Abstract
The extracellular concentrations of adenosine are elevated during sepsis and adenosine receptors regulate the host’s response to sepsis. Here, we investigated the role of the adenosine generating ectoenzyme, ecto-5′-nucleotidase (CD73) in regulating immune and organ function during sepsis. Polymicrobial sepsis was induced by subjecting CD73 knockout (KO) and wild type (WT) mice to cecal ligation and puncture. CD73 KO mice showed increased mortality in comparison with WT mice, which was associated with increased bacterial counts and elevated inflammatory cytokine and chemokine concentrations in the blood and peritoneum. CD73 deficiency promoted lung injury as indicated by increased myeloperoxidase activity and neutrophil infiltration, and elevated pulmonary cytokine levels. CD73 KO mice had increased apoptosis in the thymus, as evidenced by increased activation of caspase-3, poly(ADP-ribose) polymerase and NF-κB. Septic CD73 KO mice had higher blood urea nitrogen levels and elevated cytokine levels in the kidney, indicating increased renal dysfunction. The increased kidney injury of CD73 KO mice was associated with augmented activation of p38 MAPK and decreased phosphorylation of Akt. Pharmacological inactivation of CD73 in WT mice using AMPCP augmented cytokine levels in the blood and peritoneal lavage fluid. These findings suggest that CD73-derived adenosine may be beneficial in sepsis.
INTRODUCTION
Sepsis occurs when microbial invasion induces systemic illness (1). Despite advances in modern hemodynamic, antibiotic, and ventilatory clinical support, sepsis represents a major clinical problem with no effective therapy (2). There are 215,000 deaths related to sepsis in each year in the US alone (3, 4). Although the pathogenesis of sepsis-induced multiorgan injury leading to death is incompletely understood, current theory holds that an initial hyperinflammatory process and subsequent immune paralysis contribute to mortality and morbidity in sepsis (4, 5). The initial hyperinflammatory response seen in sepsis is associated with uncontrolled, exuberant cytokine production that can be deleterious to various tissues and lead to organ injury and dysfunction (6, 7). After this hyperinflammatory phase, an immune paralytic phase ensues with enhanced apoptotic cell death occurring in multiple organs including the spleen, kidney, liver, and heart (8).
Adenosine is a biologically active extracellular signaling molecule, which regulates a wide variety of immunological processes by binding to one or more of four G-protein-coupled adenosine receptors (A1, A2A, A2B, and A3) (9–18). Adenosine is produced during inflammation, hypoxia, ischemia, or trauma (10, 19–24), and since sepsis is associated with these metabolically stressful conditions, systemic adenosine levels reach high concentrations in mice and patients with sepsis and septic shock (25). There is growing evidence that adenosine receptors can regulate the host’s response to sepsis. In recent studies, A1, A2B, and A3 receptors were found to decrease mortality, inflammation, renal dysfunction, and hepatic injury in murine cecal ligation and puncture (CLP), a clinically relevant model of polymicrobial sepsis (26–28). In contrast, we showed that A2A receptor activation contributed to the lethal effect of sepsis via decreased bacterial clearance, increased splenic apoptosis, and insufficient inflammatory cytokine levels (9). These data confirm that extracellular adenosine is an important regulator of immune events in mice undergoing sepsis and also support the notion that different adenosine receptors can have different and sometimes opposing effects on immunity during sepsis (22, 29, 30).
One major pathway leading to increased extracellular adenosine levels during metabolic stress is release of precursor adenine nucleotides, mostly adenosine triphosphate (ATP) from the cell followed by extracellular catabolism to adenosine by a cascade of ectonucleotidases, including CD39 (nucleoside triphosphate diphosphorylase, NTPDase) and CD73 (ecto-5′-nucleotidase, Ecto-5′-NTase) (31–35). CD39 is a transmembrane molecule, which initiates extracellular adenosine generation by catalyzing the degradation of ATP and ADP to AMP (22, 36). CD73 is a 70-kDa glycosyl phosphatidylinositol-anchored cell surface protein with ecto-5′-nucleotidase enzyme activity that catalyzes the dephosphorylation of AMP to adenosine (37, 38). Therefore, this enzyme has a key role in the generation of extracellular adenosine by catalyzing the last step in the cascade of ATP breakdown. In fact, CD73 has been proposed to be the rate-limiting enzyme in the generation of adenosine during metabolic stress (37, 39). The immune regulatory functions of CD73 are well documented in several in vivo experimental models. The anti-inflammatory action of methotrexate has been reported to be dependent on the adenosine producing activity of CD73 (40). During LPS-induced acute lung injury, CD73-generated adenosine attenuates LPS-induced polymorphonuclear neutrophil (PMN) trafficking (41). Similarly, CD73-derived adenosine protects against bleomycin-induced lung injury (38) and ventilator–induced acute lung injury (42). In hypoxia models, CD73 activity was required to prevent vascular leak and neutrophil infiltration into various tissues, indicating that extracellular adenosine produced during hypoxia is a potent anti-inflammatory signal for PMNs in vivo (31, 43). A protective role of CD73-generated adenosine has also been shown in renal and myocardial ischemia (44, 45).
Although the role of the particular adenosine receptors in sepsis has been addressed, the global effect of CD73-derived adenosine acting at all 4 adenosine receptors remains unknown. Here, we investigated how targeted genetic deletion or pharmacological inactivation of the adenosine producing CD73 affects the course of the host’s response to sepsis using the CLP model.
MATERIALS AND METHODS
Experimental animals
CD73 knockout (KO) mice and their wild-type (WT) littermates were developed as described previously and had been backcrossed 6–8 times to a C57Bl/6J background (31). Essentially, mice with a targeted disruption of the gene for CD73 were generated by homologous recombination in which the third exon of CD73 was replaced by a neomycin resistance cassette. They were healthy, bred normally, and appeared to have normal immune system development (31). Genotyping was performed on genomic DNA by PCR to detect either exon 3 or the neomycin cassette. All mice were bred and all colonies were maintained in accordance with the recommendations of the “Guide for the Care and Use of Laboratory Animals”, and the experiments were approved by the New Jersey Medical School Animal Care Committee. WT and KO littermates of heterozygous parents were used exclusively in all studies. For pharmacological studies we used the selective and competitive inhibitor of CD73, α, β–methylene adenosine 5′-diphosphate (AMPCP), and the non-selective adenosine receptor agonist 5′-N-ethylcarboxamidoadenosine (NECA), both of which were purchased from Sigma Chemical (St. Louis, MO).
Sepsis induction by CLP
Polymicrobial sepsis was induced by subjecting mice to CLP, as we have described previously (9). CD73 KO or WT male mice between the age of eight and twelve weeks, were anesthetized with intraperitoneally injected pentobarbital (50 mg/kg). Under aseptic conditions, a 2-cm midline laparotomy was performed to allow exposure of the cecum with adjoining intestine. Approximately two-thirds of the cecum was tightly ligated with a 3.0 silk suture, and the ligated part of the cecum was perforated twice (through and through) with a 20 1/2-gauge needle (BD Biosciences). Thereafter, the ligated cecum was gently squeezed to extrude a small amount of feces through the perforation site and was then returned to the peritoneal cavity. The laparotomy was closed in two layers with 4.0 silk sutures. Following the operation, all mice were resuscitated with 1 ml of physiological saline injected subcutaneously and returned to their cages with free access to food and water. Sixteen hour after the CLP operation (the following day), the mice were reanesthetized with pentobarbital (50 mg/kg, i. p.), and blood, peritoneal lavage fluid, as well as lung, heart, spleen, thymus, and kidney were harvested.
Collection of blood, peritoneal lavage fluid, and organs
After opening the chest of mice, blood samples were obtained aseptically by cardiac puncture using heparinized syringes. Blood samples were then placed into heparinized Eppendorf tubes and kept on ice until further processing for bacteriological analysis. Serial dilutions for bacteriological analysis were made as described previously (9). The blood samples were centrifuged at 2,000 × g for 10 min and the recovered plasma was stored at −70°C until further use. To collect peritoneal lavage fluid, first the abdominal skin was cleansed with 70% ethanol and the abdominal wall was exposed by opening the skin. Two milliliters of sterile physiological saline were slowly injected into the peritoneal cavity via an 18-gauge needle. The abdomen was gently massaged for 1 min while keeping the tip of the needle in the peritoneum. In the next step, the peritoneal fluid was recovered through the needle and the recovered peritoneal lavage fluid was placed on ice until processed for bacteriological examination. After making serial dilutions of the peritoneal lavage fluid to determine the number of the CFU, the peritoneal lavage fluid was centrifuged at 5,000 × g for 10 min and the supernatant was stored at −70°C until further analysis. Spleen, thymus, lung, heart, and kidney samples were excised and snap frozen using liquid nitrogen or fixed overnight in 10% formalin.
