Abstract
Following its release into the extracellular space in response to metabolic disturbances, the endogenous nucleoside adenosine exerts a range of immunomodulatory effects and cells of the mononuclear phagocyte system are among its major targets. Adenosine governs mononuclear phagocyte functions via 4 G-protein–coupled cell membrane receptors, which are denoted A1, A2A, A2B, and A3 receptors. Adenosine promotes osteoclast differentiation via A1 receptors and alters monocyte to dendritic cell differentiation through A2B receptors. Adenosine downregulates classical macrophage activation mainly through A2A receptors. In contrast A2B receptor activation upregulates alternative macrophage activation. Adenosine promotes angiogenesis, which is mediated by inducing the production of vascular endothelial growth factor by mononuclear phagocytes through A2A, A2B, and A3 receptors. By regulating mononuclear phagocyte function adenosine dictates the course of inflammatory and vascular diseases and cancer.
Keywords: cytokines, G proteins, immune system, immunosuppressive therapy, macrophages
Monocytes, macrophages, and dendritic cells (DCs) are central components of the mononuclear phagocyte system and arise by passing through the common myeloid precursor, the granulocyte/macrophage precursor, and the macrophage/DC progenitor stages in the bone marrow.1 Monocytes and macrophages possess remarkable heterogeneity, which is related to their origin, phenotype, tissue localization, and function.2 Monocytes circulate in the blood and then enter tissues to become macrophages or DCs, where they phagocytose and kill bacteria, scavenge toxic compounds, and present antigens to T lymphocytes. Thus, cells of the mononuclear phagocyte system are major components of the inflammatory response of tissues to infection and injury. Monocytes and macrophages secrete cytokines, free radicals, prostanoids, antimicrobial peptides, proteinases, and angiogenic factors to alarm the tissue to the presence of infectious and injurious stimuli and to orchestrate the ensuing inflammatory response. This inflammatory response is central to ridding the tissue of the infectious or injurious agent and then to laying the groundwork for the restoration of tissue homeostasis. When these processes go awry, tissue homeostasis remains disturbed and chronic inflammatory diseases, such as atherosclerosis, diabetes, and autoimmune diseases, develop. In addition to orchestrating inflammation, macrophages are also important for both the development and normal cellular turnover of organs by virtue of their ability to phagocytose apoptotic cells, cellular debris, and matrix. The goal of this review article is to highlight the role of the adenosine receptor system in regulating mononuclear phagocyte function. We discuss the role of adenosine receptors in governing monocyte differentiation, classical and alternative macrophage activation, and the angiogenic switch.
Adenosine and Its Receptors
Extracellular adenosine generates a constellation of tissue responses that can be generally viewed as organ protective thereby mediating homeostasis.3 Following its release from the intracellular into the extracellular space, adenosine exerts a range of immunomodulatory effects and cells of the mononuclear phagocyte system are among its major targets.4,5 Adenosine is an essential component of intracellular metabolic pathways and can be released into the extracellular space in response to both metabolic disturbances and other types of insults, which include, inflammation, physical damage, and apoptosis.6 The release of adenosine can occur by 2 mechanisms: via cell membrane embedded adenosine/nucleoside transporters and as constituent of ATP and ADP, which can be released by a number of mechanisms, including membrane damage, through connexin/pannexin and other channels, and via protein or hormone-transporting vesicles. Once ATP and ADP are released, the phosphate groups of extracellular ATP and ADP are sequentially split off, first by nucleoside triphosphate diphosphorylases (NT-PDases, including CD39) and then by 5′-ectonucleotidase (Ecto5′Ntase, CD73).7 In addition to host cells, pathogens such as Staphylococcus aureus,8 Enteropathogenic Escherichia coli,9 and Trichomonas vaginalis10 are equipped with ectonucleotidases, and excessive adenosine generation by these ectonucleotidases represents a means for these pathogens to subvert the host immune system thereby aiding their propagation. Adenosine governs cellular functions via 4 G-protein–coupled cell membrane receptors, which have been denoted A1, A2A, A2B, and A3 receptors.6 In general, A1 and A3 receptors are coupled to pertussis toxin-inhibited Gi-coupled signal transduction proteins and their activation leads to decreased intracellular cAMP levels. A2A receptors are traditionally appreciated as GαS- or Gαolf-linked receptors that stimulate adenylyl cyclase, cAMP accumulation, and protein kinase A activation, but A2A receptors can also signal independently of G proteins. A2B receptors can couple to both GαS and Gq. It is now appreciated that adenosine receptor expression is dynamically altered during both development and activation on the surface of macrophages and DCs. The differential expression of adenosine receptors at various stages of inflammation is important for fine-tuning adenosine receptor responsiveness to maximize their ability to alter cell function in a way that generally leads to the restoration of homeostasis.
