Abstract
Background
The red cell distribution width (RDW), an automated measure of red blood cell size heterogeneity (e.g. anisocytosis) that is largely overlooked, is a newly recognized risk marker in patients with established cardiovascular disease (CVD). It is unknown whether RDW is associated with mortality in the general population, or whether this association is specific to CVD.
Methods
We examined the association of RDW with all-cause mortality, as well as cardiovascular, cancer, and chronic lower respiratory disease mortality among 15,852 adult participants in The Third National Health and Nutrition Examination Survey (1988–1994), a nationally representative sample of the United States population. Mortality status was obtained by matching to the National Death Index, with follow-up through December 31, 2000.
Results
Estimated mortality rates increased 5-fold from the lowest to highest quintile of RDW after accounting for age, and 2-fold after multivariable adjustment (each Ptrend < 0.001). A 1- standard deviation increment in RDW (0.98) was associated with a 23% greater risk of all-cause mortality (hazard ratio (HR) 1.23, 95% confidence interval (CI) 1.18–1.28) after multivariable adjustment. RDW was also associated with risk of death due to cardiovascular disease (HR 1.22, 95% CI 1.14–1.31), cancer (HR 1.28, 95% CI 1.21–1.36), and chronic lower respiratory disease (HR 1.32, 95% CI 1.17–1.49).
Conclusions
Higher RDW was associated with increased mortality risk in this large, community-based sample, an association not specific to CVD. Study of anisocytosis may therefore yield novel pathophysiological insights, and measurement of RDW may contribute to risk assessment.
Introduction
The red blood cell distribution width (RDW) is an automated measure of the heterogeneity of red blood cell sizes (e.g., anisocytosis), routinely performed as part of a complete blood count (CBC) (1–3). The RDW is utilized in the differential diagnosis of anemia, but otherwise has received little attention. In fact, in the last five years there are only ten articles indexed by PubMed with ‘red cell distribution width’ in the title (4).
In a recent exploratory analysis of a large contemporary clinical heart failure trial (The Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Program), higher RDW was found to be a strong and independent predictor of increased risk for mortality and adverse cardiovascular (CV) outcomes(5). The investigators confirmed these findings in a clinical database of patients who had undergone cardiac catheterization at Duke University Medical Center. A subsequent analysis of The Cholesterol and Recurrent Events (CARE) Trial, which included patients who had a prior myocardial infarction but no evidence of heart failure, reported that higher RDW strongly and independently predicted all-cause mortality as well as adverse CV outcomes(6). Thus, higher RDW was associated with adverse prognosis in three separate cohorts of patients with established cardiovascular disease (CVD). Whether this association is present in the general population, and whether the association of RDW with mortality risk is specific to CVD, has not been examined.
The National Health and Nutrition Examination Survey (NHANES) is a nationally representative examination of the United States population. The Third NHANES (NHANES III) has been linked to the National Death Index (NDI), allowing prospective examination of mortality risk. We used this publicly available resource to examine whether RDW is associated with mortality risk in the general population. Based upon the previously described findings we were interested in the association of RDW with all-cause and CV mortality(5, 6). Additionally, there have been cross-sectional associations of RDW with chronic lung disease and cancer(7, 8), though no prospective data is available for these outcomes. We therefore also examined the association of RDW with death due to lung disease and cancer, to explore whether an observed association of RDW with mortality would be specific to CVD. In this paper we present our findings of the association of RDW with risk for all-cause, CV, cancer, and chronic lower respiratory disease mortality in NHANES III.
Methods
The NHANES is designed to assess the health and nutritional status of adults and children in the United States. NHANES III was conducted in the years 1988–1994. A stratified, multistage sample design was used to produce a nationally representative sample of the noninstitutionalized US civilian population. The survey included questionnaires, a physical examination, and laboratory tests. Detailed documentation of the NHANES III examination procedures is available elsewhere(9). NHANES was approved by the National Center for Health Statistics (NCHS) institutional review board, and all participants gave their informed consent.
Demographic, social and economic characteristics
Participants reported their attained education level as the highest grade or year of school completed. Self-reported race/ethnicity was classified in NHANES III as non-Hispanic white, non-Hispanic black, Mexican-American, or other. Physical activity was defined by self-report of activity level relative to peers of similar age (less, same, or more). Smoking status was defined as current, former, or never, and pack-years of smoking was also calculated. Participants reported their alcohol intake in terms of the number of days of alcohol consumption over the prior 12 months and the average number of drinks consumed on these days; from this we derived an average number of drinks per week.
Laboratory procedures
The RDW, hemoglobin level and mean corpuscular volume (MCV) were determined by the Coulter Counter Model S-PLUS JR with Coulter histogram differential as part of a complete blood count(10). The reference range for RDW was 11.8 to 14.8%. A central laboratory reviewed hematology data for accuracy and completeness(11). Serum creatinine, albumin, and iron were measured on a Roche Hitachi 737 multichannel analyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN). Serum folate and B12 were measured by Bio-Rad Laboratories “Quantaphase Folate” radioassay kit (Bio-Rad Laboratories, Hercules, CA). Serum total and HDL cholesterol were measured by the Hitachi 704 Analyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN). C-reactive protein (CRP) was quantified by latex-enhanced nephelometry using a modification of the Behring latex-enhanced CRP assay on the Behring Nephelometer Analyzer System (Behring Diagnostics, Westwood, Massachusetts); the lower limit of detection was 3.0 mg/L. Detailed information regarding laboratory procedures is available elsewhere(10, 11).
