Abstract
Experience-dependent brain plasticity typically declines after an early critical period during which circuits are established. Loss of plasticity with closure of the critical period limits improvement of function in adulthood, but the mechanisms that change the brain’s plasticity remain poorly understood. Here, we identified an increase in expression of Lynx1 protein in mice that prevented plasticity in the primary visual cortex late in life. Removal of this molecular brake enhanced nicotinic acetylcholine receptor signaling. Lynx1 expression thus maintains stability of mature cortical networks in the presence of cholinergic innervation. The results suggest that modulating the balance between excitatory and inhibitory circuits reactivates visual plasticity and may present a therapeutic target.
The waxing and waning of cortical plasticity during a postnatal critical period serves to consolidate neural circuits and behavior (1), but in turn limits recovery of function in the adult brain (2). For example, discordant vision through the two eyes during an early critical period results in the enduring loss of visual acuity (amblyopia) reflecting aberrant circuit remodeling within primary visual cortex (V1). Amblyopia, which affects 2–4% of the human population, exhibits little recovery in adulthood (3). Identifying specific biological mechanisms which restrict adult plasticity would inspire potentially novel strategies for therapy.
We hypothesized that the gradual emergence of molecular “brakes” might actively prevent plasticity in the adult brain. The only molecules previously reported to play a role in closing the critical period are related to axonal growth inhibition, such as chondroitin sulphate proteoglycans and myelin-signaling proteins, NgR and PirB (4–6). To identify further targets, we analyzed the transcriptome of the binocular zone in mouse V1 to identify molecules that are expressed more in adulthood than during the critical period (7). Here, we characterize one of these, lynx1, which is an endogenous prototoxin similar to α-bungarotoxin in snake venom and binds to the nicotinic acetylcholine receptor (nAChR) (8).
Lynx1 expression increases only after the critical period for amblyopia in adult V1 both at the protein and mRNA level (Fig. 1A). Along the visual pathway, lynx1 transcripts were expressed both in V1 and the lateral geniculate nucleus (LGN) (Fig. 1B). In contrast, expression of another member of the lynx family, lynx2, declined over the critical period, and was not found in the visual pathway (Fig. S1). We therefore directly assessed lynx1 function in the binocular region by electrophysiological recordings from knockout mice.
Figure 1.
Lynx1 increases in adulthood to limit visual plasticity. (A) Expression of lynx1 protein (upper panel) and mRNA (lower panel) across critical period (Pre-CP: P18, CP: P28, Post-CP: P60). **P < 0.01, *P < 0.05 One-way ANOVA. Mean ± sem. (B) In situ hybridization of lynx1 in adult V1 (upper panel), and LGN (lower panel). Scale, 100µm. (C) Adult V1 plasticity paradigm by short-term MD (Ad-MD). (D) Ad-MD shifts the ocular dominance distribution of lynx1 KO mice (lower panel; CBI = 0.55, 216 cells, 8 mice), but not in WT mice (upper panel; CBI = 0.68, 231 cells, 9 mice). KO vs WT; P < 0.0001, χ2-test. (E) Cumulative probability of quantified spike response after Ad-MD confirms shifted ocular dominance scores for lynx1 KO (blue filled circles) compared to WT (grey filled circles) (** P < 0.005, K-S test) or no MD (blue open circles, KO, 93 cells; grey open circles, WT, 82 cells; P = 0.75, K-S test).
In mice lacking the Lynx1 gene, the eye preference of single neurons (ocular dominance) was no different from that of wild-type mice (Fig. 1E). Upon short-term (4 day) monocular deprivation (MD) in mature wild-type animals (> postnatal day, P60), there was little change in the visual spiking response (3). Instead, adult lynx1 knockout mice exhibited a robust shift in responsiveness away from the deprived eye (Fig. 1C–E). This heightened plasticity was specific to older ages, as short-term MD was equally effective in both wild-type (WT) and lynx1 knockout (KO) mice during the critical period (Fig. 2A).
Figure 2.
