requirements for non-FA substrates. Deleterious effects of cardiac ischemia could be due in part to excess cardiac lipid accumulation via the VLDL receptor (Perman et al., 2011). Similarly, in another mouse model of cardiomyocyte death adiponectin-induced activation of a ceramidase and reduction of ceramide was beneficial (Holland et al., 2011). Therefore, abnormal regulation of lipid uptake or its intracellular metabolism might play an important role in heart diseases other than metabolic dilated cardiomyopathy.
An imbalance between FA uptake and oxidation leads to accumulation of long chain FAs that are incorporated into triglyceride (TG) and phospholipids, as well as a multitude of other lipid subspecies. Although TG is the most easily detected, other lipids are more likely to be toxic. Diacylglycerols (DAGs) and ceramides are signaling lipids that are thought to be toxic when their intracellular concentrations are increased. Defective mitochondrial FA oxidation could lead to accumulation of medium chain acyl carnitines (Koves et al., 2008), another possible toxin. Finally, saturated long chain FAs, most notably palmitate, are associated with toxicity in cells either because of their direct actions or their incorporation into phospholipids (Borradaile et al., 2006).
Sources of heart lipids
All tissues obtain lipids from FFAs associated with albumin, lipoproteins, and de novo synthesis (Figure 1A). Although de novo synthesis is thought to play a minor role in heart lipid metabolism, a recent study of deletion of fatty acid synthetase in heart showed that de novo synthesis is important to maintain cardiac function during aortic constriction and aging (Razani et al., 2011). Loss of lipoprotein lipase (LpL)-derived lipids leads to increased glucose uptake in mouse hearts (Augustus et al., 2004). In humans, deficiency in CD36 is associated with increased glucose uptake (Fukuchi et al., 1999). CD36 appears most important in the setting of lower concentrations of FFAs (Coburn et al., 2000). Therefore, it is not surprising that when large amounts of FFA are generated during hydrolysis of large TG-rich lipoproteins like chylomicrons, heart uptake of lipids appears to be exclusive of this receptor (Bharadwaj et al., 2010). Lipolysis of lipoproteins is also a pathway for delivery of esterified core lipids such as cholesteryl esters and retinyl esters into the heart (Bharadwaj et al., 2010).
Cardiac storage of lipids
Excess lipid, especially TG, beyond that needed for cellular structures and ATP generation is stored in lipid droplets (Figure 1B). Within the heart, there normally is little droplet accumulation, suggesting that uptake and oxidation are finely regulated. Lipid droplets are found in hearts of patients with diabetes and metabolic syndrome (Marfella et al., 2009; McGavock et al., 2007; Sharma et al., 2004) and in those of high fat diet fed rodents and genetically altered mice (see below and Table 1). In addition, after an overnight fast, lipid droplets appear in the hearts of wild type mice (Suzuki et al., 2002).
Table 1.
Models of Cardiac Lipotoxicity
| Cardiac Lipotoxicity | Reference | Corrections | Reference |
|---|---|---|---|
| MHC-ACS1 | (Chiu et al., 2001) | Leptin treatment X MHC-DGAT1 α-Lipoic acid |
(Lee et al., 2004) (Liu et al., 2009) (Lee et al., 2006) |
| MHC-FATP1 | (Chiu et al., 2005) | ||
| Heart specific PPARδ knockout | (Cheng et al., 2004) | ||
| MHC-PPARα | (Finck et al., 2002) | X heart specific LpL ko X CD36−/− Medium chain triglyceride diet |
(Duncan et al., 2010) (Yang et al., 2007) (Finck et al., 2003) |
| MHC-PPARγ | (Son et al., 2007) | X PPARα knockout | (Son et al., 2010) |
| Leptin deficiency (ob/ob) | (Christoffersen et al., 2003) (Barouch et al., 2003) |
Leptin Infusion | (Barouch et al., 2003) |
| ATGL knockout | (Schweiger et al., 2006) | PPARα agonists | (Haemmerle et al., 2011) |
| MHC-LpLGPI | (Yagyu et al., 2003) | Ceramide synthesis inhibition | (Park et al., 2008) |
| MHC-LPL X PPARa−/− | (Nohammer et al, 2003) | ||
| MHC-GLUT1 on HFD | (Yan et al., 2009) |
Lipid droplet protein makeup in the heart is different from that of adipocytes. In the heart, there is minimal expression of perilipin (Plin1). However, the other major lipid droplet proteins, adipophilin/adipose differentiation-related protein (ADRP/Plin2), tail-interacting protein 47 (Tip47/Plin3), S3-12 (Plin4), and OXPAT/myocardial LD protein/lipid-storage droplet protein 5 (Plin5) are all expressed in the heart (Paul et al., 2008). Plin2 expression might be most upregulated in some forms of lipotoxicity and be important for non-toxic lipid storage (Son et al., 2007). Plin5 appears to regulate TG oxidation by approximating lipid droplets and mitochondria (Bosma et al., 2011; Wang et al., 2011). Of these droplet proteins, only Plin2 has been deleted, and chow-fed Plin2−/−mice do not have an obvious cardiac phenotype (Chang et al., 2006). Thus, knowledge of how and whether these proteins, and probably others, modulate heart lipid accumulation and TG oxidation is likely to be forthcoming.
Lipid droplet turnover is regulated by lipid droplet associated proteins, intracellular lipases, and acyltransferases (Meex et al., 2009). Cardiac myocytes and skeletal myocytes have similar regulatory pathways that govern lipid metabolism. Lipid droplet TG can be hydrolyzed by adipose triglyceride lipase/desnutrin (ATGL) and hormone sensitive lipase (HSL), both of which are expressed in the heart. In the adipose tissue, insulin inhibits lipolysis, whereas catecholamines, thyroid hormone, and glucagon stimulate lipolysis. Whether similar regulation occurs in the heart is not known at present.
The roles of lipolytic enzymes in the heart have been studied using genetically modified mice (Table 1). Lipid droplet accumulation in overnight fasting mice is prevented by overexpression of HSL (Suzuki et al., 2001). In the total HSL knockout mouse, cardiac TG lipase activity was decreased, but cardiac TG was not dramatically changed, and there was no overt cardiac phenotype (Osuga et al., 2000). In contrast, Atgl−/− mice have markedly reduced cardiac TG lipase activity, massive lipid accumulation, and severe cardiomyopathy (Haemmerle et al., 2006). In part, this is likely due to a defect in the hydrolysis of TG that the heart stores for potential energy (Banke et al., 2010). Treatment of Atgl−/− mice with a PPARα agonist corrected the cardiac phenotype (Haemmerle et al., 2011). Therefore, the excess accumulation of TG in the Atgl−/− hearts was at least partially due to increased lipid storage secondary to defective FA oxidation. A less dramatic phenotype but one also associated with decreased FA oxidation activation occurred with cardiac deletion of acyl CoA synthetase 1 (Ellis et al., 2011). This study and another from this group (Ellis et al., 2010) suggest that PPAR activation is via a product of the CoA synthetase reaction.
