Abstract
Protons are important signals for neuronal function. In the central nervous system (CNS), proton concentrations change locally when synaptic vesicles release their acidic contents into the synaptic cleft, and globally in ischemia, seizures, traumatic brain injury, and other neurological disorders due to lactic acid accumulation. The finding that protons gate a distinct family of ion channels, the acid-sensing ion channels (ASICs), has shed new light on the mechanism of acid signaling and acidosis-associated neuronal injury. Accumulating evidence has suggested that ASICs play important roles in physiological processes such as synaptic plasticity, learning/memory, fear conditioning, and retinal integrity, and in pathological conditions such as brain ischemia, multiple sclerosis, epileptic seizures, and malignant glioma. Thus, targeting these channels may lead to novel therapeutic interventions for neurological disorders. The goal of this review is to provide an update on recent advances in our understanding of the functions of ASICs in the CNS.
Keywords: Acid-sensing ion channel, acidosis, CNS, neuron, function, neurological disease
INTRODUCTION
Maintaining a stable proton concentration inside and outside of cells is critical for normal neuronal function. Similar to non-neuronal cells, neurons maintain their extra- and intracellular proton concentrations through H+ transporting mechanisms, for example, Na+/H+ and Cl-/HCO3− systems [1]. In normal conditions, extracellular pH in the brain is maintained at ~7.3 while intracellular pH is maintained at ~7.0 [1–3]. However, in pathological conditions such as tissue inflammation, ischemic stroke, traumatic brain injury, and epileptic seizure, marked accumulation of protons or reduction of pH, a condition termed acidosis [2;4–11], takes place.
Changes in pH are known to modulate the activity of a variety of membrane receptors and ion channels. In general, reducing pH inhibits the activities of the majority of voltage-gated and ligand-gated ion channels. For example, N-methyl-D-aspartic acid (NMDA) receptor-gated cation channels are strongly inhibited by decreased pH [12;13]. In contrast to the modulating effect of protons on other channels, recent studies have demonstrated that protons can, themselves, activate a distinct family of ion channels, the acid-sensing ion channels (ASICs) [14–25]. This finding has shed new light on acidosis-associated changes of neuronal function and provided novel targets for therapeutic inventions [26–32].
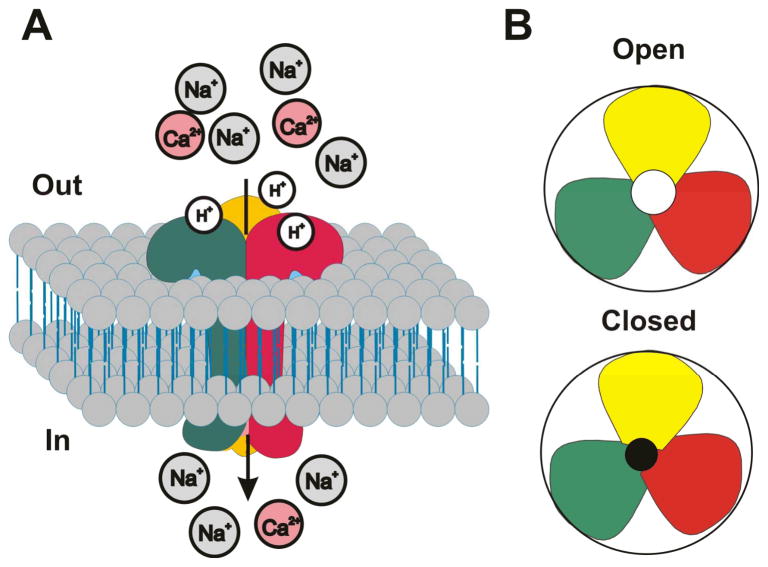
ASICs are voltage-independent, proton-gated cation-selective channels mostly permeable to Na+ ion. They belong to the degenerin/epithelial Na+ channel (DEG/ENaC) superfamily [15;33]. To date, at least seven subunits of ASICs encoded by four different genes have been identified: 1a, 1b1, 1b2, 2a, 2b, 3, and 4 [28], among which 1a, 2a, 2b, and 3 have been reported to be present in CNS neurons. The crystal structure of the chicken ASIC1a protein has illustrated that functional ASICs are trimeric assemblies [34;35](Fig. 1). Each ASIC subunit consists of two transmembrane domains (TM1 and TM2), a large, cysteine rich extracellular loop, and short intracellular N- and C-termini [15;36;37]. Among all the ASICs, homomeric ASIC1a and heteromeric ASIC1a/2b channels are known to have permeability to Ca2+ [15;29;38;39]. Thus, activation of these channels can cause accumulation of intracellular Ca2+ concentration in neurons [29;38;40]. While homomeric ASIC1a and heteromeric ASIC1a/2b channels can conduct Ca2+, several recent studies have suggested that, in neurons, a large portion of acid-evoked increases of intracellular Ca2+ are not due to Ca2+ entry directly through ASIC1a homomers [41–43]. Rather, it is likely that acidosis induced Ca2+ accumulation is due to the activation of both homomeric ASIC1a and heteromeric ASIC1a/2 channels and subsequent Ca2+ entry through voltage-gated Ca2+ channels and/or Ca2+ release from intracellular pools.
Figure 1.
Proposed structure for homomeric ASIC1a channels. A. Functional homomeric ASIC1a channels are formed by trimeric assembly of three identical subunits. Binding of protons opens the channel allowing flux of Na+ and Ca2+ ions into the cell. B. Top view of an open and closed channel.
ASICs in peripheral sensory neurons are implicated in nociception [44–50], mechanosensation [51–53], and taste transduction [54–56]. In the CNS, ASIC1a is involved in synaptic plasticity, learning/memory and fear conditioning [18;57], while ASIC2a may be required for the maintenance of retinal integrity [58] and survival of neurons following global ischemia [59]. A number of studies have also demonstrated that activation or sensitization of Ca2+-permeable ASIC1a and ASIC1a/2b channels is involved in acidosis-mediated neuronal degeneration [29;38;39;60–67]. The present review focuses on recent advances in our understanding of the functional roles of ASICs in the CNS.
PHYSIOLOLOGICAL FUNCTIONS OF ASICS IN CNS
ASIC1a in Synaptic Plasticity, Learning and Memory
ASICs are widely expressed in CNS neurons. For example, acid-activated currents have been recorded in 93–100% of cultured or acutely dissociated neurons from mouse/rat hippocampus or cortex [18;68].
Change of pH at synaptic cleft and local domain following synaptic release raised the possibility that ASICs contribute to synaptic transmission. The findings that ASICs are present at synaptic sites and can interact with post synaptic density protein 95 as well as protein interacting with C kinase 1 [18;41;69;70] further support this possibility. Indeed, studies by Wemmie and coworkers have demonstrated that ASIC1a activation is involved in synaptic plasticity, learning and memory [18]. They demonstrated that high frequency stimulation (HFS) produces long-lasting potentiation of excitatory post-synaptic potentials (EPSP) in hippocampal slices from wild-type mice. However, the potentiation of EPSP decays rapidly to the baseline in slices from ASIC1a null mice. Further study showed that the NMDA receptor antagonist D-2-Amino-5-phosphonovalerate (D-APV) inhibits EPSP summation in slices from wild-type but not in ASIC1a-knockout mice, suggesting that the loss of ASIC1a impaired NMDA-receptor function. ASIC1a disruption does not impair presynaptic vesicle release, as evidenced by normal single evoked EPSPs and paired-pulse facilitation. Interestingly, a later study by Cho and Askwith demonstrated that the presynaptic release probability is increased in cultured hippocampal neurons from the ASIC1 knockout mice [71]. Although localizations of ASICs at neuronal cell body and postsynaptic sites have been clearly demonstrated [18;41], it remains to be determined whether ASICs are also expressed at presynaptic sites.
