Abstract
Purpose of Review
Asthma is a global burden, affecting 5% of the general adult population, of whom approximately 5-10% suffer from severe asthma. Severe asthma is a complex heterogeneous disease entity, with high morbidity and mortality. Increasingly novel techniques in computerised tomography are being used to understand the pathophysiology of severe asthma. The utility and clinical implications of these CT techniques are the focus of this review.
Recent Findings
Novel qualitative and quantitative CT imaging techniques have enabled us to study the large airway architecture in detail, assess the small airway structure, and perform functional analysis of regional ventilation.
Summary
Despite advances in CT imaging techniques, there is an urgent need for both proof-of-concept studies, large cross-sectional and longitudinal clinical trials in severe asthma to validate and clinically correlate imaging derived measures. This will extend our current understanding of the pathophysiology of severe asthma, and unravel the structure-function relationship, with the potential to discover novel severe asthma phenotypes, predict mortality, morbidity, and response to existing and novel pharmacological and non-pharmacological therapies.
Keywords: Severe Asthma, Airway disease, Computed Tomography
INTRODUCTION
Over 300 million people worldwide have asthma, with the prevalence in the UK being amongst the highest in the developed world. [1] Asthma affects approximately 5% of the general adult population, of whom approximately 5-10% suffer from severe asthma. This group of patients are of particular importance, as they contribute to the vast majority of the economic burden of asthma in Europe due to frequent exacerbations, hospitalisations, and death.
Computed tomography (CT) scans in asthma are typically indicated to either identify associated conditions, such as, bronchopulmonary aspergillosis or detect conditions that mimic asthma, such as, hypersensitivity pneumonitis. In severe asthma, the potential role for CT is considerably wider. CT provides detailed macroscopic anatomical examination of the lung parenchyma. Multi-detector row CT (MDCT) scanners facilitate isotropic acquisition of the whole chest with sub-millimetre resolution within a single breath hold. Furthermore, rapid advances in post-processing software for CT images now permits multi-planar reconstructions, three-dimensional surface and volume images of the airway tree and lung parenchyma, detailed quantatitive analysis, and virtual bronchoscopy. Quantitative imaging techniques have given us the ability to obtain direct measurements by three-dimensional assessment of the large airways as well as indirect assessment of the small airways by densitometric measures of paired inspiratory and expiratory scans. In addition to the acquisition of detailed anatomical data of the lungs, CT can now also provide functional assessment of ventilation and perfusion. Current guidelines for severe asthma [2, 3] highlights CT as a tool for disease evaluation and its applications are likely to increase. In particular, the identification of airway remodelling patterns in various phenotypes of severe asthma and the ability to relate airway structure using CT, to important clinical outcomes, airway physiology and inflammation, may help to close the gap in our understanding of severe asthma phenotypes, and to target treatment more effectively.
Qualitative Assessment of CT scans in asthma
Qualitative assessments of CT findings in asthma subjects, have been performed by a number of studies [4-10]. Gupta et al [4], found that in a cohort of 185 severe asthmatics, the prevalence of bronchiectasis was 40%, which was similar to the median of the reported studies of 31%. Bronchiectasis in asthma subjects, was associated with longer disease duration [4, 5, 7, 9] and poorer lung function, [4, 10] however, association with disease severity was found in some studies [5] but not others. [5, 6, 8] Whether or not bronchiectasis in severe asthma is a co-morbidity, resulting in “difficult” to manage asthma or represents structural change or remodelling with natural progression of the disease [8] is not known, and longitudinal studies are required to ascertain this differentiation.
Bronchial wall thickening is a common feature of severe asthma. Gupta et al reported a prevalence of 62% [5] and others have found bronchial wall thickening more prevalent in asthmatics compared to healthy controls [6, 9] and was associated with asthma severity and longer disease duration.[5-7, 10]
Quantitative assessment of large airways in asthma
Advances in CT technology and post-processing software, have made quantitative assessments of the airway tree and lung parenchyma possible. However, the exact measurement of the airway wall, which involves the identification of lumen-wall and wall-parenchyma boundaries on CT images, is still an inexact science with a number of algorithms being proposed by researchers. One of the earliest and most widely used techniques is the ‘Full-Width at Half Maximum’ principle(figure 1). [11] This technique can cause systematic errors in airway wall and lumen estimation, [12] due to the blurring of edges by the CT scanner’s point spread function, oblique orientation of the airways, algorithm used for image reconstruction and/or size of airway analysed. Methods that have been devised to overcome such problems include, ‘Laplacian and Gaussian’ algorithm, [13] which utilises smoothening and edge detection filters to segment airways; ‘integral based method’, which minimises the CT’s blurring effect; and ‘phase congruency method’, which use multiple reconstruction algorithms to localise airway wall, to name but a few. Most of these new software platforms, however, have been designed for volumetric CT scans, and not for the standard HRCT scans, which limits their application to retrospective analysis of archived scans.