Quantification of bacterial CFUs from blood and peritoneal lavage fluid
Blood or peritoneal lavage fluid (100 μl) was diluted serially in sterile physiological saline. Fifty μl of each dilution was aseptically plated and cultured on trypticase blood agar plates (BD Biosciences) at 37°C. After 24 h of incubation, the number of bacterial colonies was counted. The results are expressed as CFUs per milliliter of blood or peritoneal lavage fluid.
Determination of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and blood urea nitrogen (BUN)
Plasma concentrations of ALT, AST, and BUN were analyzed using a clinical chemistry analyzer system (VetTest8008, IDEXX Laboratories) (46).
Determination of cytokine and chemokine levels
Concentrations of IL-6, IL-10, IL-1β, IL-12p40, tumor necrosis factor-α (TNF-α), MIP-1α, MIP-2, and MCP-1 were determined in blood and peritoneal lavage fluid obtained 6 or 16 h after CLP. We also measured IL-6, IL-10, MIP-2, and MCP-1 levels in protein extracts from heart and kidney 16 h after sepsis induction. In addition, IL-1β, IL-6 and MCP-1 levels were assessed in lung protein extracts 6 h after CLP. All of the above cytokines and chemokines were determined using commercially available ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Protein extraction and Western blot analysis
For Western blot analysis, organs were homogenized in a Dounce homogenizer in modified radioimmunoprecipitation assay buffer (50 mM Tris HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.25% sodium deoxycholate, 1% Nonidet P-40, 1 μg/ml pepstatin, 1 μg/ml leupeptin, 1 mM PMSF, 1 mM Na3VO4). The lysates were centrifuged at 15,000 × g for 15 min, and the supernatant was recovered. The Bio-Rad protein assay kit was used to determine the protein concentrations. Protein samples (40 μg from each organ) were separated on 8–12% Tris-glycine gel (Invitrogen Life Technologies) and transferred to nitrocellulose membrane. The membranes were probed with polyclonal rabbit anti-cleaved caspase-3, anti-cleaved poly(ADP-ribose) polymerase (PARP), and anti-inhibitory subunit of NF-κB (IκBα), (all from Cell Signaling Technology). Following several wash cycles, the nitrocellulose membranes were incubated with a secondary HRP-conjugated anti-rabbit antibody (Santa Cruz Biotechnology). To assess equal protein loading, HRP-conjugated polyclonal goat anti-β actin antibody was used from Santa Cruz Biotechnology. Bands were detected using ECL Western Blotting Detection Reagent (Amersham Biosciences, Piscataway, NJ).
Isolation of nuclear protein extracts from the thymus and detection of NF-κB activation
Protein extracts from the cytosol and nucleus were isolated using NE-PER Nuclear and Cytosolic Extraction Reagent (Thermo Fisher Scientific, Waltham, MA). We measured NF-κB activation in the nuclear protein fraction using Trans AM NF-κB p65 Chemi Transcription Factor Assay Kit (Active Motif, Carlsbad, CA) using 2 μg of thymic nuclear cell extract according to the manufacturer’s protocol.
Assessment of myeloperoxidase (MPO) activity and histology of the lung
Lung samples obtained 6 h following CLP were homogenized in extraction buffer (20 mM acetate buffer (pH 4.7) containing 0.2 M NaCl, 0.5 % cetyltrimethylammonium bromide, 10 μg/ml phenylmethylsulfonyl fluoride and 1 mM EDTA) at a concentration of 100 mg/ml. NWLSS Myeloperoxidase Activity Assay kit (Northwest Life Science Specialties, Vancouver, WA) was used for MPO determination, according to the instructions provided with the kit.
For histological analysis, lung specimens were fixed in 10% formalin solution overnight. Following automated dehydration through a graded ethyl alcohol series, transverse lung slices were embedded in paraffin, sectioned at 5 μm, and stained with hematoxylineosin (H&E). Morphological assessment was performed by an experienced pathologist who was blinded to the origin (WT or KO mice) of lungs.
Flow cytometric analysis of leukocyte subsets
Cytometric detection of leukocyte subsets was performed using flow cytometry, as previously described (47). Both total numbers and percent distribution of leukocyte subsets in whole blood and spleen cell suspensions were determined by staining CD3+CD4+, CD3+CD8+ T cells, CD19+ B cells, and CD11b+ myeloid cells using Abs against CD markers conjugated with FITC, allophycocyanin, PerCP, or PE (BD Biosciences, San Jose, CA). Aliquots of 0.1 ml whole blood or splenocyte suspension were incubated with the respective markers for 15 min followed by incubation with BD FACS lysing solution (BD Biosciences) for 7 min at 37°C. Cells were washed twice with BD FACS wash buffer and then fixed with 1% methanol-free formaldehyde. Flow cytometric acquisitions were performed at the centralized flow cytometry facility of UMDNJ-New Jersey Medical School. At least 10,000 events were collected for each analysis.
Statistical Analysis
To statistically compare cytokine/chemokine concentrations, CFU numbers, and all other laboratory parameters as well as densitometry read-outs, unpaired, two-tailed student t test or Mann Whitney test were used. Statistical significance was assigned to p values less than 0.05.
RESULTS
CD73 deficiency decreases survival and bacterial clearance in CLP-induced sepsis
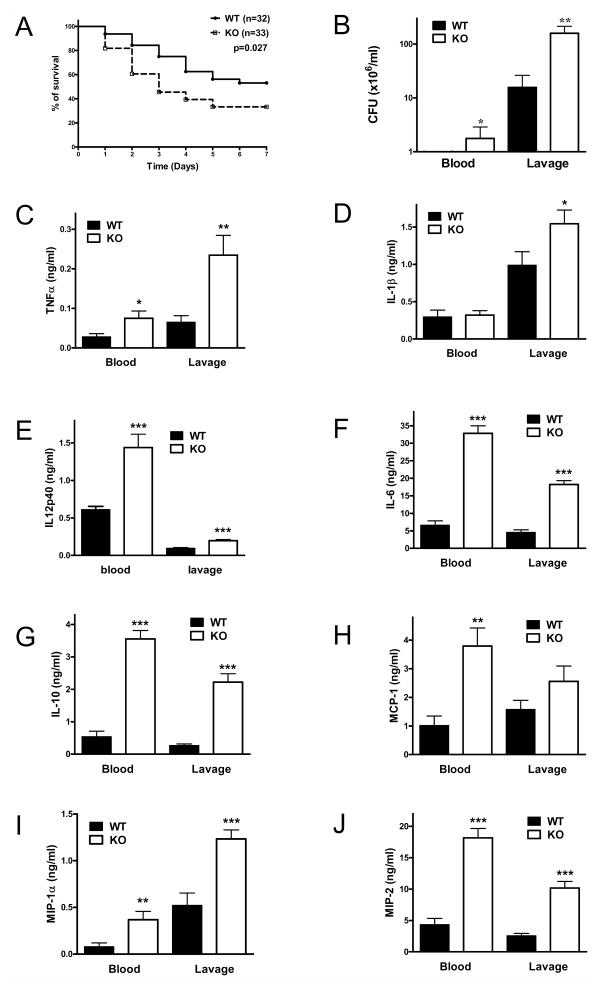
To begin to examine the role of CD73, we first investigated the effect of CD73 deficiency on the survival of CLP-challenged septic mice. We observed that the survival rate of CD73 KO mice was significantly lower than that of CD73 WT animals (Figure 1A). Because persistence of local bacterial infection and bloodstream microbial invasion contribute to death in the CLP model (9, 27, 47), we assessed the impact of CD73 on bacterial levels in the blood stream and at the primary site of peritoneal infection. CD73 KO mice had significantly higher bacterial load in both the blood and peritoneal lavage fluid when compared to WT animals 16 hours after CLP (Figure 1B). Thus, CD73 limits mortality and bacterial load in sepsis.
Figure 1. Loss of CD73 decreases survival, and increases bacterial load and inflammation in polymicrobial sepsis.