Based on its established role in synaptic plasticity and learning/memory, a recent preliminary study has suggested that a reduced function of ASIC1a channels may contribute to the learning and memory deficit associated with Alzheimer’s disease [72]. In this study, Maysami et al showed that acid-activated currents in mouse cortical neurons and in CHO cells expressing ASIC1a are inhibited by nanomolar concentrations of amyloid beta peptide, a critical player for the pathology of Alzheimer’s disease. In addition to a reduction of current amplitude, amyloid beta peptide also slows the activation of the channels. Thus, restoring the activity of ASIC1a channels could be a new intervention for Alzheimer’s disease.
ASIC1a in Fear Conditioning
ASIC1a is enriched in the structures of fear circuit (e.g. amygdala). Thus, ASIC1a may influence fear responses. Indeed, Wemmie and colleagues demonstrated that ASIC1-null mice display significant deficits in cue and context fear conditioning [57]. The loss of ASIC1a also reduces unconditioned fear in the open field test, during acoustic startle, and in response to predator odor [73]. Overexpressing ASIC1a, on the other hand, increases fear conditioning [74], but not unconditioned fear responses [75].
Further studies by Wemmie’s group suggest that activation of ASIC1a in brain chemosensor contribute to CO2 induced fear-related behavior [76]. It has long been known that breathing CO2 triggers panic attacks in patients with panic disorder, and that these patients show an increased sensitivity to CO2 inhalation [77]. In addition, patients with increasing hypercarbia due to respiratory failure become extremely anxious. How can CO2 inhalation contribute to fear behavior and related panic disorders? Wemmie and colleagues have provided evidence that ASIC1a is involved [76]. They showed that inhaled CO2 triggers a drop in brain pH and induces fear behavior in mice. Eliminating or inhibiting ASIC1a significantly limits this activity. Overexpressing ASIC1a in the amygadala, a key structure in fear circuit, rescues the CO2-induced fear deficit in ASIC1a null mice. Buffering brain pH, on the other hand, attenuates fear behavior, whereas lowering pH with microinjections in amygdale reproduces the effect of CO2. These studies provide a novel molecular mechanism underlying CO2-induced intense fear and related anxiety/panic disorders and define the amygdale as an important chemosensor that detects hypercarbia/acidosis and initiates behavioral responses [76].
ASICs and Retinal Integrity
pH variations in the retina are involved in the fine-tuning of visual perception. One study by Ettaiche suggested that ASIC2 is important for retinal function and likely protects against light-induced retinal degeneration. They showed that both photoreceptors and neurons of the mouse retina express ASIC2a and ASIC2b. Inactivation of the ASIC2 gene in mice leads to an increased rod electroretinogram of a- and b-waves, indicating an enhanced gain of visual transduction. ASIC2 knock-out mice also show more sensitivity to light-induced retinal degeneration. Thus, ASIC2 is likely a negative modulator of rod phototransduction, and that functional ASIC2 channels are beneficial for the maintenance of retinal integrity [58]. However, since homomeric ASIC2a channels have an extremely low-sensitivity to protons (i.e. pH50 of 4.4), it is not clear whether active channel activity is required for this role.
Further studies by Ettaiche and colleagues also suggested an involvement of ASIC1a in retinal physiology [78]. In situ hybridization and immunohistochemistry detected the expression of ASIC1a in the outer and inner nuclear layers (cone photoreceptors, horizontal cells, some amacrine and bipolar cells) and in the ganglion cell layer. ASIC1a knockdown by antisense oligonucleotides and ASIC1a blockade by specific inhibitor PcTX1 decreased the photopic a-and b-waves and oscillatory potentials. This finding suggests that ASIC1a is involved in normal retinal activity. Interestingly, a recent study by Render and colleagues did not detect any remarkable morphological changes in cone photoreceptors in ASIC1a−/− mice, at least at 5 or 22–27 weeks of age [79]. Thus, the exact role of this subunit in retinal integrity and/or function remains to be determined.
In addition to ASIC1a and ASIC2, potential role of ASIC3 in retinal function and survival has been reported by a recent study. Ettaiche and colleagues demonstrated the presence of ASIC3 in the rod inner segment of photoreceptors, in horizontal and some amacrine cells. ASIC3 is also detected in retinal ganglion cells (RGCs) but contributes little to ASIC currents recorded in cultured RGCs. At 2 to 3 months, knockout mice experienced a moderate enhancement of scotopic electroretinogram a-wave amplitude and a concomitant increase of b-wave amplitude without alteration of retinal structure. Older (8-month-old) mice had large reductions in scotopic a- and b-waves, respectively, and reductions in oscillatory potential amplitudes associated with complete disorganization of the retina and degenerating rod inner segments. At 8 and 12 months of age, GFAP and TUNEL staining revealed an up-regulation of GFAP expression in Müller cells and the presence of apoptotic cells in the inner and outer retina [80]. Thus, ASIC3 also appears to be required for the maintenance of retina integrity.
PATHOLOGICAL FUNCTIONS OF ASICS
ASIC1a Activation in Acidosis-Mediated Ischemic Neuronal Injury
During neurological conditions such as brain ischemia, increased anaerobic glycolysis due to reduced oxygen supply leads to lactic acid accumulation [6]. Accumulation of lactic acid, alone with increased H+ release from ATP hydrolysis, causes a decrease in pH, resulting in brain acidosis. During brain ischemia, for example, extracellular pH falls to 6.5 or lower [2;6].
For many years, acidosis was recognized to play an important role in ischemic brain injury [8;9], however, the cellular and molecular mechanism remained unclear. The widespread expression of ASIC1a in the brain, its activation by pH drops to the level commonly seen during ischemia, and its demonstrated role in intracellular Ca2+ accumulation suggested a potential involvement of these channels in the pathology of brain injury. Indeed, a number of recent studies have demonstrated an important role for ASIC1a activation in acidosis-mediated neuronal injury [29;38;40;60–62;64;65;67;81–83]. In cultured mouse and human cortical neurons, for example, activation of ASICs by acid incubation induced glutamate receptor-independent neuronal injury inhibited by specific ASIC1a blockade, and/or by ASIC1 gene knockout [29;67]. In rodent models of brain ischemia, intracerebroventricular injection of ASIC1a blocker/inhibitor reduced the infarct volume from transient or permanent focal ischemia by up to 60% [29;60]. Similarly, ASIC1 gene knockout produced significant neuroprotection in mice [29]. The protection by ASIC1a blockade had an time window of efficacy of up to 5 hours, and the protection persists for at least 7 days [60]. In addition to ASIC1a blockade, a reduced expression of ASIC1a subunit is effective in reducing ischemic brain injury. For example, Pignataro and colleagues have recently shown that a reduced level of ASIC1a expression contributes to neuroprotection elicited by ischemic preconditioning and postconditioning in rats [83].
