Abstract
Aims
Several bacteriocins (BCNs) that were identified from chicken commensal bacteria dramatically reduced Campylobacter colonization in poultry and are being directed toward on-farm control of this important foodborne human pathogen. A recent study has shown that BCN resistance in C. jejuni is very difficult to develop in vitro. In this study, in vivo development and stability of BCN resistance in Campylobacter was examined.
Methods and Results
Chickens infected with C. jejuni NCTC 11168 were treated with BCN E-760 at the dose of 5 mg/kg body weight/day via oral gavages for three consecutive days, which selected BCN-resistant (BCNr) mutants in the treated birds. However, all the in vivo-selected mutants only displayed low-levels of resistance to BCN (MIC = 2–8 mg/L) when compared to parent strain (MIC = 0.5 mg/L). Inactivation of CmeABC efflux pump of the BCNr mutants led to increased susceptibility to BCN (8–32 fold MIC reduction). Three different BCNr Campylobacter strains (in vitro- or in vivo-derived) were examined for the stability of BCN resistance using both in vitro and in vivo systems. The low-level of BCN resistance in these strains was not stable in vitro or in vivo in the absence of BCN selection pressure.
Conclusions
Usage of BCN E-760 only selected low-level BCNr C. jejuni mutants in vivo and the low-level BCN resistance was not stable in vitro and in vivo.
Significance and Impact of the Study
The study provides helpful information for risk assessment of the future practical application of the anti-Campylobacter BCNs in animals.
Keywords: Campylobacter, bacteriocin, resistance
INTRODUCTION
Campylobacter species, the epsilon class of proteobacteria, are the leading bacterial causes of human gastroenteritis in developed countries (Allos, 2001). In addition to watery diarrhea and/or hemorrhagic colitis, infection with Campylobacter spp can result in post-infectious manifestations such as Guillain Barre syndrome, an acute immune mediated disorder that may lead to respiratory muscle compromise and death (Nachamkin et al., 1998). Campylobacter spp are considered to be commensal organisms in the intestinal tracts of wild and domestic animals including chickens and other avian species (Diker et al., 2000). Epidemiological studies demostrated that consumption of contaminated poultry meat is the major cause of human campylobacteriosis (Stern et al., 2001, Stern et al., 2004). Thus, on-farm control of Campylobacter spp could reduce the risk of human Campylobacter infections. Of the several proposed strategies to reduce this risk, anti-Campylobacter bacteriocins (BCNs) are considered a promising strategy to protect food safety and public health (Lin, 2009).
BCNs are short cationic antimicrobial peptides (AMPs) naturally produced by diverse microbes in different environments (Willey and van der Donk, 2007). Despite significant structural and characteristic differences, BCNs display potent antimicrobial activities against a wide range of viruses, bacteria, and fungi and have been recognized as a novel class of antimicrobials to control food borne pathogens (Settanni and Corsetti, 2008, Zasloff, 2002, Hugas et al., 1998, Galvez et al., 2007). As a group of naturally nontoxic antimicrobials, some BCNs, such as nisin, have long been applied for food preservation (Willey and van der Donk, 2007). Many bacteria including intestinal commensals could make at least one bacteriocin (Klaenhammer, 1988, Riley and Gordon, 1992). Therefore, the intestinal BCN-producing bacteria may achieve a competitive advantage and function as innate barriers against pathogens in the hosts. In addition, the natural and low-toxic BCNs have been proposed as promising candidates for novel antimicrobials against microbial infections (Joerger, 2003, Asaduzzaman and Sonomoto, 2009). Several anti-Campylobacter BCNs have been isolated and characterized from chicken commensal bacteria, which includes OR-7 from Lactobacillus salivarius (Stern et al., 2006), E-760 and E50–52 from Enterococcus faecium (Line et al., 2008b, Svetoch et al., 2008), and bacillocins from Paenibacillus polymyxa (Stern et al., 2005). Animal studies have demonstrated that these BCNs greatly reduced C. jejuni colonization in chicken intestine. Therefore, these natural anti-Campylobacter BCNs are being developed for on-farm control of Campylobacter to protect public health.
To develop the BCN-based intervention strategy against Campylobacter, several important issues (e.g. BCN resistance development, mechanism, stability) need to be addressed for future regulatory approval and public acceptability. Recently, we examined prevalence, development, and molecular mechanisms of BCN resistance in Campylobacter using molecular and genomic approaches (Hoang et al., 2011). In this study, susceptibilities of 137 C. jejuni and 20 C. coli isolates to the anti-Campylobacter BCNs OR-7 and E-760 were examined. Only one C. coli strain displayed resistance to the BCNs (MIC = 64mg/L) while others were susceptible with MIC ranging from 0.25 to 4 mg/L. The C. coli mutants resistant to BCN OR-7 also were obtained by in vitro selection but all displayed only low-level resistance to OR-7 (MIC = 8 to 16 mg/L). We also observed that in vitro BCN resistance in C. jejuni is very difficult to develop. However, it is still unknown if usage of bacteriocins will promote the emergence of BCN-resistant (BCNr) Campylobacter mutants in vivo. In addition, it is unclear if the BCNr Campylobacter can persist in the absence of selective pressure. To address these questions, in this study, in vivo emergence of BCNr Campylobacter was examined using a chicken model system and the stability of BCN resistance was determined using both in vitro and in vivo systems. This study provides helpful information for risk assessment of the future practical application of the anti-Campylobacter BCNs in poultry.