(A) The number of surviving CD73 WT and KO mice were counted daily for 7 days after inducing sepsis by cecal ligation and puncture (CLP). (B) Blood and peritoneal lavage fluid obtained from CD73 WT and KO mice 16 h post CLP were cultured on soy-trypticase agar plates for 24 h and then the bacterial colonies were counted (n = 13 mice per group). Serum and peritoneal lavage fluid concentration of TNF-α (C), IL-1β (D), IL12p40 (E), IL-6 (F), IL-10 (G), MCP-1 (H), MIP-1α (I) and MIP-2 (J) were determined in samples collected 16 h post CLP using ELISA. All results (mean ± SEM) shown are representative of three separate experiments. * p < 0.05 versus WT; ** p < 0.01 versus WT; * ** p < 0.001 versus WT.
Loss of CD73 increases cytokine and chemokine production in CLP-induced sepsis
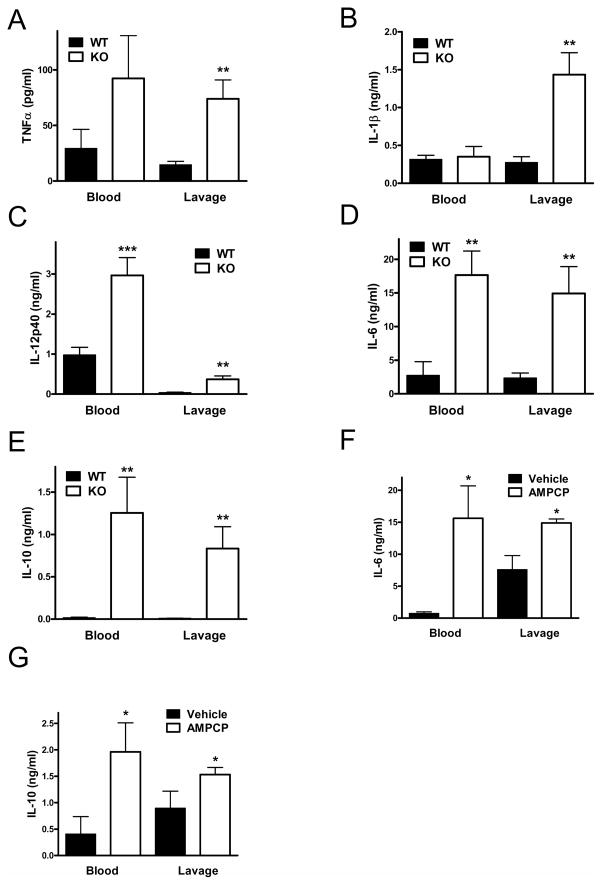
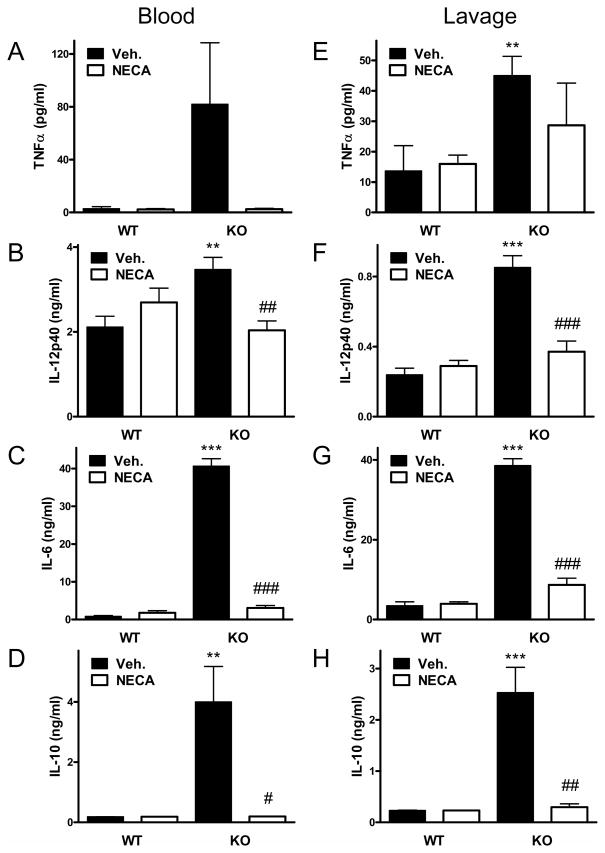
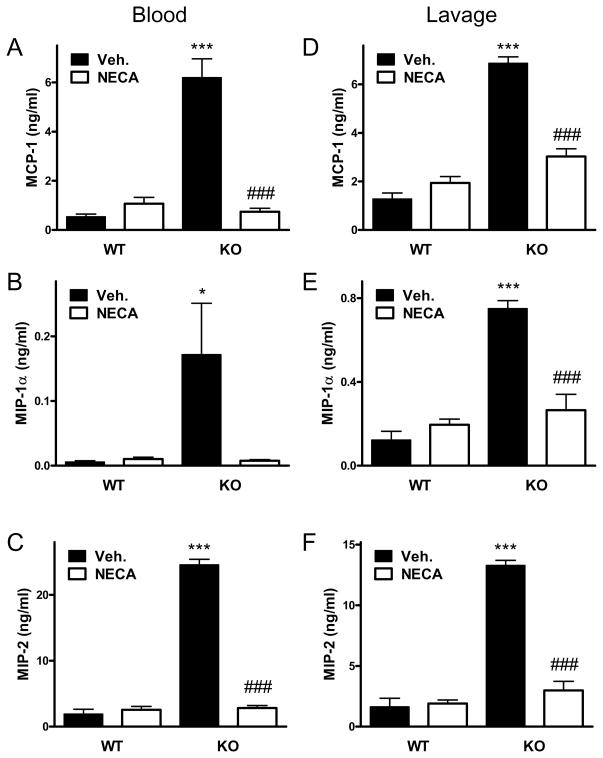
Elevated systemic and local levels of of cytokines and chemokines are associated with dysregulated immune responses and mortality in sepsis (9, 28, 47). We, therefore, determined concentrations of TNF-α, IL-1β, IL-12p40, IL-6, IL-10, MCP-1, MIP-1α, and MIP-2 in both the plasma and peritoneal lavage fluid. We found that with the exception of IL-1β the concentrations of all these cytokines and chemokines were markedly elevated in the blood and peritoneal fluid of CD73 KO vs. WT mice at 16 h after CLP (Figure 1, C–J). In addition, we detected increased levels of TNF-α, IL-1β, IL-12p40, IL-6, and IL-10 in CD73KO vs. WT mice already at 6 h following the CLP procedure (Figure 2, A–E).
Figure 2. Genetic disruption of CD73 increases cytokine and chemokine levels at 6 h after sepsis induction. Treatment with AMPCP, a selective inhibitor of CD73, increases the levels of IL-10 and IL-6 in blood and peritoneal lavage fluid.
Blood and peritoneal lavage fluid were obtained from CD73 WT and KO mice at 6 h post CLP and TNF-α (A), IL-1β (B), IL12p40 (C), IL-6 (D), and IL-10 (E) concentrations were determined using ELISA. In another set of experiments, mice were injected with AMPCP (2×2 mg/kg/day) or its vehicle (DMSO) and IL-6 (F) and IL-10 (G) levels were determined in blood and peritoneal lavage fluid 16 h post CLP. All results (mean ± SEM) shown are representative of three separate experiments. Data are the mean ± SEM of n = 7–9 mice in each group. * p < 0.05 versus WT; ** p < 0.01 versus WT; *** p < 0.001 versus WT.
Pharmacological inactivation of CD73 enhances CLP-induced cytokine production
We next examined the role of CD73 in modulating CLP-induced sepsis using a pharmacological approach. C57Bl/6J mice were treated with the selective CD73 antagonist AMPCP (2 × 2 mg/kg daily) or its vehicle, and cytokine levels were measured in the blood and peritoneal lavage fluid 16 h after the CLP procedure. Similar to the genetic inactivation of CD73, the concentrations of IL-6 and IL-10 were higher in both the blood and peritoneal lavage fluid of mice with pharmacological inhibition of CD73 enzyme (Figure 2, F and G). These results confirm that the inactivation of CD73 increases inflammatory responses in polymicrobial sepsis.
