Abstract
Background
Little information is available about factors associated with racial differences across a broad spectrum of post–myocardial infarction outcomes, including patients’ symptoms and quality of life.
Objective
To determine racial differences in mortality, rehospitalization, angina, and quality of life after myocardial infarction and identify the factors associated with these differences.
Design
Prospective cohort study.
Setting
10 hospitals in the United States.
Patients
1849 patients who had myocardial infarction, 28% of whom were black.
Measurements
Demographic, economic, clinical, psychosocial, and treatment characteristics and outcomes were prospectively collected. Outcomes included time to 2-year all-cause mortality, 1-year rehospitalization, and Seattle Angina Questionnaire–assessed angina and quality of life.
Results
Black patients had higher unadjusted mortality (19.9% vs. 9.3%; P < 0.001) and rehospitalization rates (45.4% vs. 40.4%; P = 0.130), more angina (28.0% vs. 17.8%; P < 0.001), and worse mean quality of life (80.6 [SD, 22.5] vs. 85.9 [SD, 17.2]; P < 0.001). After adjusting for patient characteristics, black patients trended toward greater mortality (hazard ratio, 1.29 [95% CI, 0.92 to 1.81]; P = 0.142), fewer rehospitalizations (hazard ratio, 0.82 [CI, 0.66 to 1.02]; P = 0.071), higher likelihood of angina at 1 year (odds ratio, 1.41 [CI, 1.03 to 1.94]; P = 0.032), but similar quality of life (mean difference, −0.6 [CI, −3.4 to 2.2]). Adjusting for site further attenuated mortality differences (hazard ratio, 1.04 [CI, 0.71 to 1.52]; P = 0.84). Adjustment for treatments had minimal effect on any association.
Limitation
Residual confounding and missing data may have introduced bias.
Conclusion
Although black patients with myocardial infarction have worse outcomes than white patients, these differences did not persist after adjustment for patient factors and site of care. Further adjustment for treatments minimally influenced observed differences. Strategies that focus on improving baseline cardiac risk and hospital factors may do more to attenuate racial differences in myocardial infarction outcomes than treatment-focused strategies.
Consistent with the national priority of eradicating racial disparities in health care (1–3), many studies have examined differences in treatment and outcomes between black and white patients with acute coronary syndromes (4–7). Such studies are important for documenting differences in care and disparities in outcomes; however, they have not provided much insight into what patient or treatment characteristics are most associated with the observed differences in outcomes. Although causation is difficult to establish from observational studies, such studies can illuminate patient characteristics or processes of care that attenuate observed disparities in outcomes and form an important basis for the design and testing of subsequent interventions that can minimize such disparities.
Moreover, death and readmission after a myocardial infarction are not the only relevant outcomes. From the patients’ perspectives, their health status (symptoms, function, and quality of life) is equally, or more, important (8–12). Although most previous studies that examined mortality outcomes have not found differences in survival after adjusting for demographic, socioeconomic, clinical, and treatment factors (13–20), the only study to examine health status outcomes found worse fully adjusted 1-year health status outcomes among black patients (21). Underscoring the importance of evaluating the magnitude and mediators of racial differences in outcomes, a recent study of U.S. residents found that health status differences in black patients with and without coronary heart disease were greater than those of white patients, which emphasizes the need for more research to “address predictors and determinants of optimum [health-related quality of life] as a guide to developing interventions aimed at minimizing impairments in … health status” (22).
Achieving racial equity in outcomes requires research to illuminate the root cause of observed disparities in outcomes, including factors that explain the differences. We conducted a multicenter observational study to investigate racial differences in outcomes, including health status, in the year after an acute myocardial infarction. We further analyzed patient, hospital, and treatment characteristics that may mediate the association between race and outcome to determine potential factors that might account for observed disparities and to serve as a foundation for future studies that seek to eradicate such disparities.
Methods
Patient Population
A detailed discussion of the purpose, conceptual framework of data collection, patient selection, generalizability, and site characteristics of the PREMIER (Prospective Registry Evaluating Myocardial Infarction: Events and Recovery) study has been published (23). Patients were eligible for participation if they were age 18 years or older, had myocardial infarction, and were admitted with biochemical evidence of myocardial necrosis and prolonged (>20 minutes) symptoms of myocardial ischemia or diagnostic electrocardiography changes to 1 of 19 hospitals. An institutional review board approved the study at each participating center and patients signed informed consent for baseline and follow-up interviews. We restricted our analyses to white and black patients and only included the 10 centers that enrolled at least 10 patients in each racial group. In addition, we excluded any patients who died during the initial hospitalization, to focus on long-term outcomes as a target for future quality improvement efforts.
Data Collection
During the index myocardial infarction admission, trained data collectors performed chart abstractions and a detailed baseline patient interview that included the patient’s self-identified racial category, our primary independent variable. Racial categories mirrored the classifications used by the Congressional Office of Management and Budget. The PREMIER study was designed to quantify a broad range of potential mediators of outcomes, including demographic characteristics, patients’ health, economic and psychosocial status, comorbid medical conditions, disease severity, site, treatments (both quality of care [24] and invasive procedures), discharge medications, and discharge instructions (Table). For such domains as depression (25), optimism (26, 27), social support (28, 29), and economic status (30), validated instruments were used (23).
Table.