Recent studies have elucidated the role of autophagy in hepatic TG lipolysis. In the rodent heart, autophagy has been investigated as a stress response mechanism in myocardial infarction and pressure induced cardiac hypertrophy. Fasting induces autophagy in the heart (Ogata et al., 2010), yet characterization of autophagy in the heart has not focused on lipid metabolic derangements. Inducible heart-specific autophagy knockouts develop cardiomyopathy (Nakai et al., 2007); whether this is associated with increased lipid accumulation, as has been found in other organs (Singh et al., 2009), is unknown.
Creation of cardiac lipotoxicity in mice
A number of reasons for the association between diabetes and heart dysfunction in the absence of underlying vascular disease have been proposed; one of these is excess accumulation of lipids in cardiomyocytes (Boudina and Abel, 2007). This possible cause of cardiomyopathy has been modeled by creating genetically modified animals in which lipid accumulation without generalized metabolic derangements leads to contractile impairment (Figure 2, Table 1). These animals have an imbalance between lipid uptake and oxidation due to either increased lipid uptake or decreased oxidation. Increased uptake of circulating FFAs or lipoprotein-derived lipids as occurs with transgenic expression of LpL (Nohammer et al., 2003; Yagyu et al., 2003) leads to reduced heart function. Transgenic mice with cardiomyocyte specific expression of fatty acid transport protein 1 (Chiu et al., 2005) and acyl CoA synthetase 1 (Chiu et al., 2001) are thought to have increased FFA uptake or trapping in the heart leading to heart failure. PPAR transcription factors drive FA oxidation; however, the increased lipoprotein-lipid uptake in PPARα transgenic mice (Duncan et al., 2010) must exceed the increased FA oxidation found in this model because the hearts have excess stored lipids. Cardiomyocyte PPARγ overexpression leads to a similar phenotype (Son et al., 2007). Surprisingly, PPARδ expression does not lead to cardiac dysfunction or toxicity (Burkart et al., 2007), presumably because upregulation of the LpL inhibitor angiopoietin-like protein 4 (Angptl4) prevents excess lipid uptake (Georgiadi et al., 2010).
Figure 2. Lipotoxicity is created by an imbalance of lipid uptake and oxidation.
Genetically modified mice have been created that have either increased lipid uptake or decreased oxidation. Uptake occurs via the cell surface molecules lipoprotein lipase (LpL) and perhaps the fatty acid transporter CD36 and/or FATPs. More fatty acids are “trapped” by complexing to CoA. Stored triglyceride accumulates with defective hydrolysis due to deletion of ATGL. Two interventions, loss of PPARδ and high fat feeding in mice overexpressing Glut1, lead to reduced lipid oxidation. Transgenic expression of PPARα and PPARγ, which induce lipid oxidation genes, also cause lipid accumulation, presumably because uptake exceeds oxidation.
LpL is the key enzyme for distribution of circulating lipids between organs. Perhaps the most clinically relevant model of cardiac lipotoxicity is one created by accident. Wang et al. deleted LpL using a skeletal muscle specific promoter (Wang et al., 2009). The mice were meant to model physically inactive humans who also have reduced muscle LpL and FA oxidation. These mice have increased insulin sensitivity in skeletal muscle, as would be expected with reduced FA uptake, but develop insulin resistance in the heart, often a precursor of eventual heart dysfunction.
Reduced lipid oxidation can also lead to lipid accumulation and cardiomyopathy. This occurs with cardiac specific knockout of PPARδ (Cheng et al., 2004) and a cardiac LpL transgene crossed onto the Ppara−/− background (Nohammer et al., 2003). Similarly heart dysfunction with excess lipid accumulation is found when heart-specific GLUT1 overexpressing mice are placed on a high fat diet (Yan et al., 2009). In contrast, when these animals eat chow their function is improved in the presence of hypertension (Liao et al., 2002). So in some situations, dietary driven lipid uptake – a likely accompaniment of our western diet – needs to be added to create a lipotoxic environment.
Perhaps of most interest for understanding heart lipid metabolism and toxicity are the situations where reduced FA oxidation does not lead to lipid accumulation. In many cases the reason for this has not been investigated, but we would presume that there is a compensation, such as a reduction in lipid uptake. This occurs with genetic or pharmacologic deficiency of DGAT1, which markedly reduces CD36 expression (Liu et al., 2011). Pharmacologic FA oxidation inhibitors proposed for reduction of ischemia (Lopaschuk et al., 2010) and genetically engineered defects in FA oxidation defects (Dyck et al., 2006) would be expected to also create cardiac lipid accumulation and toxicity. That this does not occur indicates the existence of some processes that balance reduced oxidation or that lead to non-toxic lipid storage.
Lipid stores and the causes of toxicity
Although the most obvious and easiest to measure accumulated lipid is TG, TG itself might not be toxic. Consistent with cellular studies (Listenberger et al., 2003), several experimental situations have dissociated TG accumulation from toxicity. Total body knockout of HSL was associated with more re-feeding TG accumulation, but no toxicity (Suzuki et al., 2009). A cross of the PPARγ transgene onto the Ppara−/− background corrected toxicity without reducing heart TG, ceramide or DAGs, but redistributed the lipids into larger droplets (Son et al., 2010).
Although the preferred substrate for the heart is FA, a case has been made that excess reliance on FA oxidation is harmful, even under non-ischemic conditions. Some experimental data suggest the opposite. High fat diets, which usually increase reliance of FA oxidation, may be beneficial in the setting of non-ischemic heart failure (Okere et al., 2006b). Increased FA oxidation, has been found with a Pparγ transgene crossed onto the Ppara−/− background (Son et al., 2010), Dgat1 transgenic expression (Liu et al., 2009), and most recently with PPARα agonist treatment of Atgl−/− mice (Haemmerle et al., 2011) all of which improve heart failure.