MATERIALS AND METHODS
Bacteriocin, bacterial strains and growth conditions
The E-760 was purified from E. faecium NRRL B-30745 as described in a recent publication (Line et al. 2008), which includes two general purification steps: 1) crude E-760 (~9% purity) preparation from the supernatant using ammonium sulfate precipitation, and 2) E-760 purification from the crude preparation using two different chromatography columns which finally results in E-760 purity up to 98.8%. The E-760 was dissolved in sterile distilled H2O and stored at −20°C prior to use. The amino acid sequences of E-760 (62 aa residues) was consistent with class IIa bacteriocins based on its N-terminal region of the peptide and the E-760 were also resistant to high temperature (e.g. 100 °C, 5 min) and a wide pH range (e.g. 5.0–8.7) (Line, Svetoch et al. 2008).
C. jejuni NCTC 11168, a BCN sensitive (BCNs) strain (E-760 MIC = 0.5 mg/L), was purchased from NCTC and was used as a parent strain in this study for selecting BCNr mutants in vitro. JL106, a natural BCNr C. coli strain isolated from human (E-760 MIC = 64 mg/L), was examined in a recent study (Hoang et al, 2011) and was also used for stability testing in this study. JL 341, a BCNr mutant (E-760 MIC = 8 mg/L) derived from NCTC 11168 by natural transformation using genomic DNA of C. coli JL106 (Hoang et al, 2011), was also chosen for stability testing in this study. The Campylobacter strains were grown routinely on MH agar plates or in MH broth at 42 °C under microaerobic conditions generated by using CampyGen Plus gas pack (Oxoid, Lenxa, KS) in an enclosed jar. Campylobacter-specific growth supplements and selective agents (SR084E and SR117E; Oxoid) were added to the media when needed. When required, the MH media were also supplemented with various amounts of E-760.
In vivo development of BCN resistance in Campylobacter
The in vivo development of BCN resistance in Campylobacter was examined using a chicken model system. In this experiment, day-old broiler chicks (a kind gift from commercial company Hubbard Hatchery, Pikeville, TN) were randomly assigned to either treatment (10 chicks) or control groups (10 chicks). All birds were placed in sanitized wire cages with unlimited access to feed and water. Nutritionally complete feed was prepared in the feed mill at the Johnson Animal Research and Teaching Unit. Prior to inoculation with C. jejuni NCTC 11168, all birds were confirmed to be free of Campylobacter by culture of cloacal swabs. However, these birds have not been tested to examine which BCNs are present naturally in the intestine. At 2 days of age, all birds in treatment and control groups were inoculated with fresh C. jejuni NCTC 11168 cultures (107 CFU/bird) via oral gavages. For the treatment group, at 9 days of age when each bird was fully colonized by the C. jejuni 11168, all birds were treated with BCN E-760 at the dose of 5 mg/kg body weight/day via oral gavages for three consecutive days. Birds in the control group were gavaged with water. Cloacal swabs were collected from all birds in both groups at day 1, 2, 3, 5, and 7 after the initial BCN treatment. Samples from each bird were spread onto MH agar selective plates containing 8 mg/L of E-760 to select for in vivo emerged BCNr mutants. Individual colonies from BCN E-760 containing plates were randomly selected to identify level of BCN E-760 resistance using MIC testing as described below. Multiple isolates with different E-760 MICs were analyzed by PCR to confirm their genetic identities. The PCR was done using primers specific for the cmp gene encoding the major outer membrane protein as previously described by (Huang et al., 2005) which revealed no difference between input strain and output isolates.
In vitro stability of BCN resistance
Three BCNr mutants were examined for the in vitro stability of acquired BCN resistance, which include the human clinical isolate JL106, the in vitro-derived mutant JL341, and the in vivo-selected mutant K58 (E-760 MIC = 8 mg/L) obtained from the above chicken experiment using C. jejuni NCTC 11168 as a parent strain. Briefly, the three strains were inoculated in BCN-free MH broth and grown under microaerobic conditions at 42°C. The Campylobacter cultures were sub-cultured every 2 days in fresh MH broth (1:400 dilutions) for 70 days in the absence of any antimicrobials. Following passages 10, 15, 20, 25, 30, and 35, the cultures were serially diluted (10-fold dilutions) in MH broth and plated onto both MH agar plates and MH agar plates supplemented with E-760 at a concentration of 8 mg/L. The plates were then incubated under microaerobic conditions at 42°C for two days. The total numbers of colonies on each type of plates were counted and compared at each time point. In addition, for passage 35, 20 colonies for each mutant were randomly selected from BCN-free MH agar plates and were subjected to MIC testing.
