Abstract
BACKGROUND
Lidocaine is a local anesthetic that has multiple pharmacological effects including antiarrhythmia, antinociception, and neuroprotection. Acid sensing ion channels (ASICs) are proton-gated cation channels that belong to the epithelial sodium channel/degenerin superfamily. Activation of ASICs by protons results in sodium and calcium influx. ASICs have been implicated in various physiological processes including learning/memory, nociception, and in acidosis-mediated neuron injury. In this study, we examined the effect of lidocaine on ASICs in cultured mouse cortical neurons.
METHODS
ASIC currents were activated and recorded using a whole-cell patch-clamp technique in cultured mouse cortical neurons. The effects of lidocaine at different concentrations were examined. To determine whether the inhibition of lidocaine on ASIC currents is subunit specific, we examined the effect of lidocaine on homomeric ASIC1a and ASIC2a currents expressed in Chinese hamster ovary cells.
RESULTS
Lidocaine significantly inhibits the ASIC currents in mouse cortical neurons. The inhibition was reversible and dose dependent. A detectable effect was noticed at a concentration of 0.3 mM lidocaine. At 30 mM, ASIC current was inhibited by approximately 90%. Analysis of the complete dose-response relationship yielded a half-maximal inhibitory concentration of 11.79 ± 1.74 mM and a Hill coefficient of 2.7 ± 0.5 (n = 10). The effect is rapid and does not depend on pH. In Chinese hamster ovary cells expressing different ASIC subunits, lidocaine inhibits the ASIC1a current without affecting the ASIC2a current.
CONCLUSION
ASIC currents are significantly inhibited by lidocaine. Our finding reveals a new pharmacological effect of lidocaine in neurons.
Lidocaine is a widely used local anesthetic for topical anesthesia, local infiltration, nerve block, and spinal and epidural anesthesia. The primary mechanism of action as local anesthetics is the blockade of voltage-dependent sodium channels.1 Lidocaine can be administered IV for ventricular tachyarrhythmia and for suppression of laryngeal reflex during tracheal intubation and extubation. Previous studies have shown that lidocaine is protective against ischemia or hypoxia-induced neuronal injury.2,3 Clinical trials indicate that lidocaine improves cognitive function for patients undergoing cardiopulmonary bypass.4 Extended IV lidocaine infusion has been shown to be effective for relief of neuropathic pain in humans.5,6 The underlying mechanism for the effects of lidocaine other than blocking nerve conductance is not fully understood.
Acid sensing ion channels (ASICs) belong to the epithelial sodium channel/degenerin superfamily. ASICs are widely expressed in neurons of the central nervous system (CNS) as well as in peripheral sensory neurons. Activation of ASICs by its ligand proton induces sodium and calcium influx, resulting in membrane depolarization and neuronal excitation. Several studies have suggested that activation of ASICs is involved in various physiological and pathological processes, including synaptic plasticity, learning/ memory,7 mechanosensation and nociception,8,9 and in acidosis-mediated neuronal injury.10
Because ASICs are widely expressed and involved in various physiological processes that lidocaine may interact with, we examined the effect of lidocaine on ASICs. Using the whole-cell patch-clamp recording technique, we tested the effect of lidocaine on ASICs in cultured mouse cortical neurons. We demonstrated a significant inhibition of the ASIC currents by lidocaine. Our finding suggests a new pharmacological effect for lidocaine.
METHODS
Primary Cell Culture
The method for culturing mouse cortical neurons has been described previously.10 The use of mice for neuronal cell culturing was approved by the Institutional Animal Care and Use Committee of the Legacy Clinical Research and Technology Center. Swiss mice at embryonic day 16 were anesthetized with halothane followed by decapitation. Brains were rapidly removed and placed in ice-cold Ca2+- and Mg2+-free Hanks solution. Cerebral cortices were dissected and digested with 0.05% trypsin-EDTA for 10 minutes at 37°C, followed by trituration with fire-polished glass pipettes, and plated on poly-l-ornithine–coated culture dishes (35 mm in diameter) at a density of 1 × 106 cells per dish. Neurons were cultured with Neurobasal medium supplemented with B27 (Invitrogen, San Diego, CA) and maintained at 37°C in a humidified 5% CO2 atmosphere incubator. Cultures were fed twice per week. In general, neurons were used for electrophysiological recording 10 to 14 days after plating.