Nicotinic receptors mediate adult plasticity in lynx1 KO mice. (A) Mice without MD (open circles, grey KO / blue WT) shift equally after MD during the CP (light blue, KO: mean CBI = 0.48, 6 mice; light grey, WT: CBI = 0.50, 8 mice; P > 0.5, t-test). Adult plasticity (blue, KOMD: CBI = 0.55, 12 mice vs grey, WTMD: CBI = 0.68, 9 mice; *** P < 0.0001, t-test) is abolished by concurrent nAChR antagonists (red, KOMD + mecamylamine: CBI = 0.68, 9 mice vs KOMD, *** P < 0.0001; vs grey, WTMD + mecamylamine: CBI = 0.69, 4 mice, P > 0.7; vs no MD KO + mecamylamine: CBI = 0.68, 7 mice, P > 0.9, t-test; orange, KOMD + DHβE / MLA: CBI = 0.68, 7 mice vs KOMD, *** P < 0.0001, t-test). Darker circles represent cortical mini-pump infusion. (B) Enhanced nicotine response in lynx1 KO mice. Averaged VEP traces (mean ± sem) before (light grey) and 10 minutes after (black) subcutaneous nicotine injection (+ nic) in WT (left) and lynx1 KO mice (right). (C) Integrated VEP (area of first negative peak) for WT (empty bars, 6 mice) and lynx1 KO mice (filled bars, 11 mice). * P < 0.05, t-test; n.s., not significant.
Lynx1 protein directly binds to nAChRs (9), such as the major central subunits α4β2 heteromers or α7 homomers, to reduce their sensitivity to acetylcholine. We directly assessed the response to systemic nicotine injection in lynx1 knockout mice by measuring visual evoked potential (VEP) response in anesthetized V1. Enhancement of VEP response was only observed in lynx1 knockout mice (Fig. 2B,C). To test whether nAChR signaling mediates adult plasticity in lynx1 knockout mice, we applied the broad spectrum antagonist mecamylamine concurrent with short-term MD. Either systemic injection or restricted infusion directly into V1 by osmotic mini-pump were sufficient to prevent adult plasticity. These results were corroborated by systemic treatment with a mixture of α4- and α7-subunit selective nAChR antagonists (10), dihydro-β-eryhtroidine (DHβE) plus methyllycaconitine (MLA) (Fig. 2A).
To establish clinical relevance of these findings, we directly measured recovery from amblyopia in adulthood. In wild-type mice, long-term MD spanning the entire critical period results in significant reduction of visual acuity as measured directly in V1 by VEP (3). Notably, this reduction persists into adulthood even if the closed eye was reopened for more than one month after the critical period (Fig. 3A,C). Dramatically, lynx1 knockout mice spontaneously recovered visual acuity to normal levels simply by reopening the closed eye (Fig. 3A,C), exhibiting VEPs even at higher spatial frequencies (Fig. 3B). Given the cholinergic basis of this plasticity, we further attempted to induce recovery even in adult wild-type mice by enhancing endogenous ACh signaling. Injection of an acetylcholinesterase inhibitor, physostigmine, during the period of eye re-opening similarly restored vision to wild-type mice initially rendered amblyopic (Fig. 3C).
Figure 3.
Recovery from amblyopia in lynx1 KO mice. (A) After long-term MD (LTMD) spanning the critical period (P19-P33), the deprived eye was reopened (>1 month) until VEP acuity was measured in V1 (>P60). For AChEI experiments, daily injections were made starting at P45. (B) Averaged VEP traces (left, mean ± sem; scale: 20 µV, 0.1 sec) and amplitudes (right) of first negative peak (mean ± sem) reveal acuity recovery after reopening an eye (blue, 6 mice) initially deprived during the critical period (grey, 5 mice). (C) Visual acuity in WT mice (white bars) without deprivation (no MD: 0.48 ± 0.03 cyc/deg, 6 mice) decreases after LTMD spanning the critical period (LTMD: 0.28 ± 0.01 cyc/deg, 3 mice) and endures (+eye open: 0.30 ± 0.02 cyc/deg, 5 mice; vs LTMD, P > 0.45; vs no MD, P < 0.0005, t-test). In contrast, reopening the deprived eye together with cholinesterase inhibitor restores vision (grey bar, AChEI: 0.48 ± 0.06 cyc/deg, 4 mice; vs WT + eye open, * P < 0.05; vs WT no MD + AChEI: 0.47 ± 0.02 cyc/deg, 6mice, P > 0.8, t-test). Lynx1 KO mice (black bars) spontaneously recover from LTMD (0.28 ± 0.03 cyc/deg, 5 mice) simply by reopening the deprived eye (0.56 ± 0.02 cyc/deg, 6 mice; *** P < 0.0001, t-test) to reach normal levels (no MD: 0.56 ± 0.04 cyc/deg, 3 mice).