Medical history and examination
We defined CVD by self report of a physician diagnosis of myocardial infarction, heart failure, or stroke; cancer by self report of a diagnosis of non-skin cancer; and lung disease by self report of a diagnosis of asthma, chronic bronchitis or emphysema. A diagnosis of hypertension was assigned if the subject reported a physician diagnosis of hypertension, reported taking prescription medications for hypertension, or if, upon examination, the systolic blood pressure was ≥ 140 or the diastolic blood pressure was ≥ 90 mm Hg. A diagnosis of hypercholesterolemia was assigned if the subject reported a physician diagnosis of hypercholesterolemia, reported taking prescription medications for hypercholesterolemia, or if the total cholesterol level was ≥ 6.2 mmol/L (240 mg/dL). A diagnosis of diabetes mellitus was assigned if the subject reported a physician diagnosis of diabetes, reported taking prescription medications (either insulin or oral agents) for diabetes, if a non-fasting plasma glucose was ≥ 11.1 mmol/L (200 mg/dL), or if a fasting plasma glucose was ≥ 7.0 mmol/L (126 mg/dL). We estimated glomerular filtration rate (eGFR) from serum creatinine using the Modification of Diet in Renal Disease Study equation, and chronic kidney disease (CKD) was defined as an eGFR < 60 ml/min(12, 13). Body mass index (BMI) was calculated as the weight (kilograms) divided by the height (meters) squared and was categorized as normal weight (BMI < 25), overweight (BMI 25.0–29.9), or obese (BMI ≥ 30). We defined anemia as hemoglobin < 13.9 g/dL in men and < 12.0 g/dL in women.
Outcomes
The National Death Index is a central computerized database of all certified death in the United States since 1979. Probabilistic matching based on 12 identifiers for each participant (e.g., Social Security Number, name) was used to link NHANES III and NDI records. Mortality follow-up occurred through December 31, 2000. A calibration study applying identical matching methodology to the NHANES I Follow-up Survey found an overall correct classification of vital status of 98.5%(14).
The International Classification of Diseases (ICD) revision 9 (ICD-9) was used to classify deaths that occurred through 1998, otherwise ICD-10 was used. Cause-specific mortality was categorized as CVD (ICD-9 codes 390 to 448; ICD-10 codes I00-I99), malignant neoplasm (herein referred to as cancer) (ICD-9 codes 140 to 208; ICD-10 codes C00-C97), and chronic lower respiratory disease (ICD-9 codes 490–496; ICD-10 codes J40-J47). External-cause deaths (e.g., accidental, violence-related) were classified as ICD-9 codes E800-E999 or ICD-10 codes U01-U03, V01-Y89.
There were 18,512 non-pregnant participants age ≥ 20 years with mortality follow up. Of these, 15,852 participants had RDW measurements and are included in this analysis.
Statistical methods
NHANES uses a multistage, probability-sampling design to select participants representative of the civilian, non-institutionalized US population(15). All our analyses accounted for the sampling design and use sample weights; these account for unequal probabilities of selection and include adjustments for noncoverage and nonresponse. For age-standardized results, we used the 1990 United States census population as the reference standard (age groups 20–29, 30–39, 40–49, 50–59, 60–69, > 69 years). We used SAS- (version 9.1; SAS Institute Inc, NC) callable SUDAAN version 9.01 (Research Triangle Institute, NC).
The primary outcome was all-cause mortality. Secondary outcomes were death due to CVD, cancer, and chronic lower respiratory disease. We used Cox proportional hazards models to estimate the relative risk for mortality corresponding to a 1-standard deviation (sd) increment in RDW (0.98)(16). We did not find evidence of important deviations from linearity in the association between RDW and mortality risk. The association of RDW with mortality was also estimated across quintiles of RDW. We used time on study as the time scale for analysis, calculated as the time from the NHANES examination to the earlier of death or December 31, 2000. We repeated the analyses using age as the time scale for analysis and obtained nearly identical results, which is expected for an outcome, such as death, whose baseline hazard varies exponentially with age(17). Neither RDW nor any covariate violated the proportional hazards assumption, tested by including time-dependent covariates in the models. Relative risks are expressed as hazard ratios (HR) with 95% confidence intervals (CI). For cause-specific analyses, we censored participants at the time of death from causes other than the cause of interest.