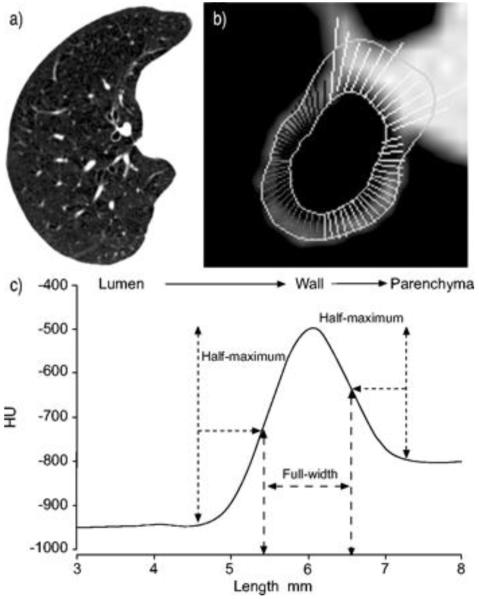
Figure 1.
Airway dimension estimation using full-width at half maximum principle. (A) A representative computed tomography image is shown. (B) A magnified view of an airway is shown. Rays can be seen between the inner and outer boundary of the airway wall. Some rays extend into the pulmonary artery because the artery has similar x-ray attenuation values as the airway wall. Those rays are manually eliminated and the airway dimension calculated using the remaining rays. (C) A representative x-ray attenuation profile for a ray that passes from airway lumen to the lung parenchyma through the airway wall is shown. The inner and outer airway wall boundary is estimated based on points at which x-ray attenuation value is half way to the local maximum and half way to the local minimum respectively. [15**]
More recently software can be used on volumetric CT scans for 3D analysis of the airway tree, which allows lumen extraction, generation of the airway centreline, segmentation of the airway tree up to 5th and 6th generation, and measurement of airway dimensions on reformatted images orthogonal to the long axis to minimise errors due to oblique orientation.
Various studies have utilised CT for non-invasive quantitative assessment of proximal airway structural changes in asthmatic adults [14-21] and children. [22, 23] De Blic et al demonstrated, in children with severe asthma a correlation between bronchial wall thickening and reticular basement membrane thickness; [22] Saglani et al [23] did not. Other studies have shown that asthma patients have increased airway wall thickness regardless of disease severity, [4, 16, 18, 21] whilst others have demonstrated a correlation between CT assessed airway remodelling and asthma severity. [17, 19, 20] However, there is some inconsistency between researchers on the airway lumen in asthma. Niimi et al reported no significant difference in lumen area of asthma subjects of varying severity and healthy controls. [20] Gupta et al, on the other hand, demonstrated that the right upper lobe apical bronchus (RB1) lumen area (LA) corrected for body surface area (BSA) was significantly narrowed, in 99 severe asthmatics, compared to healthy subjects. [14] In asthma, airway wall thickening has been correlated with airflow limitation, [14, 17, 20] airway hyperresponsiveness, [17, 21, 24] and air trapping on expiratory CT.