Heart lipid content can also be increased by non-genetic means. While in some ways these models may more closely reflect human pathology, they suffer from the many other systemic effects of over-nutrition or diabetes. In addition, the heart phenotypes are relatively mild compared to those found with genetic modifications. Mice fed a high fat diet rapidly develop cardiac insulin resistance, suggesting that lipid accumulation rapidly causes changes in heart metabolism allowing it to rely more on FAs as its fuel (Park et al., 2005). But the effects of high fat on cardiac function are mixed. Hypertensive rats fed a 60% fat diet had less left ventricular hypertrophy and systolic dysfunction than animals fed 10% fat (Okere et al., 2006b). Similarly, rats fed a high-fat diet appeared to compensate by increasing FA oxidation, whereas those eating a lower fat but higher carbohydrate western diet developed dysfunction (Wilson et al., 2007). Not all fats are equal. Saturated FA-rich diets alone increased cardiomyocyte apoptosis, perhaps due to accumulation of ceramide (Okere et al., 2006a). In contrast, medium chain FAs are protective against cardiac dysfunction in some situations (Irie et al., 2003; Labarthe et al., 2005).
Other pharmacologic and genetic alterations change heart TG content. Using oxfenicine to block CPT-1, mice fed a diet enriched in long-chain saturated FA accumulated TG, but had no changes in left ventricular dimensions or systolic function, while PPAR-regulated genes were upregulated (Okere et al., 2007). Ob/ob and db/db mice have increased heart FA oxidation that develops prior to hyperglycemia (Buchanan et al., 2005). Eventually these mice develop decreased contractile function (Boudina et al., 2005).
Correction of lipotoxic heart disease
A role of animal models is to test interventions that might be beneficial in human disease. Genetic approaches define targets, which might be amenable to pharmacologic or dietary interventions. Although a common underlying theme of lipotoxic cardiomyopathy is that it is created via an alteration in lipid metabolism, a single underlying toxic lipid species might not be causative in all case. Thus, interventions in one model might not prove to be beneficial in another. Similarly, the genetic and dietary variation amongst humans might also lead to multiple causes of cardiomyopathy associated with lipid accumulation.
Altering the amount or type of FAs acquired by the heart will prevent toxicity both in genetic and dietary models of lipid toxicity (Table 1). Deletion of either CD36 or LpL corrected the cardiac toxicity associated with cardiomyocyte overexpression of PPARα (Duncan et al., 2010; Yang et al., 2007). CD36 deletion was also reported to improve heart function in aged mice that were eating a diet enriched in medium chain fatty acids (Koonen et al., 2007). Medium chain fatty acid-rich diets were also beneficial in PPARα transgenic mice (Finck et al., 2003), presumably because of a reduction in saturated long chain fat-enriched lipid accumulation. Other methods to reduce heart lipid content may also correct or prevent toxicity. The approaches to do that have included transgenic expression of HSL to increase lipolysis of stored lipids (Ueno et al., 2008), reduced expression glycerol-3-phosphate acyltransferase-1 to decrease TG accumulation (Lewin et al., 2008), and overexpression of apolipoproteinB to increase cardiac lipid secretion (Yokoyama et al., 2004) .
A targeted approach to modify one lipid species would seem to be an ideal way to both treat the disease and also define the toxic lipid species. Such an approach was taken by reducing ceramide levels in LpL-overexpressing mice using the serine palmitoyl transferase (SPT) inhibitor myriocin and SPT deficient mice (Park et al., 2008). A similar attempt to specifically modify another toxic lipid, DAG was attempted by overexpressing diacylglycerol acyl transferase 1 (DGAT1) in cardiomyocytes. Although this intervention reduced DAG levels and heart dysfunction, ceramide was also reduced (Liu et al., 2009). This study suggests that alteration of a single lipid species in the heart might be difficult due to the inter-relationship of lipid metabolism pathways.
Evidence that human cardiac dysfunction is associated with excess lipid
Unger has speculated that heart dysfunction due to excess accumulation of lipid, termed lipotoxic cardiomyopathy or fatty heart, is an unappreciated clinical entity (Szczepaniak et al., 2007). While this claim is controversial due to the confounding effects of classical risk factors coexisting in obese people such as physical inactivity, hypertension, hyperlipidemia and diabetes, cardiac lipotoxicity alone or in combination with other risk factors might be an additional pathophysiologic abnormality that develops in obesity. It is argued that patients with type 2 diabetes, metabolic syndrome, and obesity accumulate excess intramyocardial lipid and exhibit decreased systolic or diastolic function (Ernande et al., 2010; Ng et al., 2009). Clinical data show that both obesity and diabetes markedly increase risk of heart failure even in the absence of ischemic vascular disease (Kannel et al., 1974; Regan et al., 1977). The underlying molecular mechanisms could be either increased lipid uptake or impaired mitochondrial oxidative function leading to accumulation of TGs and toxic lipid species such as ceramides, which cause myocyte loss through apoptosis, induction of iNOS and pro-hypertrophic signaling (Unger and Orci, 2001). More recent data have, however, found conflicting results with TG accumulation being detectable both in patients with and without systolic and diastolic cardiac dysfunction and it has been claimed that levels of TG are rather a phenomenon defined by the type of specific cardiomyopathy and not directly related to cardiac dysfunction itself (Ernande et al., 2010; Kankaanpaa et al., 2006; Nakae et al., 2010; Ng et al., 2009). Therefore, the specific form of excess cardiac lipid compounds (TG, ceramides, DAG), their cellular compartmentalization and storage form (lipid droplets), and the specific cause of heart failure are likely to determine the importance of lipotoxicity in human disease.
Studies of human cardiac function and metabolism rely on imaging methods that are relatively non-invasive. PET scanning assesses the uptake of various tracers into the heart. This technique is well standardized for both glucose and FFA uptake. Myocardial PET imaging has consistently shown increased FFA uptake and oxidation, as well as impaired glucose uptake, in diabetic patients with normal systolic and mildly impaired diastolic function (Rijzewijk et al., 2009). More recently, magnetic resonance protocols have been developed to track TG metabolism such as 1H magnetic resonance spectroscopy (MRS) (O'Connor et al., 2011).
Although hypertension and coronary artery disease are common in obese and diabetic patients, reduced heart function independent of these underlying disorders may relate to toxicities from excess metabolic substrates and defective insulin action. Some studies in patients with obesity and diabetes correlated TG accumulation with left ventricular hypertrophy (Herrero et al., 2006; Szczepaniak et al., 2003). More TG has also been found in failing hearts of patients with obesity or diabetes at the time of transplantation (Sharma et al., 2004). Similarly, a modest correlation exists between plasma FFA levels and reduced diastolic function (Leichman et al., 2006). Limited data suggest that weight loss leads to reduced cardiac TG levels and reduced FA uptake as well as improved diastolic function (Hammer et al., 2008; Viljanen et al., 2009).