Baseline Characteristics
| Characteristic | Race Category | P Value | |
|---|---|---|---|
| White (n = 1335) | Black (n = 514) | ||
| Mean age (SD), y | 61.7 (12.9) | 57.3 (13.2) | <0.001 |
| Men, n (%) | 942 (70.6) | 283 (55.1) | <0.001 |
| Socioeconomic status | |||
| High school education, n (%) | 1078 (82.3) | 309 (61.8) | <0.001 |
| Health insurance, n (%) | 1158 (90.5) | 369 (77.2) | <0.001 |
| Insurance coverage for medications, n (%) | 1026 (78.0) | 325 (64.4) | <0.001 |
| Monthly financial situation, n (%) | <0.001 | ||
| Some money left over | 768 (60.4) | 137 (27.8) | |
| Just enough to make ends meet | 363 (28.6) | 197 (40.0) | |
| Not enough to make ends meet | 140 (11.0) | 159 (32.3) | |
| Medical costs have been an economic burden, n (%) | 367 (27.9) | 203 (40.4) | <0.001 |
| Have avoided getting health care due to cost, n (%) | 230 (17.7) | 124 (24.8) | <0.001 |
| Have not taken medication due to cost, n (%) | 156 (11.9) | 103 (20.4) | <0.001 |
| Social support | |||
| Married, n (%) | 897 (68.0) | 178 (35.7) | <0.001 |
| Mean ENRICHD social support score (SD) | 29.7 (5.5) | 28.4 (6.4) | <0.001 |
| Psychological status | |||
| Mean Patient Health Questionnaire depression score (SD) | 5.2 (5.2) | 6.7 (6.0) | <0.001 |
| Mean Life Orientation Test-Revised optimism score (SD) | 15.9 (3.7) | 15.3 (3.4) | 0.004 |
| Medical history | |||
| Mean body mass index (SD), kg/m2 | 29.2 (6.4) | 29.1 (7.1) | 0.87 |
| Diabetes, n (%) | 335 (25.1) | 207 (40.3) | <0.001 |
| Chronic heart failure, n (%) | 109 (8.2) | 135 (26.3) | <0.001 |
| Chronic lung disease, n (%) | 188 (14.1) | 79 (15.4) | 0.48 |
| Current smoker, n (%)* | 431 (32.6) | 208 (41.1) | <0.001 |
| Chronic renal failure, n (%) | 79 (5.9) | 138 (26.8) | <0.001 |
| Arthritis, n (%) | 166 (12.4) | 61 (11.9) | 0.74 |
| Cancer (other than skin), n (%) | 114 (8.5) | 29 (5.6) | 0.037 |
| Family history of coronary artery disease, n (%) | 490 (36.7) | 135 (26.3) | <0.001 |
| Hypertension, n (%) | 801 (60.0) | 406 (79.0) | <0.001 |
| Hypercholesterolemia, n (%) | 681 (51.0) | 212 (41.2) | <0.001 |
| History of vascular disease, n (%)† | 428 (32.1) | 181 (35.2) | 0.196 |
| Previous cerebrovascular accident, n (%) | 73 (5.5) | 57 (11.1) | <0.001 |
| Disease severity | |||
| ST-segment elevation myocardial infarction on presentation, n (%) | 652 (48.8) | 124 (24.1) | <0.001 |
| Mean peak troponin level (SD), µg/L | 104.3 (260.3) | 29.8 (105.7) | <0.001 |
| Multivessel coronary artery disease, n (%) | 558 (45.1) | 124 (34.8) | <0.001 |
| Moderate or severe left ventricular systolic dysfunction, n (%) | 324 (24.3) | 155 (30.3) | 0.009 |
| Mean TIMI ST-segment elevation myocardial infarction risk score (SD) | 3.2 (2.3) | 3.5 (2.1) | 0.22 |
| Mean TIMI unstable angina/non-ST-segment elevation myocardial infarction risk score (SD) | 3.3 (1.4) | 2.9 (1.3) | <0.001 |
| Mean heart rate on admission (SD), beats/min | 79.1 (20.3) | 85.9 (22.3) | <0.001 |
| Mean systolic blood pressure on admission (SD), mm Hg | 136.7 (29.5) | 143.8 (33.5) | <0.001 |
| Mean estimated glomerular filtration rate on admission (SD), ml/min per 1.73 m2 | 74.5 (26.5) | 68.3 (38.0) | <0.001 |
| Mean hematocrit on admission (SD) | 40.4 (5.8) | 38.4 (6.6) | <0.001 |
| Mean glucose on admission (SD) | 0.047 | ||
| mmol/L | 8.3 (4.1) | 8.8 (5.8) | |
| mg/dL | 149.4 (74.3) | 158.1 (104.6) | |
| Invasive procedures | |||
| Diagnostic catheterization, n (%) | 1236 (92.6) | 356 (69.3) | <0.001 |
| Revascularization | <0.001 | ||
| None, n (%) | 255 (19.1) | 296 (57.6) | |
| Percutaneous coronary intervention, n (%) | 922 (69.1) | 195 (37.9) | |
| Coronary artery bypass grafting, n (%) | 158 (11.8) | 23 (4.5) | |
| Quality of care§ | |||
| Aspirin within 24 hours | 1270 (97.3) | 479 (96.2) | 0.21 |
| β-blocker within 24 hours | 1139 (93.7) | 406 (89.8) | 0.006 |
| Acute reperfusion‡ | 527 (74.9) | 79 (46.2) | <0.001 |
| Aspirin at discharge | 1237 (95.3) | 434 (89.7) | <0.001 |
| β-blocker at discharge | 1188 (93.3) | 403 (88.4) | <0.001 |
| ACE inhibitor or angiotensin-receptor blocker for LVSD at discharge | 303 (90.4) | 121 (81.8) | 0.007 |
| Smoking cessation counseling | 352 (76.5) | 122 (58.1) | <0.001 |
| Mean eligible quality-of-care indicators (SD), n | 5.3 (1.3) | 4.8 (1.3) | <0.001 |
| Mean eligible quality-of-care indicators received (SD), % | 89.4 (15.0) | 83.6 (20.4) | <0.001 |
| Other discharge medications | |||
| Statin, % | 1110 (83.1) | 371 (72.2) | <0.001 |
| Thienopyridine, % | 1002 (75.1) | 249 (48.4) | <0.001 |
| Diuretic, % | 237 (17.8) | 150 (29.2) | <0.001 |
| Nitrate, % | 352 (26.4) | 192 (37.4) | <0.001 |
| Discharge instructions | |||
| Discharge medication instructions, % | 1197 (89.7) | 458 (89.1) | 0.73 |
| Whom to call if symptoms worsen, % | 912 (68.3) | 341 (66.3) | 0.42 |
| Exercise counseling, % | 817 (61.2) | 168 (32.7) | <0.001 |
| Diet counseling, % | 1071 (80.2) | 395 (76.8) | 0.108 |
| Follow-up appointment scheduled, % | 1275 (95.5) | 487 (94.7) | 0.49 |
ACE = angiotensin-converting enzyme; ENRICHD = ENhanced Recovery In Coronary Heart Disease; TIMI = Thrombolysis in Myocardial Infarction.
<30 d.
Myocardial infarction, percutaneous coronary intervention, or coronary artery bypass grafting.
Among patients with ST-segment elevation myocardial infarction.
Among patients eligible for each measure.
Outcome Assessment
Telephone interviews were conducted at 1, 6, and 12 months. Interviews included the Seattle Angina Questionnaire (SAQ), a 19-item health status measure that quantifies a patient’s coronary artery disease–specific health status, including frequency of angina and quality of life (31, 32). Scores on these domains range from 0 to 100, with higher scores representing fewer symptoms and better quality of life. The SAQ is valid, reliable, responsive, and prognostic of subsequent mortality and acute coronary syndromes (33). In addition, the interviewers asked about interval hospitalizations since the patient’s last contact. Mortality was assessed through the Social Security Administration Death Master File as of 30 June 2006.