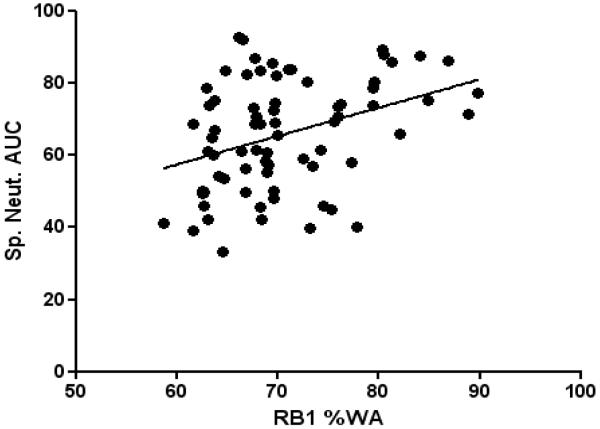
Only very few studies have explored the link between CT-assessed remodelling and airway inflammation in asthma. Little et al failed to find an association between airway wall geometry and sputum neutrophils.[19] Niimi et al failed to find an association between sputum eosinophilia and airway wall geometry in two quantitative airway imaging studies, [20, 24] in contrast De Blic et al demonstrated an association between broncho-alveolar lavage eosinophil cationic protein levels and airway wall thickening in a childhood severe asthma cohort. [22] Gupta et al have demonstrated an increase in RB1 % wall area (WA) in subjects with and without eosinophilic inflammation and was associated with the preceding burden of neutrophilic inflammation over time measured by repeated sputum analysis (Figure 2).[4] Furthermore, Siddiqui et al have demonstrated that although severe asthma and eosinophilic bronchitis have a similar degree of airway eosinophilic inflammation, eosinophilic bronchitis subjects lacked airway wall thickening with maintained patency of the RB1 lumen area as compared to severe asthmatics. [21]
Figure 2.
Correlation between right upper lobe apical segmental bronchus (RB1) percentage wall area (%WA) and sputum neutrophil area under the curve (AUC %) in patients with severe asthma (r = 0.36, p<0.005). [14]
CT assessment of airway remodelling has proven to be a sensitive measure to detect and quantify change after the administration of treatments. Three different studies have demonstrated a significant decrease in airway wall thickness/area after the administration of inhaled corticosteroids. [25-27] Halder et al demonstrated a decrease in RB1 wall area after 1 year of treatment with anti-IL-5 compared to placebo, of ~10 %, in 52 severe refractory asthmatic subjects with eosinophilic airway inflammation, [28] which is similar to the ~11% WA/BSA change demonstrated by Niimi et al. [27] These findings support the view that changes in proximal airway geometry are clinically important. This altered geometry in severe asthma patients compared to healthy controls, may be the key to understanding the physiological characteristics of asthma, such as, airway hyperresponsiveness and airflow obstruction, however at present results are conflicting. [14, 17, 20, 21, 24] This discrepancy between CT studies may be due to methodological variation, difficulties in measuring proximal airways dimensions, inability of CT to identify all components of airway remodelling, and the mere complexity of structural and functional interactions in asthma, which only accentuates the need for further prospective interventional and longitudinal studies.
Importantly, to date most studies have been undertaken in single centres and there are a number of potential issues for multi-centre trials. Standardization across centres should include not only the software, but also the CT parameters of acquisition and reconstruction of images, and the parameters used for assessing the dimensions of the airways (wall area, wall thickness, wall area % and WA/BSA). This can in part be addressed with the appropriate use of phantoms to validate different scanners. [29] More importantly the choice of airways to be measured should also be standardized: one or two bronchi (upper and lower lobes) or multiple segmental or sub-segmental bronchi. Current data suggest that RB1 correlates well with non-RB1 proximal airways in severe asthma, despite heterogeneity of remodelling. [14, 17, 21] but volumetric measurements of multiple airways would provide a more comprehensive assessment. The compromise between simplicity of outcome measure and detail has not been fully explored and will need to be addressed if this measure has a place in clinical trials. Similarly, to use such CT quantitative assessments of airway dimensions to measure outcomes after interventions, the reproducibility of these measurements between two successive acquisitions (interscan variability) need to be fully established. [30] This is critical to define the threshold required to consider modifications in airway dimensions as significant.
