Abstract
Acid-sensing ion channel 1b (ASIC1b) is a proton-gated Na+ channel mostly expressed in peripheral sensory neurons. To date, the functional significance of ASIC1b in these cells is unclear due to the lack of a specific inhibitor/blocker. A better understanding of the regulation of ASIC1b may provide a clue for future investigation of its functional importance. One important regulator of acid-sensing ion channels (ASICs) is zinc. In this study, we examined the detailed zinc inhibition of ASIC1b currents and specific amino acid(s) involved in the inhibition. In CHO cells expressing rat ASIC1b subunit, pretreatment with zinc concentration-dependently inhibited the ASIC1b currents triggered by pH dropping from 7.4 to 6.0 with a half-maximum inhibitory concentration of 26 μM. The inhibition of ASIC1b currents by pre-applied zinc was independent of pH, voltage, or extracellular Ca2+. Further, we showed that the effect of zinc is dependent on the extracellular cysteine, but not histidine residue. Mutating cysteine 149, but not cysteine 58 or cysteine 162, located in the extracellular domain of the ASIC1b subunit abolished the zinc inhibition. These findings suggest that cysteine 149 in the extracellular finger domain of ASIC1b subunit is critical for zinc-mediated inhibition and provide the basis for future mechanistic studies addressing the functional significance of zinc inhibition of ASIC1b.
Keywords: acid-sensing ion channels, zinc, ASIC1b, patch-clamp
Acid-sensing ion channels (ASICs) are proton-gated, trimeric cationic channels that belong to the degenerin/epithelial Na+ channel superfamily (Kellenberger and Schild, 2002; Jasti et al., 2007), and are expressed primarily in central and peripheral neurons (Waldmann et al., 1997a, 1997b; Wemmie et al., 2003, 2006; Askwith et al., 2004; Xiong et al., 2004, 2008; Zha et al., 2006, 2009; Grunder & Chen., 2010). At least seven ASIC subunits have been identified so far, encoded by four genes (ASIC1 - ASIC4). All ASIC subunits share a similar topology with two transmembrane domains, a large ectodomain with 14 highly conserved cysteine residues, and short cytoplasmic N- and C-termini (Waldmann and Lazdunski, 1998; Waldmann et al., 1997b; Krishtal O, 2003). Acid-sensing ion channel 1b (ASIC1b) (Bassler et al., 2001), originally named ASICβ (Chen et al., 1998), a splice variant of the amiloride-sensitive cation channel 2, neuronal (ACCN2) gene (Waldmann et al., 1997b), has distinct properties when compared with other ASICs. For example, the following properties distinguish rodent ASIC1b from ASIC1a: (1), although the amino acid sequence of approximately 2/3 of these proteins are identical, there are significant differences in the sequence for the first one third (about 172 amino acids) beginning at the N terminal; this sequence includes the intracellular N-terminus, the first transmembrane domain, and the proximal part of the ectodomain (Chen et al., 1998; Bassler et al., 2001); (2), the expression of ASIC1b in the nervous system is limited to peripheral sensory neurons, while ASIC1a is also expressed in the central nervous system (CNS); (3), rodent ASIC1b is impermeable to Ca2+ while ASIC1a channels have significant Ca2+ permeability; (4), the threshold for activation of ASIC1b current is lower (approximately at 6.5 by a drop in pH from 7.4) than ASIC1a and it has lower half-maximal activation of pH (pH50) to protons (5.9); (5), ASIC1b is potentiated by PcTx1(Chen et al., 2006), which is a specific inhibitor of ASIC1a.
Although electrophysiological and pharmacological studies show that ASIC1b subunits function as homomeric or heteromeric channels (Chen et al., 1998; Bassler et al., 2001; Benson et al., 2002; Chu et al., 2004, 2006; Hesselager et al., 2004; Poirot et al., 2006; Ugawa et al., 2008; Sherwood & Askwith, 2009; Chung et al., 2010; Hoagland et al., 2010; Jiang et al., 2010), the physiological role of ASIC1b is not fully understood. ASIC1b is expressed primarily in sensory neurons such as dorsal root ganglia (DRG) neurons (Chen et al., 1998; Bassler et al., 2001; Hoagland et al., 2010), and is also found in stereocilia of mouse cochlear hair cells (Ugawa, et al., 2006) and in vascular smooth muscle cells (VSMCs) of the cerebral artery (Chung et al., 2010). The relatively selective expression of ASIC1b by these cells suggests its potential importance in mediating acid-activated responses in these cells. Interestingly, electrophysiological studies show that ASIC1b expressed in oocytes are sensitive to mechanical stimuli (Ugawa et al., 2008). If it is similarly stimulated on cochlear hair cells, it is possible that ASIC1b might participate in cochlear mechanoelectrical transduction.
ASICs are regulated by zinc, but different ASICs respond differently to zinc, and zinc may either potentiate or inhibit a given ASIC, depending on the zinc concentration. For example, zinc potentiates ASIC2a and ASIC2a-containing channels at micromolar concentrations with half maximal effective zinc concentrations (EC50) of approximately 110 μM (Baron et al., 2001). At nanomolar concentrations, however, zinc inhibits ASIC1a and ASIC1a/2a with an IC50 of approximately 10 nM (Chu et al., 2004). Zinc inhibits ASIC3 at micromolar concentrations, with an IC50 of 61 μM (Jiang et al., 2010). The differential effects of zinc on ASICs may have important pathophysiological implications because zinc plays a critical role in the pathogenesis of several neurological diseases, such as brain ischemia, epilepsy, Alzheimer’s and Parkinson’s diseases (Frederickson et al., 2005; Paoletti et al., 2009; Zatta et al., 2009).
Unlike ASIC1a, ASIC1b is not affected by zinc at nanomolar concentrations (Chu et al., 2004). However, profound inhibition of ASIC1b currents by high non-physiological concentration of zinc (300 μM) has been reported (Poirot et al., 2006). Here we found that zinc inhibits ASIC1b currents with an IC50 of 26 μM. Further, using a combination of whole-cell patch-clamp recording, amino acid modifiers and ASIC1b mutants, we found that cysteine149 located in the extracellular finger domain of ASIC1b subunit is responsible for the identified effect.
EXPERIMENTAL PROCEDURES
ASICs Transient Expression in Chinese Hamster Ovary Cells
Tissue culture and transfection of Chinese hamster ovary (CHO) cells with various ASIC subunits were described in detail previously (Chu et al., 2004, 2006; Jiang et al., 2010). Briefly, CHO cells were maintained in standard F12 medium (American Type Culture Collection, Manassas, VA) supplemented with 10% fetal bovine serum at 37°C in a CO2 incubator. Cells were split with trypsin-EDTA, plated on a 35-mm culture dish at 10% confluence, and allowed to recover for 24 h at 37°C. At ~50 to 70% confluence, cells were transiently transfected with expression vectors containing rat ASIC1b (ASICβ) cDNA (Chen et al., 1998) and enhanced green fluorescent protein (eGFP) at a 1:0.25 molar ratio (Invitrogen, San Diego, CA) using Fugene HD transfection reagent (Roche Diagnostics, Indianapolis, IN). Cultures were used for electrophysiological recording 48 h after transfection. The cDNA of the rat ASIC1b clone was a gift from Drs. R. Waldmann and M. Lazdunski (Institut de Pharmacologie Moleculaire et Cellulaire, Centre National de Scientifique, Valbonne, France).
