Abstract
The realization of epidermal chemical sensing requires a fabrication methodology compatible with the non-planarity and irregularities of the human anatomy. This Communication describes the development of printed temporary transfer tattoo (T3) electrochemical sensors for physiological and security monitoring of chemical constituents leading towards the demonstration of ‘electronic skin’.
The advent of hybrid fabrication techniques has recently resulted in the demonstration of advanced electronic devices that can be mated directly with the skin for the measurement of physiological parameters including heart rate, temperature, and brain activity.1a–d In order to augment this capability, the analysis of the chemical constituents residing on the surface of the skin can provide useful insight into the overall health of the individual2a,b and their exposure to chemical agents/hazards residing in their local environment3a–c. While flexible screen-printed electrochemical sensors have been proposed recently3b,4, these devices cannot easily be attached to the body owing to incompatible elasticity between the substrate and the skin, thereby precluding them from direct epidermal integration. Recognizing this limitation as a fabrication challenge, the adaptation of printable electrodes to direct sensing on the skin would require a different fabrication methodology, leading to devices able to conform to the non-planar features and surface irregularities that are characteristic of the human anatomy.
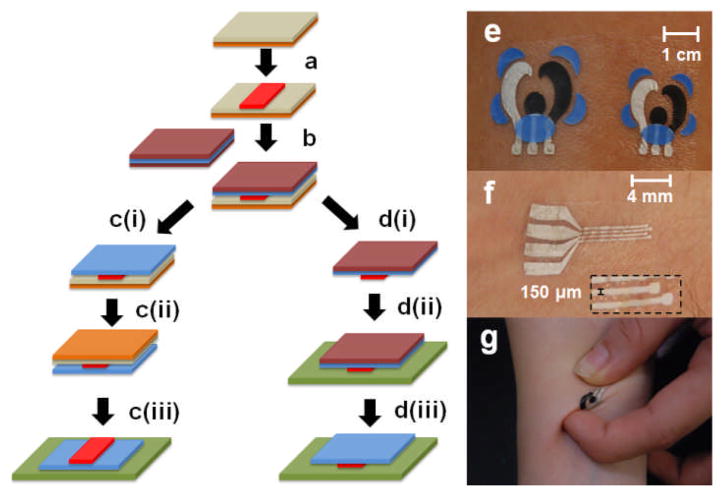
This Communication reports on the first example of a functioning electrode system based on temporary transfer on-skin tattoos, realized by a judicious integration of tattoo-transfer and thick-film fabrication protocols. Fig. 1 (left) illustrates the methodology employed for sensor fabrication and its subsequent transfer onto the skin for electrochemical sensing. A representation of the steps involved in the transfer of sensor onto the epidermis is provided in Figs. S1a–d (ESI†). The novel approach thus yields body-worn electrochemical sensors that are compliant with the skin for the realization of non-invasive chemical monitoring. Nearly any artistic tattoo design can be implemented, allowing the sensors to be concealed in rather “inconspicuous” tattoo artwork, without compromising the favorable resolution and performance inherent to printable sensors. Figs. 1e and f exhibit various T3 designs applied to the epidermis and substantiates that well-defined patterns possessing micrometer-scale resolution can be realized with the fabrication methodology. Similarly, Fig. 1g illustrates the twisting operation applied to a T3 sensor. Such fabrication of tattoo-based electrochemical systems has broad implications beyond skin-based chemical sensing.
Fig. 1.
Protocol involved in the formation of T3 electrochemical sensors (left). Step (a): The electrode design (in red) is patterned on the release agent-coated (olive) base paper (orange) via screen printing. Step (b): The adhesive sheet (blue) with protective coating (maroon) is applied to the printed electrochemical sensor. Step (c): The protective sheet is removed (i), the tattoo is flipped and applied to the skin (green) and dabbed with water (ii), and the release agent-coated base paper is removed, thereby exposing the adhered sensor pattern to the wearer’s external environment for remote sensing. In the case that physiological monitoring is desired, the routine illustrated in (d) is followed: the T3 paper is removed (i) and the tattoo pattern is applied to the skin (ii); the protective coating is then removed (iii). (e) – (g) Various T3 sensor designs on human skin; g illustrates the application of twisting. The T3 sensors are subsequently interfaced with a handheld 3-electrode potentiostat (CH Instruments 1232B) via pressure contact in conjunction with a medical-grade skin adhesive. For further integration, a self-powered micropotentiostat (a 2.6 g, 19 × 19 mm)5 can be mated with the electrode via an adhesive patch and comfortably worn on the skin for extended operation.
