Many innovative cell therapies that directly relate to hematopoietic stem cell transplantation are at an early stage of development and until recently, most were not ready to progress to later phase multicenter studies. However as the field has matured, it is likely that some strategies will be ready to test in later phase studies and pathways will need to be established for undertaking such trials.
The Blood and Marrow Transplant Clinical Trials Network (BMT CTN) anticipates being able to provide infrastructural support for a limited number of such studies when the goal of the novel cell therapy is to improve the outcome of transplant by reconstituting antitumor or anti-infectious immunity or modulating alloreactivity or to use transplant as a means of genetic correction for inherited diseases. This brief report is intended to assist investigators by outlining the process by which such studies can be submitted, and reviewed and implemented by the BMT CTN.
The BMT CTN was established in 2001 by the National Heart, Lung, and Blood Institute (NHLBI) and the National Cancer Institute (NCI) as an infrastructure to conduct multi-institutional Phase II and Phase III trials focused on improving outcomes in blood and marrow transplantation (BMT). The BMT CTN is a network of 16 Core Transplant Centers/Consortia, more than 60 Affiliated Transplant Centers, and a Data Coordinating Center (DCC). Its activities are overseen by a Steering Committee composed of representatives from the Core and Affiliate Centers, the DCC, NHLBI and NCI (www.bmtctn.net).
Many prominent advances in clinical oncology have resulted from cooperative group trials, underscoring the importance of collaborative clinical trials to the medical community. The BMT CTN is conducting more than 20 Phase II and Phase III trials to address issues critical to BMT patients. Although the results of most of these trials are still forthcoming, these studies have the potential to change practice and establish a new standard of care. Eleven BMT CTN trials have completed enrollment; patient follow-up continues on five of these, with data analysis in progress or recently completed for the others. Accrual continues on 11 trials with four additional trials expected to open in 2010. Although the BMT CTN considers issues relevant to both autologous and allogeneic BMT, it has a major focus on allogeneic transplantation. Of the 400–600 patients enrolled on BMT CTN trials annually, more than 80% are allogeneic transplant recipients. The BMT CTN infrastructure may be used to conduct multi-institutional Phase II studies that would either establish the efficacy of novel cell therapies to a level that would justify a large Phase III trial. For rare diseases or indications, licensure may be possible with a smaller study - for example European investigators have received orphan drug designation from the European Medicines Agency for gene transfer to hemopoietic stem cells to treat ADA deficiency based on studies with small numbers of patients. Study requirements for specific indications should be discussed with the FDA. A number of steps need to be followed for a cell therapy phase II/III trial to be supported by the BMT-CTN.
1. PRIOR TO CONCEPT SUBMISSION TO THE BMT CTN
Phase I Clinical Trial Data
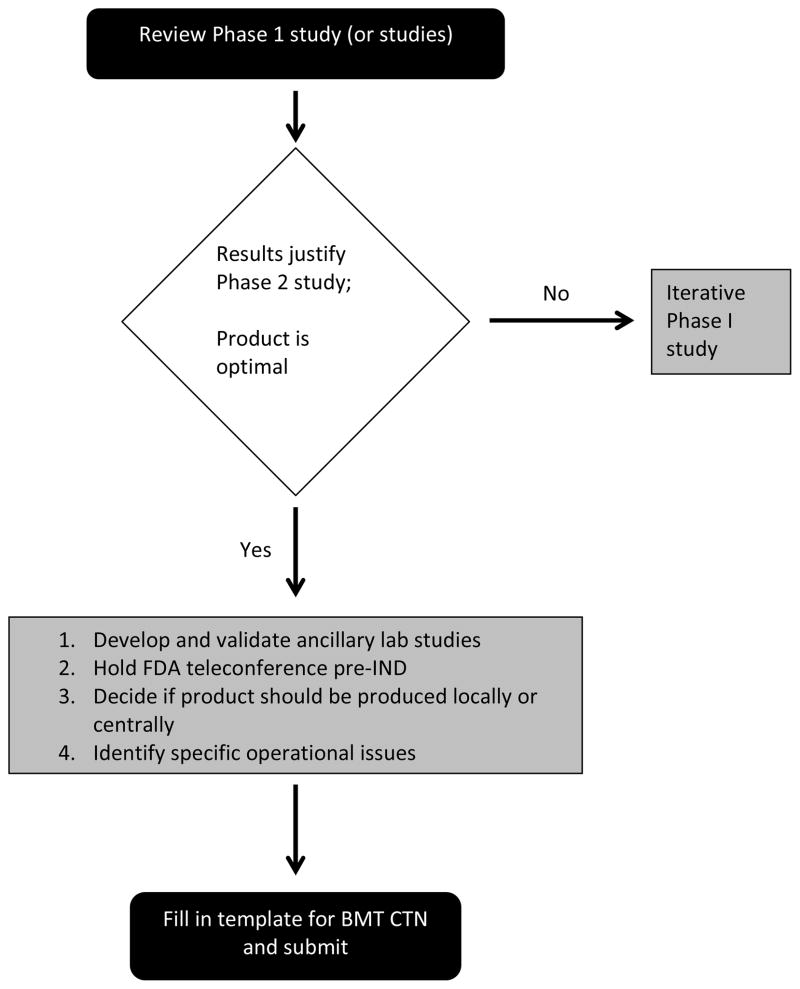