In vivo stability of BCN resistance using a chicken model system
The same BCN resistant mutants (JL106, JL341, and K58) were used for in vivo stability test using chicken model system. Bird source and maintenance were the same as those used in the in vivo development of BCN resistance experiment described above. Forty one-day old chicken were randomly assigned into four groups (10 chickens each group). Prior to inoculation with Campylobacter, all birds were confirmed free of Campylobacter by cultured cloacal swabs. Birds in each group received corresponding Campylobacter strain at a dose of 107 CFU/bird via oral gavages at 3 days old. Birds in control group were inoculated with BCNs C. jejuni NCTC11168. Birds in the other three treatment groups were inoculated with JL106, JL341, and K58, respectively. Birds in all groups received BCN-free feed and water throughout the trial. Cloacal swabs were collected from birds in all groups at day 6, 22, and 42 after Campylobacter inoculation. Samples from each bird were serially diluted and spread onto two different types of MH agar plates to recover total C. jejuni 11168 populations (normal selective plates), and BCNr populations (selective plates containing 8 mg/L of E-760). Campylobacter colonies were enumerated following 48 hours of incubation at 42°C under microaerobic conditions. At each time point, representative colonies from chicken in each group were chosen for E-760 MIC test.
The detection limit of the plating method was 100 CFUg−1 of feces. The significant difference in Campylobacter colonization levels (log10 transformed CFUg−1 of feces) at each sampling point between groups was calculated using Student’s t test. A P-value of <0.05 was considered significant.
E-760 susceptibility test
The susceptibilities of Campylobacter strains to BCN E-760 was determined by standard microtitre broth dilution method in MH broth with an inocula of 106 bacterial cells per mL as described by Jorgensen and Turnidge (2003). Minimum inhibitory concentration (MIC) was determined by the lowest concentration of E-760 showing complete inhibition of Campylobacter growth after 24 hours of incubation at 42°C. Duplicate experiments were performed to confirm the consistency of MIC results.
Inactivation of the cmeB gene in E-760 resistant mutants
Chromosomal DNA was isolated from a cmeB mutant (Lin et al., 2002) using the Wizard Genomic Purification Kit (Promega) according to the manufacturer’s instructions. The insertional mutation of cmeB in the extracted genomic DNA was transferred to the in vivo-selected E-760 resistant mutants by natural transformation. Natural transformation (biphasic method) was performed following standard procedure (Davis et al., 2008).
RESULTS
Effect of BCN E-760 treatment on the emergence of E-760 resistant Campylobacter in chickens
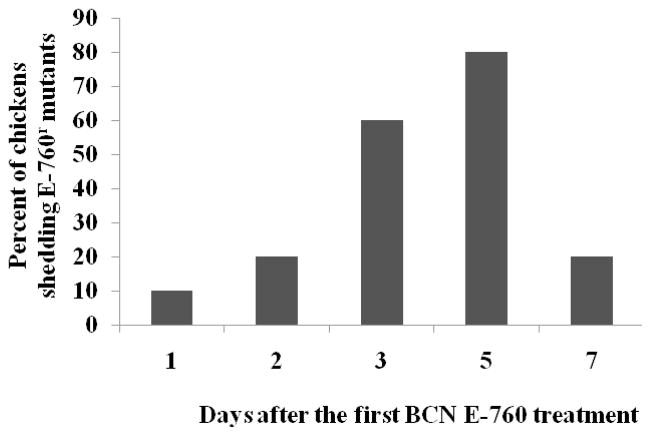
All chickens in both groups were successfully colonized by C. jejuni NCTC 11168 prior to E-760 treatment at 9 days of age. BCNr mutants were emerged in only one chicken one day after the first E-760 treatment (Fig. 1). Total 80% of chickens were observed to shed E-760r mutants at 5 days after the first treatment (Fig 1). However, BCNr mutants were soon cleared in majority of chickens (80%) after cessation of E-760 treatment (7 days after the first treatment), strongly suggesting the instability of BCN resistance in vivo. MIC test of 17 randomly selected E-760r resistant mutants indicated that all the selected mutants only displayed low-level resistance to E-760 with MIC ranging from 2 mg/L to 8 mg/L. No E-760r mutants were selected on the selective plates containing 8 mg/L E-760 for the fecal samples from all chickens in control group.
Figure 1.