Statistical Analysis
We compared 59 patient, hospital, and treatment factors between black and white patients, classified into 11 domains (Table) according to 3 phases of myocardial infarction care: patient characteristics present before seeking care (demographic characteristics, socioeconomic status, social support, medical history, psychological factors, and disease severity), the presenting hospital, and processes of care received in-hospital (invasive treatments, quality of care performance measures, discharge medications, and discharge instructions). We assessed 4 outcomes: time to mortality from any cause through 2 years, time to rehospitalization for any cause through 1 year, presence of angina at 1 year (defined by an SAQ angina frequency score <100), and 1-year SAQ Quality of Life score. We compared continuous variables by using t tests and categorical variables by using chi-square tests. We summarized time to mortality and rehospitalization by using Kaplan–Meier methods and compared them by using log-rank tests.
We evaluated the contribution of each of the 11 domains to observed racial differences in outcomes by sequential, cumulative adjustment. In the first step, we adjusted only for demographic characteristics; in the second, for demographic characteristics and socioeconomic status; and so on up to the final step, in which we simultaneously adjusted for all domains. Comparing the adjusted race estimates between adjacent steps thus indicates the incremental contribution of a given domain after adjusting for all previous variables (for example, the contribution of presenting hospital after adjusting for all patient factors). We designed the order of the domains to reflect the temporal relationship of the factors, adjusting first for patient characteristics, then location of care, and finally processes of care.
We used propensity score methods to perform the adjustments. At each step, we estimated propensity scores for being black by using nonparsimonious logistic regression on all variables up to and including the current domain. We entered continuous variables into the propensity model nonlinearly by using cubic splines. We evaluated overlap of propensity scores between groups by using histograms and scatterplots. We examined 3 methods of adjusting for propensity score: regression (inclusion of the propensity scores as a covariate in the outcome models), stratification by first digit of the propensity score, and matching. For regression adjustment, we used the logit of the propensity score and allowed for nonlinearity by using restricted cubic splines. For matching, we used the method of optimal full matching implemented in the R package optmatch, which allows for a variable number of black and white patients in matched strata (34, 35). We matched on the logit of the propensity score by using a caliper width of 0.2 times the pooled standard deviation of the logits (36). Of these 3 methods, regression adjustment had the best balancing properties, as assessed by adjusted chi-square values and P values for each of the 59 covariates on race (mean P = 0.92 [range, 0.37 to 0.99] versus 0.90 [range, 0.41 to 0.99] for stratification and 0.79 [range, 0.23 to 0.99] for matching in the final model). Furthermore, even though stratification and matching yielded similar results to those obtained by using regression adjustment, they also produced more erratic race effect estimates from step to step because of the variability in the matched or stratified groups obtained at each step. We therefore chose regression adjustment as our primary method of analysis.
We estimated race effects (black versus white) by using proportional hazards models for time to mortality and time to rehospitalization, logistic regression for angina, and linear models for SAQ Quality of Life. We included the propensity score as a covariate at each step of adjustment. For the angina and quality of life models, we also included the associated baseline SAQ score.
The first 6 adjustment steps included patient-related factors only, not location or processes of care, and thus represent population-average estimates of racial differences across all sites in the study. We used robust standard errors to account for within-site correlations. Beginning with the seventh step, all models adjusted for hospital by using stratified proportional hazards regression for mortality and rehospitalization, conditional logistic regression for angina, and a hierarchical linear model for quality of life, including within-center effects for race. Thus, the race effect estimates in the last 5 steps (presenting hospital, invasive treatments, quality of care, discharge medications, and discharge instructions) are hospital-specific (37).
Because previous research (38, 39) has documented the association of lower socioeconomic status (which is known to be more prevalent in black patients with myocardial infarction) with worse clinical outcomes, we repeated our analyses using only those with a self-reported annual household income of <$30 000 and formally tested race-by-income interaction terms in the entire cohort. We also replicated the analyses stratifying by type of myocardial infarction (ST-segment elevation vs. non–ST-segment elevation myocardial infarctions) and found no differences compared with the primary analysis.
Approximately 39% of patients had missing covariate data (26% were missing only 1 value, 10% were missing 2, and 3% were missing 3 or more; the highest missing rate for any single variable was 14%). Twenty-two percent of the 1-year assessments were incomplete because the patient died (8%), declined to participate (3%), or was lost to follow-up (11%). To correct for biases due to observed factors, we used multiple imputation methods in all analyses, incorporating all variables listed in the Table; site of enrollment; 1-, 6-, and 12-month health status scores; rehospitalization; and death during follow-up. We generated 10 imputed data sets, replicated our analyses on each, and pooled the results. The primary analyses presented here include imputation of baseline covariates but are restricted to patients with complete follow-up data. We also conducted sensitivity analyses that included full imputation of all baseline and outcome data; these yielded results that were similar to those presented.
We used SAS, version 9.1.3 (SAS Institute, Cary, North Carolina), and R, version 2.7.0 (Foundation for Statistical Computing, Vienna, Austria), to conduct our analyses. All analyses were prespecified and a 2-sided P value less than 0.05 denoted statistical significance.
Role of the Funding Source
This study was supported by the National Heart, Lung, and Blood Institute Specialized Center of Clinically Oriented Research in Cardiac Dysfunction and Disease (grant no. P50 HL077113); CV Therapeutics, Palo Alto, California; and Cardiovascular Outcomes, Kansas City, Missouri. The study was designed, executed, analyzed, interpreted, and reported by the investigators without input from the sponsors.
Results
Between 1 January 2003 and 28 June 2004, we prospectively screened 2498 patients with myocardial infarction and enrolled them into PREMIER. We excluded 129 patients of other or unknown race, as well as sites that enrolled fewer than 10 black or 10 white patients (9 sites, comprising 491 white patients and 15 black patients). In addition, we excluded 14 patients (0.8%)—1 black, 13 white—who died in the hospital. Thus, the present analyses include 1849 patients with myocardial infarction from 10 centers, of whom 514 (27.8%) were black (range, 5% to 93% per site).
The Table shows the differences in patient and treatment characteristics between white and black patients. On average, black patients were younger, more likely to be female, had more comorbid conditions, were more likely to present with non–ST-segment elevation myocardial infarction, and had worse socioeconomic and psychosocial status than white patients. Black patients were also less likely to be treated with current quality of care indicators and invasive therapy.
