Abstract
Introduction
The aim of this study was to determine whether the degree of fluid responsiveness in critically ill septic patients is related to baseline mixed venous oxygen saturation (SvO2) levels. We also sought to define whether fluid responsiveness would be less likely in the presence of a high SvO2 (>70%).
Methods
This observational study was conducted in a 32-bed university hospital medicosurgical ICU. The hemodynamic response to a fluid challenge was evaluated in 65 critically ill patients with severe sepsis. Patients were divided into two groups (responders and nonresponders) according to their cardiac index (CI) response to the challenge (>10% or <10%).
Results
Of the 65 patients, 34 (52%) were fluid responders. Baseline SvO2, CI, heart rate (HR) and mean arterial pressure (MAP) were not statistically different between groups. The responders had lower pulmonary artery occlusion pressure (PAOP) and central venous pressure (CVP) at baseline than the nonresponders. After the fluid challenge, there were no differences between the two groups in MAP, CVP, PAOP or HR. There was no correlation between changes in CI or stroke volume index and baseline SvO2. Receiver operating characteristic analysis showed that SvO2 was not a predictor of fluid responsiveness.
Conclusions
The response of septic patients to a fluid challenge is independent of baseline SvO2. The presence of a high SvO2 does not necessarily exclude the need for further fluid administration.
Introduction
Patients with severe sepsis and septic shock typically have decreased vascular tone, with a high cardiac index (CI), low systemic vascular resistance and elevated mixed venous oxygen saturation (SvO2). Fluid resuscitation is essential for the restoration and maintenance of adequate intravascular volume to improve and maintain organ perfusion [1-4]. Natural or artificial colloids or crystalloids may be used for this purpose, as no differences in outcome have been reported related to the type of fluid [5]. As fluid requirements are not easily determined, a fluid challenge technique should be used on a repeated basis according to the patient's response (for example, an increase in blood pressure) and tolerance (for example, excessive increase in cardiac filling pressure) [6-8].
By rearranging the Fick equation, SvO2 can be defined as the balance between four variables: CI, hemoglobin, oxygen saturation and oxygen consumption (VO2). Monitoring of SvO2 therefore allows assessment of total tissue oxygen balance and helps the clinician to determine whether CI and oxygen delivery (DO2) are high enough to meet the patient's needs [9,10]. The well-known study by Rivers et al. [4] indicated that targeting a mixed central venous oxygen saturation (ScvO2) level greater than 70% during early resuscitation of patients with severe sepsis may improve outcomes. However, the measurement of ScvO2 or SvO2 cannot provide complete information about the reason for the inadequacy between systemic oxygen delivery and demands, and whether these measures can guide therapy in septic patients is unclear. A normal or high SvO2 level suggests an adequate CI for tissue energy demands, but may not always indicate adequate fluid resuscitation. Further fluids may be administered in the presence of a normal or high SvO2 level to further increase the CI, but whether a fluid challenge is still worth trying when SvO2 or ScvO2 has reached 70% has not been well defined.
The aim of the present study was to test the hypothesis that preinfusion SvO2 values could help predict the response to fluid challenge in critically ill septic patients. We also wanted to define fluid responsiveness in the presence of high SvO2 (>70%).
Materials and methods
In this retrospective study, we reviewed prospectively collected data from patients who had been admitted to a 32-bed university hospital mixed medical-surgical ICU between January 2006 and December 2009. Approval was obtained from the Ethics Committee of Erasme Hospital, and informed consent was waived because of the observational nature of the study. Patients were included if they had met standard criteria for severe sepsis [11], received a fluid challenge during their ICU stay, had a pulmonary artery catheter placed in situ and had complete hemodynamic data in our computerized database of hemodynamic profiles. Patients with acute coronary syndrome or a history of cardiac disease were not included, and patients younger than 18 years of age were also excluded.