More recently, Sherwood et al., found that ASIC2b subunit can form functional channels with ASIC1a in cultured hippocampal neurons, and that the heteromeric ASIC1a/2b channels are calcium-permeable [39]. Further, activation of heteromeric ASIC1a/2b channels contributes to acidosis-induced neuronal death. These data indicate that the heteromeric ASIC1a/2b channels, like homomeric ASIC1a channels, may serve as a novel pharmacological target to prevent neuronal injury after stroke.
Since activation of NMDA receptors and subsequent Ca2+ toxicity has been known to play an important role in ischemic brain injury, the outcome of co-application of both antagonists has also been investigated. Compared to ASIC1a or NMDA blockade alone, co-application of NMDA and ASIC antagonists produced additional neuroprotection, and the presence of ASIC1a blockade prolonged the time window of effectiveness of NMDA blockade [60]. Thus, ASIC1a represents a novel pharmacological target for ischemic brain injury.
In contrast to ASIC1a, a study by Johnson and colleagues suggests that an increased ASIC2a expression could provide protection against ischemic injury [59]. They showed an increased ASIC2a expression in neurons that survived global ischemia. This may be explained by the possibility that increased ASIC2a expression favors the formation of heteromeric ASIC1a/ASIC2a channels with reduced acid-sensitivity and no Ca2+ permeability.
ASIC1 Is Involved in Axonal Degeneration in Autoimmune Inflammation of the CNS
Multiple sclerosis is a neuroinflammatory disease associated with axonal degeneration. Although inflammation and demyelination are the primary features of CNS lesions, axonal degeneration correlates best with clinical deficits in individuals with this disease. It has been suggested that the inflammatory insult leads to axonal degeneration by causing neuronal mitochondrial dysfunction, energy failure and alteration of ion exchange mechanisms [84]. Since excessive accumulation of Na+ and Ca2+ ions is associated with axonal degeneration [85], Friese et al determined whether ASIC1a activation, which is known to cause accumulation of Na+ and Ca2+ ions, contributes to such process in inflammatory lesions of the CNS [63]. They showed that in an experimental model of autoimmune encephalomyelitis (EAE), ASIC1 null mice exhibit a significantly reduced clinical deficit and axonal degeneration as compared to wild-type mice. Further, pH measurements in the spinal cord of EAE mice display tissue acidosis sufficient to open ASIC1. The ASIC1 gene disruption also shows protective effect in nerve explants in vitro. ASIC blockade by amiloride is equally neuroprotective in nerve explants and in EAE. Thus, ASIC1a may be a potential target for axon degeneration associated with multiple sclerosis.
More recently, Vergo et al., from the same group studied acute and chronic EAE and multiple sclerosis spinal cord and optic nerve tissues to examine the distribution of ASIC1 and its relationship with neuronal and glial damage [86]. They found that ASIC1 was upregulated in axons and oligodendrocytes within lesions from mice with acute EAE and from patients with active multiple sclerosis. The expression of ASIC1 was associated with axonal damage as indicated by co-localization with the axonal injury marker beta amyloid precursor protein. Moreover, blocking ASIC1 with amiloride protected both myelin and neurons from damage in the acute model, and when given either at disease onset or, more clinically relevant, at first relapse, ameliorated disability in mice with chronic-relapsing EAE. Together these findings suggest that blockade of ASIC1 has the potential to provide both neuro- and myelo-protective benefits in multiple sclerosis [86].
ASICs and Parkinson’s Disease (PD)
PD is characterized by motor impairments and a loss of dopaminergic neurons in the substantia nigra (SNc)[87]. However, the mechanism of neuronal injury is not entirely clear. Previous studies have shown that the vulnerable neurons in this region also express ASIC1a [57;88]. Given that PD, like ischemia, is associated with cerebral lactic acidosis, Arias et al tested the effect of ASIC blockade in a mouse model of PD induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treatment [66]. As expected, amiloride was found to protect SNc neurons from MPTP-induced degeneration, and to preserve dopaminergic cell bodies in the SNc. Administration of PcTX venom resulted in a modest effect, attenuating the deficits in striatal DAT binding and dopamine. These findings suggest a potential role for ASICs in the pathogenesis of Parkinson’s disease.
ASICs and Huntington’s Disease (HD)
HD is a fatal neurodegenerative disorder. Energy metabolism deficit and acidosis have been observed in both in vitro and in vivo models of HD as well as in the brains of HD patients [89]. To examine the potential involvement of ASICs in the pathology of HD, Wong et al tested effect of amiloride derivative benzamil both in vitro and in vivo [89]. They showed that benzamil markedly reduced the huntingtin-polyglutamine (htt-polyQ) aggregation in an inducible cellular system. In addition, the effect of benzamil was recapitulated in the R6/2 animal model of HD. Further experimentation showed that benzamil alleviated the inhibition of ubiquitin-proteasome system (UPS) activity, resulting in enhanced degradation of soluble htt-polyQ specifically in its pathological range. Blocking the expression of ASIC1a with siRNA also enhanced UPS activity, resulting in decreased htt-polyQ aggregation in the striatum of R6/2 mice. Thus, targeting ASIC1a might be an alternative approach to combat HD and other polyQ-related disorders.
ASICs in Depression-Related Behavior
Depression disorders are a highly prevalent condition among adults in general population but the molecular pathways underlying depression are poorly understood. Recent studies by Coryell and colleagues have linked ASIC function to depression-related behavior [90]. They demonstrated that genetically disrupting ASIC1a in mice produced antidepressant-like effects in the forced swim test, the tail suspension test, and following unpredictable mild stress. Pharmacologically inhibiting ASIC1a also had antidepressant-like effects. The effects of ASIC1a disruption in the forced swim test were independent and additive to those of several commonly used antidepressants. Restoring ASIC1a to the amygdale of ASIC1a null mice reversed the forced swim test effects. The mechanism underlying the involvement of ASIC1a in depression-related behavior is not clear. It is likely that brain-derived neurotrophic factor (BDNF) is involved since both ASIC1a disruption and inhibition interfere with the ability of stress to reduce BDNF in the hippocampus. Thus, antagonists of ASIC1a channels may have potential for combating human depression.
ASICs and Anxiety Disorders
Anxiety disorders are debilitating neuropsychiatric disorders. Current treatments for anxiety disorders include pharmacological agents such as benzodiazepines and selective serotonin reuptake inhibitors. These agents, while effective in many patients, can induce a variety of side effects. Thus, it is necessary to develop a new generation of effective and better-tolerated anxiolytic agents. In this regard, Dwyer et al have shown that ASIC1a inhibitors have an effect in preclinical rodent models of autonomic and behavioral parameters of anxiety [91]. In the stress-induced hyperthermia model, acute administration of ASIC inhibitors PcTX1, A-317567, and amiloride prevented stress-induced elevations in core body temperature. In the four-plate test, acute treatment with PcTX1 and A-317567 produced dose-dependent increases in the number of punished crossings. Further experiment showed that infusion of A-317567 into the amygdala significantly elevated the extracellular levels of GABA, but not glutamate, in this brain region. These findings suggest that ASIC inhibition has anxiolytic-like effects in some behavioral models and that GABAergic mechanisms are involved in the effects.