Effect of BCN E-760 usage on the emergence of E-760 resistant C. jejuni. Each bar represents the percentage of chickens that shed E-760 resistant mutants in the treatment group.
CmeABC multidrug efflux system contributes to in vivo acquired BCN resistance
Our recent study (Hoang et al., 2011) has shown that the multidrug efflux pump CmeABC contributed to both intrinsic and in vitro acquired BCN resistance in Campylobacter. In this study, we examined the role of CmeABC in the in vivo acquired BCN resistance using the mutants obtained from above chicken experiment. As shown in Table 1, regardless of resistance level, inactivation of cmeB significantly reduced E-760 MIC of all mutants to the level of 0.125 mg/L, which is also lower than the MIC level of wild-type parent strain 11168 for E-760 (Table 1).
Table 1.
E-760 MICs of the in vivo-selected BCNr C. jejuni isolates and their isogenic cmeB mutants
| Strain | E-760 MIC (mg/L)a | E-760 MIC of isogenic cmeB mutant(mg/L) |
|---|---|---|
| C. jejuni NCTC 11168 | 0.5 | 0.125 |
|
| ||
| E-760r mutants selected in vivo | 2 (3) | 0.125 |
| 4 (4) | 0.125 | |
| 8 (10) | 0.125 | |
Numbers in parentheses indicate the total number of isolates corresponding to each MIC.
E-760 resistance is not stable in vitro
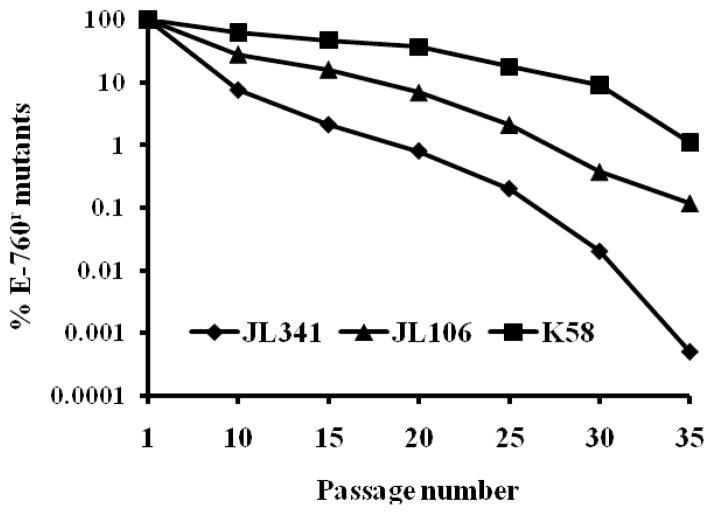
As shown in Fig 2, less than 10% of JL341 populations could be still selected on MH agar plates containing 8 mg/L of E-760 after 10 passages in the absence of E-760 selective pressure. Following 35 passages, only a very small population of JL341 (0.0005%) were recovered on the E-760-containing plates. Although JL106 and K58 showed higher stability than JL341 in vitro, less than 0.1% and 1% of JL106 and K58, respectively, were selected on the E-760-containing plates after 35 passages. Consistent with the result from differential plating, MIC tests of randomly selected colonies (20 for each mutant) after 35 passages showed all strains displayed significantly lower MIC for E-760 MIC (≤ 1 mg/L) than parent strain JL341.
Figure 2.
Stability of BCN E-760 resistance in vitro. Three strains were passed in MH broth without E-760 selection pressure as described in Materials and Methods. The percentage of BCNr population was calculated based on differential plating using plates containing 8 mg/L of BCN E-760.