Reducing plasma lipids to reduce lipid uptake and converting oxidation to more glucose and less FA might be a method to treat patients with lipotoxic and ischemic heart failure. Agents that inhibit FA oxidation have been used for angina. Perhexiline is a drug that blocks carnitine palmitoyl transferase-1 (CPT-1) and CPT-2 and mitochondrial FA uptake. Heart failure patients with both ischemic and non-ischemic heart failure treated with perhexiline had improved cardiac function and symptoms (Lee et al., 2005; Tuunanen et al., 2008). Trimetazidine, another drug thought to reduce FA oxidation, modestly improved heart function and also improved overall insulin sensitivity in patients with idiopathic dilated ischemia (Tuunanen et al., 2008). In contrast, depletion of circulating FAs in order to reduce FA uptake and storage into TGs did not affect cardiac function in patients with heart failure (Halbirk et al., 2010), and in one study actually was harmful (Tuunanen et al., 2006). This latter intervention study measuring myocardial function and metabolism before and after administration of acipimox, a nicotinic acid-like inhibitor of lipolysis, was an acute study in which in each subject served as his or her own control. Therefore, the overall benefit of reducing FA oxidation in heart failure is still unclear and might require studies in which the etiology of the heart failure is more precisely defined.
Conclusions
Although lipid-induced cardiac toxicity clearly can be created, a number of clinical and experimental issues await clarification. We do not know about the exact clinical implications of this pathophysiologic phenomenon. Is it really a primary cause of cardiac dysfunction in patients with type 2 diabetes and metabolic syndrome? If so, does this occur because of unregulated cardiac uptake of FAs or reduced FA oxidation? If the latter, one could then postulate that in the setting of ischemia and afterload-induced heart failure, reduced FA oxidation should also promote accumulation of toxic lipids. Or, as has been postulated in ischemia, does a disproportionate use of FAs create toxicity? Which lipids are really toxic and which pathways are induced that lead to cellular dysfunction? A marriage of human and animal experimentation should sort out the answers to many of these questions in the next decade.
Acknowledgments
Work from the authors’ laboratories was supported by grants HL45095 and 73029 (ijg) and P30 HL101272 (pcs). We would like to thank Dr. Heinrich Taegtmeyer and Dr. Jean Schaffer for review and critique of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Augustus A, Yagyu H, Haemmerle G, Bensadoun A, Vikramadithyan RK, Park SY, Kim JK, Zechner R, Goldberg IJ. Cardiac-specific knock-out of lipoprotein lipase alters plasma lipoprotein triglyceride metabolism and cardiac gene expression. J Biol Chem. 2004;279:25050–25057. doi: 10.1074/jbc.M401028200. [DOI] [PubMed] [Google Scholar]
- Ballard FB, Danforth WH, Naegle S, Bing RJ. Myocardial metabolism of fatty acids. The Journal of clinical investigation. 1960;39:717–723. doi: 10.1172/JCI104088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banke NH, Wende AR, Leone TC, O'Donnell JM, Abel ED, Kelly DP, Lewandowski ED. Preferential oxidation of triacylglyceride-derived fatty acids in heart is augmented by the nuclear receptor PPARalpha. Circ Res. 2010;107:233–241. doi: 10.1161/CIRCRESAHA.110.221713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch LA, Berkowitz DE, Harrison RW, O'Donnell CP, Hare JM. Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice. Circulation. 2003;108:754–759. doi: 10.1161/01.CIR.0000083716.82622.FD. [DOI] [PubMed] [Google Scholar]
- Bartels ED, Nielsen JM, Hellgren LI, Ploug T, Nielsen LB. Cardiac expression of microsomal triglyceride transfer protein is increased in obesity and serves to attenuate cardiac triglyceride accumulation. PLoS One. 2009;4:e5300. doi: 10.1371/journal.pone.0005300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj KG, Hiyama Y, Hu Y, Huggins LA, Ramakrishnan R, Abumrad NA, Shulman GI, Blaner WS, Goldberg IJ. Chylomicron- and VLDL-derived lipids enter the heart through different pathways: in vivo evidence for receptor- and non-receptor-mediated fatty acid uptake. J Biol Chem. 2010;285:37976–37986. doi: 10.1074/jbc.M110.174458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. Journal of lipid research. 2006;47:2726–2737. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- Bosma M, Minnaard R, Sparks LM, Schaart G, Losen M, de Baets MH, Duimel H, Kersten S, Bickel PE, Schrauwen P, et al. The lipid droplet coat protein perilipin 5 also localizes to muscle mitochondria. Histochemistry and cell biology. 2011 doi: 10.1007/s00418-011-0888-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- Boudina S, Sena S, O'Neill BT, Tathireddy P, Young ME, Abel ED. Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation. 2005;112:2686–2695. doi: 10.1161/CIRCULATIONAHA.105.554360. [DOI] [PubMed] [Google Scholar]
- Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, Yun UJ, Cooksey RC, Litwin SE, Abel ED. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology. 2005;146:5341–5349. doi: 10.1210/en.2005-0938. [DOI] [PubMed] [Google Scholar]
- Burkart EM, Sambandam N, Han X, Gross RW, Courtois M, Gierasch CM, Shoghi K, Welch MJ, Kelly DP. Nuclear receptors PPARbeta/delta and PPARalpha direct distinct metabolic regulatory programs in the mouse heart. The Journal of clinical investigation. 2007;117:3930–3939. doi: 10.1172/JCI32578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang BH, Li L, Paul A, Taniguchi S, Nannegari V, Heird WC, Chan L. Protection against fatty liver but normal adipogenesis in mice lacking adipose differentiation-related protein. Molecular and cellular biology. 2006;26:1063–1076. doi: 10.1128/MCB.26.3.1063-1076.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Ding G, Qin Q, Huang Y, Lewis W, He N, Evans RM, Schneider MD, Brako FA, Xiao Y, et al. Cardiomyocyte-restricted peroxisome proliferator-activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat Med. 2004;10:1245–1250. doi: 10.1038/nm1116. [DOI] [PubMed] [Google Scholar]