A recent study also suggests an involvement of ASIC3 in anxiety-like behavior [92]. Although it is widely accepted that ASIC3 is predominately distributed in the peripheral nervous system, its expression has been found in rat hypothalamus [93]. Study by Wu and colleagues also reported the expression of ASIC3 in the sensory mesencephalic trigeminal nucleus of mouse brain [92]. However, whether ASIC3 plays any functional role in the brain was unclear. Wu et al showed that, in anxiety behavior tasks, ASIC3 null mice spent more time in the open arms of an elevated plus maze than did their wild-type littermates. ASIC3 null mice also displayed less aggressiveness toward intruders but more stereotypic repetitive behaviors during resident-intruder testing than did wild-type littermates. Therefore, loss of ASIC3 produces behavioral changes in anxiety and aggression in mice, which suggests that ASIC3-dependent sensory activities might be related to the central process of emotion modulation [92].
Although the studies from ASIC1a and ASIC3 knockout mice indicated that ASICs contribute to neuropsychiatric disorders such as depression and anxiety, whether these neurological conditions are associated with significant change in local or global pH in the CNS remains to be determined.
ASIC Activation and Epileptic Seizure Activity
A significant drop of brain pH during intense neuronal excitation or seizure activity [94–99], suggests that ASIC activation might play a role in the generation/maintenance of epileptic seizures. However, the exact role of ASIC activation in seizure generation, propagation, and termination has been controversial.
Babinski and colleagues first reported a change of ASIC1a and ASIC2b expression in hippocampal area following pilocarpine induced epilepticus [100], suggesting that the channels containing ASIC1a and ASIC2b subunits might play a role in the pathology of epilepsy. However, what role these channels may play in epileptic seizures was unclear.
Later on, a number of studies showed that amiloride, a commonly used non-selective ASIC blocker, has anticonvulsant effect in vivo in pilocarpine and pentylenetetrazole models of seizures [101–103], suggesting that ASIC activation might be proconvulsant. However, since amiloride also inhibits a number of other channels and ion exchange systems (e.g. Na+-Ca2+ and Na+-H+ exchanges), these findings do not provide unarguable evidence that ASICs are indeed proconvulsant.
Using a number of in vitro epilepsy models, a preliminary study by Chang et al provided additional evidence that ASIC1a activation might be proconvulsant [104]. In a cell culture model of epilepsy, brief withdrawal of the NMDA antagonist kynurenic acid induces a dramatic increase in the firing of action potentials, in addition to a sustained membrane depolarization. ASIC blockade by amiloride and the selective ASIC1a blocker PcTX1 significantly inhibited the increase of neuronal firing and the sustained membrane depolarization. In hippocampal slices, high frequency electrical stimulation or removal of extracellular Mg2+ triggers spontaneous seizure-like bursting. Bath perfusion of amiloride and PcTX1 decreased the amplitude and the frequency of these seizure-like bursting activities. Similarly, slices prepared from the brains of ASIC1a knockout mice demonstrated a reduced sensitivity to low extracellular Mg2+-induced or stimulation-evoked seizure activities [104].
In contrast, studies by Ziemann and colleagues, performed largely in vivo, have suggested that activation of ASIC1a channels is involved in the termination of epileptic seizure activity [105]. One interesting finding by Ziemann and colleagues was that the level of ASIC1a expression is higher in GABAergic interneurons than in excitatory neurons [105]. Therefore, acidosis generated during seizures might produce more ASIC activation in inhibitory interneurons and facilitate GABAergic transmission, resulting in seizure termination.
Thus, the exact role for ASICs in epileptic seizures may depend on the models of epilepsy used. It is also possible that the age of animals used affects the outcome measures since the level of expression and the properties of ASICs in CNS neurons undergo dramatic changes with neuronal development [106]. The finding that hippocampal interneurons are highly diverse with dramatically different expression level of ASICs [107] adds additional complexity to this subject.
ASIC1 and Malignant Glioma
Gliomas are primary brain tumors with a complex biology and a propensity for invasion into normal brain tissue. These tumors are of astrocyte cell origin. Studies by Benos’s group suggest an important role for ASIC1 and ASIC2 in the growth and migration of glioblastoma cells. They demonstrated that in grade IV gliomas, also called glioblastoma multiforme (GBM), there exists a constitutively activated, amiloride-sensitive inward Na+ current [108]. This glioma cation current is mediated by mixed ASIC and ENaC components, including ASIC1 and ASIC2. Inhibition of this conductance decreases glioma growth and cell migration. Knockdown of ASIC1 and ENaC, using dominant negative constructs, also produced a significant inhibition of glioblastoma cell migration [109]. In contrast to ASIC1, increasing surface expression of ASIC2, using glycerol and sodium 4-phenylbutyrate, suppressed the proliferation and migration of GBM cells [110].
CONCLUSION
ASICs represent new biological components in peripheral sensory and CNS neurons. Increasing evidence indicates the involvement of these channels in both physiological and pathological processes of central nervous systems (Table 1). Therefore, targeting these channels may provide novel and effective therapeutic interventions for a number of CNS diseases. In addition to establishing ASIC-specific small molecule antagonists that can easily pass through the blood brain barrier, alternative strategies may consider targeting endogenous modulators that are known to enhance the express and/or activity of these channels [111].
Table 1.
Physiological and pathological functions of ASICs in central nervous systems
| Function | ASIC subunit involved | Reference |
|---|---|---|
| Synaptic plasticity, learning and memory | 1a | [18;71] |
| Fear conditioning | 1a | [57;73–76] |
| Retinal integrity | 1a, 2, 3 | [58;78–80] |
| Acidosis-mediated neuronal injury | 1a, 2b | [29;38–40;60–62;64;65;67; 81–83] |
| Multiple sclerosis | 1a | [63;86] |
| Parkinson’s disease | 1a | [66] |
| Huntington’s disease | 1a | [89] |
| Depression | 1a, 3 | [90] |
| Anxiety | 1a, 3 | [91;92] |
| Epileptic seizure | 1a, 2b | [100–105] |
| Malignant glioma | 1, 2 | [108–110] |
Future studies may explore additional physiological functions of ASICs in CNS and to determine the detailed mechanisms underlying the involvement of ASICs in neurological diseases.
Acknowledgments
The work in author’s laboratories has been supported by National Institute of Health (R01NS49470 and R01NS47506 to Z-G Xiong), American Heart Association Established Investigator Award (0840132N to Z-G Xiong) and Scientist Development Grant (0735092N to X-P Chu), University of Missouri Research Board and University of Missouri-Kansas City School of Medicine Start-up Funding (X-P Chu).