Quantitative Assessment of Small Airways in Asthma
The small airways (peripheral membranous bronchioles <2mm in diameter) are also significantly affected in asthma, [31] with the presence of significant inflammation [32] and airway remodelling [33] being demonstrated in the small airways of asthmatic subjects. CT scanners, however, cannot be used for the direct evaluation of the dimensions of the small airways as they are beyond the currently available resolution. Indirect changes caused by the small airways on the lung parenchyma can however be detected on CT, as small airways dysfunction results in reduced ventilation of part of the lung, which results in a reflex reduction in perfusion highlighted as areas of decreased attenuation on CT images. [34] Heterogeneity of lung attenuation in asthma can be noticeably accentuated in expiratory scans compared to inspiratory CT scans, due to regional differences in small airway closure or emptying rate. Low attenuation areas can then be quantified using the ‘density mask’ technique. [35] Although asymptomatic individuals with no lung function abnormalities also demonstrate low attenuation regions on CT scans, [36] asthmatic patients have significantly more air-trapping which correlates well with lung function abnormalities. [37-40] Various indices to quantify air trapping in asthma on CT have been developed, including −850 HU attenuation threshold at functional residual capacity, [38] percentage of pixels below −900 HU in expiratory scans, [40] mean lung density expiratory to inspiratory ratio, [39] difference between inspiratory and expiratory lung attenuation, [41] and median lung attenuation or lowest 10th percentile lung attenuation frequency distribution. [42]
Air trapping has been shown to correlate with asthma severity, [43] yet the relationship is not fully understood. Busacker et al [38] found that subjects with air trapping were significantly more likely to have a history of asthma related hospitalisation and intensive care visits compared to those without air-trapping, which suggests that CT assessed air-trapping could potentially be used to identify the ‘at-risk’ asthma phenotype. CT assessed air-trapping has also been associated with airway hyper-responsiveness, [40, 43] disease duration, [38] airflow limitation, [38-40] and used for evaluation of response to inhaled corticosteroid therapy.[41]
Similar to the proximal airways assessment, there is a wide variation of scanning protocols and indices of air trapping utilised by researchers. Yet, quantitative CT assessment of small airways in asthma will undoubtedly further our understanding of disease pathogenesis in asthma.
Synchrotron Radiation CT and Dual-Energy CT
To evaluate asthma it is necessary to perform both a morphological assessment of the airways and a functional analysis of regional ventilation. This can be achieved by using synchrotron radiation CT / dual-energy CT. Ventilation imaging can be accomplished through non-radioactive Xenon (Xe) enhanced CT. Xenon is radiodense which makes it possible, using CT, to track the density changes occurring regionally at the bronchoalveolar levels after inhalation of Xenon. Two Xenon CT techniques have been developed to assess regional ventilation: single breath and multi breath. In the former, imaging is performed after the subject takes a single deep breath of high Xenon concentration mixture. [44]. As a result of its simplicity and lower radiation burden there is more interest in single breath technique. [44] Synchrotron radiation CT and dual energy CT can both be used for single breath technique.
Chae et al. showed that ventilation defects can be demonstrated in stable asthmatics by Xenon ventilation dual energy CT. [45] They also showed that the ventilation defect score correlated with FEV1/FVC ratio, diffusion capacity (DLco) and residual lung volumes. The configuration and location of regional ventilation defects on dual energy CT were similar to those on Helium MRI.[45] Lung perfusion imaging can also be performed using dual energy CT scanning as it can provide an iodine map of the lung microcirculation.[46] Although true perfusion imaging is not performed using dual energy CT as it visualises only blood volume and not blood flow, it may help in assessment of perfusion alterations due to vascular remodelling, an important feature of asthma. Apart from demonstrating morphology and regional perfusion or ventilation defects, dual energy CT can potentially be helpful in understanding the pathogenesis of the disease especially helping in phenotyping patients with severe asthma and evaluating response to treatment.
Conclusion
Severe asthma is a complex heterogeneous disease entity, with high morbidity and mortality. CT imaging technology, with its unprecedented diagnostic proficiency has provided us with an opportunity to extend our understanding of this heterogeneous disease. Novel qualitative and quantitative imaging techniques have enabled us to study the large airway architecture in detail, assess the small airway structure, and perform functional physiological evaluations. Despite these spectacular advances in CT imaging techniques, there is an urgent need for both proof-of-concept studies, large cross-sectional and longitudinal clinical trials in severe asthma to validate and clinically correlate imaging derived measures. This will extend our current understanding of severe asthma pathophysiology and unravel the structure-function-relationship, which has the unprecedented potential to discover novel severe asthma phenotypes, predict mortality, morbidity and response to existing and novel pharmacological and non-pharmacological therapies.
Summary.
-
■
Asthma is a global burden, affecting 5% of the adult population, of whom 5-10% suffer from severe asthma, with high morbidity and mortality.