Transfection of Chinese Hamster Ovary Cells
Transfection of Chinese hamster ovary (CHO) cells with ASIC subunit 1a and 2a was described previously.11 Briefly, CHO cells were cultured in F12 medium (American Type Culture Collection, Manassas, VA) supplemented with 10% fetal bovine serum. Cells were cotransfected with pcDNA3 constructors (Invitrogen, Carlsbad, CA) encoding individual ASICs and green fluorescent protein (GFP), using Fugene 6 transfection reagent (Roche Diagnostics, Indianapolis, IN). cDNA for individual ASICs (0.75 μg) and cDNA for GFP (0.25 μg) were used for each culture dish. All recordings were made 48 to 72 hours after the transfection. GFP-positive cells were viewed under a fluorescent microscope for patch-clamp recording.
Electrophysiology
Patch-clamp recordings were performed as described previously.10 Patch electrodes, with resistances of 2 to 3 MΩ when filled with intracellular solution, were constructed from thin-walled borosilicated glass (1.5-mm diameter; World Precision Instruments, Sarasota, FL) on a 2-stage puller (PP83; Narishige, Tokyo, Japan). A multibarrel perfusion system (SF-77; Warner Instruments, Hamden, CT) was used to achieve a rapid exchange of extracellular solutions. Whole-cell currents or membrane potentials were recorded using Axopatch 1D or 200B amplifiers (Molecular Devices, Union City, CA). Data were filtered at 2 kHz and digitized at 5 Hz using a Digidata 1320 DAC unit (Molecular Devices). The on-line acquisition was done using pCLAMP software (version 8; Molecular Devices). In general, ASICs were activated by acid perfusion every 2 minutes to allow for a complete recovery of the channels from desensitization. During each experiment, a voltage step of −10 mV from the holding potential was applied periodically to monitor cell capacitance and access resistance. Tetrodotoxin (0.5–1 μM) was included in the extracellular solution to suppress the voltage-gated Na+ channels and the firing of action potentials. To determine the effect of lidocaine, extracellular solutions containing various concentrations of lidocaine were perfused onto neurons.
Statistical Analysis
Dose response was fit with the Hill equation to obtain the half-maximal inhibitory concentration value and Hill coefficient. Mean values and standard deviation or error of the means were calculated. Statistical significance was defined as P < 0.05 by paired t test using GraphPad Prism 4.0 (GraphPad Software, La Jolla, CA).
RESULTS
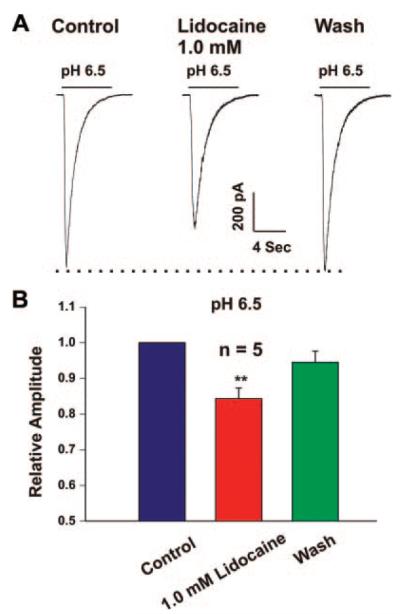
ASIC currents in cultured mouse cortical neurons were recorded as detailed in previous studies.10 In all neurons recorded, decreasing the extracellular pH to below 7.0 resulted in typical transient inward ASIC current. The amplitude of the current increases with decreasing pH value, as has been shown previously.10 We first studied the effect of lidocaine on the ASIC current activated by decreasing pH to 6.5. Addition of lidocaine (1 mM) to the extracellular solution (in both pH 7.4 and 6.5 solutions) for 2 minutes produced a significant reduction of the amplitude of ASIC currents (Fig. 1). The peak amplitude of the ASIC currents was reduced by 15.67% ± 2.96% (n = 5, P < 0.01). The effect of lidocaine was reversible upon washout.