ABBREVIATIONS
- ASICs
acid-sensing ion channels
- BDNF
brain-derived neurotrophic factor
- CNS
central nervous system
- D-APV
D-2-Amino-5-phosphonovalerate
- DEG/ENaC
degenerin/epithelial Na+ channel
- EAE
autoimmune encephalomyelitis
- EPSP
excitatory post-synaptic potentials
- GABA
γ-Aminobutyric acid
- GBM
glioblastoma multiforme
- HD
Huntington’s disease
- HFS
high frequency stimulation
- htt-polyQ
huntingtin-polyglutamine
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NMDA
N-methyl-D-aspartic acid
- PcTX1
psalmotoxin 1
- PD
Parkinson’s disease
- RGCs
retinal ganglion cells
- SNc
substantia nigra
- TM
transmembrane domain
- UPS
ubiquitin-proteasome system
References
- 1.Chesler M. The regulation and modulation of pH in the nervous system. Prog Neurobiol. 1990;34:401–27. doi: 10.1016/0301-0082(90)90034-e. [DOI] [PubMed] [Google Scholar]
- 2.Nedergaard M, Kraig RP, Tanabe J, Pulsinelli WA. Dynamics of interstitial and intracellular pH in evolving brain infarct. Am J Physiol. 1991;260:R581–R588. doi: 10.1152/ajpregu.1991.260.3.R581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Back T, Hoehn M, Mies G, Busch E, Schmitz B, Kohno K, Hossmann KA. Penumbral tissue alkalosis in focal cerebral ischemia: relationship to energy metabolism, blood flow, and steady potential. Ann Neurol. 2000;47:485–92. [PubMed] [Google Scholar]
- 4.Crowell JW, Kaufmann BN. Changes in tissue pH after circulatory arrest. Am J Physiol. 1961;200:743–5. doi: 10.1152/ajplegacy.1961.200.4.743. [DOI] [PubMed] [Google Scholar]
- 5.Ljunggren B, Norberg K, Siesjo BK. Influence of tissue acidosis upon restitution of brain energy metabolism following total ischemia. Brain Res. 1974;77:173–86. doi: 10.1016/0006-8993(74)90782-3. [DOI] [PubMed] [Google Scholar]
- 6.Rehncrona S. Brain acidosis. Ann Emerg Med. 1985;14:770–6. doi: 10.1016/s0196-0644(85)80055-x. [DOI] [PubMed] [Google Scholar]
- 7.Siesjo BK. Acidosis and ischemic brain damage. Neurochem Pathol. 1988;9:31–88. doi: 10.1007/BF03160355. [DOI] [PubMed] [Google Scholar]
- 8.Siesjo BK, Katsura K, Kristian T. Acidosis-related damage. Adv Neurol. 1996;71:209–33. [PubMed] [Google Scholar]
- 9.Tombaugh GC, Sapolsky RM. Evolving concepts about the role of acidosis in ischemic neuropathology. J Neurochem. 1993;61:793–803. doi: 10.1111/j.1471-4159.1993.tb03589.x. [DOI] [PubMed] [Google Scholar]
- 10.Revici E, Stoopen E, Frenk E, Ravich RA. The painful focus. II. The relation of pain to local physico-chemical changes. Bull Inst Appl Biol. 1949;1:21. [Google Scholar]
- 11.Sutherland SP, Cook SP, McCleskey EW. Chemical mediators of pain due to tissue damage and ischemia. Prog Brain Res. 2000;129:21–38. doi: 10.1016/S0079-6123(00)29003-1. [DOI] [PubMed] [Google Scholar]
- 12.Tang CM, Dichter M, Morad M. Modulation of the N-methyl-D-aspartate channel by extracellular H+ Proc Natl Acad Sci U S A. 1990;87:6445–9. doi: 10.1073/pnas.87.16.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Traynelis SF, Cull-Candy SG. Proton inhibition of N-methyl-D-aspartate receptors in cerebellar neurons. Nature. 1990;345:347–50. doi: 10.1038/345347a0. [DOI] [PubMed] [Google Scholar]
- 14.Waldmann R, Lazdunski M. H(+)-gated cation channels: neuronal acid sensors in the ENaC/DEG family of ion channels. Curr Opin Neurobiol. 1998;8:418–24. doi: 10.1016/s0959-4388(98)80070-6. [DOI] [PubMed] [Google Scholar]
- 15.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–7. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 16.Baron A, Waldmann R, Lazdunski M. ASIC-like, proton-activated currents in rat hippocampal neurons. J Physiol. 2002;539:485–94. doi: 10.1113/jphysiol.2001.014837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price MP, Snyder PM, Welsh MJ. Cloning and expression of a novel human brain Na+ channel. J Biol Chem. 1996;271:7879–82. doi: 10.1074/jbc.271.14.7879. [DOI] [PubMed] [Google Scholar]
- 18.Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, et al. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. 2002;34:463–77. doi: 10.1016/s0896-6273(02)00661-x. [DOI] [PubMed] [Google Scholar]
- 19.De La Rosa DA, Krueger SR, Kolar A, Shao D, Fitzsimonds RM, Canessa CM. Distribution, subcellular localization and ontogeny of ASIC1 in the mammalian central nervous system. J Physiol. 2003;546:77–87. doi: 10.1113/jphysiol.2002.030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krishtal OA, Pidoplichko VI. A receptor for protons in the nerve cell membrane. Neuroscience. 1980;5:2325–7. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- 21.Kovalchuk Y, Krishtal OA, Nowycky MC. The proton-activated inward current of rat sensory neurons includes a calcium component. Neurosci Lett. 1990;115:237–42. doi: 10.1016/0304-3940(90)90461-h. [DOI] [PubMed] [Google Scholar]
- 22.Grantyn R, Perouansky M, Rodriguez-Tebar A, Lux HD. Expression of depolarizing voltage- and transmitter-activated currents in neuronal precursor cells from the rat brain is preceded by a proton- activated sodium current. Brain Res Dev Brain Res. 1989;49:150–5. doi: 10.1016/0165-3806(89)90070-9. [DOI] [PubMed] [Google Scholar]
- 23.Ueno S, Nakaye T, Akaike N. Proton-induced sodium current in freshly dissociated hypothalamic neurones of the rat. J Physiol (Lond) 1992;447:309–27. doi: 10.1113/jphysiol.1992.sp019004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varming T. Proton-gated ion channels in cultured mouse cortical neurons. Neuropharmacology. 1999;38:1875–81. doi: 10.1016/s0028-3908(99)00079-9. [DOI] [PubMed] [Google Scholar]
- 25.Alvarez dlR, Krueger SR, Kolar A, Shao D, Fitzsimonds RM, Canessa CM. Distribution, subcellular localization and ontogeny of ASIC1 in the mammalian central nervous system. J Physiol. 2003;546:77–87. doi: 10.1113/jphysiol.2002.030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voilley N. Acid-sensing ion channels (ASICs): new targets for the analgesic effects of non-steroid anti-Inflammatory drugs (NSAIDs) Curr Drug Targets Inflamm Allergy. 2004;3:71–9. doi: 10.2174/1568010043483980. [DOI] [PubMed] [Google Scholar]
- 27.Xiong ZG, Chu XP, Simon RP. Acid sensing ion channels--novel therapeutic targets for ischemic brain injury. Front Biosci. 2007;12:1376–86. doi: 10.2741/2154. [DOI] [PubMed] [Google Scholar]