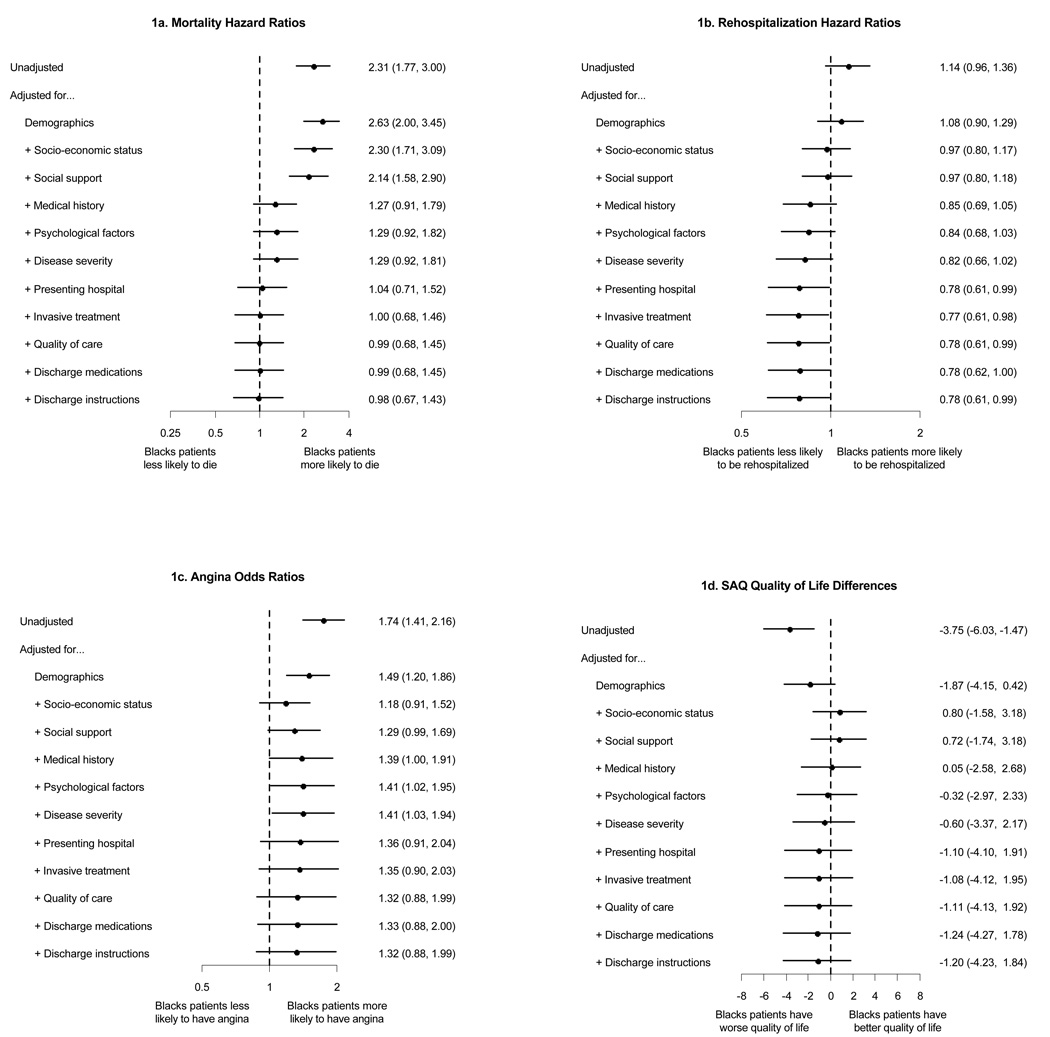
Two-year survival status was available for 99% of patients, and mean follow-up time was 26 months (SD, 8 months). Unadjusted Kaplan–Meier estimates of 2-year mortality were 19.9% for black patients versus 9.3% for white patients, and the crude hazard ratio (HR) was 2.31 (CI, 1.77 to 3.00) (Figure, A). The HR decreased to 1.29 (CI, 0.92 to 1.81) after we adjusted for patient factors, and was nearly completely attenuated after we adjusted for presenting hospital (HR, 1.04 [CI, 0.71 to 1.52]). In-hospital treatments had no further influence on racial differences in mortality (fully adjusted HR, 0.98 [CI, 0.67 to 1.43]). Although adjusting for demographic characteristics slightly increased the estimated mortality risk in black patients because of their younger age, the 2 most significant shifts in the risk estimates (attenuations of racial differences in mortality) occurred when we adjusted for comorbid medical conditions and presenting hospital.
Figure 1.
Racial differences in post–myocardial infarction outcomes. SAQ = Seattle Angina Questionnaire.
Unadjusted Kaplan–Meier estimates of 1-year rehospitalization rates were 45.4% for black patients versus 40.4% for white patients (HR, 1.14 [CI, 0.96 to 1.36]) (Figure, B). After adjusting for patient factors, however, blacks were less likely than whites to be hospitalized within 1 year (HR, 0.82 [CI, 0.66 to 1.02]). Socioeconomic status and comorbid medical conditions were associated with the largest attenuations. Neither the presenting hospital nor treatment had much effect on racial differences in rehospitalization rates (fully adjusted HR, 0.78 [CI, 0.61 to 0.99]).
In unadjusted analyses, black patients were significantly more likely to have angina 1 year after their myocardial infarction than white patients (28.0% versus 17.8%; odds ratio [OR], 1.80 [CI, 1.35 to 2.39]) (Figure, C). The risk was similar after we adjusted for angina symptoms at baseline (SAQ angina frequency score) (OR, 1.74 [CI, 1.41 to 2.16]). Adjusting for patient factors attenuated the difference by about 40% (OR, 1.41 [CI, 1.02 to 1.95]), which mostly persisted even after adjusting for hospital and treatments, although the fully adjusted effect was not statistically significant (OR, 1.32 [CI, 0.88 to 1.99]). We observed the largest attenuation after we adjusted for racial differences in socioeconomic status.
The mean unadjusted 1-year quality of life of black patients was significantly worse than that of white patients (SAQ Quality of Life score, 80.6 [SD, 22.5] vs. 85.9 [SD, 17.2]; mean difference, −5.3 [CI, −7.6 to −2.9]) (Figure, D). The difference was similar after we adjusted for baseline quality of life (mean, −3.8 [CI, −6.0 to −1.5]). Patient factors accounted for most of the difference (adjusted mean difference, −0.6 [CI, −3.4 to 2.2]), and further adjustment for hospital and treatments did not influence this (fully adjusted mean difference, −1.2 [CI, −4.2 to 1.8]). Demographic characteristics and socioeconomic status attenuated racial differences in quality of life the most.
Given the importance of socioeconomic status in attenuating the unadjusted racial differences in outcomes, we conducted a secondary analysis restricted to the 625 patients (260 black and 365 white) with a self-reported annual household income <$30 000. The unadjusted differences in outcomes in this cohort were nearly identical to those observed in the overall population. Specifically, the 2-year mortality rate for black patients was 21.5% versus 10.4% for white patients (HR, 2.19 [CI, 1.45 to 3.31]), the rehospitalization rate was 51.5% versus 45.1% (HR, 1.10 [CI, 0.85 to 1.43]), the rate of angina at 1 year was 32.5% versus 21.8% (OR, 1.73 [CI, 1.12 to 2.67]), and the mean 1-year SAQ Quality of Life scores were 78.5 (SD, 22.6) versus 83.5 (SD, 19.8) (mean difference, −5.0 [CI, −9.1 to 0.8]). Fully adjusted models showed a trend toward greater residual mortality among black patients (HR, 1.49 [CI, 0.80 to 2.75]), as well as significant residual angina (OR, 1.96 [CI, 1.03 to 3.75]), although no outcome showed a significant race-by-income interaction in the overall cohort (P > 0.26 for all).