We first fit a model (model 1) adjusting for the demographic factors of age (continuous, in years), sex, and race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican-American, other) to describe the association of RDW with mortality risk. We noted that RDW was associated with behavioral and other potential mortality risk factors; we therefore constructed additional models to explore to what extent RDW might be associated with mortality risk after accounting for commonly considered risk factors. The first of these models (model 2) additionally adjusted for the behavior and social factors of physical activity level, education level (continuous), smoking (both smoking status and pack-years), and body mass index, and was used to model the outcomes of cancer and respiratory mortality. The second of these models (model 3) additionally adjusted for the cardiovascular risk factors of systolic blood pressure, hypertension, hemoglobin A1c, diabetes mellitus, total:HDL cholesterol ratio, hypercholesterolemia, estimated GFR, and chronic kidney disease, and was used to model all-cause and cardiovascular mortality. In adjusting for these cardiovascular risk factors we included both continuous variables (e.g. SBP) to minimize residual confounding and categorical variables to utilize information obtained in the medical history (e.g. use of antihypertensive medication).
We also performed analyses of all-cause mortality stratified by age (< 50 years, ≥ 50 years), sex, race/ethnicity, smoking status, diabetes mellitus, anemia, and chronic kidney disease. We excluded the category of “other” from the analyses of race/ethnicity due to the small size and heterogeneous nature of this group. For each of these stratified analyses, we formally tested variation in RDW’s association with mortality across the subgroup levels by fitting models containing cross-products between the subgroup levels and RDW. We also fit models adjusting for hemoglobin level and adjusting for iron, folate, and B12 levels to address whether the association of RDW with mortality risk might be due to confounding by anemia or anemia-related nutrient deficiencies, respectively. A change in the estimated regression coefficient for RDW of ≥ 20% was considered evidence of significant confounding.
Hypothesis testing was two-tailed, with a P value < 0.05 considered statistically significant. To reduce the impact of chance findings in our multiple (7) exploratory interaction analyses, we considered a P value < 0.007 (0.05/7) statistically significant.
Results
The demographic and lifestyle factors of older age, non-Hispanic black race/ethnicity, lower education level, physical inactivity, body mass index and active smoking were associated with higher RDW (Table 1). The cardiovascular risk factors of hypertension, hypercholesterolemia, and chronic kidney disease were also associated with higher RDW, as was prevalent cardiovascular disease. Lung disease was more common among those with higher RDW, while a diagnosis of cancer was not associated with RDW. Hemoglobin, mean corpuscular volume, serum folate and iron levels were inversely associated with RDW, while B12 level was not associated with RDW.
Table 1.
Age-standardizeda baseline characteristics by red cell distribution width (RDW) quintile.
| RDW Quintile | ||||||
|---|---|---|---|---|---|---|
| Lowest | 2 | 3 | 4 | Highest | P valueb | |
| n | 2895 | 3289 | 3243 | 3121 | 3304 | 15852 |
| RDW, range | 10.90–12.35 | 12.50–12.65 | 12.80–13.15 | 13.20–13.75 | 13.80–30.60 | |
| RDW, median (interquartile range) | 12.10 (11.95–12.25) | 12.60 (12.50–12.65) | 12.95 (12.85–13.05) | 13.40 (13.30–13.55) | 14.35 (14.00–15.05) | |
| Age (years), mean | 39.7 | 42.0 | 45.9 | 49.0 | 54.1 | < 0.001 |
| Female sex, % | 53.2 | 50.5 | 49.0 | 52.4 | 61.2 | 0.002 |
| Race/ethnicity, % | < 0.001 | |||||
| Non-Hispanic white | 84.2 | 82.7 | 76.0 | 72.5 | 56.7 | |
| Non-Hispanic black | 5.0 | 6.1 | 9.2 | 14.0 | 29.5 | |
| Mexican American | 3.8 | 4.7 | 5.6 | 5.3 | 5.7 | |
| Other | 7.1 | 6.5 | 9.2 | 8.2 | 8.0 | |
| Highest year of education achieved, mean | 12.8 | 12.6 | 12.3 | 12.0 | 11.4 | < 0.001 |
| Graduated high school, % | 80.2 | 80.0 | 73.9 | 69.6 | 61.7 | < 0.001 |
| Pack-years of smoking, mean | 8.9 | 9.9 | 10.8 | 12.4 | 13.4 | < 0.001 |
| Active smoking, % | 18.5 | 24.6 | 30.7 | 36.5 | 37.9 | < 0.001 |
| Physical activity relative to peers, % | < 0.001 | |||||
| Less than peers | 18.4 | 19.3 | 19.6 | 24.5 | 29.3 | |
| More than peers | 39.2 | 36.4 | 36.6 | 30.2 | 27.9 | |
| Estimated glomerular filtration rate (mL/min), mean | 92.1 | 90.8 | 92.6 | 92.9 | 95.2 | < 0.001 |
| Chronic kidney disease, % | 5.2 | 4.4 | 5.4 | 6.1 | 10.6 | < 0.001 |
| Systolic blood pressure (mm Hg), mean | 123 | 123 | 123 | 125 | 126 | < 0.001 |
| Hypertension, % | 30.4 | 32.2 | 31.1 | 34.4 | 40.9 | < 0.001 |
| Hemoglobin A1c (%), mean | 5.3 | 5.4 | 5.4 | 5.5 | 5.6 | < 0.001 |
| Diabetes, % | 7.4 | 7.3 | 6.3 | 8.6 | 11.0 | 0.07 |
| Total:HDL cholesterol ratio, mean | 4.4 | 4.5 | 4.5 | 4.4 | 4.4 | 0.13 |
| Hypercholesterolemia, % | 36.3 | 35.0 | 32.0 | 32.1 | 28.6 | < 0.001 |
| Body mass index (kg/m*m), mean | 25.7 | 26.4 | 26.6 | 27.5 | 28.1 | < 0.001 |
| Obesity (BMI ≥ 30.0 kg/m*m), % | 16.5 | 21.2 | 23.3 | 27.9 | 32.3 | < 0.001 |
| Hemoglobin (g/L), mean | 143 | 144 | 143 | 142 | 132 | < 0.001 |
| Mean corpuscular volume (fL), mean | 91.0 | 90.6 | 90.3 | 89.8 | 86.9 | < 0.001 |
| Serum folate (nmol/L), mean | 17.9 | 17.1 | 15.1 | 14.0 | 13.1 | < 0.001 |
| Serum B12 (pmol/L] (n = 8,068c), mean | 360 | 356 | 354 | 360 | 359 | 0.97 |
| Serum iron (ug/dL), mean | 105 | 102 | 102 | 93 | 84 | < 0.001 |
| Cardiovascular disease, % | 4.1 | 4.6 | 5.9 | 7.7 | 9.9 | < 0.001 |
| Cancer, % | 4.3 | 4.4 | 4.0 | 3.6 | 4.3 | 0.77 |
| Lung disease, % | 12.0 | 12.0 | 13.3 | 14.5 | 14.7 | 0.01 |
Standardized to the United States Census 1990.