- Chiu HC, Kovacs A, Blanton RM, Han X, Courtois M, Weinheimer CJ, Yamada KA, Brunet S, Xu H, Nerbonne JM, et al. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ Res. 2005;96:225–233. doi: 10.1161/01.RES.0000154079.20681.B9. [DOI] [PubMed] [Google Scholar]
- Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P, Saffitz JE, Schaffer JE. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001;107:813–822. doi: 10.1172/JCI10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffersen C, Bollano E, Lindegaard ML, Bartels ED, Goetze JP, Andersen CB, Nielsen LB. Cardiac lipid accumulation associated with diastolic dysfunction in obese mice. Endocrinology. 2003;144:3483–3490. doi: 10.1210/en.2003-0242. [DOI] [PubMed] [Google Scholar]
- Coburn CT, Knapp FF, Jr, Febbraio M, Beets AL, Silverstein RL, Abumrad NA. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem. 2000;275:32523–32529. doi: 10.1074/jbc.M003826200. [DOI] [PubMed] [Google Scholar]
- Duncan JG, Bharadwaj KG, Fong JL, Mitra R, Sambandam N, Courtois MR, Lavine KJ, Goldberg IJ, Kelly DP. Rescue of cardiomyopathy in peroxisome proliferator-activated receptor-alpha transgenic mice by deletion of lipoprotein lipase identifies sources of cardiac lipids and peroxisome proliferator-activated receptor-alpha activators. Circulation. 2010;121:426–435. doi: 10.1161/CIRCULATIONAHA.109.888735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck JR, Hopkins TA, Bonnet S, Michelakis ED, Young ME, Watanabe M, Kawase Y, Jishage K, Lopaschuk GD. Absence of malonyl coenzyme A decarboxylase in mice increases cardiac glucose oxidation and protects the heart from ischemic injury. Circulation. 2006;114:1721–1728. doi: 10.1161/CIRCULATIONAHA.106.642009. [DOI] [PubMed] [Google Scholar]
- Ellis JM, Li LO, Wu PC, Koves TR, Ilkayeva O, Stevens RD, Watkins SM, Muoio DM, Coleman RA. Adipose acyl-CoA synthetase-1 directs fatty acids toward beta-oxidation and is required for cold thermogenesis. Cell Metab. 2010;12:53–64. doi: 10.1016/j.cmet.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis JM, Mentock SM, Depetrillo MA, Koves TR, Sen S, Watkins SM, Muoio DM, Cline GW, Taegtmeyer H, Shulman GI, et al. Mouse cardiac acyl coenzyme a synthetase 1 deficiency impairs Fatty Acid oxidation and induces cardiac hypertrophy. Mol Cell Biol. 2011;31:1252–1262. doi: 10.1128/MCB.01085-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernande L, Rietzschel ER, Bergerot C, De Buyzere ML, Schnell F, Groisne L, Ovize M, Croisille P, Moulin P, Gillebert TC, et al. Impaired myocardial radial function in asymptomatic patients with type 2 diabetes mellitus: a speckle-tracking imaging study. J Am Soc Echocardiogr. 2010;23:1266–1272. doi: 10.1016/j.echo.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Finck BN, Han X, Courtois M, Aimond F, Nerbonne JM, Kovacs A, Gross RW, Kelly DP. A critical role for PPARalpha-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc Natl Acad Sci U S A. 2003;100:1226–1231. doi: 10.1073/pnas.0336724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, Han X, Gross RW, Kozak R, Lopaschuk GD, et al. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:121–130. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi K, Nozaki S, Yoshizumi T, Hasegawa S, Uehara T, Nakagawa T, Kobayashi T, Tomiyama Y, Yamashita S, Matsuzawa Y, et al. Enhanced myocardial glucose use in patients with a deficiency in long-chain fatty acid transport (CD36 deficiency) J Nucl Med. 1999;40:239–243. [PubMed] [Google Scholar]
- Georgiadi A, Lichtenstein L, Degenhardt T, Boekschoten MV, van Bilsen M, Desvergne B, Muller M, Kersten S. Induction of cardiac Angptl4 by dietary fatty acids is mediated by peroxisome proliferator-activated receptor beta/delta and protects against fatty acid-induced oxidative stress. Circ Res. 2010;106:1712–1721. doi: 10.1161/CIRCRESAHA.110.217380. [DOI] [PubMed] [Google Scholar]
- Goodwin GW, Taylor CS, Taegtmeyer H. Regulation of energy metabolism of the heart during acute increase in heart work. J Biol Chem. 1998;273:29530–29539. doi: 10.1074/jbc.273.45.29530. [DOI] [PubMed] [Google Scholar]
- Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- Haemmerle G, Moustafa T, Woelkart G, Buttner S, Schmidt A, van de Weijer T, Hesselink M, Jaeger D, Kienesberger PC, Zierler K, et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nature medicine. 2011;17:1076–1085. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbirk M, Norrelund H, Moller N, Schmitz O, Gotzsche L, Nielsen R, Nielsen-Kudsk JE, Nielsen SS, Nielsen TT, Eiskjaer H, et al. Suppression of circulating free fatty acids with acipimox in chronic heart failure patients changes whole body metabolism but does not affect cardiac function. American journal of physiology. Heart and circulatory physiology. 2010;299:H1220–1225. doi: 10.1152/ajpheart.00475.2010. [DOI] [PubMed] [Google Scholar]
- Hammer S, Snel M, Lamb HJ, Jazet IM, van der Meer RW, Pijl H, Meinders EA, Romijn JA, de Roos A, Smit JW. Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases myocardial triglyceride content and improves myocardial function. Journal of the American College of Cardiology. 2008;52:1006–1012. doi: 10.1016/j.jacc.2008.04.068. [DOI] [PubMed] [Google Scholar]