Whole-cell Patch-clamp Recording
Whole-cell patch-clamp recordings were performed as described previously (Xiong et al., 2004; Chu et al., 2004, 2006; Jiang et al., 2009, 2010). Patch electrodes, whose resistance ranged from 3 to 6 MΩ when filled with intracellular solution, were constructed from thin-walled borosilicated glass (1.5 mm diameter, WPI, Sarasota, FL) on a two-stage puller (PC-10, Narishige, Tokyo, Japan). Whole-cell currents were elicited by a drop in pH from 7.4 to various levels at a holding potential of −60 mV and recorded using Axopatch 200B amplifiers (Axon CNS, Molecular Devices, Foster City, CA). Data were filtered at 2 kHz and digitized at 5 Hz using Digidata 1440 DAC units (Axon CNS, Molecular Devices, Foster City, CA). The on-line acquisition was done using pCLAMP software (Version 10.2, Axon CNS, Molecular Devices, Foster City, CA).
In general, ASIC channels were activated by a drop in pH from 7.4 to specific target levels every 2 min to allow for a complete recovery of the channel from desensitization. During each experiment, a voltage step of −10 mV from the holding potential (−60 mV unless specified otherwise) was applied periodically to monitor the cell capacitance and the access resistance. Recordings in which either the access resistance or the capacitance changed by more than 10% during the experiment were excluded from data analysis.
Site-directed mutagenesis
The site-directed mutagenesis was conducted as described previously (Chu et al., 2004, 2006). Briefly, rat ASIC1b point mutations were made using the Quick-Change Site-Directed Mutagenesis system (Stratagene, La Jolla, CA) in accordance with the manufacture’s protocol. The primers were obtained from Sigma-Genosys (The Woodlands, TX). Mutations were confirmed by restriction enzyme digest and DNA sequence analysis. In all cases, the entire ASIC1b cDNA was sequenced to determine whether any nonspecific mutations were introduced.
Solutions and Compounds
Standard extracellular fluid (ECF) contained (mM) 140 NaCl, 5.4 KCl, 2.0 CaCl2, 1.0 MgCl2, 20 HEPES, and 10 glucose (pH 7.4; 320 ~ 330 mOsm). For solutions with pH of 6.0 or lower, MES was used instead of HEPES for more reliable pH buffering (Chu et al., 2004, 2006; Jiang et al., 2009, 2010). The pipette solution contained (mM) 140 K-Gluconate, 10 HEPES, 11 EGTA, 2 TEA, 1 CaCl2, 2 MgCl2, and 4 K2ATP (pH 7.2 ~ 7.3; 290 ~ 300 mOsm). Methanethiosulfonate-ethyltrimethylammonium (MTSET) and N-bromosuccinimide (N-BrSuc) were purchased from Toronto Research Chemicals (Toronto, Canada) and MP Biomedicals (Solon, OH), respectively. Other chemicals were purchased from Sigma-Aldrich (St. Louis, MO). A multi-barrel perfusion system (SF-77, Warner Instrument Co., CT) was employed to achieve a rapid exchange of extracellular solutions. For the pretreatment with zinc, zinc is present in the ECF of both pH 7.4 and lower pH (e.g. 6.0); for the increasing of concentrations of extracellular calcium (e.g. from 2 to 5 or 10 mM), the same concentration of calcium is present in the ECF of both pH 7.4 and lower pH (e.g. 6.0); for the co-application of zinc, zinc is only present in the ECF of lower pH (e.g. 6.0) (only in Fig. 1C, D).
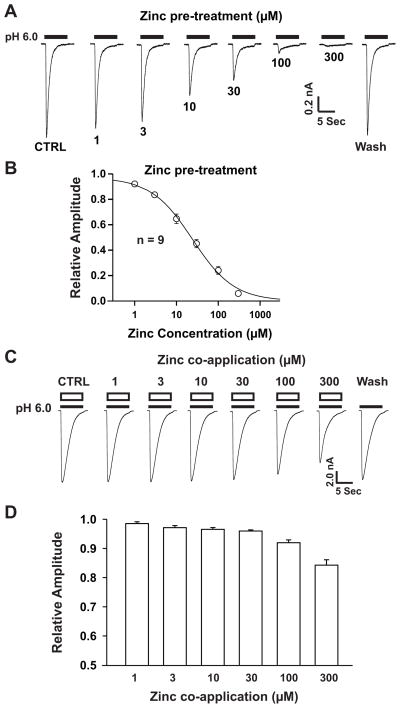
Fig. 1. Pretreatment with zinc, but not co-application, inhibits ASIC1b currents concentration-dependently in ASIC1b transfected CHO cells.
(A) Representative traces showing the concentration-dependent blockade of ASIC1b currents triggered by dropping the pH from 7.4 to 6.0 in the presence of different concentrations of zinc with pretreatment. Transient inward ASIC1b currents in CHO cells were recorded with drops in pH from 7.4 to 6.0 in whole-cell configurations at −60 mV. (B) Concentration-inhibition curve of pH 6.0-induced currents by zinc pretreatment. The IC50 of zinc blockade is 26 ± 2.8 μM. Each point represents the average responses of nine cells. (C) Representative traces showing pH-6.0 triggered ASIC1b currents with co-application of different concentrations of zinc in CHO cells. The duration of co-application with pH drops is 7 seconds. (D) Bar graphs showing relative amplitudes of ASIC1b currents at pH 6.0 with co-application of zinc at different concentrations. Each point represents the average responses of six to eleven cells. There are no significant differences between each group (p > 0.05, ANOVA). CTRL represents control.
The histidine-modifying reagents, diethylpyrocarbonate (DEPC) and N-BrSuc, were dissolved in ECF to 0.6 and 1.0 mM concentration, respectively. The membrane-impermeable cysteine modifier MTSET was made as a 10 mM stock in ECF, held on ice for less than 1 h, then diluted to a 1.0 mM working concentration in ECF at room temperature immediately before application.
Data analysis
All data were analyzed using Clampfit 10.2 software (Axon CNS, Molecular Devices, Foster City, CA). For half-maximum inhibitory concentration (IC50) curves of zinc, a pH 6.0-triggered ASIC1b current, without zinc treatment, was used as a control. For treatment with different concentrations of zinc (1, 3, 10, 30, 100, and 300 μM), ASIC1b currents were normalized to control values without zinc treatment. Normalized values were fitted to the Hill equation to obtain IC50 values and Hill coefficients.
Statistics
Statistical analyses were carried out using SigmaPlot software. Significant differences between mean values from each experimental group were tested using a Student’s t-test for two groups and one-way analysis of variance (ANOVA) for multiple comparisons. Differences were considered significant if p < 0.05.