Recovery of function in lynx1 knockout mice is likely due to an enhanced visual responsiveness during arousal. We did not observe structural changes at the level of perineuronal nets (4) or myelination (5) in lynx1 knockout mice (Fig. S2). Aging-related neurodegeneration reported previously in these animals (11) was confirmed to occur only past nine months of age. Instead, local excitatory-inhibitory circuit balance might have been affected earlier (3) (Fig. 4A). Previous reports across various species have localized nAChRs to thalamocortical terminals presynaptic to principal cells (12–16), facilitating excitation in V1 (17, 18). Activation of nAChRs upon specific inhibitory neurons could further modulate excitatory-inhibitory balance by disinhibition (19, 20), as in the case of congenital nAChR mutation that disrupts GABAergic transmission (21).
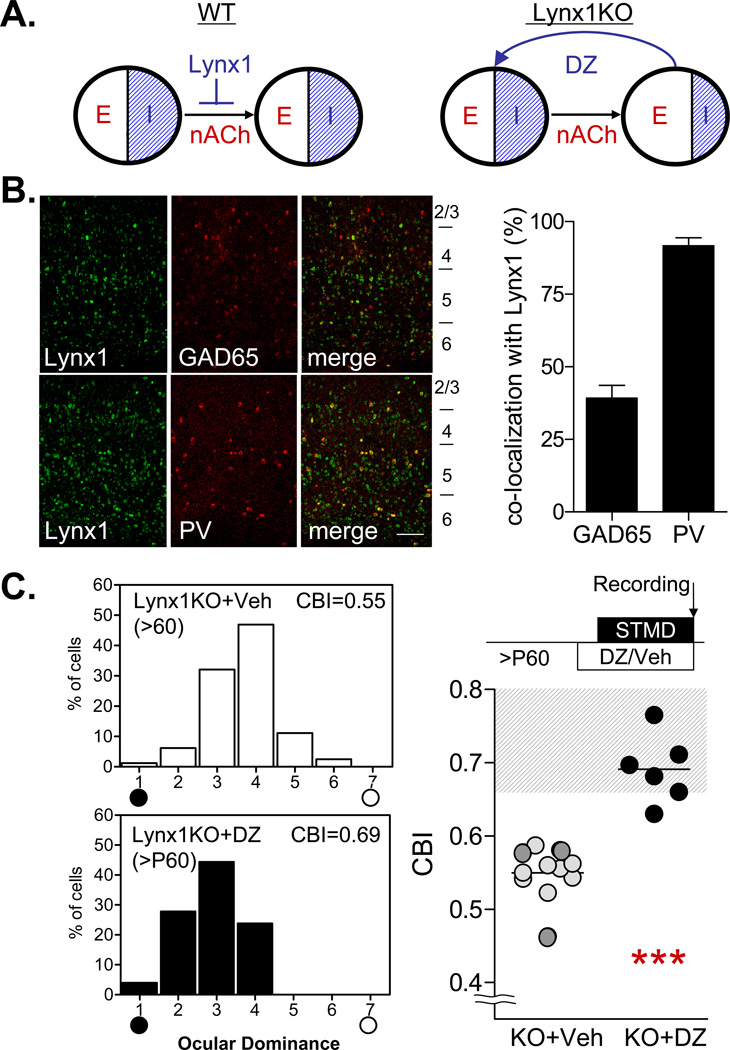
Figure 4.
Lynx1 may adjust cortical excitatory-inhibitory balance to regulate adult plasticity. (A) In WT animals (left), mature excitatory-inhibitory balance is maintained by lynx1 that limits nAChR response. In lynx1 KO mice (right), enhanced nAChR signaling may lead to excitatory-inhibitory imbalance and adult plasticity, which could be sensitive to acute restoration of inhibition with diazepam (DZ). (B) Double in situ hybridization of lynx1 (green) with GAD65 (red, upper panel) or parvalbumin (PV, lower panel) in adult V1 (left). Scale, 100µm. Quantification of overlapping pixels (right) indicates selective expression of lynx1 in a subset (40%) of GAD65-positive interneurons, most likely PV-positive cells (> 90% co-localization). (C) Focal diazepam infusion during adult MD in lynx1 KO mice abolishes ocular dominance plasticity (black, DZ: CBI = 0.67, 6 mice vs grey, vehicle: CBI = 0.54, 14 mice; *** P < 0.001, t-test). Dark circles represent cortical mini-pump infusion.