in vivo instability of E-760 resistance in Campylobacter
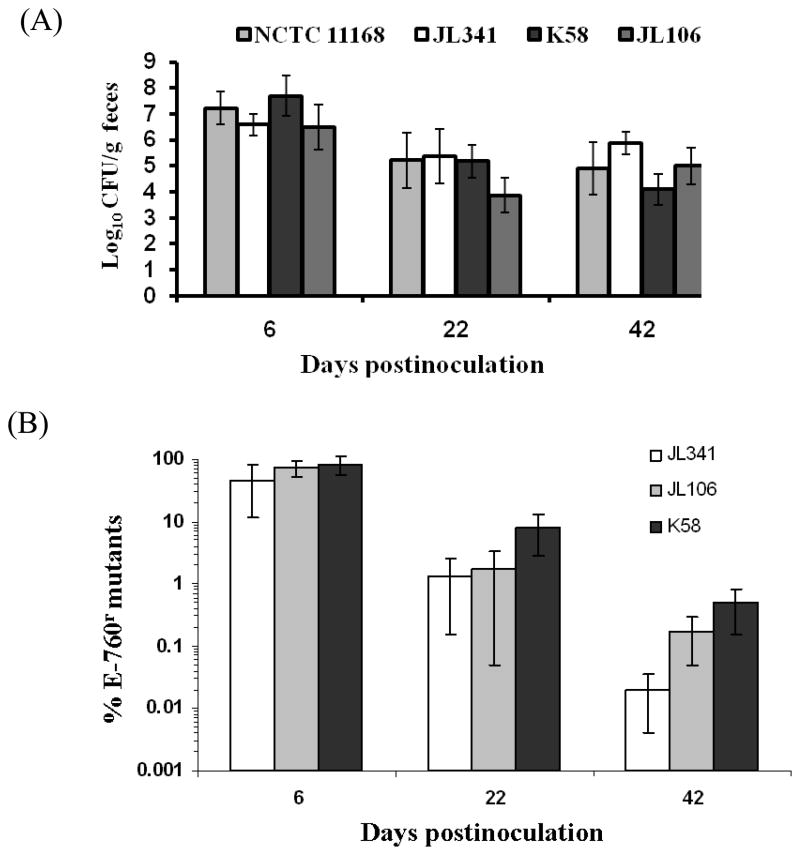
All chickens were successfully colonized by Campylobacter in either control group inoculated with C. jejuni NCTC 11168 or in treatment groups inoculated with C. jejuni JL341, K58, or C. coli JL106; the shedding level is approximately 7 log10 units per gram feces at 6 days post inoculation (Fig. 3A). The shedding levels of Campylobacter in colonized chickens were also slightly reduced in both control and treatment groups at 22 and 42 days post inoculation when compared to those at 6 days post inoculation (Fig. 3A).
Figure 3.
Stability of BCN E-760 resistance in vivo. (A) Shedding levels of various Campylobacter strains in chickens. Chickens in each group (10 birds/group) were inoculated with E-760 sensitive strain NCTC 11168, or one of E-760r mutants (JL341, K58, or JL106). All chickens received non medicated feed throughout the study. Each bar represents the mean of log10 CFUgram−1 feces ± standard deviation of Campylobacter colonized chickens in each group; (B) Instability of E-760 resistance in vivo. The percentage of E-760r population was calculated based on differential plating as detailed in Materials and Methods. Each bar represents the mean of percentage of the E-760r population of individual chickens ± standard deviation in each group.
The in vivo stability of BCN E-760 resistance was monitored by differential plating method as well as MIC test of randomly selected colonies for E-760 (40 colonies per time point per group). Differential plating method indicated that percentage of E-760 resistant mutants in total Campylobacter population in fecal sample from individual chicken dramatically decreased over the long-term growth of three E-760 resistant Campylobacter strains in chickens without E-760 selection pressure (Fig. 3B). By 42 days postinoculation, approximately 0.02%, 0.17%, and 0.49% of Campylobacter populations from chickens inoculated with JL341, JL106, and K58, respectively, grew on E-760-containing selective plates (Fig. 3B). MIC test of selected isolates confirmed the same trend of instability of E-760 resistance in vivo. The resistance levels of all randomly selected colonies were reduced to ≤ 1 mg/L at 42 days postinoculation.
DISCUSSION
Several anti-Campylobacter BCNs have successfully been identified and characterized from chicken commensal bacteria (Stern et al., 2006, Line et al., 2008a, Stern et al., 2005, Svetoch et al., 2008, Svetoch et al., 2005). Feeding these anti-Campylobacter BCNs to poultry at pre-slaughter stage eliminated Campylobacter colonization and these BCNs have been proposed to control Campylobacter spp in poultry (Stern et al., 2006, Line et al., 2008a, Stern et al., 2005, Svetoch et al., 2008, Svetoch et al., 2005). Although these BCNs are effective in reducing Campylobacter spp colonization in poultry, the use of these anti-Campylobacter BCNs in poultry may lead to emergence of BCNr isolates which may affect sustainable application of BCNs in poultry for Campylobacter control. Therefore, studying BCN resistance including development and stability of resistance are crucially important for future regulatory approval and public acceptability of this intervention measure.