The most important single element in the development of a Phase II trial proposal is the acquisition of appropriate Phase I data to support the safety of the cell product and its therapeutic application, as well as to provide at least preliminary evidence of activity sufficient to warrant the investment of time and resources required for larger multi-institutional studies. The body of data required will vary among trials; however, greater clinical risk or greater financial burden will mandate more substantial Phase I data. In general, the Phase I data should include a minimum of 15 patients in an adult study or 12 patients in pediatrics, with an acceptable safety profile for the cell therapy product although smaller number may suffice for orphan diseases if response rates are high and endpoints for safety are met. Because of the resources required to initiate and complete a multicenter Phase II study are considerable, it is important that investigators be confident that they have identified the precise product with which they will proceed. Complex biological therapies such as cell therapies require multiple parameters to be optimized, such as the type of target cell, manufacturing processes, and choice of vector. It may be necessary to perform iterative Phase I studies or review the results of multiple Phase I studies from different centers to ensure that an optimal product is chosen before proceeding to later phase studies. It is also advantageous if the investigators develop and conduct ancillary laboratory studies, for example by using validated biomarkers of efficacy.
Regulatory Considerations
It is recommended that the Phase I study sponsor hold a End-of-Phase 1 (EOP1) conference with the FDA review team to document that the Agency does not identify any major barriers to proceeding to a Phase II trial based on the available Phase I data. The concept for the proposed Phase II study should be discussed at that time to allow the Agency an opportunity to advise whether a new IND will be needed, or whether the current IND can be amended. It may be appropriate to submit a clinical development plan for FDA review. Although the resources of the BMT CTN are substantial they are not unlimited and so the leadership of the BMT CTN and the NHLBI/NCI will only be able to accept the most promising studies. Hence, FDA approval does not guarantee that the BMT CTN will decide to undertake a particular trial.
Product Preparation and Distribution
In most cases, cell therapy trials will involve processing human cells in the laboratory. Many trials include genetic modification of cells by viral or non-viral DNA transfer. Although establishing manufacturing processes is necessary for Phase I trials, many new manufacturing challenges exist when a multicenter trial is planned. Hence, investigators are strongly encouraged to consider using the Production Assistance for Cellular Therapies (PACT), an NHLBI-supported cell processing program (http://www.pactgroup.net/index2.htm). This program provides a central manufacturing site for trials and can assist with technical aspects of manufacturing, such as scale-up, SOP development, assay development, and phenotype determination as well as quality assurance and quality control. Once an application is accepted by PACT, all agreed upon services are provided free of charge to the investigators. If the study involves gene transfer, the NHLBI Gene Therapy Resource Program (GTRP) is a resource for providing clinical grade lentiviral and AAV vectors (http://www.gtrp.org/). For other vectors, investigators must identify and secure funding for manufacture by an academic center (see http://www.aabmonline.org/ for a listing) or a commercial supplier.
Operational Issues
Most operational issues will be addressed during the protocol development process after a proposal is accepted by the BMT CTN. However, proposals should discuss any potential problems, the expense of replication competent retrovirus (RCR) testing if a product is genetically modified with retroviral vectors or the need for long term follow up needed for subjects receiving integrating vectors.