Indeed, lynx1 and nAChRs were co-expressed not only in the LGN (Fig. S3), but also in a sub-population of GABA cells, primarily parvalbumin-positive interneurons (Fig. 4B, S3B). Activation of nAChRs may also exert chronic epigenetic effects on GABA synthesis (22). To probe whether excitatory-inhibitory imbalance may contribute to adult plasticity in Lynx1 knockout mice, we directly restored intracortical inhibition by focal benzodiazepine infusion from osmotic minipumps. Diazepam treatment of V1 abolished adult plasticity in lynx1 knockout mice (Fig. 4C) as did nAChR blockade above (Fig. 2A). Thus, lynx1 reduces adult plasticity through cholinergic signaling mechanisms that may adjust excitatory-inhibitory balance later in life (3).
Taken together, lynx1 provides both a valuable endogenous tool with which to probe critical period closure and offers novel therapeutic and conceptual insight. In contrast to muscarinic receptors engaged during the critical period (23), our results highlight a nicotinic component for adult V1 plasticity. While we do not rule out a role for muscarinic receptors (24), deletion of Lynx1 alone is sufficient to rescue visual acuity. Recovery strategies aimed at the lynx1-nAChR interaction (8, 9) could be fruitful in conjunction with attentional tasks that stimulate cholinergic release (e.g. perceptual learning, video game training) (25–28). Clinically approved cholinesterase inhibitors that boost the afferent response in human visual cortex (29) may be useful for treating some amblyopes (Fig. 3C), including those with sub-cortical changes (30). Amblyopia might further serve as a diagnostic measure to identify tobacco exposure (31) or schizophrenia (32).
While a permissive role for cholinergic input has long been appreciated during the critical period (33), it has remained a mystery why V1 plasticity is severely restricted in adulthood even in the presence of massive innervation from the basal forebrain. Lynx1 expression not only contributes to nAChR agonist binding and desensitization kinetics (8), but also may respond to changes in network activity (34). Local regulation of Lynx1 levels may allow cholinergic activation to induce islands of plasticity while maintaining overall circuit stability. Visual attention tasks in fact preferentially modulate fast-spiking inhibitory neurons (35,36), consistent with a convergence of top-down influences upon local excitatory-inhibitory circuit balance.
Supplementary Material
Acknowledgments
We thank H.A. Lester and A. Takesian for helpful comments on the manuscript and M. Marcotrigiano for animal maintenance. Supported by James S. McDonnell Foundation ‘Recovery from Amblyopia’ network (T.K.H.), NIH Director’s Pioneer Award (1 DP1 OD 003699-01 to T.K.H.), the Ellison Medical Foundation (T.K.H.), HHMI (N.H.), DA-17279 and TRDRP (J.M.M.), and the Japanese Society for Promotion of Science (H.M.)
Footnotes
Supporting Online Material
Materials and Methods
Figs. S1–3
References
- 1.Hensch TK. Annu Rev Neurosci. 2004;27:549. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- 2.Wandell BA, Smirnakis SM. Nat Rev Neurosci. 2009 Dec;10:873. doi: 10.1038/nrn2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morishita H, Hensch TK. Curr Opin Neurobiol. 2008 Jun 3;18:101. doi: 10.1016/j.conb.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Pizzorusso T, et al. Science. 2002 Nov 8;298:1248. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- 5.McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Science. 2005 Sep 30;309:2222. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Syken J, Grandpre T, Kanold PO, Shatz CJ. Science. 2006 Sep 22;313:1795. doi: 10.1126/science.1128232. [DOI] [PubMed] [Google Scholar]