RESULTS
Pretreatment with zinc, but not co-application, concentration-dependently inhibits ASIC1b currents
In contrast to ASIC1a currents, ASIC1b currents are not affected by zinc at nanomolar concentrations (Chu et al., 2004). However, it has been demonstrated that, at micromolar concentrations, zinc significant inhibited the ASIC1b currents (Poirot et al., 2006). To determine the detailed concentration-response relationship for zinc effect on ASIC1b current, CHO cells expressing ASIC1b were pretreated with varying zinc concentrations for 2 minutes before decreasing the pH, under a whole-cell voltage clamp configuration. As shown in Fig. 1A, a large inward ASIC1b channel current was activated by dropping the extracellular pH from 7.4 to 6.0. Pretreatment with 1 or 3 μM zinc produced slight decreases in ASIC1b currents, but these changes were not significant (Figs. 1A and 1B). Pretreatment with zinc at concentrations of 10, 30, 100 or 300 μM, however, significantly and concentration-dependently decreased ASIC1b currents. At 10, 30, 100 and 300 μM, ASIC1b currents were inhibited by 35%, 57%, 78% and 97%, respectively. The inhibitory effect of zinc on ASIC1b currents was rapidly reversed after washout (Fig. 1A). The concentration-inhibition curve for zinc is shown in Fig. 1B, with an IC50 value of 26 ± 2.8 μM and a Hill coefficient of 0.97 ± 0.01. These data support the concept that ASIC1b activity is inhibited by pretreatment with zinc, at micromolar concentrations, and that this inhibitory effect is concentration dependent.
We next examined whether co-application of zinc with acidic solution had a similar inhibitory effect on ASIC1b currents. Co-application of zinc, at concentrations between 1 to 300 μM, did not result in significant inhibition of ASIC1b currents induced by a pH drop from 7.4 to 6.0 (7 seconds in duration) (Fig. 1C, D). These data support the concept that ASIC1b activity, induced by a drop in pH from 7.4 to 6.0, is not significantly inhibited by co-application of zinc at concentrations between 1 and 300 μM.
Zinc inhibition of ASIC1b is independent of pH activation, steady-state desensitization, and membrane potential
We previously showed that inhibition of ASIC1a channels by nanomolar zinc is pH dependent (Chu et al., 2004). To determine whether inhibition of ASIC1b channels by micromolar zinc is also pH dependent, we generated pH concentration-response curves before and after pretreatment with 30 μM zinc; this concentration was selected because it approximates the IC50 calculated in figure 1B. Pretreatment with zinc (30 μM) inhibited ASIC1b currents induced by pH drops from 7.4 to 6.5, 6.0, 5.0, 4.0, and 3.0 to 44.2 ± 2.1%; 44.7 ± 2.9 %, 46.3 ± 3.1%, 48.3 ± 3.0%, and 51.5 ± 2.8% of the control value, respectively (Figs. 2A and 2B). The capacity of zinc to inhibit ASIC1b currents induced by drops in pH from 7.4 to 6.0 did not differ significantly from the level of inhibition observed with other pH endpoints (p > 0.05; ANOVA). These results support the conclusion that the extent of inhibition of ASIC1b currents by pre-applied zinc does not depend on the magnitude of the pH change.
Fig. 2. Zinc inhibition of ASIC1b currents in ASIC1b transfected CHO cells is independent of pH activation and steady-state desensitization.
(A) Original current traces showing inhibitory effects of 30 μM zinc with pretreatment on the pH-dependent activation of ASIC1b currents in CHO cells. The starting pH for all experiments was 7.4, the activating pH is indicated by bars above the trace and CHO cells were clamped at −60 mV. (B) Bar graphs showing relative amplitude of ASIC1b current inhibition by pre-applied 30 μM zinc. Each point represents the average response of eight to twelve cells. There are no significant differences between different groups in the presence of 30 μM zinc (p > 0.05, ANOVA). (C) Original current traces showing inhibitory effects of 30 μM zinc with pretreatment on steady-state desensitization of ASIC1b currents in CHO cells. Steady-state desensitization was induced by various conditioning pH values between 7.8 and 6.8 for 36 min before application of pH 5.0. CHO cells were clamped at −60 mV. (D) Bar graphs showing relative amplitude of ASIC1b current inhibition on steady-state desensitization by pre-applied 30 μM zinc at various conditioning pH values. ASIC1b current was evoked by application of pH 5.0. Each point represents the average response of five cells. There are no significant differences between each group in the presence of 30 μM zinc (p > 0.05, ANOVA). CTRL represents control.
To further explore the mechanism underlying the inhibition of the ASIC1b current by zinc, we determined the effect of 30 μM zinc with pretreatment on steady-state desensitization of ASIC1b. CHO cells expressing ASIC1b subunit were incubated in extracellular solutions at various conditioning pH values between 7.8 and 6.8 for ~6 min before the ASIC currents were activated by a drop in pH to 5.0. As shown in Figs. 2C and 2D, bath application of 30 μM zinc inhibited ASIC1b currents induced by pH drops from different conditioning values to 5.0. The capacity of zinc to inhibit ASIC1b currents induced by drops in pH to 5.0 from different conditioning values did not differ significantly (p>0.05; ANOVA). These results demonstrate that the degree of inhibition of ASIC1b currents by pre-applied zinc is independent of the steady-state desensitization of the ASIC1b channels.
Next, we examined whether inhibition of ASIC1b currents by zinc depends on the membrane potential. Currents were induced by reductions in pH from 7.4 to 6.0, while membrane potentials were held at different values (between +40mV and −60 mV) before and during the channel activation. The extent to which pre-applied 30 μM zinc inhibited ASIC1b currents was comparable at all membrane potentials tested, indicating the lack of a voltage-dependent effect (Fig. 3A, B). In addition, the reversal potential of ASIC1b currents remained unchanged in the presence of zinc with pretreatment (Fig. 3B), suggesting that pre-applied zinc inhibits ASIC1b currents without altering Na+ selectivity of the channels.
Fig. 3. Zinc inhibition of ASIC1b currents in ASIC1b transfected CHO cells is independent of voltage and calcium.
(A) Original current traces showing ASIC1b currents activated at different holding potentials ranging from −60 to +40 mV in the absence (left panel) and presence (right panel) of pre-applied 30 μM zinc. Currents were triggered by a drop in pH from 7.4 to 6.0. (B) Current-voltage relationship (I–V curve) before (●) and after (▲) 30 μM zinc. (C) Original current traces showing inhibitory effect of pre-applied zinc on ASIC1b currents in the presence of 2, 5 and 10 mM Ca2+. ASIC currents were activated by a drop in pH from 7.4 to 6.0 at a membrane potential of −60 mV. (D) Summary data showing relative amplitude of zinc inhibition at different concentrations of Ca2+. Each point represents the average response of five cells. There are no significant differences between different groups (p > 0.05, ANOVA). CTRL represents control.
Extracellular Ca2+ does not compete with zinc inhibition
It has been reported that extracellular Ca2+ also has an inhibitory effect on ASIC1b currents (Babini et al., 2002). Consequently, our next series of experiments were designed to determine whether zinc-induced inhibition of ASIC1b currents was affected by alterations in the concentration of extracellular Ca2+. Increasing the extracellular Ca2+ concentration from 2 to 5 or 10 mM (in the ECF of both pHs 7.4 and 6.0) had a significant inhibitory effect on ASIC1b currents, as expected (Fig. 3C). However, the capacity of 30 μM zinc with pretreatment to inhibit ASIC1b currents triggered by a drop in pH from 7.4 to 6.0 was not significantly affected by increasing the extracellular Ca2+ concentration from 2 to 5 or 10 mM (Figs. 3C and 3D; p > 0.05). The percent inhibition of ASIC1b currents by zinc was 44.7 ± 2.8%, 49.8 ± 3.2%, and 51.7 ± 3.2% in the presence of 2.0, 5.0 and 10 mM Ca2+, respectively (Fig. 3D). The failure of extracellular Ca2+, at concentrations between 2.0 and 10 mM, to significantly impact zinc-mediated inhibition of ASIC1b currents, in conjunction with the capacity of Ca2+ to inhibit ASIC1b currents, suggests that zinc and calcium inhibit ASIC1b by binding to distinct sites on the ASIC1b subunit, and that they do not compete with one another for these binding sites.