In order to counteract cracking and alleviate mechanical degradation associated with routine skin-based wear, carbon fiber (CF) segments are dispersed within the tattoo ink, hence augmenting the electrode’s tensile strength and providing an interlinked conductive backbone while enhancing the electrochemical behavior, reflecting the properties of the CF constituents.6a–d Details regarding T3 sensor fabrication and CF-reinforced ink formulation are discussed in ESI†.
To validate the structural resiliency of the T3 sensors to extreme mechanical deformation, various strain permutations were applied to the sensors onto human skin. By harnessing CF-dispersed inks for mechanical reinforcement, the sensor exhibits noteworthy resiliency against deformation such as repeated pinching, bending, and twisting. The resulting wearable epidermal sensing devices thus couple favorable substrate-skin elasticity along with high-fidelity electrochemical performance. Such resiliency of the T3 sensor against severe deformation has been illustrated when pinched with the forefingers (Fig. S2 a), upon stretching the skin (Fig. S2 b), and upon twisting operation (Fig. 1g). As can be observed, the application of these strain permutations exhibited minimal effect on the appearance of the T3 sensor. Further studies, discussed below, investigated the impact of such strain permutations upon the electrochemical performance of these printable epidermal sensors.
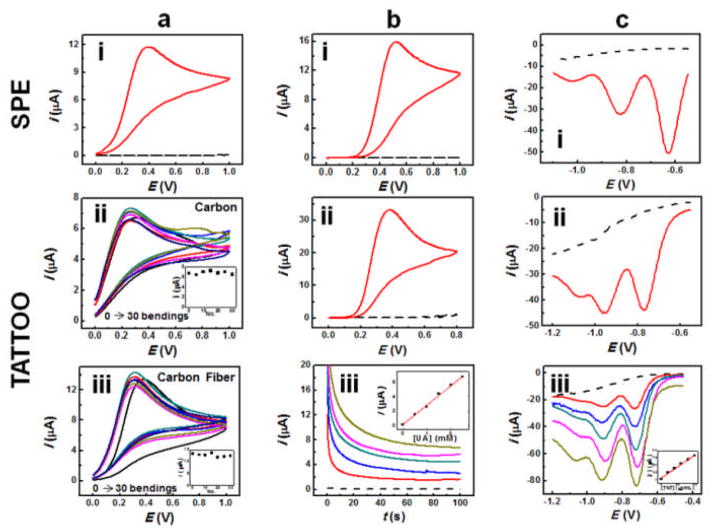
Electrochemical characterization of the T3 sensing methodology was aimed at comparing this new sensing paradigm with conventional screen printed electrodes (SPEs) on solid alumina substrates. Accordingly, cyclic voltammograms (CVs) were contrasted between the two systems, and a GORE-TEX fabric was used for the tattoo investigations in order to emulate the viscoelastic properties of the epidermis3a in connection with spiked buffer samples applied to the T3 surface. Fig. 2a (left column) compares the CVs obtained for 2.5 mM ascorbic acid (AA) at an SPE (i), a T3 on GORE-TEX (ii), and a CF-reinforced T3 on GORE-TEX (iii). As is evident from the figures provided, the T3 sensor embodied favorable electrochemical properties when compared with the conventional SPE. As an additional benefit, the incorporation of CFs into the ink matrix enhanced the electrochemical response of the tattoo device notably, leading to better-defined oxidation peaks that emulated the response obtained at the conventional SPE. Moreover, Figs. 2a(ii) and (iii) (center column) depict the voltammetric response of the CF-reinforced and unreinforced T3 sensors under severe repetitive bending operations. Fig. 2b illustrates the CV response obtained for the detection of 2.5 mM uric acid (UA) at a SPE (i) and the CF-reinforced T3 sensor on porcine skin (ii). Furthermore, the amperometric response generated at the CF-reinforced T3 electrochemical sensor for increasing UA concentration is displayed in Fig. 2b(iii). A highly linear calibration is observed at the skin-based electrode, corroborating its use not only as a viable alternative to SPEs but also as an epidermal sensor.
Fig. 2.
(a) CV of AA on (i) SPE; (ii) unreinforced T3 sensor on GORE-TEX; (iii) CF-reinforced T3 sensor on GORE-TEX. (b) CV of UA on (i) SPE; (ii) CF-reinforced T3 sensor (porcine skin); (iii) amperometry on CF-reinforced T3 sensor (porcine skin); (c) SWV on (i) SPE; (ii) and (iii) CF-reinforced T3 sensor (porcine skin).