- 7.Plessy C, et al. PLoS One. 2008;3:e3012. doi: 10.1371/journal.pone.0003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miwa JM, et al. Neuron. 1999 May;23:105. doi: 10.1016/s0896-6273(00)80757-6. [DOI] [PubMed] [Google Scholar]
- 9.Ibanez-Tallon I, et al. Neuron. 2002 Mar 14;33:893. doi: 10.1016/s0896-6273(02)00632-3. [DOI] [PubMed] [Google Scholar]
- 10.Davis JA, Gould TJ. Psychopharmacol. 2006 Mar;184:345. doi: 10.1007/s00213-005-0047-y. [DOI] [PubMed] [Google Scholar]
- 11.Miwa JM, et al. Neuron. 2006 Sep 7;51:587. doi: 10.1016/j.neuron.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 12.Disney AA, Aoki C, Hawken MJ. Neuron. 2007 Nov 21;56:701. doi: 10.1016/j.neuron.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prusky GT, Shaw C, Cynader MS. Brain Res. 1987 May 26;412:131. doi: 10.1016/0006-8993(87)91447-8. [DOI] [PubMed] [Google Scholar]
- 14.Parkinson D, Kratz KE, Daw NW. Exp Brain Res. 1988;73:553. doi: 10.1007/BF00406614. [DOI] [PubMed] [Google Scholar]
- 15.Gil Z, Connors BW, Amitai Y. Neuron. 1997 Sep;19:679. doi: 10.1016/s0896-6273(00)80380-3. [DOI] [PubMed] [Google Scholar]
- 16.Kruglikov I, Rudy B. Neuron. 2008 Jun 26;58:911. doi: 10.1016/j.neuron.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lucas-Meunier E, et al. Cereb Cortex. 2009 Jan 28;19:2411. doi: 10.1093/cercor/bhn258. [DOI] [PubMed] [Google Scholar]
- 18.Kuo MC, Rasmusson DD, Dringenberg HC. Neuroscience. 2009 Sep 29;163:430. doi: 10.1016/j.neuroscience.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 19.Aracri P, et al. Cereb Cortex. 2009 Oct 7;20:1539. doi: 10.1093/cercor/bhp214. [DOI] [PubMed] [Google Scholar]
- 20.Alkondon M, Pereira EFR, Eisenberg HM, Albuquerque EX. J Neurosci. 2000 Jan 1;20:66. doi: 10.1523/JNEUROSCI.20-01-00066.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mann EO, Mody I. Curr Opin Neurol. 2008;21:155. doi: 10.1097/WCO.0b013e3282f52f5f. [DOI] [PubMed] [Google Scholar]
- 22.Satta R, et al. Proc Natl Acad Sci USA. 2008 Oct 21;105:16356. doi: 10.1073/pnas.0808699105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu Q, Singer W. Eur J Neurosci. 1993 May 1;5:475. doi: 10.1111/j.1460-9568.1993.tb00514.x. [DOI] [PubMed] [Google Scholar]
- 24.Herrero JL, et al. Nature. 2008 Aug 28;454:1110. doi: 10.1038/nature07141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goard M, Dan Y. Nat Neurosci. 2009 Nov;12:1444. doi: 10.1038/nn.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang JI, Vaucher E. PLoS One. 2009;4:e5995. doi: 10.1371/journal.pone.0005995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levi DM, Li RW. Philos Trans R Soc Lond B Biol Sci. 2009 Feb 12;364:399. doi: 10.1098/rstb.2008.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dye MW, Green CS, Bavelier D. Neuropsychologia. 2009 Feb 7;47:1780. doi: 10.1016/j.neuropsychologia.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silver MA, Shenhav A, D'Esposito M. Neuron. 2008 Dec 10;60:904. doi: 10.1016/j.neuron.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hess RF, Thompson B, Gole G, Mullen KT. Eur J Neurosci. 2009 Mar;29:1064. doi: 10.1111/j.1460-9568.2009.06650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lempert P. Ophthalmic Physiol Opt. 2005 Nov;25:592. doi: 10.1111/j.1475-1313.2005.00329.x. [DOI] [PubMed] [Google Scholar]
- 32.Sarter M, Hasselmo ME, Bruno JP, Givens B. Brain Res Brain Res Rev. 2005 Feb;48:98. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Bear MF, Singer W. Nature. 1986 Mar 13–19;320:172. doi: 10.1038/320172a0. [DOI] [PubMed] [Google Scholar]
- 34.Pfeffer CK, et al. J Neurosci. 2009 Mar 18;29:3419. doi: 10.1523/JNEUROSCI.1377-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchell JF, Sundberg KA, Reynolds JH. Neuron. 2007 Jul 5;55:131. doi: 10.1016/j.neuron.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, et al. Nat Neurosci. 2008 Jul 6;11:974. doi: 10.1038/nn.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.