Extracellular domain of ASIC1b subunit is involved in the zinc-mediated inhibition
Biological membranes are relatively impermeable to cations, including zinc, so we hypothesize that zinc-mediated inhibition of the ASIC1b channel is likely to be dependent upon interactions with the extracellular domain of the channel. To determine whether zinc suppresses ASIC1b by binding to extracellular vs. intracellular domains, we included zinc at a concentration of 30 μM in the recording pipette before testing the effect of extracellular zinc in the bath solution. After formation of whole-cell configurations, zinc was allowed to diffuse from the recording pipette into cells for 20 min. Zinc, at the same concentration as that used in recording pipettes (30 μM), was then added to the bath solution to examine whether it could still inhibit the ASIC1b current. As expected, even under conditions in which intracellular zinc concentrations were 30 μM, extracellular zinc (30 μM) inhibited ASIC1b currents triggered by dropping the pH from 7.4 to 6.0 (Fig. 4; **p < 0.01). Further, the extent of inhibition by extracellular zinc in the presence of intracellular zinc is similar to the percent inhibition without intracellular zinc (Fig. 2B vs. Fig. 4B). These data support the concept that zinc inhibits ASIC1b currents by binding to the extracellular domain(s) of the channel.
Fig. 4. Extracellular zinc is responsible for inhibition of the ASIC1b current in ASIC1b transfected CHO cells.
(A) Representative traces showing that inhibition of ASIC1b currents by bath application of 30 μM zinc (extracellularzinc) was not affected by inclusion of 30 μM zinc in the pipette solution (i.e. intracellular zinc). ASIC currents were activated by a drop in pH from 7.4 to 6.0. (B) Summary data showing relative amplitude of zinc inhibition in the presence of 30 μM zinc in the pipette. Each point represents the average response of six cells. Asterisk indicates values significantly different from the control, t-test, **p < 0.01. CTRL represents control.
Histidine residues are not involved in zinc-mediated inhibition of ASIC1b
It has previously been demonstrated that histidine or cysteine residues mediate zinc-induced effects on ion channels (Glusker, 1991; Paoletti et al., 1997; Fayyazuddin et al., 2000; Baron et al., 2001; Seebungkert and Lynch, 2001; Nevin et al., 2003; Connolly and Wafford, 2004). For example, potentiation of ASIC2a currents by zinc involves interactions with histidine 162 and/or 339 residues in the extracellular domain (Baron et al., 2001). Using a similar strategy, we determined whether inhibition of ASIC1b by zinc is also mediated by interactions with histidine residues. Specifically, we compared zinc-mediated (30 μM) inhibition of ASIC1b currents induced by a drop in pH from 7.4 to either 6.0 or 5.0 in the presence and absence of two histidine-modifying agents, diethylpyrocarbonate (DEPC) and N-bromosuccinimide (N-BrSuc). As shown in Figs. 5A and 5B, ASIC1b currents triggered by a drop in pH from 7.4 to 5.0, were not affected by the application of DEPC (0.6 mM for 20 min) to the bath. In contrast, ASIC1b currents were significantly inhibited by application of both DEPC (0.6 mM for 20 min) and zinc (30 μM) to the bath (**p < 0.01), and the degree of inhibition was comparable to that attained by zinc alone (compare Figs 5A and 2B). Similarly, DEPC at a concentration of 0.6 mM did not affect zinc-induced inhibition of ASIC1b currents elicited by a drop from 7.4 to endpoints other than 5.0 (e.g. 6.5, and 4.0; data not shown).
Fig. 5. Modification of histidine has no effect on zinc inhibition of ASIC1b currents.
(A) Representative traces showing inhibition of ASIC1b currents in ASIC1b transfected CHO cells by 30 μM zinc. Cysteine modifying agent, DEPC (0.6 mM), had no effect on ASIC1b current, and zinc still inhibited ASIC1b current in the presence of 0.6 mM DEPC. ASIC currents were activated by dropping the pH from 7.4 to 5.0. (B) Bar graph showing relative amplitude of zinc inhibition with or without 0.6 mM DEPC. Asterisk indicates values significantly different from CTRL, t-test, **p < 0.01. Each point represents the average of seven cells. (C) Representative traces showing inhibition of ASIC1b current by 30 μM zinc. Another histidine modifying agent, N-BrSuc (1.0 mM), had no effect on ASIC1b current by itself, and zinc still inhibited ASIC1b currents in the presence of N-BrSuc. ASIC currents were triggered by dropping the pH from 7.4 to 6.0. (D) Bar graph showing relative amplitude of zinc inhibition with or without N-BrSuc. Asterisk indicates values significantly different from the control, t-test, ** p < 0.01. Each point represents the average response of eight ASIC1b transfected cells. CTRL represents control.
Next, we examined the effect of N-BrSuc on ASIC1b channels. N-BrSuc has been reported to block the zinc-mediated ASIC2a potentiation (Jiang et al., 2010). As shown in Figs. 5C and 5D, ASIC1b currents triggered by a drop in pH from 7.4 to 5.0, were not affected by the application of N-BrSuc alone (1.0 mM for 30 min) to the bath. In contrast, ASIC1b currents were significantly inhibited by application of both N-BrSuc (1.0 mM for 30 min) and zinc (30 μM) to the bath (**p < 0.01), and the degree of inhibition was comparable to that attained by zinc alone (compare Figs. 5C and 2B). Similarly, N-BrSuc at a concentration of 1.0 mM did not affect zinc-induced inhibition of ASIC1b currents elicited by a drop from 7.4 to endpoints other than 5.0 (e.g. 6.5, and 4.0; data not shown).
The collective results of the experiments presented in Figure 5 demonstrate that zinc mediated inhibition of ASIC1b currents is not dependent upon its interaction with histidine residues.
Cysteine residues are critical to the function of ASIC1b channels and to zinc-mediated inhibition of these channels
MTSET, a cysteine modifying agent, has been widely used to assess secondary structure, membrane topology and conformational changes of cloned ion channels (Holmgren et al., 1996; Chu et al., 2006; Jiang et al., 2010). Cysteine residues have been shown to be involved in the modulation of ASIC1a channels (Chu et al., 2006; Pfister et al., 2006). In order to determine whether cysteine residues are involved in zinc-mediated inhibition of ASIC1b channels, we examined the effect of MTSET on zinc-induced inhibition of ASIC1b currents. To our surprise, ASIC1b currents triggered by a drop in pH from 7.4 to 6.0 were significantly inhibited by the application of 1.0 mM MTSET alone to the bath; the degree of inhibition increased over time, but stabilized after approximately 10 minutes (Fig. 6A); After MTSET mediated inhibition of ASIC1b currents had stabilized, 30 μM zinc was added to the bath. As shown in Fig. 6A, addition of zinc did not induce further inhibition of ASIC1b currents (Fig. 6A). Statistical analysis revealed that there were no significant differences between the MTSET vs. MTSET plus zinc groups (Fig. 6B; p > 0.05). The capacity of MTSET, by itself, to inhibit ASIC1b currents indicates that cysteine residues are critical to function of ASIC1b channels. The failure of zinc to induce further inhibition of ASIC1b channels that had been pretreated with MTSET indicates that cysteine residue(s) are involved in zinc-mediated inhibition of these channels.