- 28.Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29:578–86. doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, et al. Neuroprotection in ischemia: blocking calcium-permeable Acid-sensing ion channels. Cell. 2004;118:687–98. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 30.Benveniste M, Dingledine R. Limiting stroke-induced damage by targeting an acid channel. N Engl J Med. 2005;352:85–6. doi: 10.1056/NEJMcibr045010. [DOI] [PubMed] [Google Scholar]
- 31.Huang Y, McNamara JO. Ischemic stroke: “acidotoxicity” is a perpetrator. Cell. 2004;118:665–6. doi: 10.1016/j.cell.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Sluka KA, Winter OC, Wemmie JA. Acid-sensing ion channels: A new target for pain and CNS diseases. Curr Opin Drug Discov Devel. 2009;12:693–704. [PMC free article] [PubMed] [Google Scholar]
- 33.Alvarez dlR, Canessa CM, Fyfe GK, Zhang P. Structure and regulation of amiloride-sensitive sodium channels. Annu Rev Physiol. 2000;62:573–94. doi: 10.1146/annurev.physiol.62.1.573. [DOI] [PubMed] [Google Scholar]
- 34.Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1. 9 A resolution and low pH. Nature. 2007;449:316–23. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- 35.Gonzales EB, Kawate T, Gouaux E. Pore architecture and ion sites in acid-sensing ion channels and P2X receptors. Nature. 2009;460:599–604. doi: 10.1038/nature08218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krishtal O. The ASICs: signaling molecules? Modulators? Trends Neurosci. 2003;26:477–83. doi: 10.1016/S0166-2236(03)00210-8. [DOI] [PubMed] [Google Scholar]
- 37.Grunder S, Chen X. Structure, function, and pharmacology of acid-sensing ion channels (ASICs): focus on ASIC1a. Int J Physiol Pathophysiol Pharmacol. 2010;2:73–94. [PMC free article] [PubMed] [Google Scholar]
- 38.Yermolaieva O, Leonard AS, Schnizler MK, Abboud FM, Welsh MJ. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc Natl Acad Sci U S A. 2004;101:6752–7. doi: 10.1073/pnas.0308636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sherwood TW, Lee KG, Gormley MG, Askwith CC. Heteromeric acid-sensing ion channels (ASICs) composed of ASIC2b and ASIC1a display novel channel properties and contribute to acidosis-induced neuronal death. J Neurosci. 2011;31:9723–34. doi: 10.1523/JNEUROSCI.1665-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mari Y, Katnik C, Cuevas J. ASIC1a channels are activated by endogenous protons during ischemia and contribute to synergistic potentiation of intracellular Ca(2+) overload during ischemia and acidosis. Cell Calcium. 2010;48:70–82. doi: 10.1016/j.ceca.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Zha XM, Wemmie JA, Green SH, Welsh MJ. Acid-sensing ion channel 1a is a postsynaptic proton receptor that affects the density of dendritic spines. Proc Natl Acad Sci U S A. 2006;103:16556–61. doi: 10.1073/pnas.0608018103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samways DS, Harkins AB, Egan TM. Native and recombinant ASIC1a receptors conduct negligible Ca2+ entry. Cell Calcium. 2009;45:319–25. doi: 10.1016/j.ceca.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herrera Y, Katnik C, Rodriguez JD, Hall AA, Willing A, Pennypacker KR, Cuevas J. sigma-1 receptor modulation of acid-sensing ion channel a (ASIC1a) and ASIC1a-induced Ca2+ influx in rat cortical neurons. J Pharmacol Exp Ther. 2008;327:491–502. doi: 10.1124/jpet.108.143974. [DOI] [PubMed] [Google Scholar]
- 44.Bevan S, Yeats J. Protons activate a cation conductance in a sub-population of rat dorsal root ganglion neurones. J Physiol (Lond) 1991;433:145–61. doi: 10.1113/jphysiol.1991.sp018419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krishtal OA, Pidoplichko VI. A receptor for protons in the membrane of sensory neurons may participate in nociception. Neuroscience. 1981;6:2599–601. doi: 10.1016/0306-4522(81)90105-6. [DOI] [PubMed] [Google Scholar]
- 46.Ugawa S, Ueda T, Ishida Y, Nishigaki M, Shibata Y, Shimada S. Amiloride-blockable acid-sensing ion channels are leading acid sensors expressed in human nociceptors. J Clin Invest. 2002;110:1185–90. doi: 10.1172/JCI15709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106:229–39. doi: 10.1016/S0304-3959(03)00269-0. [DOI] [PubMed] [Google Scholar]
- 48.Chen CC, Zimmer A, Sun WH, Hall J, Brownstein MJ, Zimmer A. A role for ASIC3 in the modulation of high-intensity pain stimuli. Proc Natl Acad Sci U S A. 2002;99:8992–7. doi: 10.1073/pnas.122245999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu LJ, Duan B, Mei YD, Gao J, Chen JG, Zhuo M, et al. Characterization of acid-sensing ion channels in dorsal horn neurons of rat spinal cord. J Biol Chem. 2004;279:43716–24. doi: 10.1074/jbc.M403557200. [DOI] [PubMed] [Google Scholar]
- 50.Benson CJ, Eckert SP, McCleskey EW. Acid-evoked currents in cardiac sensory neurons: A possible mediator of myocardial ischemic sensation. Circ Res. 1999;84:921–8. doi: 10.1161/01.res.84.8.921. [DOI] [PubMed] [Google Scholar]
- 51.Price MP, Lewin GR, McIlwrath SL, Cheng C, Xie J, Heppenstall PA, et al. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature. 2000;407:1007–11. doi: 10.1038/35039512. [DOI] [PubMed] [Google Scholar]
- 52.Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, et al. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron. 2001;32:1071–83. doi: 10.1016/s0896-6273(01)00547-5. [DOI] [PubMed] [Google Scholar]
- 53.Page AJ, Brierley SM, Martin CM, Price MP, Symonds E, Butler R, et al. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut. 2005;54:1408–15. doi: 10.1136/gut.2005.071084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ugawa S, Yamamoto T, Ueda T, Ishida Y, Inagaki A, Nishigaki M, Shimada S. Amiloride-insensitive currents of the acid-sensing ion channel-2a (ASIC2a)/ASIC2b heteromeric sour-taste receptor channel. J Neurosci. 2003;23:3616–22. doi: 10.1523/JNEUROSCI.23-09-03616.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ugawa S. Identification of sour-taste receptor genes. Anat Sci Int. 2003;78:205–10. doi: 10.1046/j.0022-7722.2003.00062.x. [DOI] [PubMed] [Google Scholar]
- 56.Lin W, Ogura T, Kinnamon SC. Acid-activated cation currents in rat vallate taste receptor cells. J Neurophysiol. 2002;88:133–41. doi: 10.1152/jn.2002.88.1.133. [DOI] [PubMed] [Google Scholar]