P value corresponds to the overall age-adjusted association between the characteristic and RDW quintile, based on linear, logistic, or multinomial logistic regression models with RDW quintiles and age as the independent variables. The comparison of age and RDW quintile does not adjust for age.
Vitamin B12 level was only measured in the years 1991–1994
All-cause mortality
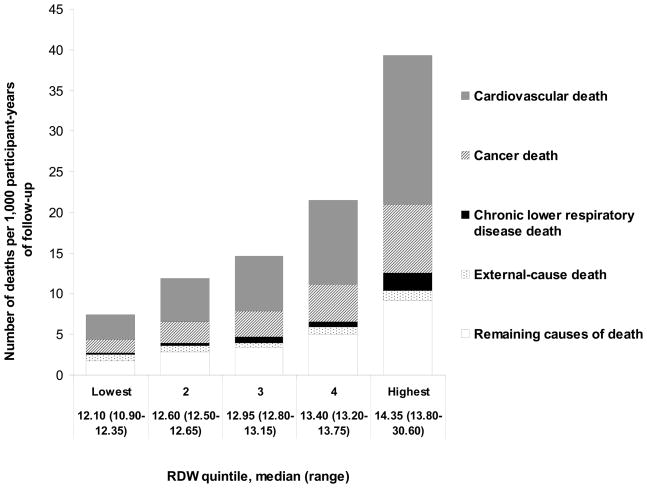
There were 2,629 deaths among the 15,852 participants over a mean of 8.7 years of follow-up. Age-standardized mortality rates progressively rose with increasing RDW quintile, and the highest quintile of RDW, compared with the lowest, was associated with a > 5-fold increased risk of death (figure 1). After adjustment for age, sex, and race/ethnicity, a 1-standard deviation increment in RDW was associated with a 27% increased risk (HR 1.27, 95% CI 1.21–1.33) for all-cause mortality (table 2). Additional adjustment for demographic and lifestyle factors partially attenuated this estimate (HR 1.24, 95% CI 1.19–1.30), while further adjustment for cardiovascular risk factors did not. Examining this relationship by RDW quintile revealed that the highest quintile of RDW, compared with the lowest, was significantly associated with a doubling of the risk of all-cause mortality after full multivariable adjustment (table 3). Excluding subjects with RDW levels outside of the reference range did not attenuate the association of RDW with mortality risk (model 3, n remaining = 13,080, HR 1.34, 95% CI 1.17–1.53).
Figure 1. The association of RDW with mortality in NHANES III.
Rates of all-cause, cardiovascular, cancer, and chronic lower respiratory disease deaths increased significantly across quintiles (all Ptrend < 0.05), while external-cause deaths (e.g. due to accident or intent) did not (Ptrend = 0.94).
Table 2.
Association of red blood cell distrubution width with all-cause and specific-cause mortality in NHANES III
| Risk associated with a 1-standard deviation increment in RDW | All-cause mortality | Cardiovascular mortality | Cancer mortality | Chronic lower respiratory disease mortality | ||||
|---|---|---|---|---|---|---|---|---|
| No. events | HR (95% CI) | No. events | HR (95% CI) | No. events | HR (95% CI) | No. events | HR (95% CI) | |
| Model 1 (n = 15,852) | 2,629 | 1.27 (1.21–1.33) | 1,220 | 1.26 (1.19–1.33) | 564 | 1.30 (1.22–1.39) | 115 | 1.36 (1.23–1.51) |
| Model 2 (n = 14,996) | 2,394 | 1.24 (1.19–1.30) | 1,102 | 1.22 (1.15–1.30) | 525 | 1.28 (1.21–1.36) | 105 | 1.32 (1.17–1.49) |
| Model 3 (n = 14,310) | 2,221 | 1.23 (1.18–1.28) | 1,024 | 1.22 (1.14–1.31) | NA | NA | NA | NA |
Abbreviations: RDW, Red cell distribution width; CI, confidence interval; HR, hazard ratio; NA, data not applicable.