Adenosine and Monocyte Differentiation
Adenosine has been shown to regulate the differentiation and maturation of cells of the mononuclear phagocyte system. In the presence of adenosine, phorbol myristate acetate-differentiated monocytes develop into multinucleated giant cells via A1 receptor stimulation, whereas A2 receptor activation prevents the generation of giant cells.11 Similarly, A1 receptor activation promotes the formation of multinucleated osteoclasts from monocytes differentiated with colony stimulating factor-1 and receptor activator of NF-κB ligand (RANKL).12 Consistent with these in vitro results, A1 receptor inactivation prevents bone loss in a murine model for postmenopausal osteoporosis.13
Recent evidence indicates that A2B receptor stimulation induces aberrant development of DCs from granulocyte-macrophage colony-stimulating factor and interleukin (IL)-4–differentiated hematopoietic progenitor cells and monocytes.14 These “adenosine-differentiated” DCs develop into a cell type that fails to downregulate its surface expression of the monocytic marker CD14 despite upregulating the DC marker CD1a. These aberrant DCs are impaired in their ability to induce T-cell proliferation and are proangiogenic and have been recently shown to contribute to tumor growth in a variety of cancer models.15,16
Adenosine Regulation of Classical Macrophage Activation
The phenotype and activation state of mature macrophage populations is defined by chemical cues encountered during the differentiation process, which include growth factors, microbial pathogen-associated molecular patterns, tumor products, extracellular matrix, as well as T helper (Th)1 or Th2 effector T cells and their secretory products. The well-established “classical activation” or proinflammatory macrophage profile occurs in a Th1 cytokine-rich environment (interferon-γ, tumor necrosis factor [TNF]-α) or on recognition of pathogen-associated molecular patterns (lipopolysaccharide, lipoproteins, dsRNA, lipoteichoic acid, etc.) and endogenous “danger” signals (heat shock proteins, etc.).17 As such, the proinflammatory phenotype of these macrophages plays an important role in protection against intracellular pathogens, and under certain conditions also cancer cells. Classically activated macrophages (caMϕ or M1) typically produce high levels of TNF-α and IL-12 and low levels of IL-10 and are consequently strong promoters of Th1 immune responses.18 In addition, these cells exert antiproliferative and cytotoxic activities, resulting partly from their ability to secrete reactive nitrogen and oxygen species (nitric oxide, peroxynitrite, hydrogen peroxide, superoxide) and proinflammatory cytokines (TNF-α, IL-1, IL-6).
Consistent with its generally restorative function in tissues, adenosine receptor activation has been repeatedly shown to have effects that prevent excessive classical macrophage activation thereby resulting in tissue protection (Figure). Although adenosine has been shown to decrease expression of several proinflammatory mediators by macrophages, including IL-12,19 macrophage inflammatory protein-1α,20 nitric oxide,21,22 and superoxide,23 the best understood of these antiinflammatory effects is the capacity of adenosine to decrease the production of TNF-α. Results of both human and murine studies confirmed that adenosine suppresses monocyte and macrophage TNF-α production.24 The dominant adenosine receptor mediating this suppressive effect is the A2A receptor,19,25–29 but based on results with both selective A2A and A2B receptor ligands and A2A and A2B receptor knockout macrophages, it is now apparent that A2B receptors can also contribute, especially in murine systems.25,30,31 These results are also consistent with observations that the expression of A2A receptor predominates over A2B in human macrophages28 and expression of both A2A and A2B receptors is highest in murine macrophages.25,26