The extension of the tattoo sensing paradigm to the identification of hazards present in the vicinity of the wearer is of noteworthy importance in the environmental and security monitoring fields. Accordingly, the concept was extended to the detection of the common explosive 2,4,6-trinitrotoluene (TNT), in connection with square wave voltammetry (SWV). Fig. 2c (left column) illustrates the SWV response obtained for the detection of 25 μg mL−1 TNT at an SPE (i) and the CF-reinforced T3 sensor on porcine skin (ii). Both (i) and (ii) exemplify a well-defined TNT response, which substantiates that the T3 electrochemical sensors contend with the performance offered by well-established SPEs fabricated on solid supports. The SWV response of the epidermal sensor for increasing TNT concentrations is also well-defined (Fig. 2c(iii)) and highly linear (inset). The T3 sensors were also evaluated for the detection of 2,4-dinitrotoluene (DNT) vapors on porcine skin (Fig. S4).
The investigation of the fundamental electrical properties of the T3 sensor is imperative for assessing its utility for integration with epidermal electronics1a. For this reason, the resistive and impedance profile were investigated under the application of mechanical deformation. Both tattoo sensors exhibited repeatable electrochemical performance following several dozen bending iterations (provided in Figs. S3), the unreinforced electrode trace rapidly increased in its intrinsic resistance until catastrophic failure occurred at the 100th bending iteration. Conversely, the CF-reinforced electrode, although possessing a slightly elevated initial resistance (~25 Ω), maintained its conductivity even following over 350 bending repetitions, reflecting its ability to withstand highly-repetitive mechanical deformation and underscores its suitability for epidermal integration(Fig. S3 a–c).
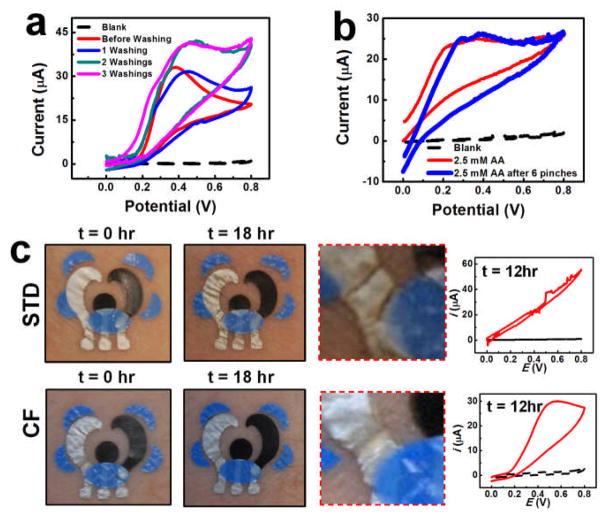
To validate the integrity of the sensing paradigm against chemical and mechanical degradation due to natural skin flexion, the T3 sensors were subjected to repetitive washing cycles (twash = 5 s with hand soap) to emulate hand-washing or bathing. Fig. 3a illustrates the effect of repetitive washing cycles upon the CV generated at the tattoo biosensor (on porcine skin) using 2.5 mM UA. Although washing did impart relatively minor degradation in the response, well-defined peaks for UA oxidation can be observed from the figure. An increase in the oxidation current is observed following washing, reflecting the exposure of a larger active electrode area. Such changes are not of major concern since these devices are low cost and easily replaceable. Further extending the T3 sensor to its performance limits, the effect of repetitive pinching of the tattoo pattern was investigated in greater detail. A CF-reinforced T3 sensor was mated to porcine skin and repetitively pinched for 2 s intervals. Fig. 3b displays the electrochemical response of the sensor after repetitive pinching operations with 2.5 mM AA. The data indicate that repeated pinching of the sensor produced minimal degradation in the electrochemical performance. Both the peak current and peak potential remained stable throughout these experiments, thereby providing evidence that the CF-reinforced electrochemical sensor is capable of high-fidelity electroanalytical operation under the severe demands imparted by epidermal wear.
Fig. 3.
CVs of UA generated at a CF-reinforced T3 sensor on porcine skin following (a) washing and (b) pinching. (C) Photographic and electrochemical evaluation of an unreinforced and CF-reinforced T3 sensor on human skin.
In addition to the previous studies, a visual examination of sensor lifetime over extended durations of routine wear was performed immediately following the application of the T3 sensors and subsequent to 18 hrs of continuous human epidermal wear (Fig. 3c). A close inspection of the images (right column) reveals that cracking, especially at the Ag/AgCl-insulator and carbon-insulator interfaces, occurred at the unreinforced T3 sensor following such prolonged epidermal wear. On the other hand, the CF-reinforced sensor exhibited no outward signs of degradation over this extended period. A CV for 0.5 mM UA was recorded following 12 hrs of continuous wear of both the unreinforced and CF-reinforced sensors (Fig. 3c (right)). As is clearly observed, the CV recorded at the unreinforced sensor exhibited substantial distortion, compared to the well-defined anodic UA oxidation peak visible at the CF-reinforced T3 sensor. A comparison with the CVs obtained from previous experiments at unperturbed electrodes supports the sensor’s ability to yield high-fidelity electroanalytical performance over extended wear.