-
■
Asthma is a complex, heterogeneous disease process with different clinical and pathophysiological characteristics.
-
■
Novel qualitative and quantitative CT imaging techniques have enabled us to study the large airway architecture in detail, assess the small airway structure, and perform functional physiological evaluations.
-
■
True understanding of the structure/function relationship in severe asthma can only be achieved by integration of all data, as well as large cross-sectional and longitudinal clinical trials to validate and clinically correlate imaging derived measures.
Acknowledgements
AirPROM (FP7-270194). Professor Brightling is also a Wellcome Trust Senior Clinical Fellow.
Abbreviations used in manuscript
- BSA
Body surface area
- CT
Computed tomography
- DLco
Diffusion capacity
- FRC
Functional residual capacity
- HRCT
High resolution computed tomography
- HU
Hounsfield units
- LA
Lumen area
- MRI
Magnetic resonance imaging
- MDCT
Multi-detector computed tomography
- RB1
Right upper lobe apical bronchus
- 3D
Three dimensional
- TA
Total area
- WA
Wall area
- WT
Wall thickness
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004 May;59(5):469–78. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 2.Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008 Jan;31(1):143–78. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 3.British Thoracic Society British guideline on the management of asthma - A national clinical guideline. Thorax. 2008 [Google Scholar]
- 4.Gupta S, Siddiqui S, Haldar P, et al. Qualitative analysis of high-resolution CT scans in severe asthma. Chest. 2009 Dec;136(6):1521–8. doi: 10.1378/chest.09-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paganin F, Seneterre E, Chanez P, et al. Computed tomography of the lungs in asthma: influence of disease severity and etiology. Am J Respir Crit Care Med. 1996 Jan;153(1):110–4. doi: 10.1164/ajrccm.153.1.8542102. [DOI] [PubMed] [Google Scholar]
- 6.Grenier P, Mourey-Gerosa I, Benali K, et al. Abnormalities of the airways and lung parenchyma in asthmatics: CT observations in 50 patients and inter- and intraobserver variability. Eur Radiol. 1996;6(2):199–206. doi: 10.1007/BF00181147. [DOI] [PubMed] [Google Scholar]
- 7.Yilmaz S, Ekici A, Ekici M, et al. High-resolution computed tomography findings in elderly patients with asthma. Eur J Radiol. 2006 Aug;59(2):238–43. doi: 10.1016/j.ejrad.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Takemura M, Niimi A, Minakuchi M, et al. Bronchial dilatation in asthma: relation to clinical and sputum indices. Chest. 2004 Apr;125(4):1352–8. doi: 10.1378/chest.125.4.1352. [DOI] [PubMed] [Google Scholar]
- 9.Lynch DA, Newell JD, Tschomper BA, et al. Uncomplicated asthma in adults: comparison of CT appearance of the lungs in asthmatic and healthy subjects. Radiology. 1993 Sep;188(3):829–33. doi: 10.1148/radiology.188.3.8351357. [DOI] [PubMed] [Google Scholar]
- 10.Bumbacea D, Campbell D, Nguyen L, et al. Parameters associated with persistent airflow obstruction in chronic severe asthma. Eur Respir J. 2004 Jul;24(1):122–8. doi: 10.1183/09031936.04.00077803. [DOI] [PubMed] [Google Scholar]
- 11.de Jong PA, Muller NL, Pare PD, et al. Computed tomographic imaging of the airways: relationship to structure and function. Eur Respir J. 2005 Jul;26(1):140–52. doi: 10.1183/09031936.05.00007105. [DOI] [PubMed] [Google Scholar]
- 12.Nakano Y, Whittall KP, Kalloger SE, et al. Development and validation of human airway analysis algorithm using multidetector row CT. Medical Imaging 2002: Physiology and Function from Multidimensional Images; 2002. pp. 460–9. [Google Scholar]
- 13.Montaudon M, Lederlin M, Reich S, et al. Bronchial measurements in patients with asthma: comparison of quantitative thin-section CT findings with those in healthy subjects and correlation with pathologic findings. Radiology. 2009 Dec;253(3):844–53. doi: 10.1148/radiol.2533090303. [DOI] [PubMed] [Google Scholar]