- Herrero P, Peterson LR, McGill JB, Matthew S, Lesniak D, Dence C, Gropler RJ. Increased myocardial fatty acid metabolism in patients with type 1 diabetes mellitus. Journal of the American College of Cardiology. 2006;47:598–604. doi: 10.1016/j.jacc.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nature medicine. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue L, Taegtmeyer H. The Randle cycle revisited: a new head for an old hat. Am J Physiol Endocrinol Metab. 2009;297:E578–591. doi: 10.1152/ajpendo.00093.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie H, Krukenkamp IB, Brinkmann JF, Gaudette GR, Saltman AE, Jou W, Glatz JF, Abumrad NA, Ibrahimi A. Myocardial recovery from ischemia is impaired in CD36-null mice and restored by myocyte CD36 expression or medium-chain fatty acids. Proc Natl Acad Sci U S A. 2003;100:6819–6824. doi: 10.1073/pnas.1132094100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankaanpaa M, Lehto HR, Parkka JP, Komu M, Viljanen A, Ferrannini E, Knuuti J, Nuutila P, Parkkola R, Iozzo P. Myocardial triglyceride content and epicardial fat mass in human obesity: relationship to left ventricular function and serum free fatty acid levels. The Journal of clinical endocrinology and metabolism. 2006;91:4689–4695. doi: 10.1210/jc.2006-0584. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. The American journal of cardiology. 1974;34:29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- Koonen DP, Febbraio M, Bonnet S, Nagendran J, Young ME, Michelakis ED, Dyck JR. CD36 expression contributes to age-induced cardiomyopathy in mice. Circulation. 2007;116:2139–2147. doi: 10.1161/CIRCULATIONAHA.107.712901. [DOI] [PubMed] [Google Scholar]
- Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Labarthe F, Khairallah M, Bouchard B, Stanley WC, Des Rosiers C. Fatty acid oxidation and its impact on response of spontaneously hypertensive rat hearts to an adrenergic stress: benefits of a medium-chain fatty acid. American journal of physiology. Heart and circulatory physiology. 2005;288:H1425–1436. doi: 10.1152/ajpheart.00722.2004. [DOI] [PubMed] [Google Scholar]
- Lee L, Campbell R, Scheuermann-Freestone M, Taylor R, Gunaruwan P, Williams L, Ashrafian H, Horowitz J, Fraser AG, Clarke K, et al. Metabolic modulation with perhexiline in chronic heart failure: a randomized, controlled trial of short-term use of a novel treatment. Circulation. 2005;112:3280–3288. doi: 10.1161/CIRCULATIONAHA.105.551457. [DOI] [PubMed] [Google Scholar]
- Lee Y, Naseem RH, Duplomb L, Park BH, Garry DJ, Richardson JA, Schaffer JE, Unger RH. Hyperleptinemia prevents lipotoxic cardiomyopathy in acyl CoA synthase transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13624–13629. doi: 10.1073/pnas.0405499101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Naseem RH, Park BH, Garry DJ, Richardson JA, Schaffer JE, Unger RH. Alpha-lipoic acid prevents lipotoxic cardiomyopathy in acyl CoA-synthase transgenic mice. Biochemical and biophysical research communications. 2006;344:446–452. doi: 10.1016/j.bbrc.2006.03.062. [DOI] [PubMed] [Google Scholar]
- Leichman JG, Aguilar D, King TM, Vlada A, Reyes M, Taegtmeyer H. Association of plasma free fatty acids and left ventricular diastolic function in patients with clinically severe obesity. The American journal of clinical nutrition. 2006;84:336–341. doi: 10.1093/ajcn/84.1.336. [DOI] [PubMed] [Google Scholar]
- Lewin TM, de Jong H, Schwerbrock NJ, Hammond LE, Watkins SM, Combs TP, Coleman RA. Mice deficient in mitochondrial glycerol-3-phosphate acyltransferase-1 have diminished myocardial triacylglycerol accumulation during lipogenic diet and altered phospholipid fatty acid composition. Biochim Biophys Acta. 2008;1781:352–358. doi: 10.1016/j.bbalip.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao R, Jain M, Cui L, D'Agostino J, Aiello F, Luptak I, Ngoy S, Mortensen RM, Tian R. Cardiac-specific overexpression of GLUT1 prevents the development of heart failure attributable to pressure overload in mice. Circulation. 2002;106:2125–2131. doi: 10.1161/01.cir.0000034049.61181.f3. [DOI] [PubMed] [Google Scholar]
- Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Shi X, Bharadwaj KG, Ikeda S, Yamashita H, Yagyu H, Schaffer JE, Yu YH, Goldberg IJ. DGAT1 expression increases heart triglyceride content but ameliorates lipotoxicity. J Biol Chem. 2009;284:36312–36323. doi: 10.1074/jbc.M109.049817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Yu S, Khan RS, Ables GP, Bharadwaj KG, Hu Y, Huggins LA, Eriksson JW, Buckett LK, Turnbull AV, et al. DGAT1 deficiency decreases PPAR expression and does not lead to lipotoxicity in cardiac and skeletal muscle. J Lipid Res. 2011;52:732–744. doi: 10.1194/jlr.M011395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- Marfella R, Di Filippo C, Portoghese M, Barbieri M, Ferraraccio F, Siniscalchi M, Cacciapuoti F, Rossi F, D'Amico M, Paolisso G. Myocardial lipid accumulation in patients with pressure-overloaded heart and metabolic syndrome. J Lipid Res. 2009;50:2314–2323. doi: 10.1194/jlr.P900032-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavock JM, Lingvay I, Zib I, Tillery T, Salas N, Unger R, Levine BD, Raskin P, Victor RG, Szczepaniak LS. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation. 2007;116:1170–1175. doi: 10.1161/CIRCULATIONAHA.106.645614. [DOI] [PubMed] [Google Scholar]
- Meex RC, Schrauwen P, Hesselink MK. Modulation of myocellular fat stores: lipid droplet dynamics in health and disease. Am J Physiol Regul Integr Comp Physiol. 2009;297:R913–924. doi: 10.1152/ajpregu.91053.2008. [DOI] [PubMed] [Google Scholar]
- Nakae I, Mitsunami K, Yoshino T, Omura T, Tsutamoto T, Matsumoto T, Morikawa S, Inubushi T, Horie M. Clinical features of myocardial triglyceride in different types of cardiomyopathy assessed by proton magnetic resonance spectroscopy: comparison with myocardial creatine. Journal of cardiac failure. 2010;16:812–822. doi: 10.1016/j.cardfail.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- Neely JR, Rovetto MJ, Oram JF. Myocardial utilization of carbohydrate and lipids. Prog Cardiovasc Dis. 1972;15:289–329. doi: 10.1016/0033-0620(72)90029-1. [DOI] [PubMed] [Google Scholar]