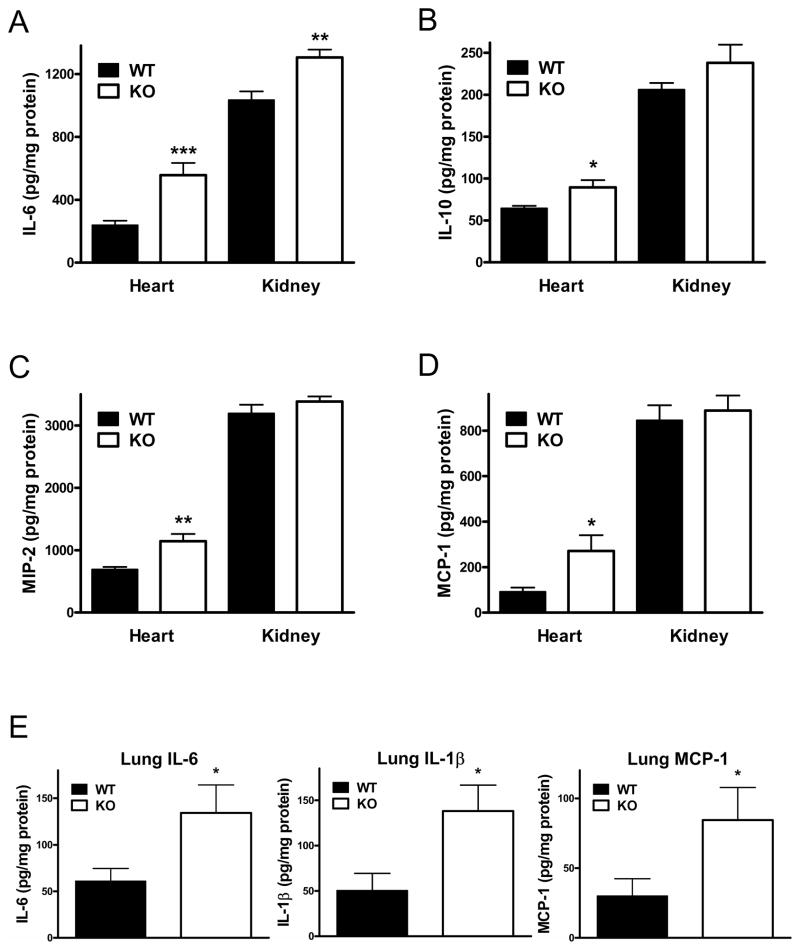
Genetic ablation of CD73 enhances the levels of inflammatory cytokines in the heart, kidney and lung of septic mice
Inflammation in the heart (48), kidney (49) and lung (50) contributes to organ dysfunction and mortality in sepsis. Therefore, we measured inflammatory mediator concentrations in these organs, in an attempt to provide insights into the protective functions of CD73 in sepsis. The levels of IL-6, IL-10, MIP-2 and MCP-1 were higher in the heart of CD73 KO than WT mice 16 h after CLP induction (Figure 3A–D). In addition, increased levels of IL-6 were detected in the kidney (Figure 3A) of septic CD73 KO vs. WT mice 16 h after the CLP procedure. Finally, we found markedly increased levels of IL-6, IL-1β, and MCP-1 in the lung of CD73 KO when compared to their WT counterparts (Figure 3E).
Figure 3. Genetic ablation of CD73 enhances sepsis-induced inflammatory cytokine and chemokine responses in the heart, lung and kidney.
Concentrations of IL-6 (A), IL-10 (B), MIP-2 (C), and MCP-1 (D) were measured from heart and kidney extracts of CD73 WT and KO mice taken at 16 h post CLP. (E) Pulmonary levels of IL-6, IL-1β, and MCP-1 were detected from lung extract of CD73 WT and KO mice taken 6 h following CLP. Data shown are the mean ± SEM of n = 7–9 mice in each group. Results are representative of three separate experiments. * p < 0.05 versus WT; ** p < 0.01 versus WT; *** p < 0.001 versus WT.
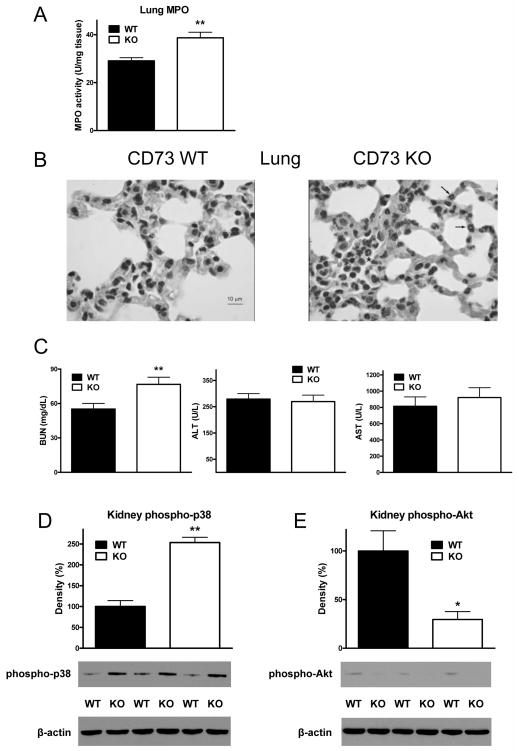
Genetic CD73 deletion augments pulmonary injury in septic mice
PMN infiltration of the lung occurs in sepsis (51) contributing to inflammation, tissue damage and organ dysfunction. Since we found elevated levels of inflammatory cytokines in the lung of septic CD73 KO mice, we investigated if this correlated with an increased accumulation of PMNs in the lungs of CD73 KO animals. We detected increased MPO activity, an indicator of PMN infiltration, in the lung of CD73 KO mice as compared with the lung of WT mice (Figure 4A). This increased PMN content was confirmed by microscopic evaluation of H&E stained lung sections, as lungs from animals lacking CD73 showed increased numbers of PMNs in comparison with lungs from WT mice (Figure 4B). Thus, we conclude that CD73 diminishes pulmonary PMN infiltration and inflammation during sepsis.
Figure 4. Loss of CD73 augments sepsis-induced inflammatory injury of the lung and kidney.
(A) Lung MPO activity was measured from lung extract collected from CD73 WT and KO mice 6 h after CLP. (B) Shown are tissue sections of lungs of septic CD73 KO and WT mice stained with H&E staining. Sections of lung of WT animal show no evident lesions. In the lung from a KO animal, there are several neutrophils present in alveolar capillaries (arrows). Neutrophils are also present together with erythrocytes and mononuclear cells in a small airspace (asterisk). A representative section is shown from slides from 3 WT and KO animals. (C) Concentrations of BUN, AST, and ALT were measured from blood of CD73 WT and KO mice that was taken at 16 h post CLP. Phosphorylation of p38 (D) and Akt (F) in kidney protein extracts of CD73 WT and KO mice was examined using Western blotting with specific antibodies. All results (mean ± SEM) shown are representative of three experiments (n = 6/group in each experiment). * p < 0.05 versus WT; ** p < 0.01 versus WT.
CD73 deletion increases kidney but not liver dysfunction in sepsis
Since we detected increased cytokine concentrations in the kidney, we next examined kidney function in septic CD73 KO and WT mice (26, 27, 49, 52–55). Serum levels of BUN, a marker of impaired kidney function, were higher in CD73 KO vs. WT mice indicating augmented renal injury in septic CD73 KO mice as compared to WT controls (Figure 4C). Because p38 MAPK activation in the kidney is an important factor leading to kidney inflammation and injury, we studied p38 activation in septic kidney specimens from CD73 KO and WT mice. As Figure 4D shows, the activation of p38 in the kidney of CD73-deficient mice was greater when compared with WT animals. Since Akt-signaling protects against the deleterious effects of kidney inflammation (56), we examined whether CD73 can regulate Akt signaling in the septic kidney. We found that Akt activation (phosphorylation) was decreased in the kidney of septic mice lacking CD73 when compared with WT littermates (Figure 4E). Finally, in contrast to the kidney, markers of liver function (ALT and AST) were not significantly different between the CD73WT and CD73 KO groups of septic animals (Figure 4C).