Model 1 adjusts for age, sex, and race/ethnicity.
Model 2 additioanlly adjusts for physical activity level, achieved education level, smoking status, pack-years of smoking, and body mass index.
Model 3 additioanlly adjusts for systolic blood pressure, hypertension, hemoglobin A1c, diabetes mellitus, total:HDL cholesterol ratio, hypercholesterolemia, chronic kidney disease, and estimated glomerular filtration.
Table 3.
Multivariable-adjusteda hazard ratios (HR) and 95% confidence intervals (CI) for all-cause and specific-cause death associated with quintiles of RDW in NHANES III.
| All-cause deaths | Cardiovascular deaths | Cancer deaths | Chronic lower respiratory disease deaths |
External-cause deaths |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RDW Quintile | RDW, median (range) |
Participant-years of follow-upb |
No. events | HR (95% CI) | No. events | HR (95% CI) | No. events | HR (95% CI) | No. events | HR (95% CI) | No. events | HR (95% CI) |
| Lowest | 12.10 (10.90–12.35) | 23,366 | 172 | (ref) | 70 | (ref) | 35 | (ref) | 3 | (ref) | 20 | (ref) |
| Second | 12.60 (12.50–12.65) | 26,248 | 302 | 1.13 (0.90–1.42) | 136 | 1.40 (0.90–2.18) | 67 | 0.84 (0.48–1.47) | 8 | 1.94 (0.48–7.81) | 20 | 0.86 (0.27–2.80) |
| Third | 12.95 (12.80–13.15) | 26,077 | 366 | 1.07 (0.85–1.36) | 162 | 1.11 (0.75–1.63) | 85 | 1.22 (0.74–2.01) | 20 | 2.74 (0.67–11.15) | 15 | 0.66 (0.21–2.12) |
| Fourth | 13.40 (13.20–13.75) | 24,478 | 500 | 1.31 (1.04–1.64) | 247 | 1.47 (1.00–2.16) | 111 | 1.31 (0.76–2.25) | 12 | 1.42 (0.19–10.39) | 18 | 0.91 (0.35–2.35) |
| Highest | 14.35 (13.80–30.60) | 24,217 | 881 | 2.00 (1.57–2.56) | 409 | 2.34 (1.50–3.66) | 188 | 1.88 (1.18–2.98) | 52 | 5.89 (1.23–28.24) | 27 | 1.07 (0.42–2.72) |
| P-trend | < 0.001 | < 0.001 | < 0.001 | 0.03 | 0.98 | |||||||
These analyses were limited to participants who had complete data on all covariates adjusted for (n = 14,310).
All models are adjusted for age, sex, race/ethnicity, education level, physical activity, smoking (smoking status and pack-years), and body mass index. We additionally adjusted analyses of all-cause and cardiovascular mortality for systolic blood pressure, hypertension, hemoglobin A1c, diabetes, total:HDL cholesterol ratio, hypercholesterolemia, estimated GFR, and chronic kidney disease.
Standardized to the United States census 1990.
Cardiovascular disease mortality
Age-standardized CV death rates significantly increased across RDW quintiles (P < 0.001) (figure 1). There were 1,220 deaths attributed to CVD. A 1-sd increment in RDW was significantly associated with 22% increased risk (table 2), and the highest quintile of RDW, compared with the lowest, was significantly associated with 134% increased risk of CV mortality after multivariable adjustment (table 3). Excluding participants with CVD at baseline (n = 1,218) did not change this result (model 3 HR 1.23, 95% CI 1.12–1.33).
Cancer mortality
There were 564 deaths attributed to cancer. A 1-sd increment in RDW was significantly associated with a 28% increased risk (table 2), and the highest quintile of RDW, compared with the lowest, was significantly associated with an 88% increased risk of death due to cancer after multivariable adjustment (table 3). Excluding participants with cancer at baseline (n = 602) partially attenuated this result (model 2 HR 1.23, 95% CI 1.14–1.33).
Chronic lower respiratory disease mortality
There were 115 deaths attributed to chronic lower respiratory disease. A 1-sd increment in RDW was associated with a 32% increased risk (table 2), and the highest quintile of RDW, compared with the lowest, was associated with a 5.9-fold increased risk of death from chronic lower respiratory disease after multivariable adjustment (table 3). Excluding participants with lung disease at baseline (n = 2,004) did not change the result (model 2 HR 1.31, 95% CI 1.19–1.45).
Death due to external causes
There were 114 deaths attributed to external causes. RDW was not associated with external-cause death (figure 1, table 3).
Subgroup analyses
We performed stratified analyses to examine the consistency of the association of RDW with mortality risk among several subgroups of interest (table 4). The association of RDW with all-cause mortality risk was consistent among subgroups defined by age, sex, smoking status, diabetes mellitus, anemia and chronic kidney disease (all Pinteraction > 0.007).
Table 4.