Figure 1.
Inhibition of acid sensing ion channel (ASIC) currents by lidocaine (1 mM) in cultured mouse cortical neurons. ASIC currents were induced by decreasing extracellular pH to 6.5. Lidocaine was added in the extracellular solution to a final concentration of 1 mM. A. Representative current traces showing the inhibition of the ASIC currents activated at pH 6.5 by 1.0 mM lidocaine. B. Summary bar graph showing the reduction in the amplitude of the ASIC current by lidocaine. n = 5, **P < 0.01.
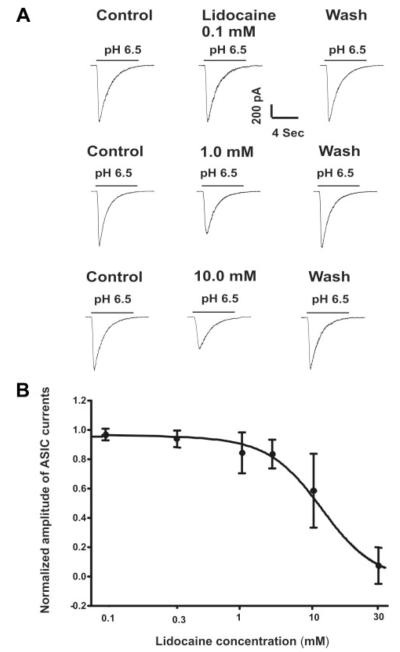
The inhibition by lidocaine was dose dependent (Fig. 2). A detectable effect was noticed at a concentration of 0.3 mM lidocaine. At 30 mM, ASIC current was inhibited by approximately 90%. Analysis of the complete dose response yielded a half-maximal inhibitory concentration of 11.79 ± 1.74 mM and a Hill coefficient of 2.7 ± 0.5 (n = 10).
Figure 2.
A, Representative current traces showing the dose-dependent inhibition of the acid sensing ion channel (ASIC) currents by lidocaine. B, Summary data showing the dose-response relationship for lidocaine inhibition of the ASIC currents in cultured mouse cortical neurons. ASIC currents were induced by decreasing the extracellular pH to 6.5. Lidocaine was added to the extracellular solution to the indicated final concentrations. The dose-response curve was fit with the Hill equation. Data are represented as mean ± SD (n = 10).
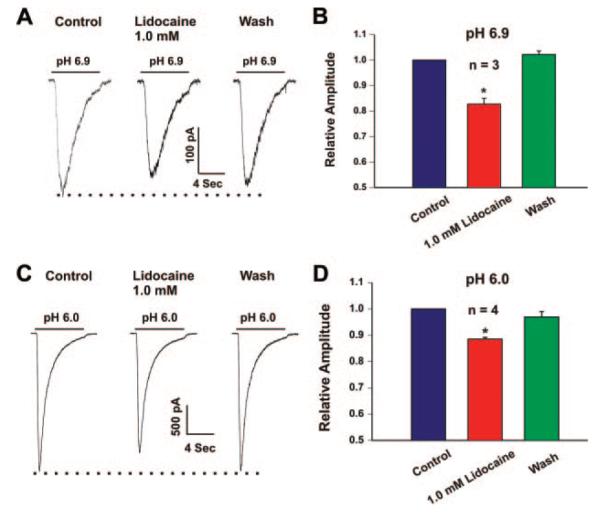
The inhibition of the ASIC current by lidocaine does not depend on the pH because a similar inhibition of the current was also observed at pH 6.9 and 6.0 (Fig. 3). In the presence of 1 mM lidocaine, the peak amplitude of the ASIC currents was reduced by 17.28% ± 2.25% at pH 6.9 (n = 3, P < 0.05) and 11.46% ± 0.69% at pH 6.0, respectively (n = 4, P < 0.05).