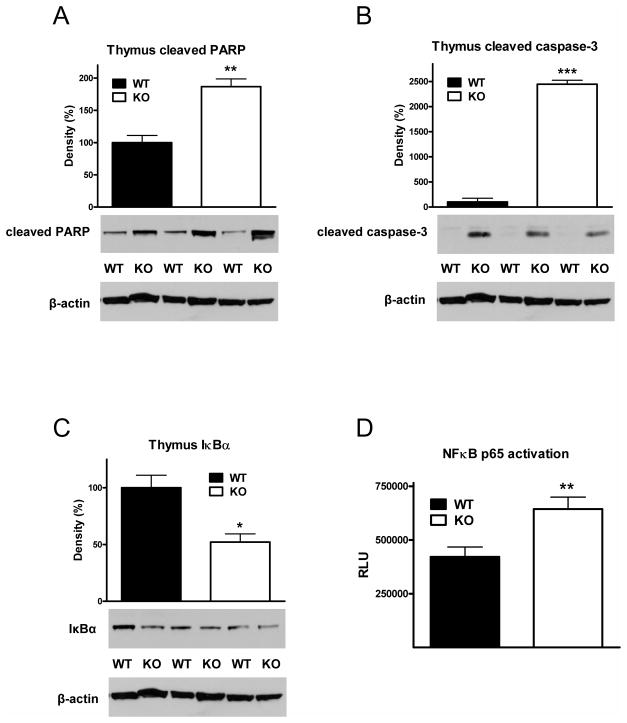
Genetic deletion of CD73 increases apoptosis in the thymus of septic mice
Sepsis induces extensive apoptosis in the thymus (57–59), which contributes to immune dysregulation and increases mortality (60). Based on previous findings indicating that the proteolytic cleavage of caspase-3 (58) and PARP (61) is a reliable molecular indicator of apoptosis, we investigated whether CD73 deficiency would affect the cleavage of these molecules in the thymus of mice subjected to CLP. We found that the cleavage of both PARP (Figure 5A) and caspase-3 (Figure 5B) was markedly increased in the thymus of CD73 KO mice. In addition, since NF-κB activation during sepsis can culminate in apoptosis (60, 62–64), we examined the effect of CD73 deletion on NF-κB activation in the thymus of septic mice. We assessed the activation state of the NF-κB system by measuring IκBα protein levels, which are inversely correlated with NF-κB activation, and nuclear p65 levels, whose abundance is indicative of activated NF-κB. We found decreased IκBα levels, and increased nuclear p65 levels in the thymus of septic CD73 KO mice when compared to WT littermates (Figure 5C and D), indicating increased NF-κB activation in CD73 KO mice.
Figure 5. Genetic CD73 deficiency increases PARP and caspase-3 cleavage, and NF-κB activation in the thymus of septic mice.
Protein extracts were prepared from thymus taken from septic CD73 WT and KO mice at 16 h post CLP challenge. The proteolytic cleavage of PARP (A), caspase-3 (B) and the cytosolic levels of IκBα (C) were examined using Western blotting with specific antibodies. Data (mean ± SEM) shown are representative of three experiments (n = 6/group in each experiment). The levels of p65 in the nuclear fractions of thymus extracts were assessed using NF-κB (p65) chemiluminescence assay kit (D). The results (mean ± SEM) shown are the summary of three experiments (WT, n = 25; KO n = 26). * p < 0.05 versus WT; ** p < 0.01 versus WT; *** p < 0.001 versus WT.
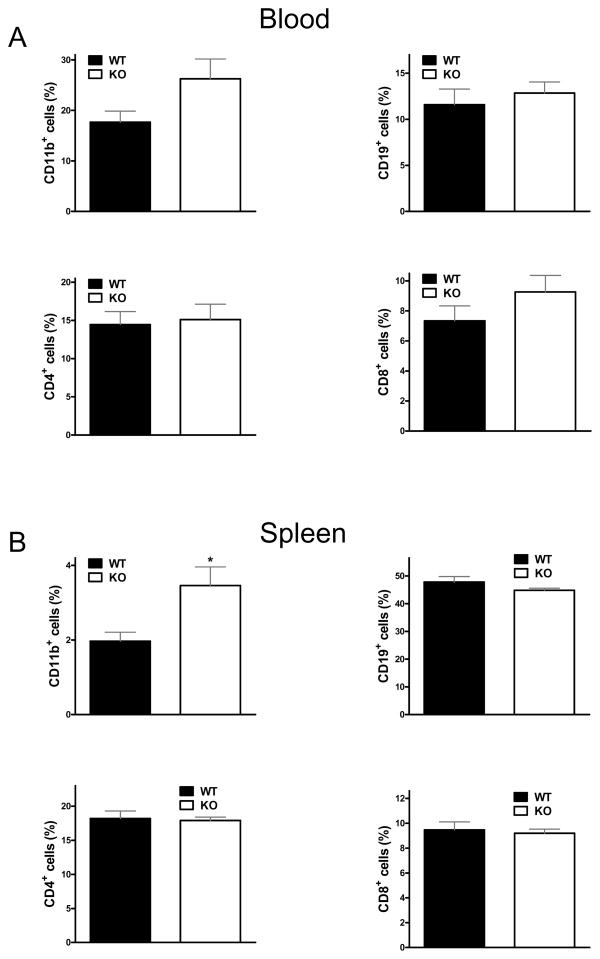
Lack of CD73 increases the number of splenic CD11b+ cells in CLP-induced sepsis
Because sepsis triggers changes in white blood cell numbers and distribution (60), we investigated white blood cell responses to sepsis in CD73 WT and KO mice. Analysis of white blood cell subsets revealed an increase in the numbers of CD11b+ cells in the spleen but not in the circulation of mice lacking CD73 when compared to WT (Figure 6A and B). The populations of CD19+, CD4+ and CD8+ cells in the blood and the spleen were similar in both (WT and KO) groups of septic mice (Figure 6A and B).
Figure 6. Loss of CD73 increases splenic CD11b+ cell accumulation.
Numbers of various lymphocyte subsets were monitored in blood (A) and spleen (B) by flow cytometric analysis after 16 h of sepsis induced by CLP. Population proportions of CD11b+, CD19+, CD4+ and CD8+ cells were analyzed. The results (mean ± SEM) shown are the summary of two separate experiments (WT, n = 22; KO, n = 20). * p < 0.05 versus WT.
NECA suppresses cytokine and chemokine levels in CD73 deficient mice
To confirm that lack of adenosine signaling is responsible for the increased production of inflammatory mediators in CD73 deficient mice, we conducted studies in which we attempted to rescue CD73 deficient mice from decreased adenosine signaling by stimulating adenosine receptors exogenously with the general adenosine receptor agonist NECA. Administration of NECA to CD73 deficient mice completely reversed the increased cytokine (Figure 7) and chemokine (Figure 8) levels, which were decreased in NECA-treated CD73 deficient mice to levels comparable to those observed in CD73 sufficient mice. We conclude that the increased inflammatory response observed in CD73 deficient mice is caused by decreased adenosine receptor activation.
Figure 7. Treatment with the adenosine receptor agonist NECA reverses the increased cytokine levels caused by CD73 deletion.
CD73 WT and KO mice underwent CLP and concentrations of various cytokines were measured from blood and peritoneal lavage fluid that were obtained 16 hours post-CLP. NECA (0.03 mg/kg) was administered intraperitoneally 30 minutes before the CLP insult. The results (mean ± SEM) shown are the summary of two separate experiments (WT, n = 19; KO, n = 18). * p < 0.05 versus WT with Veh. (Vehicle, DMSO); ** p < 0.01 versus WT with Veh.; # p < 0.05 versus KO with Veh.; ## p < 0.01 versus KO with Veh.; ### p < 0.005 versus KO with Veh.
Figure 8. Treatment with the adenosine receptor agonist NECA reverses the increased chemokine levels caused by CD73 deletion.
CD73 WT and KO mice underwent CLP and concentrations of various chemokines were measured from blood and peritoneal lavage fluid that were obtained 16 hours post-CLP. NECA (0.03 mg/kg) was administered intraperitoneally 30 minutes before the CLP insult. The results (mean ± SEM) shown are the summary of two separate experiments (WT, n = 19; KO, n = 18). * p < 0.05 versus WT with Veh.; *** p < 0.005 versus WT with Veh.; ### p < 0.005 versus KO with Veh.
DISCUSSION
This study demonstrates that CD73 improves the survival of septic mice, and this improved survival correlates with a decrease in bacterial growth and lessened lung and kidney injury.
Our finding that CD73 improved the survival of septic mice in combination with data in the literature indicates that increased adenosine levels and adenosine signaling can decrease mortality in sepsis. This idea is supported by previous studies that showed that increasing extracellular adenosine levels by interfering with adenosine metabolism downstream from CD73 improves survival in sepsis. Inhibiting adenosine deaminase, an enzyme that degrades adenosine to its inactive metabolite inosine (65), attenuated microvascular dysfunction and inflammation and improved survival in models of sepsis (66). In addition, pharmacological inhibition of adenosine kinase, an enzyme that catalyzes the phosphorylation of adenosine to nucleotides thereby decreasing extracellular adenosine levels, also increased survival in sepsis (67). Our reconstitution studies with NECA confirmed that the tissue-protective effects of CD73 in our sepsis model are related to its enzymatic function and role in elevating extracellular adenosine levels. It is also noteworthy that previous studies using adenosine receptor specific KO mice showed that endogenous adenosine via activating A1 (26), A2B (28), and A3 (27) receptors improves survival in sepsis. Although A2A receptor activation by endogenous adenosine decreases survival (9), our data with CD73 inhibition and previous results with adenosine deaminase and kinase inhibition suggest that the beneficial effects of adenosine via A1 (26), A2B (28), and A3 (27) receptor signaling can override the deleterious effect of A2A receptor signaling. We have also found decreased bacterial counts in the presence of CD73. This finding was somewhat unexpected because adenosine is, in general, an immunosuppressive mediator (22) and we, therefore, anticipated that CD73-derived adenosine would suppress anti-bacterial immune responses. It is possible that increased A1 receptor signaling leading to enhanced superoxide production (68) or augmented neutrophil chemotaxis via A3 receptors (69) contributed to the decreased bacterial load in CD73-sufficient animals. Indeed, according to Nakav et al. (70), at the onset of peritonitis, the expression of A1 receptor dominates and the expression of the anti-inflammatory A2A receptor is low. Thus, it is plausible that in the first hours after inducing peritonitis, CD73-derived adenosine augments antibacterial defense via A1 receptors on neutrophils. In addition, supporting a role for A3 receptors in controlling bacteria is the observation that A3 adenosine receptor WT mice displayed decreased aerobic bacterial counts following CLP-induced sepsis when compared to their KO counterparts (26, 27).