Disease severity was evaluated by calculation of the Sequential Organ Failure Assessment (SOFA) score [12]. Hemodynamic measurements, arterial oxygen saturation and SvO2 values taken before and after fluid challenge were recorded. In our department, fluid challenges are performed when there is a suspicion of hypovolemia based on clinical signs such as oliguria, tachycardia or hypotension. Fluid challenges are conducted according to a standard procedure [8] using 500 mL of colloid or 1,000 mL of crystalloid administered over 30 minutes. Fluids consisted of synthetic colloids (gelatin or pentastarch; Fresenius, Bad Homburg, Germany), albumin 4% (Albumex; CSL, Leuven, Belgium) or crystalloids (Hartmann solution or 0.9% saline solution; Baxter, Lessines, Belgium). The choice of fluid was left to the attending physician. The decision to stop the fluid challenge was based on predetermined safety limits for each patient according to a standard procedure. Respiratory support settings were unchanged during the fluid challenge, and no therapeutic interventions were allowed until new hemodynamic measurements were calculated. Fluid responders were defined as those patients in whom CI increased by at least 10% after fluid challenge.
Statistical analysis
Statistical analysis was performed using SPSS software (SPSS Inc., Chicago, IL, USA). Student's t-test was used for continuous variables. Pearson's correlation was applied. Receiver operating characteristic (ROC) curve analysis was used to assess the predictive ability of fluid responsiveness for SvO2, central venous pressure (CVP) and pulmonary artery occlusion pressure (PAOP) values. P < 0.05 was considered statistically significant.
Results
Patient characteristics and demographic data are presented in Table 1. Of the 65 patients, 34 (52%) were fluid responders. Among the 34 fluid responders, 17 (50%) had septic shock, and 19 (61%) of the 31 fluid nonresponders had septic shock. The source of sepsis was the respiratory system in 25 patients (38.4%), the abdomen in 16 patients (24.6%), the urinary tract in 8 patients (12.3%) and the bloodstream in 16 patients (24.6%). Sixty of the patients received mechanical ventilation (29 of 31 nonresponders and 31 of 34 responders) in volume-controlled mode (tidal volume 6 to 8 mL/kg and positive end-expiratory pressure (PEEP) 6 to 12 cm H2O) or pressure support mode with PEEP 6 to 10 cm H2O. Hemodynamic values for fluid responders and nonresponders before and after the fluid challenge are shown in Tables 2 and 3. There were no statistically significant differences between the groups at baseline, except for lower PAOP (P = 0.003) and CVP (P = 0.008) levels in the responders than in the nonresponders (Figure 1 and Table 2). There were no significant differences in any of the measured variables between the groups after the fluid challenge (Table 2).
Table 1.
Patients' characteristics and interventions during ICU stay and outcomesa
| Characteristics | Nonresponders (n = 31) | Responders (n = 34) | P value |
|---|---|---|---|
| Mean age, years | 69 ± 14 | 71 ± 9 | 0.69 |
| Medical/surgical | 22/9 | 9/25 | 0.21 |
| Admission SOFA score | 10 ± 2 | 10 ± 4 | 0.98 |
| Mechanical ventilation | 29 | 31 | 0.54 |
| Dobutamine/norepinephrine | 18/19 | 16/17 | 0.58 |
| Hartmann/hetastarch/albumin | 19/4/8 | 23/6/5 | 0.17 |
| Mortality | 18 (58%) | 18 (53%) | 0.43 |
aSOFA: Sequential Organ Failure Assessment. Data are presented as mean ± SD, n, or n (%).
Table 2.
Hemodynamic values in fluid responders and nonresponders before and after fluid challengea
| Before fluid challenge | After fluid challenge | |||||
|---|---|---|---|---|---|---|
| Parameters | Nonresponders (n = 31) | Responders (n = 34) | P value | Nonresponders (n = 31) | Responders (n = 34) | P value |
| Mean arterial pressure, mmHg | 71 ± 8 | 71 ± 9 | 0.79 | 76 ± 8 | 77 ± 9 | 0.58 |
| Heart rate, beats/minute | 105 ± 21 | 103 ± 17 | 0.71 | 102 ± 21 | 98 ± 18 | 0.71 |
| Central venous pressure, mmHg | 12 ± 4 | 9 ± 4 | 0.008 | 14 ± 3 | 12 ± 4 | 0.18 |
| Pulmonary artery occlusion pressure, mmHg | 14 ± 3 | 12 ± 3 | 0.003 | 17 ± 6 | 14 ± 5 | 0.18 |
| Mixed venous oxygen saturation, % | 67 ± 9 | 67 ± 7 | 0.80 | 67 ± 9 | 71 ± 6 | 0.08 |
| Cardiac index, L/minute/m2 | 3 ± 0.9 | 2.9 ± 0.8 | 0.73 | 3.2 ± 0.6 | 3.6 ± 0.9 | 0.11 |
aData are presented as mean ± SD.