- 57.Wemmie JA, Askwith CC, Lamani E, Cassell MD, Freeman JH, Jr, Welsh MJ. Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J Neurosci. 2003;23:5496–502. doi: 10.1523/JNEUROSCI.23-13-05496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ettaiche M, Guy N, Hofman P, Lazdunski M, Waldmann R. Acid-sensing ion channel 2 is important for retinal function and protects against light-induced retinal degeneration. J Neurosci. 2004;24:1005–12. doi: 10.1523/JNEUROSCI.4698-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnson MB, Jin K, Minami M, Chen D, Simon RP. Global ischemia induces expression of acid-sensing ion channel 2a in rat brain. J Cereb Blood Flow Metab. 2001;21:734–40. doi: 10.1097/00004647-200106000-00011. [DOI] [PubMed] [Google Scholar]
- 60.Pignataro G, Simon RP, Xiong ZG. Prolonged activation of ASIC1a and the time window for neuroprotection in cerebral ischaemia. Brain. 2007;130:151–8. doi: 10.1093/brain/awl325. [DOI] [PubMed] [Google Scholar]
- 61.Gao J, Duan B, Wang DG, Deng XH, Zhang GY, Xu L, Xu TL. Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron. 2005;48:635–46. doi: 10.1016/j.neuron.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 62.Jetti SK, Swain SM, Majumder S, Chatterjee S, Poornima V, Bera AK. Evaluation of the role of nitric oxide in acid sensing ion channel mediated cell death. Nitric Oxide. 2010;22:213–9. doi: 10.1016/j.niox.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 63.Friese MA, Craner MJ, Etzensperger R, Vergo S, Wemmie JA, Welsh MJ, et al. Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat Med. 2007;13:1483–9. doi: 10.1038/nm1668. [DOI] [PubMed] [Google Scholar]
- 64.Gu L, Liu X, Yang Y, Luo D, Zheng X. ASICs aggravate acidosis-induced injuries during ischemic reperfusion. Neurosci Lett. 2010;479:63–8. doi: 10.1016/j.neulet.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 65.Sherwood TW, Askwith CC. Dynorphin opioid peptides enhance acid-sensing ion channel 1a activity and acidosis-induced neuronal death. J Neurosci. 2009;29:14371–80. doi: 10.1523/JNEUROSCI.2186-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arias RL, Sung ML, Vasylyev D, Zhang MY, Albinson K, Kubek K, et al. Amiloride is neuroprotective in an MPTP model of Parkinson’s disease. Neurobiol Dis. 2008;31:334–41. doi: 10.1016/j.nbd.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 67.Li M, Inoue K, Branigan D, Kratzer E, Hansen JC, Chen JW, et al. Acid-sensing ion channels in acidosis-induced injury of human brain neurons. J Cereb Blood Flow Metab. 2010;30:1247–60. doi: 10.1038/jcbfm.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gao J, Wu LJ, Xu L, Xu TL. Properties of the proton-evoked currents and their modulation by Ca2+ and Zn2+ in the acutely dissociated hippocampus CA1 neurons. Brain Res. 2004;1017:197–207. doi: 10.1016/j.brainres.2004.05.046. [DOI] [PubMed] [Google Scholar]
- 69.Zha XM, Costa V, Harding AM, Reznikov L, Benson CJ, Welsh MJ. ASIC2 subunits target acid-sensing ion channels to the synapse via an association with PSD-95. J Neurosci. 2009;29:8438–46. doi: 10.1523/JNEUROSCI.1284-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hruska-Hageman AM, Wemmie JA, Price MP, Welsh MJ. Interaction of the synaptic protein PICK1 (protein interacting with C kinase 1) with the non-voltage gated sodium channels BNC1 (brain Na+ channel 1) and ASIC (acid-sensing ion channel) Biochem J. 2002;361:443–50. doi: 10.1042/0264-6021:3610443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cho JH, Askwith CC. Presynaptic Release Probability Is Increased in Hippocampal Neurons From ASIC1 Knockout Mice. J Neurophysiol. 2008;99:426–41. doi: 10.1152/jn.00940.2007. [DOI] [PubMed] [Google Scholar]
- 72.Maysami S, Branigan D, Simon RP, Xiong ZG. Amyloid beta peptide modulates the activity of acid-sensing ion channels in neurons. Society for Neuroscience Annual Meeting. 2009;237.8 [Google Scholar]
- 73.Coryell MW, Ziemann AE, Westmoreland PJ, Haenfler JM, Kurjakovic Z, Zha XM, et al. Targeting ASIC1a Reduces Innate Fear and Alters Neuronal Activity in the Fear Circuit. Biol Psychiatry. 2007;62:1140–8. doi: 10.1016/j.biopsych.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 74.Wemmie JA, Coryell MW, Askwith CC, Lamani E, Leonard AS, Sigmund CD, Welsh MJ. Overexpression of acid-sensing ion channel 1a in transgenic mice increases acquired fear-related behavior. Proc Natl Acad Sci U S A. 2004;101:3621–6. doi: 10.1073/pnas.0308753101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Coryell MW, Wunsch AM, Haenfler JM, Allen JE, McBride JL, Davidson BL, Wemmie JA. Restoring Acid-sensing ion channel-1a in the amygdala of knock-out mice rescues fear memory but not unconditioned fear responses. J Neurosci. 2008;28:13738–41. doi: 10.1523/JNEUROSCI.3907-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, et al. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–21. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Papp LA, Klein DF, Gorman JM. Carbon dioxide hypersensitivity, hyperventilation, and panic disorder. Am J Psychiatry. 1993;150:1149–57. doi: 10.1176/ajp.150.8.1149. [DOI] [PubMed] [Google Scholar]
- 78.Ettaiche M, Deval E, Cougnon M, Lazdunski M, Voilley N. Silencing acid-sensing ion channel 1a alters cone-mediated retinal function. J Neurosci. 2006;26:5800–9. doi: 10.1523/JNEUROSCI.0344-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Render JA, Howe KR, Wunsch AM, Guionaud S, Cox PJ, Wemmie JA. Histologic examination of the eye of acid-sensing ion channel 1a knockout mice. Int J Physiol Pathophysiol Pharmacol. 2010;2:69–72. [PMC free article] [PubMed] [Google Scholar]
- 80.Ettaiche M, Deval E, Pagnotta S, Lazdunski M, Lingueglia E. Acid-sensing ion channel 3 in retinal function and survival. Invest Ophthalmol Vis Sci. 2009;50:2417–26. doi: 10.1167/iovs.08-3028. [DOI] [PubMed] [Google Scholar]
- 81.Duan B, Wang YZ, Yang T, Chu XP, Yu Y, Huang Y, et al. Extracellular spermine exacerbates ischemic neuronal injury through sensitization of ASIC1a channels to extracellular acidosis. J Neurosci. 2011;31:2101–12. doi: 10.1523/JNEUROSCI.4351-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hu R, Duan B, Wang D, Yu Y, Li W, Luo H, et al. Role of Acid-sensing ion channel 1a in the secondary damage of traumatic spinal cord injury. Ann Surg. 2011;254:353–62. doi: 10.1097/SLA.0b013e31822645b4. [DOI] [PubMed] [Google Scholar]
- 83.Pignataro G, Cuomo O, Esposito E, Sirabella R, Di Renzo G, Annunziato L. ASIC1a contributes to neuroprotection elicited by ischemic preconditioning and postconditioning. Int J Physiol Pathophysiol Pharmacol. 2011;3:1–8. [PMC free article] [PubMed] [Google Scholar]
- 84.Waxman SG. Axonal conduction and injury in multiple sclerosis: the role of sodium channels. Nat Rev Neurosci. 2006;7:932–41. doi: 10.1038/nrn2023. [DOI] [PubMed] [Google Scholar]