Evidence supports the role of the tissue injurious effects of activated neutrophils infiltrating immune competent organs following infections. Consistently with this notion our findings indicated decreased numbers of neutrophils recruited into the lung and spleen in CD73-sufficient animals. This could be the result of an decreased activation of neutrophils as the result of the suppressed cytokine storm or it can also imply a direct role of adenosine in modulating neutrophil activation and transmigration (71).
Our data showing an amelioration of the sepsis-induced cytokine storm in the presence of CD73 may provide additional insight into the protective mechanisms of CD73-derived adenosine during sepsis. Similar to our study, CD73 was previously demonstrated to suppresses pro-inflammatory cytokine production in murine gastritis (72), cardiac allographt vasculopathy (73), and intestinal ischemia-reperfusion injury (74). The decreases in inflammatory cytokine levels correlated well with the tissue protective effects of CD73 in vital organs such as lung and kidney of septic mice. These organ protective effects can contribute to the improved survival in septic CD73-sufficient mice, because inflammatory tissue damage and multiorgan failure result in high mortality rates in sepsis (3–5). The lung and kidney protection we documented here is in line with previous data showing that CD73 protects against bleomycin-, ventilator- and LPS-induced lung injury (38), and ischemia-reperfusion-induced kidney injury (44).
The mechanisms by which sepsis induces parenchymal tissue damage and organ injury are multifactorial. It is well documented for example that increased activation of NF-κB (62) and p38 (75) as well as the inactivation of Akt (76) can result in tissue damage and cell death leading to impaired clinical outcome in sepsis. Our results demonstrating the down-regulation of p38 (kidney) and NF-κB (thymus) activation, and the up-regulation of Akt (kidney) activation may represent important intracellular pathways by which CD73 and adenosine lead to lessened organ injury in sepsis. Confirming an important role for adenosine signaling in decreasing p38 and increasing Akt activation in tissue stress is the observation that disrupting adenosine signaling by the systemic administration of the adenosine receptor antagonist aminophylline resulted in higher p38 and lower Akt activation in anoxia-induced brain (77). In addition to injuring non-immune organs, sepsis can induce extensive apoptosis in immune tissues like the thymus (58, 59) and the spleen (78). It has been reported that caspase-3 activity is elevated to a greater extent than other caspases in the thymus and that it plays an important role in sepsis-induced thymocyte apoptosis (58). Our data showing decreased levels of caspase-3 cleavage as well as PARP cleavage in the thymus of septic CD73 WT mice indicate that this enzyme and its product, adenosine are essential protectors against apoptotic processes.
In summary, the present study demonstrates that CD73 is a critical control point for modulation of the host’s response to sepsis.
Abbreviations
- ALT
alanine aminotransferase
- AMPCP
α, β–methylene adenosine 5′-diphosphate
- AST
aspartate aminotransferase
- BUN
blood urea nitrogen
- CLP
cecal ligation and puncture
- KO
knockout
- MPO
myeloperoxidase
- NECA
5′-N-ethylcarboxamidoadenosine
- PARP
poly(ADP-ribose) polymerase
- PMN
polymorphonuclear neutrophil
- WT
wild type
Footnotes
Support: This work was supported by the National Institutes of Health (NIH) Grants GM66189 (GH), AI18220 (LT), and GM084932 (ZS), and the Intramural Research Program of NIH, National Institute on Alcohol Abuse and Alcoholism, as well as Hungarian Research Fund OTKA (CK 78275).
References
- 1.Bone RC. Immunologic dissonance: a continuing evolution in our understanding of the systemic inflammatory response syndrome (SIRS) and the multiple organ dysfunction syndrome (MODS) Ann Intern Med. 1996;125:680–687. doi: 10.7326/0003-4819-125-8-199610150-00009. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 4.Hotchkiss RS, I, Karl E. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 5.Riedemann NC, Guo RF, Ward PA. The enigma of sepsis. J Clin Invest. 2003;112:460–467. doi: 10.1172/JCI19523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benjamim CF, Hogaboam CM, Kunkel SL. The chronic consequences of severe sepsis. J Leukoc Biol. 2004;75:408–412. doi: 10.1189/jlb.0503214. [DOI] [PubMed] [Google Scholar]
- 7.Oberholzer A, Oberholzer C, Moldawer LL. Interleukin-10: A complex role in the pathogenesis of sepsis syndromes and its potential as an anti-inflammatory drug. Crit Care Med. 2002;30:S58–S63. [PubMed] [Google Scholar]
- 8.Ayala A, I, Chaudry H. Immune dysfunction in murine polymicrobial sepsis: mediators, macrophages, lymphocytes and apoptosis. Shock. 1996;6(Suppl 1):S27–38. [PubMed] [Google Scholar]
- 9.Nemeth ZH, Csoka B, Wilmanski J, Xu D, Lu Q, Ledent C, Deitch EA, Pacher P, Spolarics Z, Hasko G. Adenosine A2A receptor inactivation increases survival in polymicrobial sepsis. J Immunol. 2006;176:5616–5626. doi: 10.4049/jimmunol.176.9.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasko G, Szabo C, Nemeth ZH, Kvetan V, Pastores SM, Vizi ES. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J Immunol. 1996;157:4634–4640. [PubMed] [Google Scholar]
- 11.Hasko G, Kuhel DG, Chen JF, Schwarzschild MA, Deitch EA, Mabley JG, Marton A, Szabo C. Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. Faseb J. 2000;14:2065–2074. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- 12.Nemeth ZH, Leibovich SJ, Deitch EA, Sperlagh B, Virag L, Vizi ES, Szabo C, Hasko G. Adenosine stimulates CREB activation in macrophages via a p38 MAPK-mediated mechanism. Biochem Biophys Res Commun. 2003;312:883–888. doi: 10.1016/j.bbrc.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Nemeth ZH, Leibovich SJ, Deitch EA, Vizi ES, Szabo C, Hasko G. cDNA microarray analysis reveals a nuclear factor-kappaB-independent regulation of macrophage function by adenosine. J Pharmacol Exp Ther. 2003;306:1042–1049. doi: 10.1124/jpet.103.052944. [DOI] [PubMed] [Google Scholar]
- 14.Nemeth ZH, Lutz CS, Csoka B, Deitch EA, Leibovich SJ, Gause WC, Tone M, Pacher P, Vizi ES, Hasko G. Adenosine augments IL-10 production by macrophages through an A2B receptor-mediated posttranscriptional mechanism. J Immunol. 2005;175:8260–8270. doi: 10.4049/jimmunol.175.12.8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nemeth ZH, Bleich D, Csoka B, Pacher P, Mabley JG, Himer L, Vizi ES, Deitch EA, Szabo C, Cronstein BN, Hasko G. Adenosine receptor activation ameliorates type 1 diabetes. FASEB J. 2007;21:2379–2388. doi: 10.1096/fj.07-8213com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fredholm BB, APIJ, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 19.Zhong H, Belardinelli L, Maa T, Zeng D. Synergy between A2B adenosine receptors and hypoxia in activating human lung fibroblasts. Am J Respir Cell Mol Biol. 2005;32:2–8. doi: 10.1165/rcmb.2004-0103OC. [DOI] [PubMed] [Google Scholar]