The multivariable-adjusteda association of RDW with all-cause mortality across certain subgroups.
| Number of participants | Number of events | Association of RDW with all-cause mortality within subgroup Hazard ratiob (95% CI) | P-interactionc | |
|---|---|---|---|---|
| Age | 0.27 | |||
| < 50 years | 7997 | 246 | 1.13 (1.04–1.22) | |
| > 50 years | 6313 | 1975 | 1.24 (1.18–1.31) | |
| Sex | 0.68 | |||
| Male | 6760 | 1208 | 1.25 (1.19–1.31) | |
| Female | 7550 | 1013 | 1.20 (1.12–1.29) | |
| Race-ethnicityd | 0.13 | |||
| Non-Hispanic white | 6068 | 1339 | 1.23 (1.16–1.30) | |
| Non-Hispanic black | 3850 | 476 | 1.21 (1.14–1.29) | |
| Mexican American | 3826 | 372 | 1.15 (1.07–1.24) | |
| Smoking status | 0.07 | |||
| Never | 7224 | 959 | 1.16 (1.07–1.24) | |
| Former | 3437 | 796 | 1.27 (1.20–1.34) | |
| Active | 3649 | 466 | 1.28 (1.17–1.39) | |
| Diabetes | 0.19 | |||
| No | 12783 | 1694 | 1.20 (1.15–1.26) | |
| Yes | 1527 | 527 | 1.30 (1.18–1.44) | |
| Anemia | 0.21 | |||
| No | 13285 | 1834 | 1.22 (1.13–1.32) | |
| Yes | 2268 | 652 | 1.16 (1.10–1.23) | |
| Chronic kidney disease | 0.51 | |||
| No | 12042 | 1569 | 1.21 (1.15–1.27) | |
| Yes | 1165 | 647 | 1.27 (1.19–1.35) |
Adjusted, as appropriate, for age, sex, race/ethnicity, physical activity level, achieved education level, smoking status, pack-years of smoking, and body mass index, systolic blood pressure, hypertension, hemoglobin A1c, hemoglobin A1c, diabetes mellitus, total:HDL cholesterol ratio, hypercholesterolemia, chronic kidney disease, and estimated glomerular filtration rate.
Hazard ratio is for a 1-standard deviation increment in RDW
P interaction refers to the interaction with RDW.
The category of ‘Other’ was excluded due to its small size (n = 589) and heterogenous nature
Additional sensitivity analyses for all-cause mortality
We found no evidence that the association between RDW and all-cause mortality risk was substantially changed by further adjustment for hemoglobin, MCV, iron folate, or vitamin B12 levels (n = 7536) in those participants who had these data available. Additional adjustment for serum albumin level or alcohol intake also did not change the results.
A possible role for inflammation
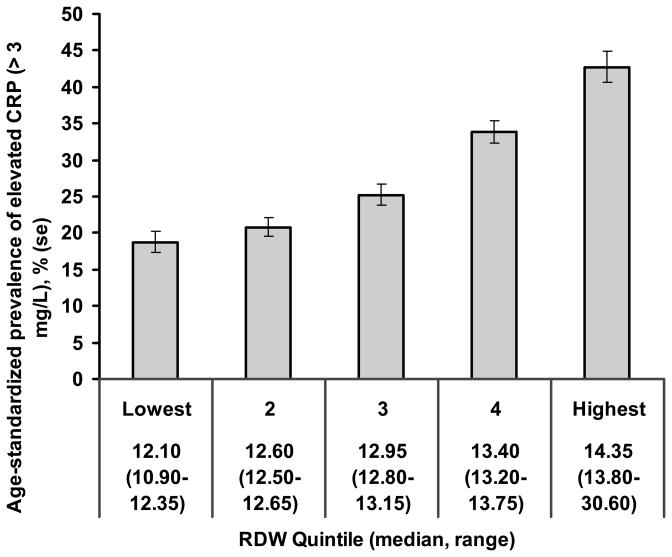
We hypothesized that the association of RDW with mortality risk may in part be due to inflammation promoting both anisocytosis and chronic disease. We therefore examined the association of RDW with the C-reactive protein (CRP) level, a marker of inflammation. The age-standardized prevalence of a high CRP level (> 3 mg/L) increased 2.7-fold from the lowest to the highest RDW quintile (figure 2). Fitting a model in which we regressed RDW on all of the covariates in model 3, as well as CRP level, revealed an independent association between CRP and RDW (P < 0.001). Further, elevated CRP was associated with a 45% increased mortality risk (model 1 HR 1.45, 95% CI 1.30–1.62), an association that was attenuated somewhat by additional adjustment for RDW (HR 1.34, 95% CI 1.20–1.50). Finally, there was no apparent difference in the risk estimates for RDW in subjects with elevated CRP (HR 1.23, 95% CI 1.16–1.30) compared to those with low CRP (HR1.20, 95% CI 1.13–1.27), suggesting that the association of RDW with mortality was not dependent upon the presence of inflammation.
Figure 2. The association of RDW with inflammation in NHANES III.
The age-standardized prevalence of a C-reactive protein level of > 3 mg/L increased significantly across quintiles of RDW (Ptrend < 0.001).