In our previous study, we demonstrated that Campylobacter spp could develop low level BCN resistance in vitro but high-level of BCN resistance failed to develop in vitro despite extensive efforts (Hoang et al. 2011). In this study, we examined the in vivo development E-760 resistance in Campylobacter using a chicken model. We have used the similar chicken model system to study in vivo development of Campylobacter resistance to various clinical antibiotics (Lin et al., 2007, Luo et al., 2003, Han et al., 2008, Caldwell et al., 2008). Our data showed that in response to E-760 treatment, limited BCNr C. jejuni emerged as early as one day after the E-760 treatment. However, E-760 resistance level in the in vivo-selected Campylobacter was low throughout the whole study. This emergence pattern is different from in vivo development of fluoroquinolone resistance in Campylobacter (Luo et al, 2003) in which the mutants with high-level resistance emerged rapidly in all treated chickens as early as 1 day after the initiation of enrofloxacin treatment and also was different from macrolide resistance development (Lin, Yan et al. 2007) in which in vivo development of macrolide resistance was only observed after long-term exposure to tylosin (> 31 days). Our findings suggest that among Campylobacter there is limited development of resistance to the anti-Campylobacter BCNs, such as E-760. These findings support a recent theory that bacteria have not developed a highly effective mechanism to resist BCNs and other endogenous AMPs during evolution, which is likely due to multiple targets of natural AMPs (Preschel and Sahl, 2006). However, the results from this study should be interpreted cautiously because the experiment was conducted in a laboratory environment using a small number of chickens which may not represent the production conditions in poultry farms. In addition, only one representative C. jejuni strain alongside one BCN (E-760) was chosen to study in vivo emergence of BCN resistance in this study. The development of antimicrobial resistance development on farms is complex and influenced by multiple factors such as animal species, production environment, genetic backgrounds of bacterial species, and management practices. Furthermore, due to the lack of sufficient E-760 for a dosing experiment, it is still unknown if higher selection pressure would promote the development of mutants with high-level resistance to BCN E-760.
To obtain important information for the risk assessment of on-farm control of Campylobacter using the anti-Campylobacter BCNs, it is important to examine whether BCNr Campylobacter can persist in the absence of selection pressure. Antibiotic resistance in Campylobacter has displayed unique features with respect to fitness cost and stability of resistance when compared with other microorganisms. Our previous studies demonstrated that low levels of macrolide resistance in Campylobacter which was associated with mutations in ribosomal proteins L4 and L22 were not stable in vitro and in vivo. In contrast, high levels of macrolide resistance due to mutations at A2074G and A2075G in 23S rRNA were very stable in the absence of macrolide selection pressure in both in vitro and in vivo systems (Lin et al., 2007, Caldwell et al., 2008). Fluoroquilonone (FQ) resistance in Campylobacter associated with gyrA mutation is more intriguing (Luo et al., 2005). Specifically, the FQ resistant strains did not show fitness cost and the FQ resistance is highly persistent in vitro and in vivo (Luo et al., 2005). Notably, FQ resistance even enhances ecological fitness of Campylobacter and make FQ-resistant C. jejuni outcompete parent sensitive strain in the absence of selective pressure (Luo et al., 2005). Using both in vitro and in vivo systems, in this study we showed that E-760 resistance was not stable in Campylobacter regardless of specific species (C. jejuni or C. coli) and resistance levels (E-760 MIC = 8 or 64 mg/L). This information provides additional information supporting feasibility of practical application of BCN for Campylobacter control in poultry. Based on the findings from this study, the BCN resistance trait of resistant mutants could be dramatically lost after long-term colonization of the mutants in new flocks that receive BCN-free feed. Consequently, emergence of low-level BCN resistance in Campylobacter may have little effect on the efficacy of later BCN treatment prior to slaughter.
Revealing molecular mechanisms of BCN resistance in Campylobacter may facilitate us to develop more effective BCN-based intervention measure to reduce Campylobacter load in poultry. Multidrug resistance efflux pump CmeABC plays a critical role in Campylobacter resistance to structurally diverse antimicrobials (Lin et al., 2002, 2003). Our recent study (Hoang et al., 2011) has revealed that CmeABC also contributed to both intrinsic and acquired resistance to BCN for the mutants selected in vitro. In this study, we further demonstrated that active transport of CmeABC efflux pump confers resistance of in vivo-selected mutants to BCN (Table 1). Given a limited number of strains examined in this study, it is suggested that a greater number of strains should be used for cmeABC sequencing and function analyses in the future. Based on these findings, inhibition of the CmeABC pump by efflux pump inhibitors should significantly increase susceptibility of Campylobacter to BCN and reduce the frequency of emergence of BCN resistance in Campylobacter, as what we have observed for the effect of efflux pump inhibitors on Campylobacter resistance to clinical antibiotics (Martinez and Lin, 2006). Consequently, specific efflux pump inhibitors may be used in a combination treatment to enhance the efficacy of oral administration of the anti-Campylobacter BCNs for reducing Campylobacter load in poultry. This speculation remains to be examined in the future.
Acknowledgments
We thank Ximin Zeng, Fuzhou Xu, and Andree A. Hunkapiller for technical support. This study was supported by grant 1 R21 AI069133-01A2 from NIH.