Black patients were significantly more likely than whites to have incomplete 1-year assessments (37.2% versus 16.4%; P < 0.001), primarily because of greater 1-year mortality (14.2% vs. 5.5%) and losses to follow-up (19.1% versus 7.9%). To examine the effect of incomplete follow-up on racial differences in rehospitalization and health status outcomes (we established death on the basis of social security number queries, which were 99% complete), we expanded our multiple imputation analyses to include imputation of outcomes as well. In these fully imputed analyses, the odds ratios for angina at 1 year among black versus white patients were similar to those in the complete data cohort: 2.26 (CI, 1.68 to 3.04) unadjusted, 1.41 (CI, 1.03 to 1.94) after adjustment for patient factors, and 1.20 (CI, 0.87 to 1.66) after full adjustment. Quality of life and rehospitalization results were nearly identical to those among patients with complete 1-year assessments.
Discussion
We found that black patients had higher crude rates of death and rehospitalization, greater frequency of angina, and worse quality of life after a myocardial infarction than did white patients. The unadjusted outcome differences were clinically significant and demonstrate that, on average, black patients bear a disproportionate burden of adverse outcomes after myocardial infarction. We also determined that the characteristics and treatment of black and white patients differ significantly. After full adjustment for these differences, black patients had similar mortality, quality of life, and frequency of angina and a trend toward lower rates of rehospitalization. In general, the outcome differences between black and white patients were most attenuated by adjusting for patient characteristics present before admission (such as socioeconomic status and comorbid conditions) and were only marginally affected by adjusting for differences in treatment. Our findings suggest that racial disparities in outcome are associated with a myriad of racial differences in risk factors for adverse outcomes and that focusing on the processes of care for myocardial infarction may not be the most effective strategy for achieving equity in outcomes.
In contrast to the racial disparities in other outcomes, we also found that admitting hospital explained nearly all of the residual racial differences in 2-year mortality after we adjusted for patient characteristics—an effect previously described by Skinner and colleagues (40). Although this may be attributable to unmeasured patient characteristics that vary by geographic region, it may also indicate unmeasured variations in hospital quality and performance or disparities in outpatient management. Larger multicenter studies should further explore between-center differences in care that may be modified to improve racial disparities in mortality.
Our study expands the traditional outcomes used to evaluate racial disparities by including assessments of patients’ symptoms and quality of life. Whereas previous studies of racial disparities have focused on survival (13–20), patients are often equally, if not more, interested in their symptoms, function, and quality of life (9, 10, 41–43). We demonstrate that not only do black patients with myocardial infarction have poorer survival rates, but those who do survive are more likely to have angina and worse quality of life after their myocardial infarction. The persistent disparities in quality of life that we observed in a previous study (21) were attenuated in this multicenter study, which included more centers and a broader spectrum of characteristics that differ by race. Fortunately, several interventions exist to improve the health status of patients with coronary artery disease (44–46). In particular, the recent results of the COURAGE trial (44) suggest that optimal medical therapy, with or without revascularization, can have a dramatic effect on improving symptoms and quality of life in patients with chronic angina. Although this study did not explicitly examine differences in access to care after myocardial infarction discharge, for which black and white patients are known to differ (47–49), it seems logical that following patients after a myocardial infarction for persistent symptoms or diminished quality of life could identify candidates for more intensive treatment and potentially reduce the unadjusted differences in health status that we observed.
An observational study cannot conclusively demonstrate causation when associating a patient’s characteristics with outcomes. This is particularly true for race, which could be a marker for a myriad of potential risk factors that contribute to outcomes after a myocardial infarction. After sequentially adjusting for a broad range of clinical, socioeconomic, and psychosocial differences between black and white patients who had myocardial infarction, we found few residual disparities in outcome, which suggests that the worse outcomes of black patients can probably be attributed to a greater prevalence of other risk factors for poorer outcomes, rather than an inherent characteristic of race itself. Because some of these patient characteristics (such as socioeconomic disparities or comorbid conditions) may be partially attributable to race, these analyses serve to illuminate potential explanations for the observed differences in outcomes rather than to provide unconfounded estimates of the effect of race on outcome. For example, it has previously been noted that poor socioeconomic status is both more prevalent among black persons and associated with worse outcome (38, 39). Because lower socioeconomic status may be associated with poorer access to care or adherence to secondary prevention, it is possible that improving access and adherence may reduce racial disparities in outcomes. However, even among patients in the lowest economic class, black patients had worse unadjusted survival, angina control, and quality of life than similarly disadvantaged white patients, which suggests that racial differences, even among the poorest patients, will require additional interventions to achieve equity.
Our study suggests that no single omission in the care offered black patients would, if overcome, eradicate the crude differences in observed outcomes. Previous investigators (4–7) have extensively documented racial differences in the use of invasive treatments; however, adjusting for these procedures had little effect on the relative risk for adverse outcomes among black patients. Although a recent study (50) suggested that differences in the transfer of black Medicare patients and the use of revascularization may account for the adverse mortality observed among black patients, their investigation, which was based on administrative claims data, could not include the depth of clinical and socioeconomic data of our prospectively conducted study. Given the importance of socioeconomic status and comorbid medical conditions in attenuating the racial differences in outcomes that we observed, further research is needed to identify the mechanisms of these associations and achieve greater equity in clinical outcomes. For example, interventions directed at primary prevention and prevention of comorbid conditions, such as diabetes and renal disease, may be effective in minimizing observed racial disparities in outcomes. Therefore, although we do not address the complexity of integrating socioeconomic and clinical considerations when selecting care for individual patients, we do suggest that public policies that merely address in-hospital treatments may not achieve their desired intent of eliminating disparities in outcomes.
Several aspects of our study warrant consideration when interpreting the results. A fair comparison of the outcomes of black and white patients requires adjusting for characteristics that differ between these populations. We used propensity-based methods to balance the differences in characteristics between black and white patients (36, 51–54). We found, however, that the distributions of the 11 estimated propensity scores exhibited increasing degrees of nonoverlap between black and white patients as we added additional variables, from 0.5% of black or white patients who could not be matched with a member of the opposite race in the first step (demographic characteristics only) to 17% in the final step that included all variables. This reflects the marked differences in patient characteristics between racial groups. We retained all patients throughout each of our analyses by performing regression adjustment on propensity scores to maximize the generalizability of our findings. A consequence of this approach is the extrapolation of effects; that is, the assumption that our observed results apply equally to patients with nonoverlapping propensity scores. While we cannot conclusively test this assumption, we detected no significant interaction between propensity score and race for any outcome, which at least suggests that the effects of race on outcome may be similar throughout the range of patients.