Discussion
The principal finding of this examination of 15,852 community-dwelling adults is that greater red blood cell size heterogeneity, as reflected by higher RDW, is strongly associated with risk of all-cause mortality. In addition, we found that higher RDW predicted CV, cancer, and chronic lower respiratory disease mortality. These results did not appear to be confounded by anemia or anemia-related nutrient deficiencies. Also, the association of RDW with mortality risk was observed across the entire range of RDW and was not driven by RDW levels outside the normal range, as excluding subjects with an abnormal RDW did not attenuate the association of RDW with mortality risk.
The magnitude of risk associated with higher RDW that we observed is similar to that observed in previous examinations of patients with pre-existing cardiovascular disease (5, 6, 18). On average, a 1-sd increment in RDW was associated with a relative risk of all-cause mortality of 1.2, and the highest quintile of RDW and highest quartile in the CARE trial were associated with an approximately 2-fold increased mortality risk.
Our examination of the relation of RDW to cancer and lung disease mortality was prompted by reports of corresponding cross-sectional associations, but no prospective data for these outcomes have been reported. Grant and colleagues found that RDW was inversely associated with lung function in a community-based sample(8). Ozkalemkas et al and Seitenades et al found that elevated RDW was associated with bone marrow involvement in cancer, and Spell and colleagues observed that elevated RDW was common in colon cancer(7), (19). Our findings suggest that the relevance of higher RDW to mortality risk is not specific to a single organ system or process.
The biology underlying the association of higher RDW with mortality risk is unclear at this time. It seems unlikely that anisocytosis itself is a causal factor in risk, but we cannot eliminate this possibility. Increased anisocytosis is most commonly thought of as a consequence of anemia or anemia-related nutrient deficiency. We did not find that the association of RDW with mortality risk is dependent upon these factors. Inflammation may alter erythropoiesis, red blood cell circulation half-life, and red cell membrane deformability, factors that might increase anisocytosis(20). Anemia of chronic disease is in fact characterized by increased RDW, independent of iron status(21). We hypothesized that the association of RDW with mortality risk may be due to underlying inflammation, as inflammation is increasingly appreciated to contribute to the pathogenesis of chronic disease (22–24). Our data support an association of anisocytosis with inflammation, and suggest that the association of RDW with mortality risk may in part be due to an affect of inflammation on both anisocytosis and risk. We did not find that the association of RDW with mortality risk is entirely dependent upon inflammation, as the risk associated with RDW was not significantly diminished in participants with a low CRP level compared with those with a high CRP level. It is also important to note that this dataset cannot determine the temporal association of anisocytosis with inflammation.
Since RDW is widely available, at no additional cost to the routinely performed complete blood count, and is highly reproducible, it may serve as an important biomarker(2, 25). The strength of RDW’s association with mortality risk that we and others have observed compares favorably with established risk factors. It is unknown, however, if the risk associated with RDW is modifiable, or if RDW itself is modified by current therapies that alter prognosis.
The NHANES sample is designed to be representative of the non-institutionalized U.S. population. The comprehensive examination allowed adjustment for many important covariates. The number of events provides stable estimates of risk associated with RDW. These strengths, along with a focused analysis based upon prior work from more restrictive populations, make it less likely that our consistent results are spurious.
A few limitations warrant consideration. It may be that serial measurements of RDW would allow a better characterization of the association of RDW with risk, and our data do not address RDW’s relation to incident non-fatal disease. The association of RDW with mortality risk may be in part due to its association with prevalent disease. We did not find that excluding participants with diagnosed CVD, cancer, or lung disease affected the association of RDW with the respective disease-specific mortality risk, however, an association of RDW with occult disease may in part be responsible for our findings. Perhaps the most important limitation is the uncertainty of the biologic underpinning for greater RDW and its association with risk. We hope that our results provide a stimulus for investigations into mechanisms underlying anisocytosis.
In summary, we have made the novel observation that higher RDW is strongly and independently associated with risk of all-cause and CV, cancer, and chronic lower respiratory disease mortality in a community-based sample. These results suggest that study of anisocytosis may yield important pathophysiological insights, and that RDW may contribute to identifying individuals at higher risk.
Acknowledgments
Funding Sources: Dr. Perlstein was supported by the National Heart Lung and Blood Institute (NHLBI) Training Grant T32 HL07604 and an American College of Cardiology Foundation/Merck Research Fellowship Award. Dr. Weuve was supported by NIH ES R01-ES05257 and Robert Wood Johnson Foundation contract 045821. Dr. Beckman was supported by American Diabetes Association Career Development Award 1-06-CD-01.
Dr. Perlstein had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Conflict of interest statement: We have no conflict of interest, financial or personal, to report.