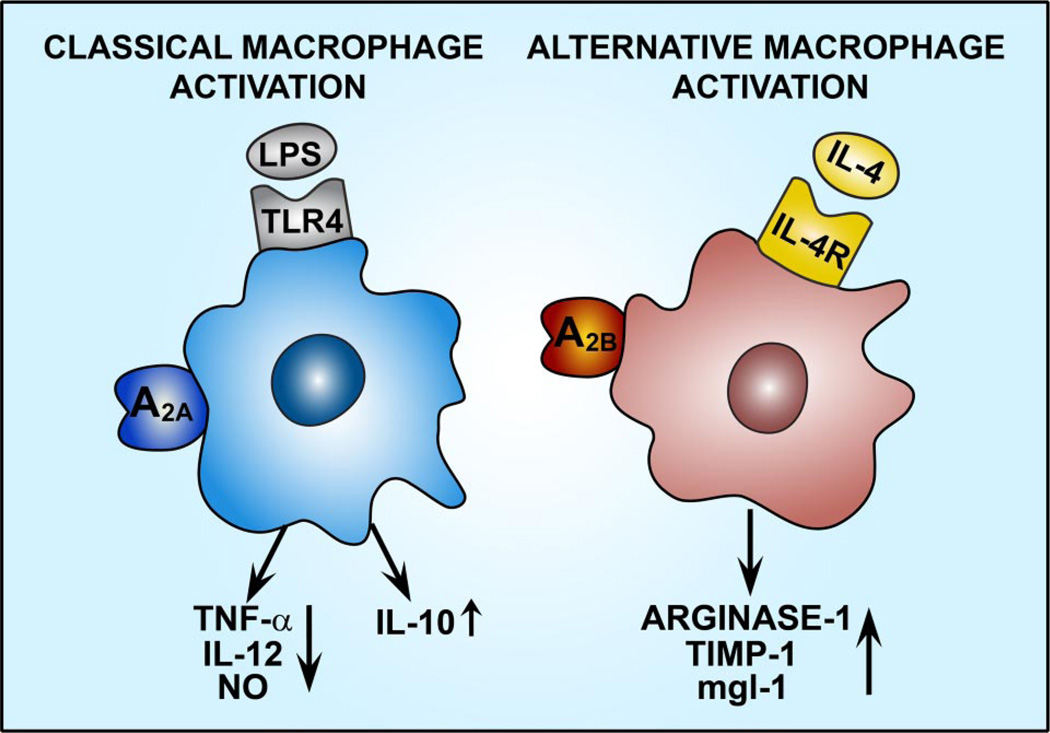
Figure.
A2A receptor activation suppresses tumor necrosis factor-α (TNF-α), interleukin (IL)-12, and nitric oxide (NO) production and augments IL-10 production by classically activated macrophages. A2B receptor activation augments arginase-1, tissue inhibitor of metalloproteinases-1 (TIMP-1), and mgl-1 expression in alternatively activated macrophages. IL-4R indicates IL-4 receptor; mgl-1, macrophage galactose-type C-type lectin; TLR4, toll-like receptor 4.
The intracellular mechanisms of suppression, however, remain incompletely understood. In murine alveolar macrophages stimulated with hyaluronan fragments, adenosine receptor activation decreased TNF-α mRNA levels via exchange protein activated by cAMP (Epac)-1, but independently of PKA.27 In contrast, in murine peritoneal macrophages stimulated with lipopolysaccharide, adenosine receptor stimulation suppressed TNF-α mRNA transcription independently of both Epac and PKA.32 Surprisingly, the adenosine suppression of TNF-α production was reversed by okadaic acid indicating involvement of phosphatases.32 Finally, a recent line of investigation uncovered an important role for heme oxygenase-1 and its product carbon monoxide in contributing to the adenosine-mediated suppression of TNF-α production by macrophages. Studies by Haschemi et al33 and Weis et al34 have revealed that heme oxygenase-1 and carbon monoxide upregulate the expression of A2A receptors on macrophages and these increased levels of A2A receptors, in turn, sensitize macrophages to the TNF-α–suppressing effect of adenosine.
In contrast to the suppressive effect of adenosine on the production of proinflammatory mediators, adenosine augments production of the antiinflammatory cytokine IL-10.3 We recently demonstrated a pivotal role for A2A receptors, as adenosine failed to upregulate E. coli-induced IL-10 secretion by murine peritoneal macrophages lacking A2A receptors but not WT macrophages.35 This upregulation of IL-10 production was achieved through a CCAAT-enhancer-binding protein β-mediated transcriptional process. In the RAW264.7 macrophage cell line, which expresses low basal levels of A2A receptors, adenosine augments IL-10 production via an A2B receptor-mediated posttranscriptional mechanism.36
In an inflammatory environment, ATP supply by anaerobic glycolysis is an essential source of energy for macrophages. In classically activated macrophages, adenosine increases glycolytic flux by upregulating expression of the pfkb3 isozyme of 6-phosphofructo-1-kinase.37 Both A2A and A2B receptors are involved in mediating the effect of adenosine and the transcription factor Specificity Protein 1 appears to play a central role in the adenosine induction of pfkb3 expression. Thus, in addition to deactivating classically activated macrophages, A2A and A2B receptors support macrophage metabolism, which may enable the macrophages to persist in the tissue and switch their phenotype to alternatively activated macrophages to participate in tissue restoration.