Figure 3.
Representative current traces (A and C) and summary bar graph (B and D) showing inhibition of the acid sensing ion channel (ASIC) currents activated at different pH levels (6.9 and 6.0) by 1 mM lidocaine in cultured mouse cortical neurons. Lidocaine inhibited the ASIC currents activated either at 6.9 or 6.0. n = 3 to 4, *P < 0.05.
The effect of lidocaine was rapid because adding lidocaine directly to the low pH solutions without adding it to the pH 7.4 solution also caused inhibition of the ASIC currents (n = 5, not shown).
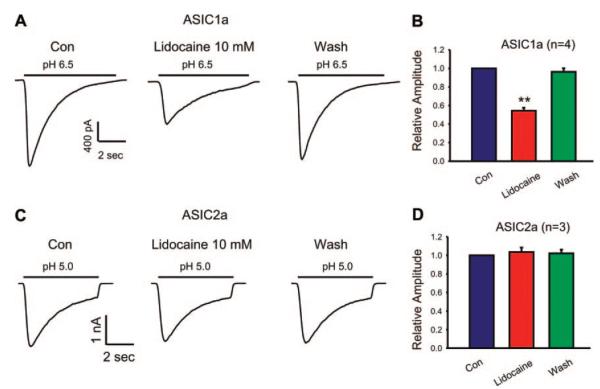
ASICs in CNS neurons are mediated by a combination of ASIC1a and ASIC2a subunits. To determine whether the inhibition of lidocaine on the ASIC current depends on the presence of a specific ASIC subunit, we examined the effect of lidocaine on homomeric ASIC1a and ASIC2a currents expressed in CHO cells. As shown in Figure 4, lidocaine significantly inhibited the ASIC1a current (n = 4, P < 0.01; Fig. 4, A and B) without affecting the ASIC2a current (n = 3, P > 0.05; Fig. 4, C and D).
Figure 4.
Representative current traces and summary bar graph showing the effect of lidocaine (10 mM) on homomeric acid sensing ion channel (ASIC)1a current (A and B) or ASIC2a current (C and D) expressed in Chinese hamster ovary cells. n = 3 to 4, **P < 0.01. Con = control.
DISCUSSION
Lidocaine is a frequently used local anesthetic whose primary action is the blockade of voltage-gated Na+ channels. In addition, it has been shown that lidocaine has multiple effects on other ion channels and receptors, including K+ and Ca2+ channels, glutamate, γ-aminobutyric acid, glycine, and vanilloid receptors.6,12 In this study, we demonstrated a new pharmacological effect of lidocaine: inhibition of ASIC currents. In our experimental condition, the involvement of voltage-gated sodium channels is unlikely. In the extracellular medium, tetrodotoxin is routinely included to prevent the activation of voltage-gated sodium channels. In addition, a similar effect of lidocaine can be repeated on ASIC1a current expressed in CHO cells where there are no voltage-gated sodium channels (Fig. 4). The inhibitory effect of lidocaine had fast onset because rapid perfusion of lidocaine in the low pH solution had a similar degree of inhibition compared with incubating lidocaine in the pH 7.4 solutions for 5 minutes. It is dose dependent and reversible. This effect seems to be pH independent because addition of lidocaine showed a similar degree of inhibition at 6.9, 6.5, and 6.0. The effect of lidocaine is specific to ASIC1a channels. As shown in Figure 4, lidocaine inhibits ASIC1a current without affecting the ASIC2a current. Furthermore, our recent preliminary study has shown that other amide local anesthetics, including mepivacaine, bupivacaine, and ropivacaine, which share the property of blocking the voltage-gated sodium channel with lidocaine, do not affect ASIC current (Lin J, et al. Effects of amide local anesthetics on acid sensing ion channels in cultured mouse cortical neurons. ASA Annual Meeting Abstract A284. October 16, 2010, San Diego.). Therefore, although this concentration of lidocaine also blocks the voltage-gated sodium channel, its effect on ASICs is significant and selective.