References
- 1.Perkins SL. Examination of blood and bone marrow. In: Greer JP, Foerster J, Lukens JN, Paraksevas F, Glader BE, editors. Wintrobe’s Clinical Hematology. Vol. 2003. Salt Lake City: Lippincott Wilkins and Williams; 2003. pp. 5–25. [Google Scholar]
- 2.Bessman JD, Hurley EL, Groves MR. Nondiscrete heterogeneity of human erythrocytes: comparison of Coulter-principle flow cytometry and Soret-hemoglobinometry image analysis. Cytometry. 1983;3(4):292–5. doi: 10.1002/cyto.990030411. [DOI] [PubMed] [Google Scholar]
- 3.England JM, Down MC. Red-cell-volume distribution curves and the measurement of anisocytosis. Lancet. 1974;1(7860):701–3. doi: 10.1016/s0140-6736(74)92904-3. [DOI] [PubMed] [Google Scholar]
- 4.PubMed search performed February 7, 2008. Search: Red cell distribution width. Field: Title. Limits: Published within the last 5 years. www.pubmed.gov.
- 5.Felker GM, Allen LA, Pocock SJ, et al. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. J Am Coll Cardiol. 2007;50(1):40–7. doi: 10.1016/j.jacc.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 6.Tonelli M, Sacks F, Arnold M, Moye L, Davis B, Pfeffer M. Relation Between Red Blood Cell Distribution Width and Cardiovascular Event Rate in People With Coronary Disease. Circulation. 2008;117(2):163–168. doi: 10.1161/CIRCULATIONAHA.107.727545. [DOI] [PubMed] [Google Scholar]
- 7.Ozkalemkas F, Ali R, Ozkocaman V, et al. The bone marrow aspirate and biopsy in the diagnosis of unsuspected nonhematologic malignancy: a clinical study of 19 cases. BMC Cancer. 2005;5:144. doi: 10.1186/1471-2407-5-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant BJ, Kudalkar DP, Muti P, et al. Relation between lung function and RBC distribution width in a population-based study. Chest. 2003;124(2):494–500. doi: 10.1378/chest.124.2.494. [DOI] [PubMed] [Google Scholar]
- 9.NHANES III Data Files, Documentation, and SAS Code. National Center for Health Statistics; [Accessed January 30, 2008]. Available at http://www.cdc.gov/nchs/about/major/nhanes/nh3data.htm. [Google Scholar]
- 10.NHANES III Laboratory Data File Documentation. U.S. Department of Health and Human Services; 2006. [Accessed February 28, 2008.11]. Available at http://www.cdc.gov/nchs/data/nhanes/nhanes3/lab-acc.pdf. [Google Scholar]
- 11.Gunter EW, Lewis BG, Konicowski SM. Laboratory Procedures Used for the Third National Health and Nutrition Survey (NHANES III), 1988–1994. U.S. Department of Health and Human Services; 1996. [Accessed February 28, 2008]. Available at http://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/labman.pdf. [Google Scholar]
- 12.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354(23):2473–83. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 13.Coresh J, Astor BC, McQuillan G, et al. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis. 2002;39(5):920–9. doi: 10.1053/ajkd.2002.32765. [DOI] [PubMed] [Google Scholar]
- 14.National Center for Health Statistics, Centers for Disease Control; [Accessed February 28, 2008]. The Third National Health and Nutrition Examination Survey (NHANES III) Linked Mortality File: Matching Methodology. Available at http://www.cdc.gov/nchs/data/datalinkage/matching_methodology_nhanes3_final.pdf. [Google Scholar]
- 15.National Center for Health Statistics, Centers for Disease Control; [Accessed February 28, 2008]. Analytical and reporting guidelines: The Third National Health and Nutrition Examination Survey, NHANES III (1988–1994) Available at http://www.cdc.gov/nchs/data/nhanes/nhanes3/nh3gui.pdf. [Google Scholar]
- 16.Cox D. Regression Models and Life Tables. Journal of the Royal Statistical Society. 1972;34:187–220. Series B. [Google Scholar]
- 17.Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol. 1997;145(1):72–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- 18.Tonelli M, Pfeffer M, Arnold M, et al. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation. doi: 10.1161/CIRCULATIONAHA.107.727545. In press. [DOI] [PubMed] [Google Scholar]
- 19.Spell DW, Jones DV, Jr, Harper WF, David Bessman J. The value of a complete blood count in predicting cancer of the colon. Cancer Detect Prev. 2004;28(1):37–42. doi: 10.1016/j.cdp.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352(10):1011–23. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen OJ, Andersen LS, Ludwigsen E, et al. Anaemia of rheumatoid arthritis: serum erythropoietin concentrations and red cell distribution width in relation to iron status. Ann Rheum Dis. 1990;49(6):349–53. doi: 10.1136/ard.49.6.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 23.Karin M. The IkappaB kinase - a bridge between inflammation and cancer. Cell Res. 2008;18(3):334–42. doi: 10.1038/cr.2008.30. [DOI] [PubMed] [Google Scholar]
- 24.Brody JS, Spira A. State of the art. Chronic obstructive pulmonary disease, inflammation, and lung cancer. Proc Am Thorac Soc. 2006;3(6):535–7. doi: 10.1513/pats.200603-089MS. [DOI] [PubMed] [Google Scholar]
- 25.Morrow DA, de Lemos JA. Benchmarks for the assessment of novel cardiovascular biomarkers. Circulation. 2007;115(8):949–52. doi: 10.1161/CIRCULATIONAHA.106.683110. [DOI] [PubMed] [Google Scholar]