- Ng AC, Delgado V, Bertini M, van der Meer RW, Rijzewijk LJ, Shanks M, Nucifora G, Smit JW, Diamant M, Romijn JA, et al. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. The American journal of cardiology. 2009;104:1398–1401. doi: 10.1016/j.amjcard.2009.06.063. [DOI] [PubMed] [Google Scholar]
- Nielsen LB, Veniant M, Boren J, Raabe M, Wong JS, Tam C, Flynn L, Vanni-Reyes T, Gunn MD, Goldberg IJ, et al. Genes for apolipoprotein B and microsomal triglyceride transfer protein are expressed in the heart: evidence that the heart has the capacity to synthesize and secrete lipoproteins. Circulation. 1998;98:13–16. doi: 10.1161/01.cir.98.1.13. [DOI] [PubMed] [Google Scholar]
- Nohammer C, Brunner F, Wolkart G, Staber PB, Steyrer E, Gonzalez FJ, Zechner R, Hoefler G. Myocardial dysfunction and male mortality in peroxisome proliferator-activated receptor alpha knockout mice overexpressing lipoprotein lipase in muscle. Lab Invest. 2003;83:259–269. doi: 10.1097/01.lab.0000053916.61772.ca. [DOI] [PubMed] [Google Scholar]
- O'Connor RD, Xu J, Ewald GA, Ackerman JJ, Peterson LR, Gropler RJ, Bashir A. Intramyocardial triglyceride quantification by magnetic resonance spectroscopy: In vivo and ex vivo correlation in human subjects. Magn Reson Med. 2011;65:1234–1238. doi: 10.1002/mrm.22734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata T, Oishi Y, Higuchi M, Muraoka I. Fasting-related autophagic response in slow- and fast-twitch skeletal muscle. Biochem Biophys Res Commun. 2010;394:136–140. doi: 10.1016/j.bbrc.2010.02.130. [DOI] [PubMed] [Google Scholar]
- Okere IC, Chandler MP, McElfresh TA, Rennison JH, Kung TA, Hoit BD, Ernsberger P, Young ME, Stanley WC. Carnitine palmitoyl transferase-I inhibition is not associated with cardiac hypertrophy in rats fed a high-fat diet. Clin Exp Pharmacol Physiol. 2007;34:113–119. doi: 10.1111/j.1440-1681.2007.04545.x. [DOI] [PubMed] [Google Scholar]
- Okere IC, Chandler MP, McElfresh TA, Rennison JH, Sharov V, Sabbah HN, Tserng KY, Hoit BD, Ernsberger P, Young ME, et al. Differential effects of saturated and unsaturated fatty acid diets on cardiomyocyte apoptosis, adipose distribution, and serum leptin. American journal of physiology. Heart and circulatory physiology. 2006a;291:H38–44. doi: 10.1152/ajpheart.01295.2005. [DOI] [PubMed] [Google Scholar]
- Okere IC, Young ME, McElfresh TA, Chess DJ, Sharov VG, Sabbah HN, Hoit BD, Ernsberger P, Chandler MP, Stanley WC. Low carbohydrate/high-fat diet attenuates cardiac hypertrophy, remodeling, and altered gene expression in hypertension. Hypertension. 2006b;48:1116–1123. doi: 10.1161/01.HYP.0000248430.26229.0f. [DOI] [PubMed] [Google Scholar]
- Osuga J, Ishibashi S, Oka T, Yagyu H, Tozawa R, Fujimoto A, Shionoiri F, Yahagi N, Kraemer FB, Tsutsumi O, et al. Targeted disruption of hormone-sensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. Proc Natl Acad Sci U S A. 2000;97:787–792. doi: 10.1073/pnas.97.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Cho YR, Kim HJ, Higashimori T, Danton C, Lee MK, Dey A, Rothermel B, Kim YB, Kalinowski A, et al. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes. 2005;54:3530–3540. doi: 10.2337/diabetes.54.12.3530. [DOI] [PubMed] [Google Scholar]
- Park TS, Hu Y, Noh HL, Drosatos K, Okajima K, Buchanan J, Tuinei J, Homma S, Jiang XC, Abel ED, et al. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J Lipid Res. 2008;49:2101–2112. doi: 10.1194/jlr.M800147-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A, Chan L, Bickel PE. The PAT family of lipid droplet proteins in heart and vascular cells. Curr Hypertens Rep. 2008;10:461–466. doi: 10.1007/s11906-008-0086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perman JC, Bostrom P, Lindbom M, Lidberg U, StAhlman M, Hagg D, Lindskog H, Scharin Tang M, Omerovic E, Mattsson Hulten L, et al. The VLDL receptor promotes lipotoxicity and increases mortality in mice following an acute myocardial infarction. J Clin Invest. 2011;121:2625–2640. doi: 10.1172/JCI43068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razani B, Zhang H, Schulze PC, Schilling JD, Verbsky J, Lodhi IJ, Topkara VK, Feng C, Coleman T, Kovacs A, et al. Fatty acid synthase modulates homeostatic responses to myocardial stress. The Journal of biological chemistry. 2011;286:30949–30961. doi: 10.1074/jbc.M111.230508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan TJ, Lyons MM, Ahmed SS, Levinson GE, Oldewurtel HA, Ahmad MR, Haider B. Evidence for cardiomyopathy in familial diabetes mellitus. The Journal of clinical investigation. 1977;60:884–899. doi: 10.1172/JCI108843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijzewijk LJ, van der Meer RW, Lamb HJ, de Jong HW, Lubberink M, Romijn JA, Bax JJ, de Roos A, Twisk JW, Heine RJ, et al. Altered myocardial substrate metabolism and decreased diastolic function in nonischemic human diabetic cardiomyopathy: studies with cardiac positron emission tomography and magnetic resonance imaging. Journal of the American College of Cardiology. 2009;54:1524–1532. doi: 10.1016/j.jacc.2009.04.074. [DOI] [PubMed] [Google Scholar]
- Schweiger M, Schreiber R, Haemmerle G, Lass A, Fledelius C, Jacobsen P, Tornqvist H, Zechner R, Zimmermann R. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem. 2006;281:40236–40241. doi: 10.1074/jbc.M608048200. [DOI] [PubMed] [Google Scholar]
- Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, Noon GP, Frazier OH, Taegtmeyer H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004;18:1692–1700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son NH, Park TS, Yamashita H, Yokoyama M, Huggins LA, Okajima K, Homma S, Szabolcs MJ, Huang LS, Goldberg IJ. Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J Clin Invest. 2007;117:2791–2801. doi: 10.1172/JCI30335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son NH, Yu S, Tuinei J, Arai K, Hamai H, Homma S, Shulman GI, Abel ED, Goldberg IJ. PPARgamma-induced cardiolipotoxicity in mice is ameliorated by PPARalpha deficiency despite increases in fatty acid oxidation. The Journal of clinical investigation. 2010;120:3443–3454. doi: 10.1172/JCI40905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- Stowe KA, Burgess SC, Merritt M, Sherry AD, Malloy CR. Storage and oxidation of long-chain fatty acids in the C57/BL6 mouse heart as measured by NMR spectroscopy. FEBS Letters. 2006;580:4282–4287. doi: 10.1016/j.febslet.2006.06.068. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Shen WJ, Nelson BD, Patel S, Veerkamp JH, Selwood SP, Murphy GM, Jr, Reaven E, Kraemer FB. Absence of cardiac lipid accumulation in transgenic mice with heart-specific HSL overexpression. Am J Physiol Endocrinol Metab. 2001;281:E857–866. doi: 10.1152/ajpendo.2001.281.4.E857. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Shen WJ, Nelson BD, Selwood SP, Murphy GM, Jr, Kanehara H, Takahashi S, Oida K, Miyamori I, Kraemer FB. Cardiac gene expression profile and lipid accumulation in response to starvation. Am J Physiol Endocrinol Metab. 2002;283:E94–E102. doi: 10.1152/ajpendo.00017.2002. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Ueno M, Uno M, Hirose Y, Zenimaru Y, Takahashi S, Osuga J-i, Ishibashi S, Takahashi M, Hirose M, et al. Effects of hormone-sensitive lipase disruption on cardiac energy metabolism in response to fasting and refeeding. American Journal of Physiology - Endocrinology And Metabolism. 2009;297:E1115–E1124. doi: 10.1152/ajpendo.91031.2008. [DOI] [PubMed] [Google Scholar]
- Szczepaniak LS, Dobbins RL, Metzger GJ, Sartoni-D'Ambrosia G, Arbique D, Vongpatanasin W, Unger R, Victor RG. Myocardial triglycerides and systolic function in humans: in vivo evaluation by localized proton spectroscopy and cardiac imaging. Magn Reson Med. 2003;49:417–423. doi: 10.1002/mrm.10372. [DOI] [PubMed] [Google Scholar]
- Szczepaniak LS, Victor RG, Orci L, Unger RH. Forgotten but not gone: the rediscovery of fatty heart, the most common unrecognized disease in America. Circ Res. 2007;101:759–767. doi: 10.1161/CIRCRESAHA.107.160457. [DOI] [PubMed] [Google Scholar]
- Tuunanen H, Engblom E, Naum A, Nagren K, Hesse B, Airaksinen KE, Nuutila P, Iozzo P, Ukkonen H, Opie LH, et al. Free fatty acid depletion acutely decreases cardiac work and efficiency in cardiomyopathic heart failure. Circulation. 2006;114:2130–2137. doi: 10.1161/CIRCULATIONAHA.106.645184. [DOI] [PubMed] [Google Scholar]
- Tuunanen H, Engblom E, Naum A, Nagren K, Scheinin M, Hesse B, Juhani Airaksinen KE, Nuutila P, Iozzo P, Ukkonen H, et al. Trimetazidine, a metabolic modulator, has cardiac and extracardiac benefits in idiopathic dilated cardiomyopathy. Circulation. 2008;118:1250–1258. doi: 10.1161/CIRCULATIONAHA.108.778019. [DOI] [PubMed] [Google Scholar]
- Ueno M, Suzuki J, Zenimaru Y, Takahashi S, Koizumi T, Noriki S, Yamaguchi O, Otsu K, Shen WJ, Kraemer FB, et al. Cardiac overexpression of hormone-sensitive lipase inhibits myocardial steatosis and fibrosis in streptozotocin diabetic mice. Am J Physiol Endocrinol Metab. 2008;294:E1109–1118. doi: 10.1152/ajpendo.00016.2008. [DOI] [PubMed] [Google Scholar]
- Unger RH, Orci L. Diseases of liporegulation: new perspective on obesity and related disorders. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2001;15:312–321. doi: 10.1096/fj.00-0590. [DOI] [PubMed] [Google Scholar]
- Viljanen AP, Karmi A, Borra R, Parkka JP, Lepomaki V, Parkkola R, Lautamaki R, Jarvisalo M, Taittonen M, Ronnemaa T, et al. Effect of caloric restriction on myocardial fatty acid uptake, left ventricular mass, and cardiac work in obese adults. The American journal of cardiology. 2009;103:1721–1726. doi: 10.1016/j.amjcard.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Wang H, Knaub LA, Jensen DR, Young Jung D, Hong EG, Ko HJ, Coates AM, Goldberg IJ, de la Houssaye BA, Janssen RC, et al. Skeletal muscle-specific deletion of lipoprotein lipase enhances insulin signaling in skeletal muscle but causes insulin resistance in liver and other tissues. Diabetes. 2009;58:116–124. doi: 10.2337/db07-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Sreenevasan U, Hu H, Saladino A, Polster BM, Lund LM, Gong DW, Stanley WC, Sztalryd C. Perilipin 5, a lipid droplet-associated protein, provides physical and metabolic linkage to mitochondria. Journal of lipid research. 2011;52:2159–2168. doi: 10.1194/jlr.M017939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CR, Tran MK, Salazar KL, Young ME, Taegtmeyer H. Western diet, but not high fat diet, causes derangements of fatty acid metabolism and contractile dysfunction in the heart of Wistar rats. Biochem J. 2007;406:457–467. doi: 10.1042/BJ20070392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagyu H, Chen G, Yokoyama M, Hirata K, Augustus A, Kako Y, Seo T, Hu Y, Lutz EP, Merkel M, et al. Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J Clin Invest. 2003;111:419–426. doi: 10.1172/JCI16751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Young ME, Cui L, Lopaschuk GD, Liao R, Tian R. Increased glucose uptake and oxidation in mouse hearts prevent high fatty acid oxidation but cause cardiac dysfunction in diet-induced obesity. Circulation. 2009;119:2818–2828. doi: 10.1161/CIRCULATIONAHA.108.832915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Sambandam N, Han X, Gross RW, Courtois M, Kovacs A, Febbraio M, Finck BN, Kelly DP. CD36 deficiency rescues lipotoxic cardiomyopathy. Circ Res. 2007;100:1208–1217. doi: 10.1161/01.RES.0000264104.25265.b6. [DOI] [PubMed] [Google Scholar]
- Yokoyama M, Yagyu H, Hu Y, Seo T, Hirata K, Homma S, Goldberg IJ. Apolipoprotein B production reduces lipotoxic cardiomyopathy: studies in heart-specific lipoprotein lipase transgenic mouse. J Biol Chem. 2004;279:4204–4211. doi: 10.1074/jbc.M311995200. [DOI] [PubMed] [Google Scholar]