We have demonstrated the first example of tattoo-based electrodes, which exhibit attractive electroanalytical performance. The realization of a new class of electrochemical sensors and biosensors for direct skin-based monitoring of relevant compounds of physiological and security importance has leveraged conventional and widely-deployed printing techniques in conjunction with commercially-available temporary transfer tattoo paper. Dispersing CF segments within the inks results in sensors that embody high tolerance against severe mechanical deformation typically encountered during epidermal wear. Moreover and most importantly, the T3 sensors exhibit equivalent electrochemical performance to their screen-printed counterparts. A distinct advantage of the technique resides in the ability of the designer to harness their creativity and conceal their sensors in artistic tattoo patterns. The new epidermal electrochemical system has broad implications beyond the scope of analytical devices and can facilitate diverse new applications. Further work will be concerned with the functionalization of the T3 electrodes with enzymes and ionophores to impart high recognition and improved selectivity towards target bioanalyes or electrolytes. Future research thrusts will endeavor to address the full integration of these tattoo-based electrochemical sensors with wearable electronics (potentiostatic control, wireless data transfer, and energy harvesting) in order to realize fully-functional ‘electronic skin’, opening a plethora of diverse applications where true bionic integration is a core requirement.
Supplementary Material
Acknowledgments
This work was supported by the ONR (N00014-08-1-1202) and the NSF (CBET-1066531). A.G.M. acknowledges support from the NIH under the Initiative for Maximizing Student Diversity. Thanks are due to Prof. R. Narayan (UNC/NCSU) for graciously providing the porcine skin samples.
Footnotes
Electronic Supplementary Information (ESI) available: See DOI: 10.1039/b000000x/
References
- 1.(a) Kim DH, Lu N, Ma R, Kim YS, Kim RH, Wang S, Wu J, Won SM, Tao H, Islam A, Yu KJ, Kim T, Chowdhury R, Ying M, Xu L, Li M, Chung HJ, Keum H, McCormick M, Liu P, Zhang YW, Omenetto FG, Huang Y, Coleman T, Rogers JA. Science. 2011;333:838–843. doi: 10.1126/science.1206157. [DOI] [PubMed] [Google Scholar]; (b) Rogers JA, Huang Y. P Natl Acad Sci USA. 2009;106:10875–10876. doi: 10.1073/pnas.0905723106. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Cheng H, Wu J, Li M, Kim DH, Kim YS, Huang Y, Kang Z, Hwang KC, Rogers JA. Appl Phys Lett. 2011;98:061902. [Google Scholar]; (d) Ma R, Kim DH, McCormick M, Coleman T, Rogers JA. Conf Proc IEEE Eng Med Biol Soc. 2010:6405–6408. doi: 10.1109/IEMBS.2010.5627315. [DOI] [PubMed] [Google Scholar]
- 2.(a) Diamond D, Coyle S, Scarmagnani S, Hayes J. Chem Rev. 2008;108:652–679. doi: 10.1021/cr0681187. [DOI] [PubMed] [Google Scholar]; (b) Coyle S, Yanzhe W, Lau KT, De Rossi D, Wallace G, Diamond D. MRS Bull. 2007;32:434–442. [Google Scholar]
- 3.(a) Chuang MC, Windmiller JR, Santhosh P, Ramirez GV, Galik M, Chou TY, Wang J. Electroanal. 2010;22:2511–2518. [Google Scholar]; (b) Malzahn K, Windmiller JR, Ramirez GV, Schöning MJ, Wang J. Analyst. 2011;136:2912–2917. doi: 10.1039/c1an15193b. [DOI] [PubMed] [Google Scholar]; (c) Romolo FS, Margot P. Forensic Sci Int. 2001;119:195–211. doi: 10.1016/s0379-0738(00)00428-x. [DOI] [PubMed] [Google Scholar]
- 4.Yang YL, Chuang MC, Lou SL, Wang J. Analyst. 2010;135:1230–1234. doi: 10.1039/b926339j. [DOI] [PubMed] [Google Scholar]
- 5.Windmiller JR, Santhosh P, Katz E, Wang J. Sensor Actuat B. 2011;155:206–213. [Google Scholar]
- 6.(a) Tibbetts GC, McHugh JJ. J Mater Res. 1999;14:2871–2880. [Google Scholar]; (b) Gutowski TG, Dillon G. J Compos Mater. 1992;26:2330–2347. [Google Scholar]; (c) Wang J, Lu J, Hocevar SB, Farias PAM. Anal Chem. 2000;72:3218–3222. doi: 10.1021/ac000108x. [DOI] [PubMed] [Google Scholar]; (d) Wang J, Angnes L. Anal Chem. 1992;64:456–459. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.