Adenosine Regulation of Alternative Macrophage Activation
In contrast to a Th1 millieu, a Th2 cytokine-rich environment, which arises during parasitic disease and wound healing imparts immunomodulatory and antiinflammatory rather than proinflammatory properties on macrophages. These macrophages generated in the presence of the Th2 cytokines IL-4 and/or IL-13 are termed alternatively activated or M2a.17,38 Exposure to Th2 cytokines, such as IL-4 and IL-13 triggers a specific gene expression pattern in macrophages, and the most prominent gene that is induced is arginase-1.39 Arginase-1 metabolizes arginine to urea and ornithine, and ornithine can then be used for proline and collagen synthesis resulting in extracellular matrix deposition and fibrosis.40 Importantly, arginase-1 can outcompete the other major arginine-utilizing enzyme, inducible nitric oxide synthase, for the substrate in alternatively activated macrophages, and, thus, decrease nitric oxide production. Other genes that are often induced are genes whose products are involved in matrix remodeling such as tissue inhibitor of metalloproteinases-1 (TIMP-1).41 TIMP-1 regulates tissue remodeling by inhibiting extracellular matrix-degrading matrix metalloproteinases. In addition, TIMP-1 has complex effects on cell growth and mutations in the matrix metalloproteinases-inhibitory domain of TIMP-1 fail to abrogate the effects of TIMP-1 on cell growth and survival. Finally, macrophage galactose-type C-type lectin (mgl)-1,42 Ym1 (chitinase-like-3), and found in inflammatory zone (Fizz)1 are also signature markers of alternatively activated macrophages.43 By virtue of their ability to produce matrix remodeling and resolution-inducing mediators, alternatively activated macrophages are essential participants in tissue remodeling and the resolution of inflammation and are prevalent in parasitic disease and during wound healing.
We recently found that adenosine enhances IL-4– and IL-13–induced alternative macrophage activation (Figure).41 The effect of adenosine on alternative macrophage activation is less broad than the wide-ranging inhibitory effects of adenosine on classical macrophage activation, as adenosine increased the expression of arginase-1, TIMP-1, and mgl-1, but not that of Ym1 and Fizz1. The stimulatory effects of adenosine on alternative macrophage activation are mediated chiefly by A2B receptors, which contrasts with the predominant role of A2A receptors in regulating classical macrophage activation.41 Of the transcription factors known to mediate alternative macrophage activation, CCAAT-enhancer-binding protein β was required, whereas cAMP response element-binding protein and signal transducer and activator of transcription 6 were dispensable in mediating the stimulatory effect of adenosine.41
The term alternative macrophage activation is sometimes more widely used and describes various anti-inflammatory macrophage phenotypes induced by immune complexes (M2b), as well as heterogeneous deactivating stimuli such as apoptotic cells, glucocorticoids, and IL-10 (M2c).38 In macrophages engulfing apoptotic cells, endogenously released adenosine activates A2A receptors and suppresses the production of the proinflammatory chemokines macrophage inflammatory protein-2 and cytokine-induced neutrophil-attracting chemokine (KC).44 In addition, glucocorticoids induce survival of antiinflammatory monocytes via upregulation and autocrine stimulation of A3 receptors.45 Thus, in macrophages exposed to apoptotic cells and glucocorticoids, adenosine shifts macrophage phenotype from proinflammatory to antiinflammatory.
Adenosine and the Angiogenic Switch
Vascular endothelial growth factor (VEGF) is a potent inducer of angiogenesis and vascular permeability and is vital for endothelial cell differentiation during vasculogenesis, and for the sprouting of new capillaries from preexisting blood vessels.46 VEGF is thus an important component of tissue repair and is critical for the resolution of injury through the process of wound healing. Macrophages are primary producers of VEGF during wound healing, as well as in chronic inflammation and cancer.47 There is an increasing line of evidence that adenosine can accelerate angiogenesis, in part, by augmenting VEGF production by macrophages.48–50 Because adenosine boosts VEGF production and decreases proinflammatory cytokine production by toll-like receptor-activated murine macrophages, thereby shifting macrophages away from a proinflammatory to an angiogenic phenotype, we termed this process angiogenic switch.50 The synergistic stimulatory effect of adenosine and TLR activation on VEGF production is A2A receptor-dependent in murine macrophages.50–52 The stimulatory effect of adenosine on VEGF production involves transcriptional induction of the VEGF promoter by the transcription factor hypoxia-inducible factor-1α through hypoxia response element.53 Unlike hypoxic stabilization of hypoxia-inducible factor-1α protein,46 adenosine increases the activation of hypoxia-inducible factor-1α by upregulating its mRNA levels through a mechanism that is mostly post-transcriptional and is controlled by upstream activation of PKC and phosphoinositide 3-kinase.52,53
Adenosine can induce VEGF mRNA level upregulation in54 and VEGF secretion by54,55 human monocytes and macrophages and pathogen-associated molecular patterns and adenosine additively or synergistically increase VEGF production by human macrophages.55 The adenosine-mediated increase in VEGF secretion by human monocytes appears to be mediated by A2A, A2B, and A3 receptors and appears to be secondary to increased hypoxia-inducible factor-1α expression.54,55
Finally, it is noteworthy that VEGF induction is likely the most important but not the only mechanism by which adenosine promotes angiogenesis. For example, adenosine has recently been shown to inhibit the macrophage production of sFlt, which is the antiangiogenic, soluble form of the VEGF receptor.56
Conclusions
As indicated above, adenosine receptors modulate disease activity in a wide range of pathophysiological processes that involve mononuclear phagocytes, such as tumors, bone disease, and wound healing. There is also emerging evidence that cells of the mononuclear phagocyte system are important components of the protective effects of adenosine receptor agonists against vascular disease. For example, recent evidence shows that A2A receptor stimulation reduces inflammation and neointimal growth in a murine carotid ligation model and that protection is associated with decreased macrophage recruitment and function.57 Similarly, A2B receptors prevented neointima formation following femoral artery injury, and the protective action of A2B receptors was paralleled by attenuated levels of macrophage-derived proinflammatory cytokines.58 In contrast to these adenosine receptor-mediated protective effects, A2A receptors were found to contribute to the formation of atherosclerotic lesions in apolipoprotein E-deficient mice, which was explained by an A2A receptor-induced decrease in macrophage (foam cell) apoptosis.59 A better understanding of how mononuclear phagocyte function is regulated by adenosine receptors will assist in the design of novel therapeutic modalities to treat vascular and inflammatory diseases as well as cancer.