Issues that should be addressed prior to submission are outlined in Table 1
Table 1.
Implementation of Phase II Cell Therapy Trial in the BMT CTN
| Area | Tasks |
|---|---|
| Production | Develop budget Decide whether central versus distributed production Submit a proposal to PACT if central production Develop and validate SOPs Validate shipping if required |
| Regulatory | Define needed reviews Decide on IND sponsor Develop MOU to assign responsibilities Establish sequence and timeline of required reviews Review of clinical protocol by the NHLBI PRC and DSMB Write and submit RAC submission if needed Write Investigator’s brochure Write and submit IND to the FDA Select Sites IRB (and IBC if needed ) review at sites |
| Operational | Map protocol and develop SOPs Develop case report forms Develop database Finalize correlative studies |
2. SUBMISSION AND REVIEW
Submission of Proposal
Investigators interested in exploring the feasibility of conducting a novel cell therapy trial in the BMT CTN should submit a proposal to the BMT CTN DCC. The template for BMT CTN proposals is simple and can be obtained by contacting the BMT CTN DCC at bmtctn@emmes.com. For all trials, investigators must provide the following elements:
Hypothesis
Specific Aims
Background
Eligibility criteria
Treatment Plan
Primary Outcome
Secondary Outcomes
Basic Study Design:
Timeline
Funding sources
In cell therapy studies investigators should also include a discussion of regulatory issues, the EOP1 minutes if available and preliminary plans for product manufacture and distribution.
Proposal Review
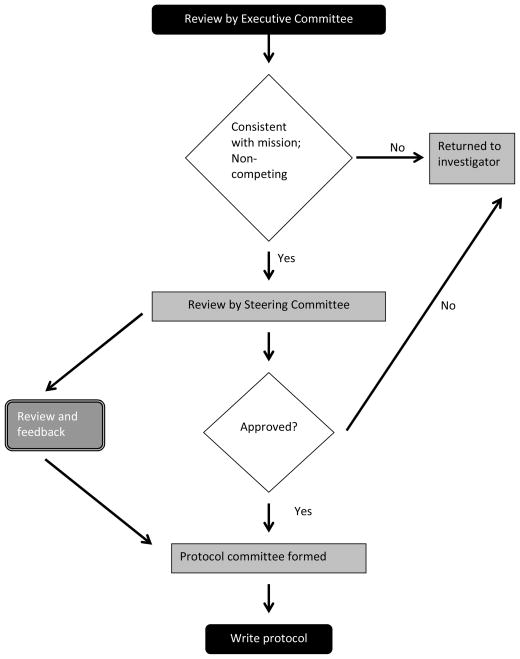
Proposals are reviewed by the BMT CTN Executive Committee which meets monthly by conference call. Proposals considered consistent with the BMT CTN’s mission and not conflicting with ongoing studies are then referred to the full Steering Committee during its in-person meetings, which occur thrice yearly, usually in February, July and October. Investigators are invited to present their proposals to the Committee, but this is not required. Proposals are approved and prioritized based on scientific merit, feasibility, expected participation of Network centers and funding availability. Cell therapy trials will be evaluated by a group of experts in the field charged with providing their recommendations to the Steering Committee. The investigator submitting the concept should expect that review will be an iterative process, in which they receive input on study design and logistics as the protocol is refined. If the proposal is selected to move forward, a protocol writing committee will be formed which will include the investigator submitting the concept, members from the steering committee and participating centers, the DCC, and individuals with particular expertise in the target disease or application. For a cell therapy application, there would be representation from PACT or the manufacturing centers involved in production of cellular products or vectors. This group would write the protocol, reporting to the steering committee and receiving feedback during the process. The protocol writing committee will simultaneously work with the DCC to consider implementation issues. The review process is summarized in Table 2.
3. IMPLEMENTATION
Regulatory
Cell therapy trials will, in most cases, require an Investigational New Drug (IND) Application approval from the FDA. The research team must determine who (individual or institution) will assume responsibility as the sponsor and hold the IND. Additionally, an individual must be designated as the Principal Investigator and accept the corresponding responsibilities. A detailed description of the responsibilities of the sponsor and site investigators for a multi-institution trial can be obtained on the FDA website (http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=312&showFR=1&subpartNode=21:5.0.1.1.3.4). While extramural funding for cell therapy trials is available, most agencies, including the NIH and the BMT CTN, might decide not to act as a study sponsor.