Fig. 6. Cysteine modifier, MESET, inhibited ASIC1b current and blocked the zinc inhibition.
(A) Representative traces showing inhibition of ASIC1b currents by bath application of 1.0 mM MTSET. Cysteine modifying agent, MTSET (1.0 mM), inhibited ASIC1b currents during the incubation; however, it blocked the zinc inhibition of ASIC1b current in the presence of MTSET. (B) Bar graph showing relative amplitude of zinc inhibition with or without 1.0 mM MTSET. Each point represents the average response of five cells. There is no significant difference between the MTSET and zinc plus MTSET groups, t-test: p > 0.05. CTRL represents control.
Cysteine 149, but not cysteine 58 or 162, residues in the extracellular domain of ASIC1b are essential for zinc-mediated inhibition
Since ASIC1a and ASIC1b respond differently to zinc, and results of the current study demonstrate that zinc mediated inhibition of ASIC1b is dependent upon interaction with cysteine residues and upon interaction with the extracellular domain of ASIC1b, we compared the amino acid sequences of ASIC1a and ASIC1b to identify potential targets of zinc. Cysteine residues present in the extracellular domain of ASIC1b, which are not present in ASIC1a, would be considered to be possible targets for zinc mediated inhibition of ASIC1b. Although the amino acid sequences of approximately 2/3 of these proteins are identical, there are significant differences in the sequence for the first one third (about 172 amino acids) beginning at the N terminal; this sequence includes the proximal part of the ectodomain (Chen et al., 1998; Bassler et al., 2001). Focusing on the proximal portion of the ectodomain, sequence alignment revealed two cysteine residues, cysteine 149 and cysteine 162 (C149 and C162), that are expressed only in ASIC1b, but not in comparable positions of ASIC1a (Fig. 7A). They are potential targets responsible for zinc inhibition. Cysteine 58 (C58), which is close to first transmembrane domain and is present in both ASIC1a and ASIC1b subunits, was chosen as a positive control (Fig. 7A). Cysteine, as a non-charged residue, was replaced with alanine (A), which is also non-charged. Site-directed mutagenesis studies were performed to identify whether these residues are responsible for zinc inhibition. Three mutants were generated, in which alanine was substituted for each of the three cysteines listed above (e.g. ASIC1b-C58A, ASIC1b-C149A, and ASIC1b-C162A).
Fig. 7. Identification of potential cysteine residues involved in zinc inhibition.
(A) Alignment of ASIC1a and ASIC1b identified potential zinc inhibition sites present in ASIC1b, but not conserved at homologous positions in ASIC1a subunit. C149 and C162 are two potential targets responsible for zinc inhibition. C58 is chosen as a positive control. (B) Representative traces showing the concentration-dependent blockade of ASIC1b-C58A mutant currents activated by dropping the pH from 7.4 to 6.0 in the presence of different concentrations of zinc with bath application. (C) Representative traces showing the concentration-dependent blockade of ASIC1b-C162A currents induced by dropping the pH from 7.4 to 6.0 in the presence of different concentrations of zinc with bath application. (D) Concentration-inhibition curve of pH 6.0-induced ASIC1b-C58A mutant currents by zinc pretreatment. The IC50 of zinc blockade is 17.5 ± 2.5 μM. Each point represents the average responses of five cells. CTRL represents control. (E) Concentration-inhibition curve of pH 6.0-induced ASIC1b-C162A currents by zinc pretreatment. The IC50 of zinc blockade is 49.1 ± 2.3 μM. Each point represents the average responses of six cells. CTRL represents control.
Each of the three mutants showed a normal response to pH activation when expressed in CHO cells, with similar pH50 values (data not shown). As expected, in CHO cells expressing ASIC1b-C58A mutants, ASIC1b currents were inhibited by zinc in a concentration-dependent manner, comparable to cells expressing wild type ASIC1b (Fig. 7B vs. 1A). A detailed concentration-response curve is presented in Fig. 7D, and the IC50 was determined to be 17.5 ± 2.5 μM. In cells expressing ASIC1b-C162A mutants, ASIC1b currents were also inhibited by zinc in a concentration-dependent manner, comparable to cells expressing wild type ASIC1b or ASIC1b-C58A mutants (Figs. 7C vs. 1A or 7B). A detailed concentration-response curve is presented in Fig. 7E, and the IC50 was determined to be 49.1 ± 2.3 μM. Although the IC50 values for these two mutants differs slightly from that of wild-type ASIC1b (26 μM), they were in a comparable range of concentrations (15 to 50 μM) indicating that these mutations had relatively minor effects on zinc mediated inhibition of ASIC1b. Both mutants were also significantly suppressed by MTSET, and the degrees of inhibition by MTSET were not significantly different from wild type ASIC1b (Fig. 8E).
Fig. 8. ASIC1b-C149A mutant abolished the zinc inhibition.
(A) Representative traces showing that ASIC1b-C149A mutation abolished the zinc inhibition in the presence of different concentrations of zinc with bath application. (B) Bar graph showing the effect of pH 6.0-induced ASIC1b-C149A currents by different concentrations of zinc pretreatment. There were no significant differences between each concentration (ANOVA, p > 0.05). Each point represents the average responses of eleven cells. (C) MTSET did not significant block the ASIC1b-C149A currents and further blocked the effect of 30 μM zinc in the presence of 1.0 mM MTSET. (D) Bar graph showing relative amplitude of ASIC1b-C149A currents to MTSET response with or without 30 μM zinc. Each point represents the average response of five cells. There is no significant difference between the MTSET and zinc plus MTSET groups, t-test, p > 0.05. (E) MTSET significantly inhibited currents from ASIC1b wild-type (n= 6), ASIC1b-C58A (n=5) and ASIC1b-C162A (n=6); there are no significant differences among three groups. WT represents ASIC1b wild-type; C58A represents ASIC1b-C58A mutant; C162A represents ASIC1b-C162A mutant. CTRL represents control.
In cells expressing ASIC1b-C149A mutants, ASIC1b currents were not significantly inhibited by zinc at any of the concentrations tested (Figs. 8A and 8B). Although MTSET induced a small inhibition, zinc did not induce further inhibition in the presence of MTSET (Figs. 8C and 8D). Collectively, these data suggest that C149, but not C58 and C162, residue at the extracellular domain of ASIC1b is essential for zinc inhibition.