Acknowledgments
Sources of Funding
This work was supported by National Institutes of Health Grant R01GM66189 (to G.H.), USAMRMC grant log #09065004 (Contract W81XWH-10-1–1015) (to G.H.), and Intramural Research Program of NIH/NIAAA (to P.P.).
Footnotes
Disclosures
None.
References
- 1.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nature Reviews Immunology. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ernst PB, Garrison JC, Thompson LF. Much ado about adenosine: Adenosine synthesis and function in regulatory t cell biology. J Immunol. 2010;185:1993–1998. doi: 10.4049/jimmunol.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine a2a receptors. Annu Rev Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 6.Fredholm BB, AP IJ, Jacobson KA, Klotz KN, Linden J. International union of pharmacology: XXV. Nomenclature and classification of adenosine receptors. Pharmacological reviews. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 7.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 8.Thammavongsa V, Kern JW, Missiakas DM, Schneewind O. Staphylococcus aureus synthesizes adenosine to escape host immune responses. Journal of Experimental Medicine. 2009;206:2417–2427. doi: 10.1084/jem.20090097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crane JK, Olson RA, Jones HM, Duffey ME. Release of ATP during host cell killing by enteropathogenic E. coli and its role as a secretory mediator. American journal of physiology Gastrointestinal and liver physiology. 2002;283:G74–G86. doi: 10.1152/ajpgi.00484.2001. [DOI] [PubMed] [Google Scholar]
- 10.Tasca T, Bonan CD, Carli GA, Battastini AM, Sarkis JJ. Characterization of an ecto-5′-nucleotidase (ec 3.1.3.5) activity in intact cells of trichomonas vaginalis. Experimental Parasitology. 2003;105:167–173. doi: 10.1016/j.exppara.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Merrill JT, Shen C, Schreibman D, Coffey D, Zakharenko O, Fisher R, Lahita RG, Salmon J, Cronstein BN. Adenosine a1 receptor promotion of multinucleated giant cell formation by human monocytes: A mechanism for methotrexate-induced nodulosis in rheumatoid arthritis. Arthritis and Rheumatism. 1997;40:1308–1315. doi: 10.1002/1529-0131(199707)40:7<1308::AID-ART16>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 12.Kara FM, Chitu V, Sloane J, Axelrod M, Fredholm BB, Stanley ER, Cronstein BN. Adenosine a1 receptors (a1rs) play a critical role in osteoclast formation and function. FASEB J. 2010;24:2325–2333. doi: 10.1096/fj.09-147447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kara FM, Doty SB, Boskey A, Goldring S, Zaidi M, Fredholm BB, Cronstein BN. Adenosine a(1) receptors regulate bone resorption in mice: Adenosine a(1) receptor blockade or deletion increases bone density and prevents ovariectomy-induced bone loss in adenosine a(1) receptor-knockout mice. Arthritis and Rheumatism. 2010;62:534–541. doi: 10.1002/art.27219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novitskiy SV, Ryzhov S, Zaynagetdinov R, Goldstein AE, Huang Y, Tikhomirov OY, Blackburn MR, Biaggioni I, Carbone DP, Feoktistov I, Dikov MM. Adenosine receptors in regulation of dendritic cell differentiation and function. Blood. 2008;112:1822–1831. doi: 10.1182/blood-2008-02-136325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryzhov S, Novitskiy SV, Zaynagetdinov R, Goldstein AE, Carbone DP, Biaggioni I, Dikov MM, Feoktistov I. Host a(2b) adenosine receptors promote carcinoma growth. Neoplasia. 2008;10:987–995. doi: 10.1593/neo.08478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cekic C, Sag D, Li Y, Theodorescu D, Strieter RM, Linden J. Adenosine a2b receptor blockade slows growth of bladder and breast tumors. Journal of Immunology. 2011 doi: 10.4049/jimmunol.1101845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon S. Alternative activation of macrophages. Nature Reviews Immunology. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 18.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nature Immunology. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 19.Hasko G, Kuhel DG, Chen JF, Schwarzschild MA, Deitch EA, Mabley JG, Marton A, Szabo C. Adenosine inhibits il-12 and tnf-[alpha] production via adenosine a2a receptor-dependent and independent mechanisms. FASEB J. 2000;14:2065–2074. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- 20.Szabo C, Scott GS, Virag L, Egnaczyk G, Salzman AL, Shanley TP, Hasko G. Suppression of macrophage inflammatory protein (mip)-1alpha production and collagen-induced arthritis by adenosine receptor agonists. Br J Pharmacol. 1998;125:379–387. doi: 10.1038/sj.bjp.0702040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasko G, Szabo C, Nemeth ZH, Kvetan V, Pastores SM, Vizi ES. Adenosine receptor agonists differentially regulate il-10, TNF-alpha, and nitric oxide production in raw 264.7 macrophages and in endotoxemic mice. J Immunol. 1996;157:4634–4640. [PubMed] [Google Scholar]
- 22.Barnholt KE, Kota RS, Aung HH, Rutledge JC. Adenosine blocks ifn-gamma-induced phosphorylation of STAT1 on serine 727 to reduce macrophage activation. Journal of Immunology. 2009;183:6767–6777. doi: 10.4049/jimmunol.0900331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends in Immunology. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Hasko G, Pacher P, Deitch EA, Vizi ES. Shaping of monocyte and macrophage function by adenosine receptors. Pharmacol Ther. 2007;113:264–275. doi: 10.1016/j.pharmthera.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kreckler LM, Wan TC, Ge ZD, Auchampach JA. Adenosine inhibits tumor necrosis factor-alpha release from mouse peritoneal macrophages via a2a and a2b but not the a3 adenosine receptor. J Pharmacol Exp Ther. 2006;317:172–180. doi: 10.1124/jpet.105.096016. [DOI] [PubMed] [Google Scholar]
- 26.Ryzhov S, Zaynagetdinov R, Goldstein AE, Novitskiy SV, Blackburn MR, Biaggioni I, Feoktistov I. Effect of A2B adenosine receptor gene ablation on adenosine-dependent regulation of proinflammatory cytokines. J Pharmacol Exp Ther. 2008;324:694–700. doi: 10.1124/jpet.107.131540. [DOI] [PubMed] [Google Scholar]
- 27.Scheibner KA, Boodoo S, Collins S, Black KE, Chan-Li Y, Zarek P, Powell JD, Horton MR. The adenosine A2A receptor inhibits matrix-induced inflammation in a novel fashion. Am J Respir Cell Mol Biol. 2009;40:251–259. doi: 10.1165/rcmb.2008-0168OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buenestado A, Grassin Delyle S, Arnould I, Besnard F, Naline E, Blouquit-Laye S, Chapelier A, Bellamy JF, Devillier P. The role of adenosine receptors in regulating production of tumour necrosis factor-alpha and chemokines by human lung macrophages. British Journal of Pharmacology. 2010;159:1304–1311. doi: 10.1111/j.1476-5381.2009.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang JG, Hepburn L, Cruz G, Borman RA, Clark KL. The role of adenosine a2a and a2b receptors in the regulation of tnf-alpha production by human monocytes. Biochemical Pharmacology. 2005;69:883–889. doi: 10.1016/j.bcp.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Chen H, Yang D, Carroll SH, Eltzschig HK, Ravid K. Activation of the macrophage a2b adenosine receptor regulates tumor necrosis factor-alpha levels following vascular injury. Experimental Hematology. 2009;37:533–538. doi: 10.1016/j.exphem.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belikoff BG, Hatfield S, Georgiev P, Ohta A, Lukashev D, Buras JA, Remick DG, Sitkovsky M. A2b adenosine receptor blockade enhances macrophage-mediated bacterial phagocytosis and improves polymicrobial sepsis survival in mice. Journal of Immunology. 2011;186:2444–2453. doi: 10.4049/jimmunol.1001567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kreckler LM, Gizewski E, Wan TC, Auchampach JA. Adenosine suppresses lipopolysaccharide-induced tumor necrosis factor-alpha production by murine macrophages through a protein kinase a- and exchange protein activated by camp-independent signaling pathway. Journal of Pharmacology and Experimental Therapeutics. 2009;331:1051–1061. doi: 10.1124/jpet.109.157651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haschemi A, Wagner O, Marculescu R, Wegiel B, Robson SC, Gagliani N, Gallo D, Chen JF, Bach FH, Otterbein LE. Cross-regulation of carbon monoxide and the adenosine A2A receptor in macrophages. Journal of Immunology. 2007;178:5921–5929. doi: 10.4049/jimmunol.178.9.5921. [DOI] [PubMed] [Google Scholar]