One of the FDA requirements for cell therapy studies is submission of a detailed data and safety monitoring plan. The DCC for the BMT CTN can take responsibility for some of these functions, such as audits and maintenance of regulatory approvals. Additionally, all BMT CTN studies are monitored by an NHLBI-appointed Data and Safety Monitoring Board. If neither the NIH nor BMT CTN elects to serve as study sponsor, a memorandum of understanding must be developed between the BMT CTN and the sponsor delineating the BMT CTN’s scope of responsibilities. This must also be clearly outlined in the data and safety monitoring plan submitted to the FDA. The exact division of responsibilities does not have to be worked out in advance but investigators are encouraged to discuss this issue with the BMT CTN leadership and to propose a tentative plan in their proposals.
For multicenter studies the FDA will also require the sponsor to develop an Investigator’s Brochure that should be reviewed annually, and updated as needed. Instructions for preparing an Investigator’s Brochure can be found on the FDA website (http://www.fdaregulatory.com/ichgcp/brochure.html). The DCC can assist with this activity for some trials.
Each participating institution will be required to obtain approval for the protocol from their local Institutional Review Board (IRB). This task can be labor intensive for many investigators so institutions should be selected, in part, based on their capacity to enroll sufficient numbers of patients to justify seeking IRB approval. Nomination of participating centers should be a part of the proposal and should be discussed with the DCC in advance. The latter can provide data on center-specific transplant activity to assist in these determinations. In some cases, the DCC can facilitate surveys of centers to determine whether they have the necessary capabilities (in addition to an adequate patient population) to participate in specific protocols. If the proposed study utilizes a gene transfer protocol, then the Recombinant DNA Advisory Committee of the Office of Biotechnology Activities and each local Institutional Biosafety Committee (IBC) must approve the final protocol. The time and sequence for these approvals must be considered in estimating a timeline for the study in the proposal and experience of centers in obtaining IBC approval should also be considered in center selection.
Production
In making final decisions on cell production, the research team must determine if the cell product is best produced locally by each participating institutions, or centrally by a single laboratory, which then distributes the product to the participating institutions. Local production will limit participation to institutions that maintain appropriate manufacturing facilities and will require standard operating protocols (SOPs) to ensure product consistency and quality at multiple production sites. Evidence that distributed production of a quality product is feasible must be provided. If the product is to be produced centrally, the site of production must be considered. The production facility must devise SOPs for distribution and validate these protocols, focusing on the integrity of the cell product after it arrives at the treating institution. A budget will be developed and funding considerations may influence decisions re centralized or distributed production. As the cost of manufacture and testing for cell products, particularly those that are genetically modified and require additional testing, is high it may be necessary to seek additional funding, or consider utilizing resource programs available through NIH for clinical grade cellular products (PACT) and vectors (GTRP).
Operational Issues
Many operational issues will be study-specific. Investigators will need to map all protocol processes and develop SOPs. Case report forms previously developed by the BMT CTN will be available for some study endpoints but study-specific forms will also be required. Investigators will also need to decide if correlative studies should be performed locally or centrally and either validate comparability of assays or shipping conditions. PACT can assist with shipping and/or assay validation.
Summary
The BMT CTN infrastructure can facilitate trials for innovative multi-institutional Phase II trials of cell therapy relevant to BMT. However, there are several key considerations for these Phase II proposals. First, the research team should have sufficient Phase I data to justify the resources required for a large multicenter clinical trial. Second, cell product preparation and distribution processes should be developed and qualified, and the various aspects of cell processing assistance should be determined. If the PACT resource is being considered, the application procedure for requesting manufacturing assistance is available on their website. Finally, a tentative plan for operation of the study by the sponsor should be developed prior to submission of the proposed study to the BMT CTN. Additional questions regarding the development of protocols intended for BMT CTN submission can be directed to bmtctn@emmes.com. Proposals can be submitted to the BMT CTN electronically to the same address. All investigators are encouraged to utilize this valuable resource.
Figure 1.
Pre Submission
Figure 2.
Review of Concept by BMT CTN
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.