Another important potential limitation is that follow-up was not complete for all patients, and black patients were less likely than whites to participate in the 1-year interviews. When we used multiple imputation to examine biases in outcomes, however, we found similar crude and adjusted differences between black and white patients for frequency of angina and quality of life. This suggests minimal bias due to observed patient characteristics and outcomes, although it does not rule out biases due to unmeasured variables associated with both incomplete follow-up and patient health status outcomes. An additional potential concern is that we included patients from only 10 centers; although they were well distributed geographically, few were rural centers or small-volume hospitals. Although we found no differences in the unadjusted differences between black and white patients across our different hospitals, site is known to be associated with both race and outcomes (40), and our findings may not be applicable to the entire country. In addition, our analyses do not include racial differences in post-discharge care. Although we found little residual difference in outcomes after we adjusted for presenting clinical profile, future efforts to quantify the processes of post–myocardial infarction care could be important. Finally, even though we used each patient’s self-designation of race, heterogeneity and misclassification certainly occurred when assigning patients to the white or black racial groups (55).
In summary, we found significant racial differences in a broad spectrum of outcomes that were attenuated after adjustment for patient factors that differed by race. Given that we observed few differences between black and white patients after adjusting for patient factors before presentation and site of care, it is unlikely that altering the processes of care alone would overcome the unadjusted differences in outcomes. Further research is needed to determine how best to address these patient-centered factors and achieve the goal of equity in U.S. health care.(1, 3)
Acknowledgment
The authors thank Peter Austin, PhD.
Grant Support: By the National Heart, Lung, and Blood Institute Specialized Center of Clinically Oriented Research in Cardiac Dysfunction and Disease (grant no. P50 HL077113); CV Therapeutics, Palo Alto, California; and Cardiovascular Outcomes, Kansas City, Missouri.
Appendix: Participating Cardiovascular Outcomes Research Consortium Sites and Investigators
Members of the Cardiovascular Outcomes Research Consortium who participated in this study included:
Mid America Heart Institute, Kansas City, Missouri: John Spertus, MD, MPH; Carole Decker, RN, PHD; Philip Jones, MS; and Kimberly Reid, MS.
Baptist Health System, Little Rock, Arkansas: Gary Collins, MD.
Barnes Jewish Hospital and Washington University, St. Louis, Missouri: Richard Bach, MD.
Beth Israel-Deaconess Medical Center and Harvard University, Boston, Massachusetts: David Cohen, MD, MSc.
Denver General Health System, Denver, Colorado: Edward Havranek, MD, and Frederick Masoudi, MD, MSPH.
Denver Veterans Affairs Medical Center, Denver, Colorado: John Rumsfeld, MD, PhD.
Duke University, Durham, North Carolina: Eric Peterson, MD, MPH.
Emory University, Atlanta, Georgia: Susmita Parashar, MD; Viola Vaccarino, MD, PhD; and William S. Weintraub, MD.
Henry Ford Medical Center, Detroit, Michigan: Sanjaya Khanal, MD, Jane Jie Cao, MD, MPH.
Kaiser Permanente, Denver, Colorado: David Magid, MD, MPH.
MeritCare, Fargo, North Dakota: Wallace Radke, MD, and Mohamed Rahman, MD.
Sentara Health System (both Sentara and Sentara Lee Hospitals), Norfolk, Virginia: John E. Brush, Jr., MD.
Stanford University and Palo Alto Veterans Affairs Medical Center, Palo Alto, Californiar: Paul Heidenreich, MD.
Swedish Medical Center, Seattle, Washington: Timothy Dewhurst, MD.
Truman Medical Center and the University of Missouri–Kansas City, Kansas City, Missouri: Annette Quick, MD.
University of Alabama, Birmingham, Alabama: John Canto, MD.
University of Colorado Health System, Denver, Colorado: John Messenger, MD.
Yale University, New Haven, Connecticut: Harlan Krumholz, MD, SM.
Footnotes
Reproducible Research Statement: Study protocol, statistical code, and data set: Available from Dr. Spertus (Spertusj@umkc.edu).
References
- 1.Institute of Medicine. Crossing the Quality Chasm: A New Health System for the Twenty-first Century. Washington, DC: National Academy Press; 2001. [Google Scholar]
- 2.Institute of Medicine. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: The National Academies Press; 2003. [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services. Healthy People 2010: Understanding and Improving Health. 2nd ed. Washington, DC: US Government Printing Office; 2000. [Google Scholar]
- 4.Kressin NR, Petersen LA. Racial differences in the use of invasive cardiovascular procedures: review of the literature and prescription for future research. Ann Intern Med. 2001;135:352–366. doi: 10.7326/0003-4819-135-5-200109040-00012. [PMID: 11529699] [DOI] [PubMed] [Google Scholar]
- 5.Henry J Kaiser Family Foundation, American College of Cardiology Foundation. Racial/Ethnic Differences in Cardiac Care: The Weight of the Evidence. Menlo Park, CA: The Henry J. Kaiser Family Foundation; 2002. [Google Scholar]
- 6.Canto JG, Allison JJ, Kiefe CI, Fincher C, Farmer R, Sekar P, et al. Relation of race and sex to the use of reperfusion therapy in Medicare beneficiaries with acute myocardial infarction. N Engl J Med. 2000;342:1094–1100. doi: 10.1056/NEJM200004133421505. [PMID: 10760310] [DOI] [PubMed] [Google Scholar]
- 7.Smedley B, Stith A, Nelson A, editors. Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Unequal Treatment: Confronting Racial and Ethnic Disparities In Health Care. Washington, DC: Nat Acad Pr; 2002. [PubMed] [Google Scholar]
- 8.Spertus J. Selecting end points in clinical trials: What evidence do we really need to evaluate a new treatment? [Editorial] Am Heart J. 2001;142:745–747. doi: 10.1067/mhj.2001.119135. [PMID: 11685157] [DOI] [PubMed] [Google Scholar]