- 34.Weis N, Weigert A, von Knethen A, Brune B. Heme oxygenase-1 contributes to an alternative macrophage activation profile induced by apoptotic cell supernatants. Molecular Biology of the Cell. 2009;20:1280–1288. doi: 10.1091/mbc.E08-10-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Csoka B, Nemeth ZH, Selmeczy Z, Koscso B, Pacher P, Vizi ES, Deitch EA, Hasko G. Role of a(2a) adenosine receptors in regulation of opsonized e. Coli-induced macrophage function. Purinergic Signal. 2007;3:447–452. doi: 10.1007/s11302-007-9075-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nemeth ZH, Lutz CS, Csoka B, Deitch EA, Leibovich SJ, Gause WC, Tone M, Pacher P, Vizi ES, Hasko G. Adenosine augments il-10 production by macrophages through an a2b receptor-mediated posttranscriptional mechanism. J Immunol. 2005;175:8260–8270. doi: 10.4049/jimmunol.175.12.8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruiz-Garcia A, Monsalve E, Novellasdemunt L, Navarro-Sabate A, Manzano A, Rivero S, Castrillo A, Casado M, Laborda J, Bartrons R, Diaz-Guerra MJ. Cooperation of adenosine with macrophage toll-4 receptor agonists leads to increased glycolytic flux through the enhanced expression of pfkfb3 gene. Journal of Biological Chemistry. 2011;286:19247–19258. doi: 10.1074/jbc.M110.190298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Frontiers in Bioscience: A Journal and Virtual Library. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 39.Murray PJ, Wynn TA. Obstacles and opportunities for understanding macrophage polarization. Journal of Leukocyte Biology. 2011;89:557–563. doi: 10.1189/jlb.0710409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Albina JE, Mills CD, Henry WL, Henry WL, Caldwell MD. Temporal expression of different pathways of 1-arginine metabolism in healing wounds. J Immunol. 1990;144:3877–3880. [PubMed] [Google Scholar]
- 41.Csoka B, Selmeczy Z, Koscso B, Nemeth ZH, Pacher P, Murray PJ, Kepka-Lenhart D, Morris SM, Jr, Gause WC, Leibovich SJ, Hasko G. Adenosine promotes alternative macrophage activation via a2a and a2b receptors. FASEB J. 2011 doi: 10.1096/fj.11-190934. PMID: 21926236 [PubMed - as supplied by publisher]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raes G, Brys L, Dahal BK, Brandt J, Grooten J, Brombacher F, Vanham G, Noel W, Bogaert P, Boonefaes T, Kindt A, Van den Bergh R, Leenen PJ, De Baetselier P, Ghassabeh GH. Macrophage galactose-type c-type lectins as novel markers for alternatively activated macrophages elicited by parasitic infections and allergic airway inflammation. J Leukoc Biol. 2005;77:321–327. doi: 10.1189/jlb.0304212. [DOI] [PubMed] [Google Scholar]
- 43.Kreider T, Anthony RM, Urban JF, Urban JF, Gause WC. Alternatively activated macrophages in helminth infections. Curr Opin Immunol. 2007;19:448–453. doi: 10.1016/j.coi.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koroskenyi K, Duro E, Pallai A, Sarang Z, Kloor D, Ucker DS, Beceiro S, Castrillo A, Chawla A, Ledent CA, Fesus L, Szondy Z. Involvement of adenosine A2A receptors in engulfment-dependent apoptotic cell suppression of inflammation. Journal of immunology. 2011;186:7144–7155. doi: 10.4049/jimmunol.1002284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barczyk K, Ehrchen J, Tenbrock K, Ahlmann M, Kneidl J, Viemann D, Roth J. Glucocorticoids promote survival of anti-inflammatory macrophages via stimulation of adenosine receptor A3. Blood. 2010;116:446–455. doi: 10.1182/blood-2009-10-247106. [DOI] [PubMed] [Google Scholar]