DISCUSSION
In the present study, we examined the effect of zinc on homomeric ASIC1b channels expressed in CHO cells. We showed that: 1) homomeric ASIC1b channels are suppressed by pre-applied zinc with an IC50 of 26 μM, but co-application of zinc with acidic solution does not have an inhibitory effect on ASIC1b currents; 2) zinc-mediated inhibition of ASIC1b channels is independent of pH activation, steady-state desensitization and membrane potential; 3) Zn2+ and Ca2+ inhibit ASIC1b by binding to distinct sites on the ASIC1b channels; 4) zinc-mediated inhibition of ASIC1b is dependent upon interactions between zinc and cysteine 149 residues in the extracellular domain of ASIC1b; 5) zinc-mediated inhibition of ASIC1b does not appear to involve binding of zinc to histidine residues. These collective findings demonstrate that zinc is a critical modulator of ASIC1b, and further implicate zinc in physiological and pathological process involving ASICs.
Zinc is recognized as a key regulator of ion channels that plays a critical role in several neurological diseases (Harrison & Gibbons, 1994; Smart et al., 1994; Koh et al., 1996; Frederickson et al., 2005; Mathie et al., 2006; Paoletti et al., 2009). In the past decade, we and others, have focused on the effects of zinc on ASICs, which are ion channels that are expressed primarily on neurons of the central and peripheral nervous system (Baron et al., 2001, 2002, 2008; Chu et al., 2004; Wu et al., 2004; Poirot et al., 2006; Hey et al., 2007; Xu and Xiong, 2007; Jiang et al., 2009, 2010). The effects of zinc on ASICs in native neurons are complex due to the fact that these channels may consist of combinations of different ASIC subunits, and the fact that zinc has differential effects on different subunits. For example, zinc potentiates ASIC2a and ASIC2a-containing channels at micromolar concentrations (EC50 ~110 μM) (Baron et al., 2001), but inhibits ASIC1a and ASIC1a-containing channels at nanomolar concentrations (IC50 ~10 nM) (Chu et al., 2004). Zinc also inhibits ASIC3 channels at micromolar (Poirot et al., 2006; Jiang et al., 2010), but not at nanomolar concentrations (Chu et al., 2004).
Zinc usually exerts its regulatory effects on ion channels through interactions with histidine, cysteine, glutamate or lysine residues (Glusker, 1991; Paoletti et al., 1997; Fayyazuddin et al., 2000; Seebungkert and Lynch, 2001; Nevin et al., 2003; Connolly and Wafford, 2004). Mutations of those residues are known to affect the capacity of zinc to modulate different ion channels, including ASIC1a and ASIC2a channels (Baron et al., 2001; Chu et al., 2004). For example, histidine-162 and -339 in the extracellular domain of ASIC2a channels are involved in low-affinity, zinc-mediated potentiation of ASIC2a and ASIC2a-containing channels (Baron et al., 2001); while lysine 133 in the extracellular domain of ASIC1a subunit is responsible for high-affinity, zinc-mediated inhibition of ASIC1a and ASIC1a-containing channels (Chu et al., 2004). In the current study, however, we found that cysteine 149, in the extracellular loop of ASIC1b, appears to be critical for low-affinity, zinc-mediated inhibition of ASIC1b channels. According to the chicken ASIC1a crystal structure (Jasti et al., 2007), cysteine 149 in rat ASIC1b subunit is next to histidine 162 in rat ASIC2a subunit. Both of them are located at extracellular finger domain. Thus, the finger domain at the extracellular loop of ASICs is critical for low-affinity zinc-mediated modulations. It is not clear whether cysteine 149 is directly involved, or closely associated, with the low-affinity zinc binding site(s) responsible for zinc-mediated inhibition of ASIC1b channels. Further studies are needed to distinguish between these two possibilities.
Recently, Hoagland et al., identified a third human ACCN2 transcript variant (hVariant 3; also called human ASIC1b), which is similar to rodent ASIC1b. Different from rodent ASIC1b, hVariant 3 has significant calcium permeability and shows a small sustained current in response to a drop in pH (Hoagland et al., 2010). Cysteine 149 in rat ASIC1b is comparable to cysteine 196 in hVariant 3. Therefore, it is necessary to test in future studies whether zinc has an inhibitory effect on hVariant 3 subunit. If this is the case, it would be interesting to determine whether cysteine 196 is involved in the inhibition.
Calcium is another cation with a demonstrated regulatory effect on ASICs including ASIC1b channels (Babini et al., 2002; Immke and McCleskey, 2003; Paukert et al., 2004). Paukert et al., showed that two negatively charged residues, E425 and D432, near the entrance of the channel pore, are crucial for the calcium blockade of ASIC1a currents (Paukert et al., 2004). Our present results suggest that Zn2+ and Ca2+ probably exert their effects through different binding sites on ASIC1b channels, because the extent to which zinc inhibited ASIC1b currents was not affected by increasing extracellular Ca2+ concentrations from 2 to 5 or 10 mM.
Recently, we reported that zinc inhibits ASIC3 channels with low-affinity (Jiang et al., 2010). The inhibition of ASIC1b currents by zinc shares some similarity to zinc inhibition of ASIC3 channels: (1) both inhibitions take place at micromolar concentrations and require pretreatment, but not co-application, which suggests that zinc has no effect on ASIC1b channels in their open state (Jiang et al., 2010); (2) the maximal inhibition is reached at 300 μM for both ASIC1b and ASIC3 channels; (3) the inhibition is pH and voltage independent; (4) the inhibition is dependent on extracellular site(s); (5) histidine residues are not involved in both cases. Compared to zinc inhibition of ASIC3 channels, which has a narrow concentration range (30 to 100 μM) (Jiang et al., 2010), zinc inhibits ASIC1b with a slightly larger range of concentration (10 to 100 μM). The IC50 of zinc inhibition of ASIC1b channel was approximately 26 μM. This is approximately less than half of that of ASIC3 (IC50 is 61 μM) and is within the range of physiological concentration in neurons (Smart et al., 1994; Paoletti et al., 2009).
Both ASIC1b and ASIC3 are all expressed in DRG neurons. It is widely accepted that ASIC3 channels play critical roles in pain perceptions (Benson et al., 1999; Sluka et al., 2003, 2007; Lingueglia E, 2007; Walder et al., 2010; Deval et al., 2009, 2011). However, the functional role of ASIC1b gene, especially in sensory neurons, is little known. In addition to expression of ASIC1b in sensory neurons, ASIC1b is also expressed in cerebral VSMCs as a dominant component among all ASIC genes (Chung et al., 2010). ASIC expression is required for normal VSMC migration (Grifino et al., 2008). However, whether zinc regulates the ASIC1b activity in VSMCs needs further investigation.
Although zinc inhibits ASIC1b channels at physiological relevant concentration, how endogenous zinc regulates the ASIC1b activity in sensory neuron or non-neuronal tissue is uncertain. It will be interesting to ask in future studies whether endogenous zinc has a specific effect on ASIC1b in those organisms.
Acknowledgments
We thank X.M. Zha for critical comments on the manuscript. This work was supported in part by American Heart Association Scientist Development Grant 0735092N, University of Missouri Research Board and University of Missouri-Kansas City School of Medicine Start-up Funding (X.P.C), and NIH grant R01NS047506 (Z.G.X).