In current clinical practice, it is unlikely to achieve the concentration needed to have an inhibitory effect on ASIC currents by IV bolus or infusion of lidocaine. Therefore, it could be assumed that systemic administration of lidocaine has negligible effects on the physiological process that ASICs mediate in CNS, e.g., synaptic plasticity, and learning and memory. However, direct local exposure to lidocaine at clinical concentrations that range from 1% to 5% (equal to approximately 37–185 mM) would have a significant effect on the ASIC activity in neurons and its underlying physiological process. As shown in Figure 2, lidocaine inhibits 15% of the ASIC current at 1 mM and 90% of the current at 30 mM. Lidocaine hydrochloride is used clinically. With a molecular weight of 270.8 g/mol, 30 mM lidocaine is equivalent to 0.8%, whereas 1% or 5% lidocaine is equivalent to 37 mM or 185 mM, respectively. In our experimental condition, 1% lidocaine inhibited >90% of the ASIC currents. Therefore, it is obviously a clinically relevant concentration, at least when lidocaine is given by direct application, infiltration, and intrathecal or epidural injections. Although the effective concentration is higher than needed to block sodium channels, which is in the range of hundreds of micromoles, the effect of lidocaine on ASIC is dose dependent, reversible, and subunit specific, and would therefore interfere with the ASIC-mediated physiological and pathological processes at a clinically relevant dose.
Because ASICs in cortical neurons are associated with acidosis-mediated neuron injury,10 direct application of lidocaine in the brain tissue undergoing interruption of blood supply during trauma or stroke surgery may be beneficial. Spinal cord and retina contain similar isoforms of ASICs as the cortical neurons.13–15 Almost all isoforms of ASICs are highly expressed in peripheral neurons including dorsal root ganglion, trigeminal ganglion, and other primary sensory neurons.14,16,17 ASICs in peripheral neurons are involved in sensory perception and neuropathic pain.14,15 It may be likely that exposure to lidocaine at clinically relevant concentrations has similar inhibitory effects on ASIC currents in the peripheral neurons. The pharmacological consequence of ASIC inhibition by lidocaine in these neurons awaits for further clarification.
In summary, we have shown a new pharmacological effect of lidocaine in the CNS by inhibiting ASIC currents in neurons.
Acknowledgments
Supported by the Research Fund, Department of Anesthesiology, SUNY Downstate Medical Center (to JL), the National Institutes of Health (R01NS47506 and R01NS49470 to ZGX), and the American Heart Association (0840132N to ZGX).
Footnotes
The authors declare no conflicts of interest.
Reprints will not be available from the authors.
DISCLOSURES Name: Jun Lin, MD, PhD.
Contribution: Study design, conduct of study, data analysis, and manuscript preparation.
Name: Xiangping Chu, MD, PhD.
Contribution: Conduct of study.
Name: Samaneh Maysami, MD, PhD.
Contribution: Conduct of study.
Name: Minghua Li, PhD.
Contribution: Conduct of study.
Name: Hongfang Si, PhD.
Contribution: Conduct of study.
Name: James E. Cottrell, MD.
Contribution: Manuscript preparation.
Name: Roger P. Simon, MD.
Contribution: Manuscript preparation.
Name: Zhigang Xiong, MD, PhD.
Contribution: Study design, data analysis, and manuscript preparation.