- 14.Gupta S, Siddiqui S, Haldar P, et al. Quantitative analysis of high-resolution computed tomography scans in severe asthma subphenotypes. Thorax. 2010 Sep;65(9):775–81. doi: 10.1136/thx.2010.136374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009 Mar 5;360(10):973–84. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Awadh N, Muller NL, Park CS, et al. Airway wall thickness in patients with near fatal asthma and control groups: assessment with high resolution computed tomographic scanning. Thorax. 1998 Apr;53(4):248–53. doi: 10.1136/thx.53.4.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aysola RS, Hoffman EA, Gierada D, et al. Airway remodeling measured by multidetector CT is increased in severe asthma and correlates with pathology. Chest. 2008 Dec;134(6):1183–91. doi: 10.1378/chest.07-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasahara K, Shiba K, Ozawa T, et al. Correlation between the bronchial subepithelial layer and whole airway wall thickness in patients with asthma. Thorax. 2002 Mar;57(3):242–6. doi: 10.1136/thorax.57.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Little SA, Sproule MW, Cowan MD, et al. High resolution computed tomographic assessment of airway wall thickness in chronic asthma: reproducibility and relationship with lung function and severity. Thorax. 2002 Mar;57(3):247–53. doi: 10.1136/thorax.57.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niimi A, Matsumoto H, Amitani R, et al. Airway wall thickness in asthma assessed by computed tomography. Relation to clinical indices. Am J Respir Crit Care Med. 2000 Oct;162(4 Pt 1):1518–23. doi: 10.1164/ajrccm.162.4.9909044. [DOI] [PubMed] [Google Scholar]
- 21.Siddiqui S, Gupta S, Cruse G, et al. Airway wall geometry in asthma and nonasthmatic eosinophilic bronchitis. Allergy. 2009 Jun;64(6):951–8. doi: 10.1111/j.1398-9995.2009.01951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Blic J, Tillie-Leblond I, Emond S, et al. High-resolution computed tomography scan and airway remodeling in children with severe asthma. J Allergy Clin Immunol. 2005 Oct;116(4):750–4. doi: 10.1016/j.jaci.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Saglani S, Papaioannou G, Khoo L, et al. Can HRCT be used as a marker of airway remodelling in children with difficult asthma? Respir Res. 2006;7:46. doi: 10.1186/1465-9921-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niimi A, Matsumoto H, Takemura M, et al. Relationship of airway wall thickness to airway sensitivity and airway reactivity in asthma. Am J Respir Crit Care Med. 2003 Oct 15;168(8):983–8. doi: 10.1164/rccm.200211-1268OC. [DOI] [PubMed] [Google Scholar]
- 25.Kurashima K, Kanauchi T, Hoshi T, et al. Effect of early versus late intervention with inhaled corticosteroids on airway wall thickness in patients with asthma. Respirology. 2008 Nov;13(7):1008–13. doi: 10.1111/j.1440-1843.2008.01384.x. [DOI] [PubMed] [Google Scholar]
- 26.Lee YM, Park JS, Hwang JH, et al. High-resolution CT findings in patients with near-fatal asthma: comparison of patients with mild-to-severe asthma and normal control subjects and changes in airway abnormalities following steroid treatment. Chest. 2004 Dec;126(6):1840–8. doi: 10.1378/chest.126.6.1840. [DOI] [PubMed] [Google Scholar]
- 27.Niimi A, Matsumoto H, Amitani R, et al. Effect of short-term treatment with inhaled corticosteroid on airway wall thickening in asthma. Am J Med. 2004 Jun 1;116(11):725–31. doi: 10.1016/j.amjmed.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 28.Haldar P, Pavord ID, Shaw DE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008 Aug 1;178(3):218–24. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson TE, Long FR, Raman P, et al. An airway phantom to standardize CT acquisition in multicenter clinical trials. Acad Radiol. 2009 Sep;16(9):1134–41. doi: 10.1016/j.acra.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 30.Brillet PY, Fetita CI, Capderou A, et al. Variability of bronchial measurements obtained by sequential CT using two computer-based methods. Eur Radiol. 2009 May;19(5):1139–47. doi: 10.1007/s00330-008-1247-8. [DOI] [PubMed] [Google Scholar]