- 85.Stys PK, LoPachin RM. Mechanisms of calcium and sodium fluxes in anoxic myelinated central nervous system axons. Neuroscience. 1998;82:21–32. doi: 10.1016/s0306-4522(97)00230-3. [DOI] [PubMed] [Google Scholar]
- 86.Vergo S, Craner MJ, Etzensperger R, Attfield K, Friese MA, Newcombe J, et al. Acid-sensing ion channel 1 is involved in both axonal injury and demyelination in multiple sclerosis and its animal model. Brain. 2011;134:571–84. doi: 10.1093/brain/awq337. [DOI] [PubMed] [Google Scholar]
- 87.Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 88.Pidoplichko VI, Dani JA. Acid-sensitive ionic channels in midbrain dopamine neurons are sensitive to ammonium, which may contribute to hyperammonemia damage. Proc Natl Acad Sci U S A. 2006;103:11376–80. doi: 10.1073/pnas.0600768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wong HK, Bauer PO, Kurosawa M, Goswami A, Washizu C, Machida Y, et al. Blocking acid-sensing ion channel 1 alleviates Huntington’s disease pathology via an ubiquitin-proteasome system-dependent mechanism. Hum Mol Genet. 2008;17:3223–35. doi: 10.1093/hmg/ddn218. [DOI] [PubMed] [Google Scholar]
- 90.Coryell MW, Wunsch AM, Haenfler JM, Allen JE, Schnizler M, Ziemann AE, et al. Acid-sensing ion channel-1a in the amygdala, a novel therapeutic target in depression-related behavior. J Neurosci. 2009;29:5381–8. doi: 10.1523/JNEUROSCI.0360-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dwyer JM, Rizzo SJ, Neal SJ, Lin Q, Jow F, Arias RL, et al. Acid sensing ion channel (ASIC) inhibitors exhibit anxiolytic-like activity in preclinical pharmacological models. Psychopharmacology (Berl) 2009;203:41–52. doi: 10.1007/s00213-008-1373-7. [DOI] [PubMed] [Google Scholar]
- 92.Wu WL, Lin YW, Min MY, Chen CC. Mice lacking Asic3 show reduced anxiety-like behavior on the elevated plus maze and reduced aggression. Genes Brain Behav. 2010;9:603–14. doi: 10.1111/j.1601-183X.2010.00591.x. [DOI] [PubMed] [Google Scholar]
- 93.Meng QY, Wang W, Chen XN, Xu TL, Zhou JN. Distribution of acid-sensing ion channel 3 in the rat hypothalamus. Neuroscience. 2009;159:1126–34. doi: 10.1016/j.neuroscience.2009.01.069. [DOI] [PubMed] [Google Scholar]
- 94.Simon RP, Copeland JR, Benowitz NL, Jacob P, III, Bronstein J. Brain phenobarbital uptake during prolonged status epilepticus. J Cereb Blood Flow Metab. 1987;7:783–8. doi: 10.1038/jcbfm.1987.134. [DOI] [PubMed] [Google Scholar]
- 95.Simon RP, Benowitz N, Hedlund R, Copeland J. Influence of the blood-brain pH gradient on brain phenobarbital uptake during status epilepticus. J Pharmacol Exp Ther. 1985;234:830–5. [PubMed] [Google Scholar]
- 96.Somjen GG. Acidification of interstitial fluid in hippocampal formation caused by seizures and by spreading depression. Brain Res. 1984;311:186–8. doi: 10.1016/0006-8993(84)91416-1. [DOI] [PubMed] [Google Scholar]
- 97.Chesler M, Kaila K. Modulation of pH by neuronal activity. Trends Neurosci. 1992;15:396–402. doi: 10.1016/0166-2236(92)90191-a. [DOI] [PubMed] [Google Scholar]
- 98.Chesler M, Chan CY. Stimulus-induced extracellular pH transients in the in vitro turtle cerebellum. Neuroscience. 1988;27:941–8. doi: 10.1016/0306-4522(88)90197-2. [DOI] [PubMed] [Google Scholar]
- 99.Urbanics R, Leniger-Follert E, Lubbers DW. Time course of changes of extracellular H+ and K+ activities during and after direct electrical stimulation of the brain cortex. Pflugers Arch. 1978;378:47–53. doi: 10.1007/BF00581957. [DOI] [PubMed] [Google Scholar]
- 100.Biagini G, Babinski K, Avoli M, Marcinkiewicz M, Seguela P. Regional and subunit-specific downregulation of acid-sensing ion channels in the pilocarpine model of epilepsy. Neurobiol Dis. 2001;8:45–58. doi: 10.1006/nbdi.2000.0331. [DOI] [PubMed] [Google Scholar]
- 101.Ali A, Ahmad FJ, Pillai KK, Vohora D. Evidence of the antiepileptic potential of amiloride with neuropharmacological benefits in rodent models of epilepsy and behavior. Epilepsy Behav. 2004;5:322–8. doi: 10.1016/j.yebeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 102.Ali A, Pillai KP, Ahmad FJ, Dua Y, Vohora D. Anticonvulsant effect of amiloride in pentetrazole-induced status epilepticus in mice. Pharmacol Rep. 2006;58:242–5. [PubMed] [Google Scholar]
- 103.N’Gouemo P. Amiloride delays the onset of pilocarpine-induced seizures in rats. Brain Res. 2008;1222:230–2. doi: 10.1016/j.brainres.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chang SY, Li MH, Li TF, Chu XP, Lan JQ, Thomson S, Jessick V, Meller R, Simon RP, Xiong ZG. Involvement of acid-sensing ion channels in the generation of epileptic seizure activity. Soc Neurosci Abstr. 2007;257.5 [Google Scholar]
- 105.Ziemann AE, Schnizler MK, Albert GW, Severson MA, Howard MA, III, Welsh MJ, Wemmie JA. Seizure termination by acidosis depends on ASIC1a. Nat Neurosci. 2008;11:816–22. doi: 10.1038/nn.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li M, Kratzer E, Inoue K, Simon RP, Xiong ZG. Developmental change in the electrophysiological and pharmacological properties of acid-sensing ion channels in CNS neurons. J Physiol. 2010;588:3883–900. doi: 10.1113/jphysiol.2010.192922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Weng JY, Lin YC, Lien CC. Cell type-specific expression of acid-sensing ion channels in hippocampal interneurons. J Neurosci. 2010;30:6548–58. doi: 10.1523/JNEUROSCI.0582-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Berdiev BK, Xia J, McLean LA, Markert JM, Gillespie GY, Mapstone TB, et al. Acid-sensing ion channels in malignant gliomas. J Biol Chem. 2003;278:15023–34. doi: 10.1074/jbc.M300991200. [DOI] [PubMed] [Google Scholar]
- 109.Kapoor N, Bartoszewski R, Qadri YJ, Bebok Z, Bubien JK, Fuller CM, Benos DJ. Knockdown of ASIC1 and epithelial sodium channel subunits inhibits glioblastoma whole cell current and cell migration. J Biol Chem. 2009;284:24526–41. doi: 10.1074/jbc.M109.037390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vila-Carriles WH, Kovacs GG, Jovov B, Zhou ZH, Pahwa AK, Colby G, et al. Surface expression of ASIC2 inhibits the amiloride-sensitive current and migration of glioma cells. J Biol Chem. 2006;281:19220–32. doi: 10.1074/jbc.M603100200. [DOI] [PubMed] [Google Scholar]
- 111.Xu TL, Xiong ZG. Dynamic regulation of acid-sensing ion channels by extracellular and intracellular modulators. Curr Med Chem. 2007;14:1753–63. doi: 10.2174/092986707781058977. [DOI] [PubMed] [Google Scholar]