- 20.Chunn JL, Mohsenin A, Young HW, Lee CG, Elias JA, Kellems RE, Blackburn MR. Partially adenosine deaminase-deficient mice develop pulmonary fibrosis in association with adenosine elevations. Am J Physiol Lung Cell Mol Physiol. 2006;290:L579–587. doi: 10.1152/ajplung.00258.2005. [DOI] [PubMed] [Google Scholar]
- 21.Haskó G, Deitch EA, Szabó C, Németh ZH, Vizi ES. Adenosine: a potential mediator of immunosuppression in multiple organ failure. Current Opinion in Pharmacology. 2002;2:440–444. doi: 10.1016/s1471-4892(02)00172-8. [DOI] [PubMed] [Google Scholar]
- 22.Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Blackburn MR, Volmer JB, Thrasher JL, Zhong H, Crosby JR, Lee JJ, Kellems RE. Metabolic consequences of adenosine deaminase deficiency in mice are associated with defects in alveogenesis, pulmonary inflammation, and airway obstruction. J Exp Med. 2000;192:159–170. doi: 10.1084/jem.192.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhong H, Belardinelli L, Maa T, Feoktistov I, Biaggioni I, Zeng D. A(2B) adenosine receptors increase cytokine release by bronchial smooth muscle cells. Am J Respir Cell Mol Biol. 2004;30:118–125. doi: 10.1165/rcmb.2003-0118OC. [DOI] [PubMed] [Google Scholar]
- 25.Martin C, Leone M, Viviand X, Ayem ML, Guieu R. High adenosine plasma concentration as a prognostic index for outcome in patients with septic shock. Crit Care Med. 2000;28:3198–3202. doi: 10.1097/00003246-200009000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Gallos G, Ruyle TD, Emala CW, Lee HT. A1 adenosine receptor knockout mice exhibit increased mortality, renal dysfunction, and hepatic injury in murine septic peritonitis. Am J Physiol Renal Physiol. 2005;289:F369–376. doi: 10.1152/ajprenal.00470.2004. [DOI] [PubMed] [Google Scholar]
- 27.Lee HT, Kim M, Joo JD, Gallos G, Chen JF, Emala CW. A3 adenosine receptor activation decreases mortality and renal and hepatic injury in murine septic peritonitis. Am J Physiol Regul Integr Comp Physiol. 2006;291:R959–969. doi: 10.1152/ajpregu.00034.2006. [DOI] [PubMed] [Google Scholar]
- 28.Csoka B, Nemeth ZH, Rosenberger P, Eltzschig HK, Spolarics Z, Pacher P, Selmeczy Z, Koscso B, Himer L, Vizi ES, Blackburn MR, Deitch EA, Hasko G. A(2B) adenosine receptors protect against sepsis-induced mortality by dampening excessive inflammation. J Immunol. 2010;185:542–550. doi: 10.4049/jimmunol.0901295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasko G, Pacher P. A2A receptors in inflammation and injury: lessons learned from transgenic animals. J Leukoc Biol. 2008;83:447–455. doi: 10.1189/jlb.0607359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, Colgan SP. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eltzschig HK, Thompson LF, Karhausen J, Cotta RJ, Ibla JC, Robson SC, Colgan SP. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism. Blood. 2004;104:3986–3992. doi: 10.1182/blood-2004-06-2066. [DOI] [PubMed] [Google Scholar]
- 33.Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, Robson SC, Colgan SP. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med. 2003;198:783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gessi S, Merighi S, Varani K, Leung E, Mac Lennan S, Borea PA. The A3 adenosine receptor: an enigmatic player in cell biology. Pharmacol Ther. 2008;117:123–140. doi: 10.1016/j.pharmthera.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ernst PB, Garrison JC, Thompson LF. Much Ado about Adenosine: Adenosine Synthesis and Function in Regulatory T Cell Biology. J Immunol. 2010;185:1993–1998. doi: 10.4049/jimmunol.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lennon PF, Taylor CT, Stahl GL, Colgan SP. Neutrophil-derived 5′-adenosine monophosphate promotes endothelial barrier function via CD73-mediated conversion to adenosine and endothelial A2B receptor activation. J Exp Med. 1998;188:1433–1443. doi: 10.1084/jem.188.8.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volmer JB, Thompson LF, Blackburn MR. Ecto-5′-Nucleotidase (CD73)-Mediated Adenosine Production Is Tissue Protective in a Model of Bleomycin-Induced Lung Injury. J Immunol. 2006;176:4449–4458. doi: 10.4049/jimmunol.176.7.4449. [DOI] [PubMed] [Google Scholar]
- 39.Eckle T, Fullbier L, Wehrmann M, Khoury J, Mittelbronn M, Ibla J, Rosenberger P, Eltzschig HK. Identification of Ectonucleotidases CD39 and CD73 in Innate Protection during Acute Lung Injury. J Immunol. 2007;178:8127–8137. doi: 10.4049/jimmunol.178.12.8127. [DOI] [PubMed] [Google Scholar]
- 40.Montesinos MC, Takedachi M, Thompson LF, Wilder TF, Fernandez P, Cronstein BN. The antiinflammatory mechanism of methotrexate depends on extracellular conversion of adenine nucleotides to adenosine by ecto-5′-nucleotidase: findings in a study of ecto-5′-nucleotidase gene-deficient mice. Arthritis Rheum. 2007;56:1440–1445. doi: 10.1002/art.22643. [DOI] [PubMed] [Google Scholar]
- 41.Reutershan J, Vollmer I, Stark S, Wagner R, Ngamsri K-C, Eltzschig HK. Adenosine and inflammation: CD39 and CD73 are critical mediators in LPS-induced PMN trafficking into the lungs. FASEB J. 2009;23:473–482. doi: 10.1096/fj.08-119701. [DOI] [PubMed] [Google Scholar]
- 42.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest. 2008;118:3301–3315. doi: 10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grenz A, Zhang H, Eckle T, Mittelbronn M, Wehrmann M, Kohle C, Kloor D, Thompson LF, Osswald H, Eltzschig HK. Protective Role of Ecto-5′-Nucleotidase (CD73) in Renal Ischemia. J Am Soc Nephrol. 2007;18:833–845. doi: 10.1681/ASN.2006101141. [DOI] [PubMed] [Google Scholar]
- 45.Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, Eltzschig HK. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 46.Senthil M, Watkins A, Barlos D, Xu DZ, Lu Q, Abungu B, Caputo F, Feinman R, Deitch EA. Intravenous injection of trauma-hemorrhagic shock mesenteric lymph causes lung injury that is dependent upon activation of the inducible nitric oxide synthase pathway. Ann Surg. 2007;246:822–830. doi: 10.1097/SLA.0b013e3180caa3af. [DOI] [PubMed] [Google Scholar]
- 47.Csoka B, Nemeth ZH, Mukhopadhyay P, Spolarics Z, Rajesh M, Federici S, Deitch EA, Batkai S, Pacher P, Hasko G. CB2 cannabinoid receptors contribute to bacterial invasion and mortality in polymicrobial sepsis. PLoS One. 2009;4:e6409. doi: 10.1371/journal.pone.0006409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nevière RFH, Chopin C, Formstecher P, Marchetti P. Caspase Inhibition Prevents Cardiac Dysfunction and Heart Apoptosis in a Rat Model of Sepsis. Am J Respir Crit Care Med. 2001;163:218–225. doi: 10.1164/ajrccm.163.1.2003109. [DOI] [PubMed] [Google Scholar]
- 49.Jo SKCD, Cho WY, Kim HK, Chang KH, Yun SY, Won NH. Inflammatory cytokines and lipopolysaccharide induce Fas-mediated apoptosis in renal tubular cells. Nephron. 2002;91:406–415. doi: 10.1159/000064280. [DOI] [PubMed] [Google Scholar]
- 50.Hiramatsu MHR, Karl IE, Buchman TG. Cecal ligation and puncture (CLP) induces apoptosis in thymus, spleen, lung, and gut by an endotoxin and TNF-independent pathway. Shock. 1997;7:247–253. doi: 10.1097/00024382-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Xu N, Gao XP, Minshall RD, Rahman A, Malik AB. Time-dependent reversal of sepsis-induced PMN uptake and lung vascular injury by expression of CD18 antagonist. Am J Physiol Lung Cell Mol Physiol. 2002;282:L796–802. doi: 10.1152/ajplung.00298.2001. [DOI] [PubMed] [Google Scholar]