- 31.Kraft M. The distal airways: are they important in asthma? Eur Respir J. 1999 Dec;14(6):1403–17. doi: 10.1183/09031936.99.14614039. [DOI] [PubMed] [Google Scholar]
- 32.Hamid Q, Song Y, Kotsimbos TC, et al. Inflammation of small airways in asthma. J Allergy Clin Immunol. 1997 Jul;100(1):44–51. doi: 10.1016/s0091-6749(97)70193-3. [DOI] [PubMed] [Google Scholar]
- 33.Carroll N, Elliot J, Morton A, et al. The structure of large and small airways in nonfatal and fatal asthma. Am Rev Respir Dis. 1993 Feb;147(2):405–10. doi: 10.1164/ajrccm/147.2.405. [DOI] [PubMed] [Google Scholar]
- 34.Guckel C, Wells AU, Taylor DA, et al. Mechanism of mosaic attenuation of the lungs on computed tomography in induced bronchospasm. J Appl Physiol. 1999 Feb;86(2):701–8. doi: 10.1152/jappl.1999.86.2.701. [DOI] [PubMed] [Google Scholar]
- 35.Muller NL, Staples CA, Miller RR, et al. Density mask”. An objective method to quantitate emphysema using computed tomography. Chest. 1988 Oct;94(4):782–7. doi: 10.1378/chest.94.4.782. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka N, Matsumoto T, Miura G, et al. Air trapping at CT: high prevalence in asymptomatic subjects with normal pulmonary function. Radiology. 2003 Jun;227(3):776–85. doi: 10.1148/radiol.2273020352. [DOI] [PubMed] [Google Scholar]
- 37.Beigelman-Aubry C, Capderou A, Grenier PA, et al. Mild intermittent asthma: CT assessment of bronchial cross-sectional area and lung attenuation at controlled lung volume. Radiology. 2002 Apr;223(1):181–7. doi: 10.1148/radiol.2231010779. [DOI] [PubMed] [Google Scholar]
- 38.Busacker A, Newell JD, Jr., Keefe T, et al. A multivariate analysis of risk factors for the air-trapping asthmatic phenotype as measured by quantitative CT analysis. Chest. 2009 Jan;135(1):48–56. doi: 10.1378/chest.08-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gono H, Fujimoto K, Kawakami S, et al. Evaluation of airway wall thickness and air trapping by HRCT in asymptomatic asthma. Eur Respir J. 2003 Dec;22(6):965–71. doi: 10.1183/09031936.03.00085302. [DOI] [PubMed] [Google Scholar]
- 40.Newman KB, Lynch DA, Newman LS, et al. Quantitative computed tomography detects air trapping due to asthma. Chest. 1994 Jul;106(1):105–9. doi: 10.1378/chest.106.1.105. [DOI] [PubMed] [Google Scholar]
- 41.Tunon-de-Lara JM, Laurent F, Giraud V, et al. Air trapping in mild and moderate asthma: effect of inhaled corticosteroids. J Allergy Clin Immunol. 2007 Mar;119(3):583–90. doi: 10.1016/j.jaci.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Goldin JG, McNitt-Gray MF, Sorenson SM, et al. Airway hyperreactivity: assessment with helical thin-section CT. Radiology. 1998 Aug;208(2):321–9. doi: 10.1148/radiology.208.2.9680554. [DOI] [PubMed] [Google Scholar]
- 43.Ueda T, Niimi A, Matsumoto H, et al. Role of small airways in asthma: investigation using high-resolution computed tomography. J Allergy Clin Immunol. 2006 Nov;118(5):1019–25. doi: 10.1016/j.jaci.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 44.van Beek EJ, Hoffman EA. Functional imaging: CT and MRI. Clin Chest Med. 2008 Mar;29(1):195–216. vii. doi: 10.1016/j.ccm.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chae EJ, Seo JB, Lee J, et al. Xenon ventilation imaging using dual-energy computed tomography in asthmatics: initial experience. Invest Radiol. 2010 Jun;45(6):354–61. doi: 10.1097/RLI.0b013e3181dfdae0. [DOI] [PubMed] [Google Scholar]
- 46.Remy-Jardin M, Faivre JB, Pontana F, et al. Thoracic applications of dual energy. Radiol Clin North Am. 2010 Jan;48(1):193–205. doi: 10.1016/j.rcl.2009.08.013. [DOI] [PubMed] [Google Scholar]