References
- Allos BM. Campylobacter jejuni Infections: update on emerging issues and trends. Clin Infect Dis. 2001;32:1201–6. doi: 10.1086/319760. [DOI] [PubMed] [Google Scholar]
- Asaduzzaman SM, Sonomoto K. Lantibiotics: diverse activities and unique modes of action. J Biosci Bioeng. 2009;107:475–87. doi: 10.1016/j.jbiosc.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Caldwell DB, Wang Y, Lin J. Development, stability, and molecular mechanisms of macrolide resistance in Campylobacter jejuni. Antimicrob Agents Chemother. 2008;52:3947–54. doi: 10.1128/AAC.00450-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox NA, Stern NJ, Hiett KL, Berrang ME. Identification of a new source of Campylobacter contamination in poultry: transmission from breeder hens to broiler chickens. Avian Dis. 2002;46:535–41. doi: 10.1637/0005-2086(2002)046[0535:IOANSO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Davis L, Young K, Dirita V. Genetic manipulation of Campylobacter jejuni. Curr Protoc Microbiol. 2008;Chapter 8(Unit 8A):2, 1–8A, 2, 17. doi: 10.1002/9780471729259.mc08a02s10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diker KS, Esendal OM, Akan M. Epidemiology of ovine Campylobacter infection determined by numerical analysis of electrophoretic protein profiles. J Vet Med B Infect Dis Vet Public Health. 2000;47:739–43. doi: 10.1046/j.1439-0450.2000.00409.x. [DOI] [PubMed] [Google Scholar]
- Galvez A, Abriouel H, Lopez RL, Ben Omar N. Bacteriocin-based strategies for food biopreservation. Int J Food Microbiol. 2007;120:51–70. doi: 10.1016/j.ijfoodmicro.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Han J, Sahin O, Barton YW, Zhang Q. Key role of Mfd in the development of fluoroquinolone resistance in Campylobacter jejuni. PLoS Pathog. 2008;4:e1000083. doi: 10.1371/journal.ppat.1000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang KV, Stern NJ, Saxton AM, Xu F, Zeng X, Lin J. Prevalence, development, and molecular mechanisms of bacteriocin resistance in Campylobacter. Appl Environ Microbiol. 2011;77:2309–16. doi: 10.1128/AEM.02094-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SX, Luangtongkum T, Morishita TY, Zhang QJ. Molecular typing of Campylobacter strains using the cmp gene encoding the major outer membrane protein. Foodborne Pathogens and Disease. 2005;2:12–23. doi: 10.1089/fpd.2005.2.12. [DOI] [PubMed] [Google Scholar]
- Hugas M, Pages F, Garriga M, Monfort JM. Application of the bacteriocinogenic Lactobacilus sakei CTC494 to prevent growth of Listeria in fresh and cooked meat products packed with different atmospheres. Food Microbiology. 1998;15:639–650. [Google Scholar]
- Joerger RD. Alternatives to antibiotics: bacteriocins, antimicrobial peptides and bacteriophages. Poult Sci. 2003;82:640–7. doi: 10.1093/ps/82.4.640. [DOI] [PubMed] [Google Scholar]
- Jorgensen JH, Turnidge JD. Susceptibility test methods:Dilution and Disk Diffusion Methods. In: Murray PR, Barton EJ, Jorgensen JH, Pfaller MA, Yolken RH, editors. Manual of clinical microbiology. American Society for Microbiology; Washington, D.C: 2003. pp. 1109–1127. [Google Scholar]
- Klaenhammer TR. Bacteriocins of lactic acid bacteria. Biochimie. 1988;70:337–49. doi: 10.1016/0300-9084(88)90206-4. [DOI] [PubMed] [Google Scholar]
- Lin J. Novel approaches for Campylobacter control in poultry. Foodborne Pathog Dis. 2009;6:755–65. doi: 10.1089/fpd.2008.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Michel LO, Zhang QJ. CmeABC functions as a multidrug efflux system in Campylobacter jejuni. Antimicrobial Agents and Chemotherapy. 2002;46:2124–2131. doi: 10.1128/AAC.46.7.2124-2131.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Sahin O, Michel LO, Zhang Q. Critical role of multidrug efflux pump CmeABC in bile resistance and in vivo colonization of Campylobacter jejuni. Infect Immun. 2003;71:4250–9. doi: 10.1128/IAI.71.8.4250-4259.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Yan M, Sahin O, Pereira S, Chang YJ, Zhang Q. Effect of macrolide usage on emergence of erythromycin-resistant Campylobacter isolates in chickens. Antimicrob Agents Chemother. 