- 9.Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant. 2001;20:1016–1024. doi: 10.1016/s1053-2498(01)00298-4. [PMID: 11557198] [DOI] [PubMed] [Google Scholar]
- 10.Tsevat J, Dawson NV, Wu AW, Lynn J, Soukup JR, Cook EF, et al. Health values of hospitalized patients 80 years or older. HELP Investigators. Hospitalized Elderly Longitudinal Project. JAMA. 1998;279:371–375. doi: 10.1001/jama.279.5.371. [PMID: 9459470] [DOI] [PubMed] [Google Scholar]
- 11.Krumholz HM, Peterson ED, Ayanian JZ, Chin MH, DeBusk RF, Goldman L, et al. National Heart, Lung, and Blood Institute working group. Report of the National Heart, Lung, and Blood Institute working group on outcomes research in cardiovascular disease. Circulation. 2005;111:3158–3166. doi: 10.1161/CIRCULATIONAHA.105.536102. [PMID: 15956152] [DOI] [PubMed] [Google Scholar]
- 12.Spertus JA, Radford MJ, Every NR, Ellerbeck EF, Peterson ED, Krumholz HM Acute Myocardial Infarction Working Group of the American Heart Association. Challenges and opportunities in quantifying the quality of care for acute myocardial infarction: summary from the Acute Myocardial Infarction Working Group of the American Heart Association/American College of Cardiology First Scientific Forum on Quality of Care and Outcomes Research in Cardiovascular Disease and Stroke. Circulation. 2003;107:1681–1691. doi: 10.1161/01.CIR.0000062026.90014.63. [PMID: 12668506] [DOI] [PubMed] [Google Scholar]
- 13.Barnato AE, Lucas FL, Staiger D, Wennberg DE, Chandra A. Hospital-level racial disparities in acute myocardial infarction treatment and outcomes. Med Care. 2005;43:308–319. doi: 10.1097/01.mlr.0000156848.62086.06. [PMID: 15778634] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnhart JM, Fang J, Alderman MH. Differential use of coronary revascularization and hospital mortality following acute myocardial infarction. Arch Intern Med. 2003;163:461–466. doi: 10.1001/archinte.163.4.461. [PMID: 12588206] [DOI] [PubMed] [Google Scholar]
- 15.Mehta RH, Marks D, Califf RM, Sohn S, Pieper KS, Van de Werf F, et al. Differences in the clinical features and outcomes in African Americans and whites with myocardial infarction. Am J Med. 2006;119:70.e1–70.e8. doi: 10.1016/j.amjmed.2005.07.043. [PMID: 16431189] [DOI] [PubMed] [Google Scholar]
- 16.Peterson ED, Wright SM, Daley J, Thibault GE. Racial variation in cardiac procedure use and survival following acute myocardial infarction in the Department of Veterans Affairs. JAMA. 1994;271:1175–1180. [PMID: 8151875] [PubMed] [Google Scholar]
- 17.Petersen LA, Wright SM, Peterson ED, Daley J. Impact of race on cardiac care and outcomes in veterans with acute myocardial infarction. Med Care. 2002;40:I86–I96. doi: 10.1097/00005650-200201001-00010. [PMID: 11789635] [DOI] [PubMed] [Google Scholar]
- 18.Sabatine MS, Blake GJ, Drazner MH, Morrow DA, Scirica BM, Murphy SA, et al. Influence of race on death and ischemic complications in patients with non-ST-elevation acute coronary syndromes despite modern, protocol-guided treatment. Circulation. 2005;111:1217–1224. doi: 10.1161/01.CIR.0000157733.50479.B9. [PMID: 15769761] [DOI] [PubMed] [Google Scholar]
- 19.Iribarren C, Tolstykh I, Somkin CP, Ackerson LM, Brown TT, Scheffler R, et al. Sex and racial/ethnic disparities in outcomes after acute myocardial infarction: a cohort study among members of a large integrated health care delivery system in northern California. Arch Intern Med. 2005;165:2105–2113. doi: 10.1001/archinte.165.18.2105. [PMID: 16217000] [DOI] [PubMed] [Google Scholar]
- 20.Ding J, Diez Roux AV, Nieto FJ, McNamara RL, Hetmanski JB, Taylor HA, Jr, et al. Racial disparity in long-term mortality rate after hospitalization for myocardial infarction: the Atherosclerosis Risk in Communities study. Am Heart J. 2003;146:459–464. doi: 10.1016/S0002-8703(03)00228-X. [PMID: 12947363] [DOI] [PubMed] [Google Scholar]
- 21.Spertus J, Safley D, Garg M, Jones P, Peterson ED. The influence of race on health status outcomes one year after an acute coronary syndrome. J Am Coll Cardiol. 2005;46:1838–1844. doi: 10.1016/j.jacc.2005.05.092. [PMID: 16286168] [DOI] [PubMed] [Google Scholar]
- 22.Xie J, Wu EQ, Zheng ZJ, Sullivan PW, Zhan L, Labarthe DR. Patient-reported health status in coronary heart disease in the United States: age, sex, racial, and ethnic differences. Circulation. 2008;118:491–497. doi: 10.1161/CIRCULATIONAHA.107.752006. [PMID: 18625894] [DOI] [PubMed] [Google Scholar]
- 23.Spertus JA, Peterson E, Rumsfeld JS, Jones PG, Decker C, Krumholz H Cardiovascular Outcomes Research Consortium. The Prospective Registry Evaluating Myocardial Infarction: Events and Recovery (PREMIER)---evaluating the impact of myocardial infarction on patient outcomes. Am Heart J. 2006;151:589–597. doi: 10.1016/j.ahj.2005.05.026. [PMID: 16504619] [DOI] [PubMed] [Google Scholar]
- 24.Center for Medicare and Medicaid Services. Overview of Specifications of Measures Displayed on Hospital Compare. Baltimore, MD: Center for Medicare and Medicaid Services; 2006. Accessed at www.cms.hhs.gov/HospitalQualityInits/downloads/HospitalOverviewOfSpecs200512.pdf on 19 December 2008. [Google Scholar]
- 25.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [PMID: 10568646] [DOI] [PubMed] [Google Scholar]
- 26.Scheier MF, Matthews KA, Owens JF, Schulz R, Bridges MW, Magovern GJ, et al. Optimism and rehospitalization after coronary artery bypass graft surgery. Arch Intern Med. 1999;159:829–835. doi: 10.1001/archinte.159.8.829. [PMID: 10219928] [DOI] [PubMed] [Google Scholar]
- 27.Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J Pers Soc Psychol. 1994;67:1063–1078. doi: 10.1037//0022-3514.67.6.1063. [PMID: 7815302] [DOI] [PubMed] [Google Scholar]
- 28.Enhancing recovery in coronary heart disease patients (ENRICHD): study design and methods. The ENRICHD investigators. Am Heart J. 2000;139:1–9. doi: 10.1016/s0002-8703(00)90301-6. [PMID: 10618555] [DOI] [PubMed] [Google Scholar]