- 52.Rosenberg IK, Gupta SL, Lucas CE, Khan AA, Rosenberg BF. Renal Insufficiency After Trauma and Sepsis: A Prospective Functional and Ultrastructural Analysis. AMA Arch Surg. 1971;103:175–183. doi: 10.1001/archsurg.1971.01350080091013. [DOI] [PubMed] [Google Scholar]
- 53.Lee HT, Xu H, Nasr SH, Schnermann J, Emala CW. A1 adenosine receptor knockout mice exhibit increased renal injury following ischemia and reperfusion. Am J Physiol Renal Physiol. 2004;286:F298–306. doi: 10.1152/ajprenal.00185.2003. [DOI] [PubMed] [Google Scholar]
- 54.Morikawa A, Sugiyama T, Kato Y, Koide N, Jiang G, Takahashi K, Tamada Y, Yokochi T. Apoptotic cell death in the response of D-galactosamine-sensitized mice to lipopolysaccharide as an experimental endotoxic shock model. Infect Immun. 1996;64:734–738. doi: 10.1128/iai.64.3.734-738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee HT, Gallos G, Nasr SH, Emala CW. A1 Adenosine Receptor Activation Inhibits Inflammation, Necrosis, and Apoptosis after Renal Ischemia-Reperfusion Injury in Mice. J Am Soc Nephrol. 2004;15:102–111. doi: 10.1097/01.asn.0000102474.68613.ae. [DOI] [PubMed] [Google Scholar]
- 56.Joo JD, Kim M, Horst P, Kim J, D’Agati VD, Emala CW, Sr, Lee HT. Acute and delayed renal protection against renal ischemia and reperfusion injury with A1 adenosine receptors. Am J Physiol Renal Physiol. 2007;293:F1847–1857. doi: 10.1152/ajprenal.00336.2007. [DOI] [PubMed] [Google Scholar]
- 57.Wang S, Huang K, Lin Y, Lei H. Sepsis-induced apoptosis of the thymocytes in mice. J Immunol. 1994;152:5014–5021. [PubMed] [Google Scholar]
- 58.Guo RF, H-LM, Wang X, Sarma V, Padgaonkar VA, Craig RA, Riedemann NC, McClintock SD, Hlaing T, Shi MM, Ward PA. Protective effects of anti-C5a in sepsis-induced thymocyte apoptosis. J Clin Invest. 2000;106:1271–1280. doi: 10.1172/JCI10793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tinsley KW, Buchman CSTG, Chang KC, Hui JJ, Swanson PE, Karl IE, Hotchkiss RS. Caspases -2, -3, -6, and -9, but not caspase-1, are activated in sepsis-induced thymocyte apoptosis. Shock. 2000;13:1–7. doi: 10.1097/00024382-200013010-00001. [DOI] [PubMed] [Google Scholar]
- 60.Hotchkiss RS, Swanson PE, Cobb JP, Jacobson A, Buchman TG, Karl IE. Apoptosis in lymphoid and parenchymal cells during sepsis: findings in normal and T- and B-cell-deficient mice. Crit Care Med. 1997;25:1298–1307. doi: 10.1097/00003246-199708000-00015. [DOI] [PubMed] [Google Scholar]
- 61.Fearnhead HO, Dinsdale D, Cohen GM. An interleukin-1[beta]-converting enzyme-like protease is a common mediator of apoptosis in thymocytes. FEBS Letters. 1995;375:283–288. doi: 10.1016/0014-5793(95)01228-7. [DOI] [PubMed] [Google Scholar]
- 62.Böhrer H, Qiu F, Zimmermann T, Zhang Y, Jllmer T, Männel D, Böttiger BW, Stern DM, Waldherr R, Saeger HD, Ziegler R, Bierhaus A, Martin E, Nawroth PP. Role of NFkappaB in the mortality of sepsis. The Journal of Clinical Investigation. 1997;100:972–985. doi: 10.1172/JCI119648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Foulds S, Galustian C, Mansfield AO, Schachter M. Transcription factor NF kappa B expression and postsurgical organ dysfunction. Ann Surg. 2001;233:70–78. doi: 10.1097/00000658-200101000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Joshi AR, Chung CS, Song GY, Lomas J, Priester RA, Ayala A. NF-kappaB activation has tissue-specific effects on immune cell apoptosis during polymicrobial sepsis. Shock. 2002;18:380–386. doi: 10.1097/00024382-200210000-00015. [DOI] [PubMed] [Google Scholar]
- 65.Hasko G, Sitkovsky MV, Szabo C. Immunomodulatory and neuroprotective effects of inosine. Trends Pharmacol Sci. 2004;25:152–157. doi: 10.1016/j.tips.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 66.Cohen ES, Law WR, Easington CR, Cruz KQ, Nardulli BA, Balk RA, Parrillo JE, Hollenberg SM. Adenosine Deaminase Inhibition Attenuates Microvascular Dysfunction and Improves Survival in Sepsis. Am J Respir Crit Care Med. 2002;166:16–20. doi: 10.1164/rccm.200109-014OC. [DOI] [PubMed] [Google Scholar]
- 67.Firestein G, Boyle D, Bullough D, Gruber H, Sajjadi F, Montag A, Sambol B, Mullane K. Protective effect of an adenosine kinase inhibitor in septic shock. J Immunol. 1994;152:5853–5859. [PubMed] [Google Scholar]
- 68.Cronstein BN, Daguma L, Nichols D, Hutchison AJ, Williams M. The adenosine/neutrophil paradox resolved: human neutrophils possess both A1 and A2 receptors that promote chemotaxis and inhibit O2 generation, respectively. J Clin Invest. 1990;85:1150–1157. doi: 10.1172/JCI114547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 70.Nakav S, Chaimovitz C, Sufaro Y, Lewis EC, Shaked G, Czeiger D, Zlotnik M, Douvdevani A. Anti-inflammatory preconditioning by agonists of adenosine A1 receptor. PLoS One. 2008;3:e2107. doi: 10.1371/journal.pone.0002107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cronstein BN, Levin RI, Philips M, Hirschhorn R, Abramson SB, Weissmann G. Neutrophil adherence to endothelium is enhanced via adenosine A1 receptors and inhibited via adenosine A2 receptors. J Immunol. 1992;148:2201–2206. [PubMed] [Google Scholar]
- 72.Alam Mohammad S, Kurtz Courtney C, Rowlett Robert M, Reuter Brian K, Wiznerowicz E, Das S, Linden J, Crowe Sheila E, Ernst Peter B. CD73 Is Expressed by Human Regulatory T Helper Cells and Suppresses Proinflammatory Cytokine Production and Helicobacter felis–Induced Gastritis in Mice. The Journal of Infectious Diseases. 2009;199:494–504. doi: 10.1086/596205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hasegawa T, Bouis D, Liao H, Visovatti SH, Pinsky DJ. Ecto-5′ Nucleotidase (CD73)-Mediated Adenosine Generation and Signaling in Murine Cardiac Allograft Vasculopathy. Circ Res. 2008;103:1410–1421. doi: 10.1161/CIRCRESAHA.108.180059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hart ML, Henn M, Kohler D, Kloor D, Mittelbronn M, Gorzolla IC, Stahl GL, Eltzschig HK. Role of extracellular nucleotide phosphohydrolysis in intestinal ischemia-reperfusion injury. FASEB J. 2008;22:2784–2797. doi: 10.1096/fj.07-103911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Song GY, Chung CS, Chaudry IH, Ayala A. Immune suppression in polymicrobial sepsis: differential regulation of Th1 and Th2 responses by p38 MAPK. J Surg Res. 2000;91:141–146. doi: 10.1006/jsre.2000.5928. [DOI] [PubMed] [Google Scholar]
- 76.Bommhardt U, Chang KC, Swanson PE, Wagner TH, Tinsley KW, Karl IE, Hotchkiss RS. Akt Decreases Lymphocyte Apoptosis and Improves Survival in Sepsis. J Immunol. 2004;172:7583–7591. doi: 10.4049/jimmunol.172.12.7583. [DOI] [PubMed] [Google Scholar]
- 77.Milton SL, Dirk LJ, Kara LF, Prentice HM. Adenosine modulates ERK1/2, PI3K/Akt, and p38MAPK activation in the brain of the anoxia-tolerant turtle Trachemys scripta. J Cereb Blood Flow Metab. 2008;28:1469–1477. doi: 10.1038/jcbfm.2008.45. [DOI] [PubMed] [Google Scholar]
- 78.Javadi PBT, Stromberg PE, Husain KD, Dunne WM, Woolsey CA, Turnbull IR, Hotchkiss RS, Karl IE, Coopersmith CM. High-dose exogenous iron following cecal ligation and puncture increases mortality rate in mice and is associated with an increase in gut epithelial and splenic apoptosis. Crit Care Med. 2004;32:1178–1185. doi: 10.1097/01.ccm.0000124878.02614.4c. [DOI] [PubMed] [Google Scholar]