2007;51:1678–86. doi: 10.1128/AAC.01411-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Line JE, Svetoch EA, Eruslanov BV, Perelygin VV, Mitsevich EV, Mitsevich IP, Levchuk VP, Svetoch OE, Seal BS, Siragusa GR, Stern NJ. Isolation and purification of enterocin E-760 with broad antimicrobial activity against gram-positive and gram-negative bacteria. Antimicrob Agents Chemother. 2008;52:1094–100. doi: 10.1128/AAC.01569-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo N, Pereira S, Sahin O, Lin J, Huang S, Michel L, Zhang Q. Enhanced in vivo fitness of fluoroquinolone-resistant Campylobacter jejuni in the absence of antibiotic selection pressure. Proc Natl Acad Sci U S A. 2005;102:541–6. doi: 10.1073/pnas.0408966102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo N, Sahin O, Lin J, Michel LO, Zhang Q. In vivo selection of Campylobacter isolates with high levels of fluoroquinolone resistance associated with gyrA mutations and the function of the CmeABC efflux pump. Antimicrob Agents Chemother. 2003;47:390–4. doi: 10.1128/AAC.47.1.390-394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A, Lin J. Effect of an efflux pump on the function of CmeABC efflux pump and antibiotic resistance in Campylobacter jejuni. Foodborne Pathogens and Disease. 2006;3:393–402. doi: 10.1089/fpd.2006.3.393. [DOI] [PubMed] [Google Scholar]
- Nachamkin I, Allos BM, Ho T. Campylobacter species and Guillain-Barre syndrome. Clin Microbiol Rev. 1998;11:555–67. doi: 10.1128/cmr.11.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley MA, Gordon DM. A Survey of Col Plasmids in Natural Isolates of Escherichia-Coli and an Investigation into the Stability of Col-Plasmid Lineages. Journal of General Microbiology. 1992;138:1345–1352. doi: 10.1099/00221287-138-7-1345. [DOI] [PubMed] [Google Scholar]
- Settanni L, Corsetti A. Application of bacteriocins in vegetable food biopreservation. Int J Food Microbiol. 2008;121:123–38. doi: 10.1016/j.ijfoodmicro.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Stern NJ, Bannov VA, Svetoch EA, Mitsevich EV, Mitsevich IP, Volozhantsev NV, Gusev VV, Perelygin VV. Distribution and characterization of Campylobacter spp. from Russian poultry. J Food Prot. 2004;67:239–45. doi: 10.4315/0362-028x-67.2.239. [DOI] [PubMed] [Google Scholar]
- Stern NJ, Fedorka-Cray P, Bailey JS, Cox NA, Craven SE, Hiett KL, Musgrove MT, Ladely S, Cosby D, Mead GC. Distribution of Campylobacter spp. in selected U.S. poultry production and processing operations. J Food Prot. 2001;64:1705–10. doi: 10.4315/0362-028x-64.11.1705. [DOI] [PubMed] [Google Scholar]
- Stern NJ, Svetoch EA, Eruslanov BV, Kovalev YN, Volodina LI, Perelygin VV, Mitsevich EV, Mitsevich IP, Levchuk VP. Paenibacillus polymyxa purified bacteriocin to control Campylobacter jejuni in chickens. J Food Prot. 2005;68:1450–3. doi: 10.4315/0362-028x-68.7.1450. [DOI] [PubMed] [Google Scholar]
- Stern NJ, Svetoch EA, Eruslanov BV, Perelygin VV, Mitsevich EV, Mitsevich IP, Pokhilenko VD, Levchuk VP, Svetoch OE, Seal BS. Isolation of a Lactobacillus salivarius strain and purification of its bacteriocin, which is inhibitory to Campylobacter jejuni in the chicken gastrointestinal system. Antimicrob Agents Chemother. 2006;50:3111–6. doi: 10.1128/AAC.00259-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svetoch EA, Eruslanov BV, Perelygin VV, Mitsevich EV, Mitsevich IP, Borzenkov VN, Levchuk VP, Svetoch OE, Kovalev YN, Stepanshin YG, Siragusa GR, Seal BS, Stern NJ. Diverse antimicrobial killing by Enterococcus faecium E 50–52 bacteriocin. J Agric Food Chem. 2008;56:1942–8. doi: 10.1021/jf073284g. [DOI] [PubMed] [Google Scholar]
- Svetoch EA, Stern NJ, Eruslanov BV, Kovalev YN, Volodina LI, Perelygin VV, Mitsevich EV, Mitsevich IP, Pokhilenko VD, Borzenkov VN, Levchuk VP, Svetoch OE, Kudriavtseva TY. Isolation of Bacillus circulans and Paenibacillus polymyxa strains inhibitory to Campylobacter jejuni and characterization of associated bacteriocins. J Food Prot. 2005;68:11–7. doi: 10.4315/0362-028x-68.1.11. [DOI] [PubMed] [Google Scholar]
- Willey JM, Van Der Donk WA. Lantibiotics: peptides of diverse structure and function. Annu Rev Microbiol. 2007;61:477–501. doi: 10.1146/annurev.micro.61.080706.093501. [DOI] [PubMed] [Google Scholar]
- Zasloff M. Antimicrobial peptides in health and disease. N Engl J Med. 2002;347:1199–200. doi: 10.1056/NEJMe020106. [DOI] [PubMed] [Google Scholar]