- 29.Vaglio J, Jr, Conard M, Poston WS, O'Keefe J, Haddock CK, House J, et al. Testing the performance of the ENRICHD Social Support Instrument in cardiac patients. Health Qual Life Outcomes. 2004;2:24. doi: 10.1186/1477-7525-2-24. [PMID: 15142277] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spertus J, Decker C, Woodman C, House J, Jones P, O'Keefe J, et al. Effect of difficulty affording health care on health status after coronary revascularization. Circulation. 2005;111:2572–2578. doi: 10.1161/CIRCULATIONAHA.104.474775. [PMID: 15883210] [DOI] [PubMed] [Google Scholar]
- 31.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Fihn SD. Monitoring the quality of life in patients with coronary artery disease. Am J Cardiol. 1994;74:1240–1244. doi: 10.1016/0002-9149(94)90555-x. [PMID: 7977097] [DOI] [PubMed] [Google Scholar]
- 32.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. doi: 10.1016/0735-1097(94)00397-9. [PMID: 7829785] [DOI] [PubMed] [Google Scholar]
- 33.Spertus JA, Jones P, McDonell M, Fan V, Fihn SD. Health status predicts long-term outcome in outpatients with coronary disease. Circulation. 2002;106:43–49. doi: 10.1161/01.cir.0000020688.24874.90. [PMID: 12093768] [DOI] [PubMed] [Google Scholar]
- 34.Hansen B, Klopfer S. Optimal full matching and related designs via network flows. JCGS. 2006;15:609–627. [Google Scholar]
- 35.Rosenbaum PR. A characterization of optimal designs for observational studies. J Royal Stats Soc. 1991;53:597–610. [Google Scholar]
- 36.Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incoporate the propensity score. Am Stat. 1985;39(1):33–38. [Google Scholar]
- 37.Localio AR, Berlin JA, Ten Have TR, Kimmel SE. Adjustments for center in multicenter studies: an overview. Ann Intern Med. 2001;135:112–123. doi: 10.7326/0003-4819-135-2-200107170-00012. [PMID: 11453711] [DOI] [PubMed] [Google Scholar]
- 38.Bernheim SM, Spertus JA, Reid KJ, Bradley EH, Desai RA, Peterson ED, et al. Socioeconomic disparities in outcomes after acute myocardial infarction. Am Heart J. 2007;153:313–319. doi: 10.1016/j.ahj.2006.10.037. [PMID: 17239695] [DOI] [PubMed] [Google Scholar]
- 39.Rahimi AR, Spertus JA, Reid KJ, Bernheim SM, Krumholz HM. Financial barriers to health care and outcomes after acute myocardial infarction. JAMA. 2007;297:1063–1072. doi: 10.1001/jama.297.10.1063. [PMID: 17356027] [DOI] [PubMed] [Google Scholar]
- 40.Skinner J, Chandra A, Staiger D, Lee J, McClellan M. Mortality after acute myocardial infarction in hospitals that disproportionately treat black patients. Circulation. 2005;112:2634–2641. doi: 10.1161/CIRCULATIONAHA.105.543231. [PMID: 16246963] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anand IS, Florea VG, Fisher L. Surrogate end points in heart failure. J Am Coll Cardiol. 2002;39:1414–1421. doi: 10.1016/s0735-1097(02)01773-4. [PMID: 11985901] [DOI] [PubMed] [Google Scholar]
- 42.Rector TS, Tschumperlin LK, Kubo SH, Bank AJ, Francis GS, McDonald KM, et al. Use of the Living With Heart Failure questionnaire to ascertain patients' perspectives on improvement in quality of life versus risk of drug-induced death. J Card Fail. 1995;1:201–206. doi: 10.1016/1071-9164(95)90025-x. [PMID: 9420652] [DOI] [PubMed] [Google Scholar]
- 43.Spertus JA, Tooley J, Jones P, Poston C, Mahoney E, Deedwania P, et al. Expanding the outcomes in clinical trials of heart failure: the quality of life and economic components of EPHESUS (EPlerenone's neuroHormonal Efficacy and SUrvival Study) Am Heart J. 2002;143:636–642. doi: 10.1067/mhj.2002.120775. [PMID: 11923800] [DOI] [PubMed] [Google Scholar]
- 44.Weintraub WS, Spertus JA, Kolm P, Maron DJ, Zhang Z, Jurkovitz C, et al. COURAGE Trial Research Group. Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med. 2008;359:677–687. doi: 10.1056/NEJMoa072771. [PMID: 18703470] [DOI] [PubMed] [Google Scholar]
- 45.Spertus JA, Salisbury AC, Jones PG, Conaway DG, Thompson RC. Predictors of quality-of-life benefit after percutaneous coronary intervention. Circulation. 2004;110:3789–3794. doi: 10.1161/01.CIR.0000150392.70749.C7. [PMID: 15596563] [DOI] [PubMed] [Google Scholar]
- 46.Gibbons RJ, Abrams J, Chatterjee K, Daley J, Deedwania PC, Douglas JS, et al. American College of Cardiology. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina---summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Chronic Stable Angina) Circulation. 2003;107:149–158. doi: 10.1161/01.cir.0000047041.66447.29. [PMID: 12515758] [DOI] [PubMed] [Google Scholar]
- 47.Bach PB, Pham HH, Schrag D, Tate RC, Hargraves JL. Primary care physicians who treat blacks and whites. N Engl J Med. 2004;351:575–584. doi: 10.1056/NEJMsa040609. [PMID: 15295050] [DOI] [PubMed] [Google Scholar]
- 48.Mukamel DB, Weimer DL, Mushlin AI. Referrals to high-quality cardiac surgeons: patients' race and characteristics of their physicians. Health Serv Res. 2006;41:1276–1295. doi: 10.1111/j.1475-6773.2006.00535.x. [PMID: 16899007] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.LaVeist TA, Arthur M, Morgan A, Rubinstein M, Kinder J, Kinney LM, et al. The cardiac access longitudinal study. A study of access to invasive cardiology among African American and white patients. J Am Coll Cardiol. 2003;41:1159–1166. doi: 10.1016/s0735-1097(03)00042-1. [PMID: 12679217] [DOI] [PubMed] [Google Scholar]
- 50.Popescu I, Vaughan-Sarrazin MS, Rosenthal GE. Certificate of need regulations and use of coronary revascularization after acute myocardial infarction. JAMA. 2006;295:2141–2147. doi: 10.1001/jama.295.18.2141. [PMID: 16684984] [DOI] [PubMed] [Google Scholar]
- 51.Austin PC. The performance of different propensity score methods for estimating marginal odds ratios. Stat Med. 2007;26:3078–3094. doi: 10.1002/sim.2781. [PMID: 17187347] [DOI] [PubMed] [Google Scholar]
- 52.Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med. 2008;27:2037–2049. doi: 10.1002/sim.3150. [PMID: 18038446] [DOI] [PubMed] [Google Scholar]
- 53.Joffe MM, Rosenbaum PR. Invited commentary: propensity scores. Am J Epidemiol. 1999;150(4):327–333. doi: 10.1093/oxfordjournals.aje.a010011. [DOI] [PubMed] [Google Scholar]
- 54.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [PMID: 9382394] [DOI] [PubMed] [Google Scholar]
- 55.Jones CP. Invited commentary: "race," racism, and the practice of epidemiology. Am J Epidemiol. 2001;154:299–304. doi: 10.1093/aje/154.4.299. discussion 305–6. [PMID: 11495851] [DOI] [PubMed] [Google Scholar]