- 46.Semenza GL. Vascular responses to hypoxia and ischemia. Arterioscler Thromb Vasc Biol. 2010;30:648–652. doi: 10.1161/ATVBAHA.108.181644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin P, Leibovich SJ. Inflammatory cells during wound repair: The good, the bad and the ugly. Trends in Cell Biology. 2005;15:599–607. doi: 10.1016/j.tcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Leibovich SJ, Chen JF, Pinhal-Enfield G, Belem PC, Elson G, Rosania A, Ramanathan M, Montesinos C, Jacobson M, Schwarzschild MA, Fink JS, Cronstein B. Synergistic up-regulation of vascular endothelial growth factor expression in murine macrophages by adenosine A(2A) receptor agonists and endotoxin. Am J Pathol. 2002;160:2231–2244. doi: 10.1016/S0002-9440(10)61170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adair TH. Growth regulation of the vascular system: An emerging role for adenosine. Am J Physiol Regul Integr Comp Physiol. 2005;289:R283–RR296. doi: 10.1152/ajpregu.00840.2004. [DOI] [PubMed] [Google Scholar]
- 50.Pinhal-Enfield G, Ramanathan M, Hasko G, Vogel SN, Salzman AL, Boons GJ, Leibovich SJ. An angiogenic switch in macrophages involving synergy between toll-like receptors 2, 4, 7, and 9 and adenosine a(2a) receptors. Am J Pathol. 2003;163:711–721. doi: 10.1016/S0002-9440(10)63698-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramanathan M, Luo W, Csoka B, Hasko G, Lukashev D, Sitkovsky MV, Leibovich SJ. Differential regulation of hif-1alpha isoforms in murine macrophages by TLR4 and adenosine A(2A) receptor agonists. Journal of Leukocyte Biology. 2009;86:681–689. doi: 10.1189/jlb.0109021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Ponti C, Carini R, Alchera E, Nitti MP, Locati M, Albano E, Cairo G, Tacchini L. Adenosine A2A receptor-mediated, normoxic induction of HIF-1 through PKC and pi-3k-dependent pathways in macrophages. Journal of Leukocyte Biology. 2007;82:392–402. doi: 10.1189/jlb.0107060. [DOI] [PubMed] [Google Scholar]
- 53.Ramanathan M, Pinhal-Enfield G, Hao I, Leibovich SJ. Synergistic up-regulation of vascular endothelial growth factor (VEGF) expression in macrophages by adenosine a2a receptor agonists and endotoxin involves transcriptional regulation via the hypoxia response element in the vegf promoter. Mol Biol Cell. 2007;18:14–23. doi: 10.1091/mbc.E06-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gessi S, Fogli E, Sacchetto V, Merighi S, Varani K, Preti D, Leung E, Maclennan S, Borea PA. Adenosine modulates HIF-1{alpha}, VEGF, IL-8, and foam cell formation in a human model of hypoxic foam cells. ATVB. 2010;30:90–97. doi: 10.1161/ATVBAHA.109.194902. [DOI] [PubMed] [Google Scholar]
- 55.Ernens I, Leonard F, Vausort M, Rolland-Turner M, Devaux Y, Wagner DR. Adenosine up-regulates vascular endothelial growth factor in human macrophages. Biochemical and Biophysical Research Communications. 2010;392:351–356. doi: 10.1016/j.bbrc.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 56.Leonard F, Devaux Y, Vausort M, Ernens I, Rolland-Turner M, Wagner DR. Adenosine modifies the balance between membrane and soluble forms of FLT-1. Journal of Leukocyte Biology. 2011;90:199–204. doi: 10.1189/jlb.0910505. [DOI] [PubMed] [Google Scholar]
- 57.McPherson JA, Barringhaus KG, Bishop GG, Sanders JM, Rieger JM, Hesselbacher SE, Gimple LW, Powers ER, Macdonald T, Sullivan G, Linden J, Sarembock IJ. Adenosine a(2a) receptor stimulation reduces inflammation and neointimal growth in a murine carotid ligation model. Arteriosclerosis, Thrombosis, and Vascular Biology. 2001;21:791–796. doi: 10.1161/01.atv.21.5.791. [DOI] [PubMed] [Google Scholar]
- 58.Yang D, Koupenova M, McCrann DJ, Kopeikina KJ, Kagan HM, Schreiber BM, Ravid K. The A2B adenosine receptor protects against vascular injury. Proc Natl Acad Sci U S A. 2008;105:792–796. doi: 10.1073/pnas.0705563105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang H, Zhang W, Zhu C, Bucher C, Blazar BR, Zhang C, Chen JF, Linden J, Wu C, Huo Y. Inactivation of the adenosine a2a receptor protects apolipoprotein e-deficient mice from atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:1046–1052. doi: 10.1161/ATVBAHA.109.188839. [DOI] [PMC free article] [PubMed] [Google Scholar]