Table 3.
Hemodynamic values before and after fluid challenge in fluid responders and nonrespondersa
| Nonresponders (n = 31) | Responders (n = 34) | |||||
|---|---|---|---|---|---|---|
| Parameters | Before | After | P value | Before | After | P value |
| Mean arterial pressure, mmHg | 71 ± 8 | 76 ± 8 | 0.01 | 71 ± 9 | 77 ± 9 | <0.01 |
| Heart rate, beats/minute | 105 ± 21 | 102 ± 21 | 0.12 | 103 ± 17 | 98 ± 18 | 0.04 |
| Central venous pressure, mmHg | 12 ± 4 | 14 ± 3 | <0.01 | 9 ± 4 | 12 ± 4 | 0.01 |
| Pulmonary artery occlusion pressure, mmHg | 14 ± 3 | 17 ± 6 | 0.02 | 12 ± 3 | 14 ± 5 | <0.01 |
| Mixed venous oxygen saturation, % | 67 ± 9 | 67 ± 9 | 0.97 | 67 ± 7 | 71 ± 6 | <0.01 |
| Cardiac index, L/minute/m2 | 3 ± 0.9 | 3.2 ± 0.6 | 0.23 | 2.9 ± 0.8 | 3.6 ± 0.9 | <0.01 |
aData are presented as mean ± SD.
Figure 1.
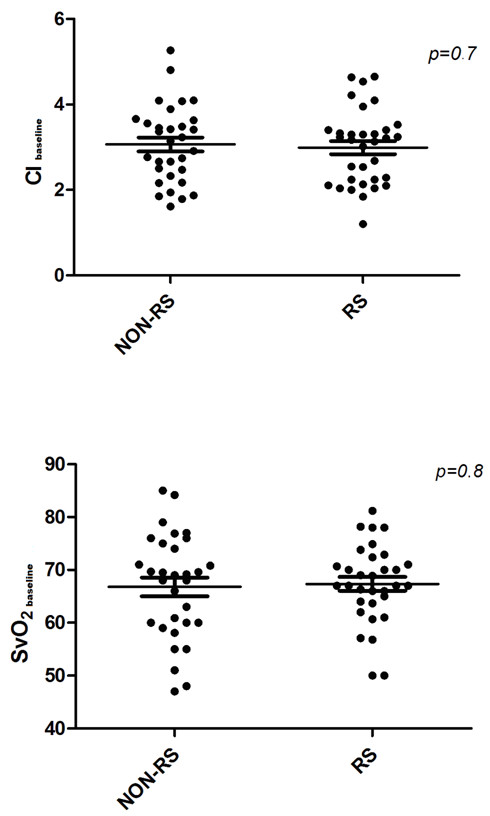
Scatterplots of baseline CI (top) and SvO2 (bottom) values in responders (RS) and nonresponders (NON-RS). CI: confidence interval; SvO2: mixed venous oxygen saturation. Thin lines represent mean values, and thick lines the standard errors.
SvO2 values in responders and nonresponders are shown in Table 4. There were no differences in baseline lactate levels between patients with higher (>70%) or lower (<70%) SvO2 levels (means ± SD: 2.1 ± 1 mg/dL vs. 2.6 mg/dL ± 1.9, respectively; P = 0.279). There were no correlations between change in CI (%DCI) or change in stroke volume index (DSVI%) and baseline SvO2 in all patients (Figure 2). In the subgroup of patients with high baseline SvO2 levels (>70%), there was also no correlation between %DCI and SvO2 levels. Similar findings were observed in the subgroup of patients with lower SvO2 values (<70%).
Table 4.