REFERENCES
- 1.Butterworth JF, Strichartz GR. Molecular mechanism of local anesthesia: a review. Anesthesiology. 1990;72:711–34. doi: 10.1097/00000542-199004000-00022. [DOI] [PubMed] [Google Scholar]
- 2.Lei B, Cottrell JE, Kass IS. Neuroprotective effect of low-dose lidocaine in a rat model of transient focal cerebral ischemia. Anesthesiology. 2001;95:445–51. doi: 10.1097/00000542-200108000-00029. [DOI] [PubMed] [Google Scholar]
- 3.Lei B, Popp S, Capuano-Waters C, Cottrell JE, Kass I. Lidocaine attenuates apoptosis in the ischemic penumbra and reduces infarct size after transient focal cerebral ischemia in rats. Neuroscience. 2004;125:691–701. doi: 10.1016/j.neuroscience.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 4.Wang D, Wu X, Li J, Xiao F, Liu X, Meng M. Effect of lidocaine on early postoperative cognitive dysfunction after coronary artery bypass surgery. Anesth Analg. 2002;95:1134–41. doi: 10.1097/00000539-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Tremont-Lukats IW, Hutson PR, Backonja MM. A randomized, double-masked, placebo-controlled pilot trial of extended IV lidocaine infusion for relief of ongoing neuropathic pain. Clin J Pain. 2006;22:266–71. doi: 10.1097/01.ajp.0000169673.57062.40. [DOI] [PubMed] [Google Scholar]
- 6.Strichartz GR. Novel ideas of local anaesthetic actions on various ion channels to ameliorate postoperative pain. Br J Anaesth. 2008;101:45–7. doi: 10.1093/bja/aen101. [DOI] [PubMed] [Google Scholar]
- 7.Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JH, Welsh MJ. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. 2002;34:463–77. doi: 10.1016/s0896-6273(02)00661-x. [DOI] [PubMed] [Google Scholar]
- 8.Krishtal O. The ASICs: signaling molecules? Modulators? Trends Neurosci. 2003;26:477–83. doi: 10.1016/S0166-2236(03)00210-8. [DOI] [PubMed] [Google Scholar]
- 9.Price MP, Lewin GR, McIlwrath SL, Cheng C, Xie J, Heppenstall PA, Stucky CL, Mannsfeldt AG, Brennan TJ, Drummond HA, Qiao J, Benson CJ, Tarr DE, Hrstka RF, Yang B, Williamson RA, Welsh MJ. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature. 2000;407:1007–11. doi: 10.1038/35039512. [DOI] [PubMed] [Google Scholar]
- 10.Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wemmie JA, Price M, Welsh MJ, Simon RP. Novel neuroprotective strategy in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–98. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 11.Chu XP, Wemmie JA, Wang WZ, Zhu XM, Saugstad JA, Price MP, Simon RP, Xiong ZG. Subunit-dependent high-affinity zinc inhibition of acid-sensing ion channels. J Neurosci. 2004;24:8678–89. doi: 10.1523/JNEUROSCI.2844-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trellakis S, Lautermann J, Lehnerdt G. Lidocaine: neurobiological targets and effects on the auditory system. Prog Brain Res. 2007;166:303–22. doi: 10.1016/S0079-6123(07)66028-2. [DOI] [PubMed] [Google Scholar]
- 13.Lilley S, LeTissier P, Robbins J. The discovery and characterization of a proton-gated sodium current in rat retinal ganglion cells. J Neurosci. 2004;24:1013–22. doi: 10.1523/JNEUROSCI.3191-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lingueglia E. Acid-sensing ion channels in sensory perception. J Biol Chem. 2007;282:17325–9. doi: 10.1074/jbc.R700011200. [DOI] [PubMed] [Google Scholar]
- 15.Ettaiche M, Deval E, Pagnotta S, Lazdunski M, Lingueglia E. Acid-sensing ion channel 3 in retinal function and survival. Invest Ophthalmol Vis Sci. 2009;50:2417–26. doi: 10.1167/iovs.08-3028. [DOI] [PubMed] [Google Scholar]
- 16.Wu LJ, Duan B, Mei YD, Gao J, Chen JG, Zhuo M, Xu L, Wu M, Xu TL. Characterization of acid-sensing ion channels in dorsal horn neurons of rat spinal cord. J Biol Chem. 2004;279:43716–34. doi: 10.1074/jbc.M403557200. [DOI] [PubMed] [Google Scholar]
- 17.Baron A, Voilley N, Lazdunski M, Lingueglia E. Acid sensing ion channels in dorsal spinal cord neurons. J Neurosci. 2008;28:1498–508. doi: 10.1523/JNEUROSCI.4975-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]