Abbreviations
- ACCN2
amiloride-sensitive cation channel 2, neuronal
- ASICβ
acid-sensing ion channelβ
- ASIC1b
acid-sensing ion channel 1b
- ASICs
acid-sensing ion channels
- CNS
central nervous system
- CTRL
control
- DEPC
diethylpyrocarbonate
- DRG
dorsal root ganglia
- ECF
extracellular solution
- EC50
half maximal effective concentration
- IC50
half maximal inhibitory concentration
- hVariant 3
third human ACCN2 transcript variant
- MTSET
Methanethiosulfonate-ethyltrimethylammonium
- N-BrSuc
N-bromosuccinimide
- pH50
pH value for half maximal activation
- VSMCs
vascular smooth muscle cells
References
- Askwith CC, Wemmie JA, Price MP, Rokhlina T, Welsh MJ. Acid-sensing ion channel 2 (ASIC2) modulates ASIC1 H+-activated currents in hippocampal neurons. J Biol Chem. 2004;279:18296–18305. doi: 10.1074/jbc.M312145200. [DOI] [PubMed] [Google Scholar]
- Babini E, Paukert M, Geisler HS, Grunder S. Alternative splicing and interaction with di-and polyvalent cations control the dynamic range of acid-sensing ion channel 1 (ASIC1) J Biol Chem. 2002;277:41597–41603. doi: 10.1074/jbc.M205877200. [DOI] [PubMed] [Google Scholar]
- Baron A, Schaefer L, Lingueglia E, Champigny G, Lazdunski M. Zn2+ and H+ are coactivators of acid-sensing ion channels. J Biol Chem. 2001;276:35361–35367. doi: 10.1074/jbc.M105208200. [DOI] [PubMed] [Google Scholar]
- Baron A, Voilley N, Lazdunski M, Lingueglia E. Acid sensing ion channels in dorsal spinal cord neurons. J Neurosci. 2008;28:1498–1508. doi: 10.1523/JNEUROSCI.4975-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron A, Waldmann R, Lazdunski M. ASIC-like, proton-activated currents in rat hippocampal neurons. J Physiol. 2002;539:485–494. doi: 10.1113/jphysiol.2001.014837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bässler EL, Ngo-Anh TJ, Geisler HS, Ruppersberg JP, Gründer S. Molecular and functional characterization of acid-sensing ion channel (ASIC) 1b. J Biol Chem. 2001;276:33782–33787. doi: 10.1074/jbc.M104030200. [DOI] [PubMed] [Google Scholar]
- Benson CJ, Eckert SP, McCleskey EW. Acid-evoked currents in cardiac sensory neurons: A possible mediator of myocardial ischemic sensation. Circ Res. 1999;84:921–928. doi: 10.1161/01.res.84.8.921. [DOI] [PubMed] [Google Scholar]
- Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci USA. 2002;99:2338–2343. doi: 10.1073/pnas.032678399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, England S, Akopian AN, Wood JN. A sensory neuron specific, proton-gated ion channel. Proc Natl Acad Sci USA. 1998;95:10240–10245. doi: 10.1073/pnas.95.17.10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu XP, Close N, Saugstad JA, Xiong ZG. ASIC1a-specific modulation of acid-sensing ion channels in mouse cortical neurons by redox reagents. J Neurosci. 2006;26:5329–5339. doi: 10.1523/JNEUROSCI.0938-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu XP, Wemmie JA, Wang WZ, Zhu XM, Saugstad JA, Price MP, Simon RP, Xiong ZG. Subunit-dependent high-affinity zinc inhibition of acid-sensing ion channels. J Neurosci. 2004;24:8678–8689. doi: 10.1523/JNEUROSCI.2844-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WS, Farley JM, Swenson A, Barnard JM, Hamilton G, Chiposi R, Drummond HA. Extracellular acidosis activates ASIC-like channels in freshly isolated cerebral artery smooth muscle cells. Am J Physiol Cell Physiol. 2010;298:C1198–1208. doi: 10.1152/ajpcell.00511.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CN, Wafford KA. The Cys-loop superfamily of ligand-gated ion channels: the impact of receptor structure on function. Biochem Soc Trans. 2004;32:529–534. doi: 10.1042/BST0320529. [DOI] [PubMed] [Google Scholar]
- Deval E, Noël J, Gasull X, Delaunay A, Alloui A, Friend V, Eschalier A, Lazdunski M, Lingueglia E. Acid-sensing ion channels in postoperative pain. J Neurosci. 2011;31:6059–6066. doi: 10.1523/JNEUROSCI.5266-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deval E, Noël J, Lay N, Alloui A, Diochot S, Friend V, Jodar M, Lazdunski M, Lingueglia E. ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J. 2008;27:3047–3055. doi: 10.1038/emboj.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayyazuddin A, Villarroel A, Le Goff A, Lerma J, Neyton J. Four residues of the extracellular N-terminal domain of the NR2A subunit control high-affinity Zn2+ binding to NMDA receptors. Neuron. 2000;25:683–694. doi: 10.1016/s0896-6273(00)81070-3. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nat Rev Neurosci. 2005;6:449–462. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- Glusker JP. Structural aspects of metal liganding to functional groups in proteins. Adv Protein Chem. 1991;42:1–76. doi: 10.1016/s0065-3233(08)60534-3. [DOI] [PubMed] [Google Scholar]
- Grifoni SC, Jernigan NL, Hamilton G, Drummond HA. ASIC proteins regulate smooth muscle cell migration. Microvasc Res. 2008;75:202–210. doi: 10.1016/j.mvr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunder S, Chen X. Structure, function, and pharmacology of acid-sensing ion channels (ASICs): focus on ASIC1a. Int J Physiol Pathophysiol Pharmacol. 2010;2:73–94. [PMC free article] [PubMed] [Google Scholar]
- Harrison NL, Gibbons SJ. Zn2+: an endogenous modulator of ligand- and voltage-gated ion channels. Neuropharmacology. 1994;33:935–952. doi: 10.1016/0028-3908(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Hesselager M, Timmermann DB, Ahring PK. pH dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing ion channel subunits. J Biol Chem. 2004;279:11006–11015. doi: 10.1074/jbc.M313507200. [DOI] [PubMed] [Google Scholar]
- Hey JG, Chu XP, Seeds J, Simon RP, Xiong ZG. Extracellular zinc protects against acidosis-induced injury of cells expressing Ca2+-permeable acid-sensing ion channels. Stroke. 2007;38:670–673. doi: 10.1161/01.STR.0000251443.68897.99. [DOI] [PubMed] [Google Scholar]
- Hoagland EN, Sherwood TW, Lee KG, Walker CJ, Askwith CC. Identification of a calcium permeable human acid-sensing ion channel 1 transcript variant. J Biol Chem. 2010;285:41852–41862. doi: 10.1074/jbc.M110.171330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A, Liu Y, Xu Y, Yellen G. On the use of thiol-modifying agents to determine channel topology. Neuropharmacology. 1996;35:797–804. doi: 10.1016/0028-3908(96)00129-3. [DOI] [PubMed] [Google Scholar]
- Immke DC, McCleskey EW. Protons open acid-sensing ion channels by catalyzing relief of Ca2+ blockade. Neuron. 2003;37:75–84. doi: 10.1016/s0896-6273(02)01130-3. [DOI] [PubMed] [Google Scholar]
- Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A° resolution and low pH. Nature. 2007;449:316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Li MH, Papasian CJ, Branigan D, Xiong ZG, Wang JQ, Chu XP. Characterization of acid-sensing ion channels in medium spiny neurons of mouse striatum. Neuroscience. 2009;162:55–66. doi: 10.1016/j.neuroscience.2009.04.029. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Papasian CJ, Wang JQ, Xiong ZG, Chu XP. Inhibitory regulation of acid-sensing ion channel 3 by zinc. Neuroscience. 2010;169:574–583. doi: 10.1016/j.neuroscience.2010.05.043. [DOI] [PubMed] [Google Scholar]
- Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Review. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272:1013–1016. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- Krishtal O. The ASICs: signaling moleculars? modulators? Trends Neurosci. 2003;26:477–483. doi: 10.1016/S0166-2236(03)00210-8. [DOI] [PubMed] [Google Scholar]
- Lingueglia E. Acid-sensing ion channels in sensory perception. J Biol Chem. 2007;282:17325–17329. doi: 10.1074/jbc.R700011200. [DOI] [PubMed] [Google Scholar]
- Mathie A, Sutton GL, Clarke CE, Veale EL. Zinc and copper: pharmacological probes and endogenous modulators of neuronal excitability. Pharmacol Ther. 2006;111:567–583. doi: 10.1016/j.pharmthera.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Nevin ST, Cromer BA, Haddrill JL, Morton CJ, Parker MW, Lynch JW. Insights into the structural basis for zinc inhibition of the glycine receptor. J Biol Chem. 2003;278:28985–28992. doi: 10.1074/jbc.M300097200. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Ascher P, Neyton J. High-affinity zinc inhibition of NMDA NR1-NR2A receptors. J Neurosci. 1997;17:5711–5725. doi: 10.1523/JNEUROSCI.17-15-05711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Vergnano AM, Barbour B, Casado M. Zinc at glutamatergic synapses. Neuroscience. 2009;158:126–136. doi: 10.1016/j.neuroscience.2008.01.061. [DOI] [PubMed] [Google Scholar]
- Paukert M, Babini E, Pusch M, Gründer S. Identification of the Ca2+ blocking site of acid-sensing ion channel (ASIC) 1: implications for channel gating. J Gen Physiol. 2004;124:383–394. doi: 10.1085/jgp.200308973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister Y, Gautschi I, Takeda AN, van Bemmelen M, Kellenberger S, Schild L. A gating mutation in the internal pore of ASIC1a. J Biol Chem. 2006;281:11787–11791. doi: 10.1074/jbc.M513692200. [DOI] [PubMed] [Google Scholar]
- Poirot O, Berta T, Decosterd I, Kellenberger S. Distinct ASIC currents are expressed in rat putative nociceptors and are modulated by nerve injury. J Physiol. 2006;576:215–234. doi: 10.1113/jphysiol.2006.113035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seebungkert B, Lynch JW. A common inhibitory binding site for zinc and odorants at the voltage-gated K+ channel of rat olfactory receptor neurons. Eur J Neurosci. 2001;14:353–362. doi: 10.1046/j.0953-816x.2001.01646.x. [DOI] [PubMed] [Google Scholar]
- Sherwood TW, Askwith CC. Dynorphin opioid peptides enhance acid-sensing ion channel 1a activity and acidosis-induced neuronal death. J Neurosci. 2009;29:14371–14380. doi: 10.1523/JNEUROSCI.2186-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106:229–239. doi: 10.1016/S0304-3959(03)00269-0. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Radhakrishnan R, Benson CJ, Eshcol JO, Price MP, Babinski K, Audette KM, Yeomans DC, Wilson SP. ASIC3 in muscle mediates mechanical, but not heat, hyperalgesia associated with muscle inflammation. Pain. 2007;129:102–112. doi: 10.1016/j.pain.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart TG, Xie X, Krishek BJ. Modulation of inhibitory and excitatory amino acid receptor ion channels by zinc. Prog Neurobiol. 1994;42:393–441. doi: 10.1016/0301-0082(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Ugawa S, Ishida Y, Ueda T, Yu Y, Shimada S. Hypotonic stimuli enhance proton-gated currents of acid-sensing ion channel-1b. Biochem Biophys Res Commun. 2008;367:530–534. doi: 10.1016/j.bbrc.2007.12.096. [DOI] [PubMed] [Google Scholar]
- Ugawa S, Inagaki A, Yamamura H, Ueda T, Ishida Y, Kajita K, Shimizu H, Shimada S. Acid-sensing ion channel-1b in the stereocilia of mammalian cochlear hair cells. Neuroreport. 2006;17:1235–239. doi: 10.1097/01.wnr.0000233093.67289.66. [DOI] [PubMed] [Google Scholar]
- Walder RY, Rasmussen LA, Rainier JD, Light AR, Wemmie JA, Sluka KA. ASIC1 and ASIC3 play different roles in the development of Hyperalgesia after inflammatory muscle injury. J Pain. 2010;11:210–218. doi: 10.1016/j.jpain.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem. 1997a;272:20975–20978. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997b;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Lazdunski M. H+-gated cation channels: neuronal acid sensors in the ENaC/DEG family of ion channels. Curr Opin Neurobiol. 1998;8:418–424. doi: 10.1016/s0959-4388(98)80070-6. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Askwith CC, Lamani E, Cassell MD, Freeman JH, Jr, Welsh MJ. Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J Neurosci. 2003;23:5496–5502. doi: 10.1523/JNEUROSCI.23-13-05496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29:578–586. doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Duan B, Mei YD, Gao J, Chen JG, Zhuo M, Xu L, Wu M, Xu T-L. Characterization of acid-sensing ion channels in dorsal horn neurons of rat spinal cord. J Biol Chem. 2004;279:43716–43724. doi: 10.1074/jbc.M403557200. [DOI] [PubMed] [Google Scholar]
- Xiong ZG, Pignataro G, Li M, Chang SY, Simon RP. Acid-sensing ion channels (ASICs) as pharmacological targets for neurodegenerative diseases. Curr Opin Pharmacol. 2008;8:25–32. doi: 10.1016/j.coph.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wemmie JA, Price M, Welsh MJ, Simon RP. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Xu TL, Xiong ZG. Dynamic regulation of acid-sensing ion channels by extracellular and intracellular modulators. Curr Med Chem. 2007;14:1753–1763. doi: 10.2174/092986707781058977. [DOI] [PubMed] [Google Scholar]
- Zatta P, Drago D, Bolognin S, Sensi SL. Alzheimer’s disease, metal ions and metal homeostatic therapy. Trends Pharmacol Sci. 2009;30:346–355. doi: 10.1016/j.tips.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Zha XM, Costa V, Harding AM, Reznikov L, Benson CJ, Welsh MJ. ASIC2 subunits target acid-sensing ion channels to the synapse via an association with PSD-95. J Neurosci. 2009;29:8438–8446. doi: 10.1523/JNEUROSCI.1284-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha XM, Wemmie JA, Green SH, Welsh MJ. Acid-sensing ion channel 1a is a postsynaptic proton receptor that affects the density of dendritic spines. Proc Natl Acad Sci U S A. 2006;103:16556–16561. doi: 10.1073/pnas.0608018103. [DOI] [PMC free article] [PubMed] [Google Scholar]