Numbers of fluid responders and nonresponders in different ranges of SvO2 valuesa
| SvO2 | Nonresponders (n = 31) | Responders (n = 34) | Proportion of responders |
|---|---|---|---|
| <50% | 3 | 2 | 40% |
| 50% to 60% | 8 | 4 | 33% |
| 60% to 70% | 8 | 15 | 65% |
| >70% | 12 | 13 | 52% |
aSvO2: mixed venous oxygen saturation.
Figure 2.
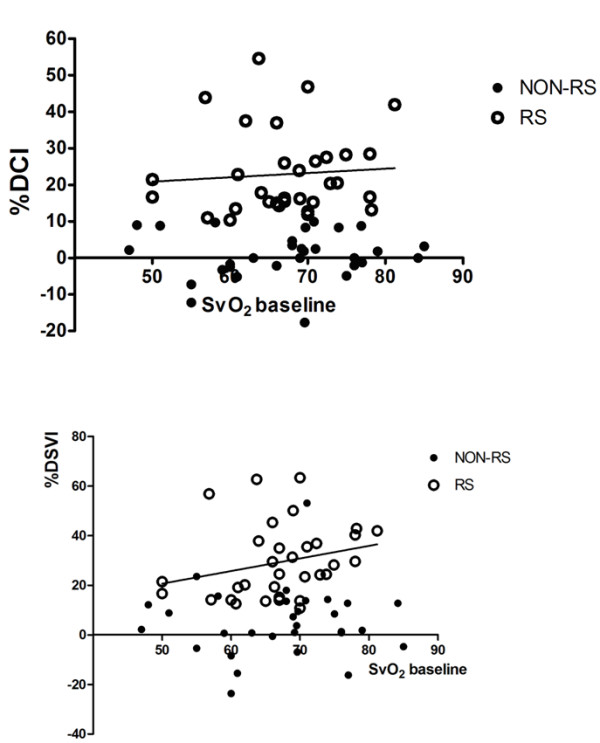
Relationship between baseline SvO2 levels and changes in cardiac index (%DCI) and stroke volume (%DSVI) in all patients (responders [RS] and nonresponders [NON-RS]). SvO2: mixed venous oxygen saturation.
ROC curves showed that baseline SvO2 level was not a good predictor of %DCI after fluid challenge (area under the curve (AUC) = 0.51, 95% confidence interval = 0.36 to 0.66; P = 0.85). Baseline CVP was an adequate predictor of %DCI (AUC = 0.68, 95% confidence interval = 0.54 to 0.83; P = 0.01), but with an unsatisfactory sensitivity and specificity (67% and 54%, respectively). Similar findings were noted for PAOP (AUC = 0.71, 95% confidence interval = 0.58 to 0.86; P = 0.04) (sensitivity and specificity, 59% and 65%, respectively) (Figure 3).
Figure 3.
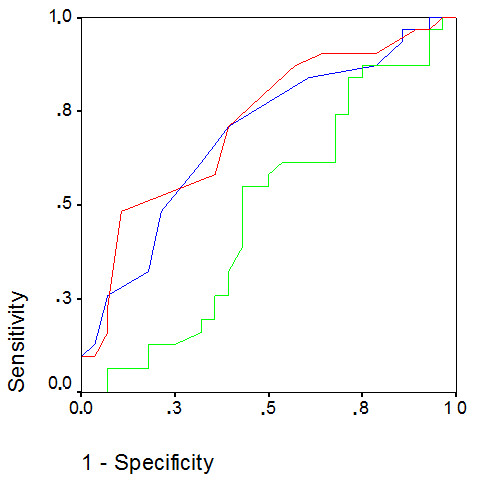
ROC curves for baseline values of SvO2 (green line), CVP (blue line) and PAOP (red line). Diagonal segments are produced by ties. AUC were 0.51, 0.68 and 0.71 for SvO2, CVP and PAOP, respectively. CVP at a cutoff of 11 mmHg had a sensitivity of 67% and a specificity of 54% for predicting fluid responsiveness. PAOP at a cutoff of 13 mmHg had a sensitivity of 59% and a specificity of 65%. AUC: area under the curve; CVP: central venous pressure; PAOP: pulmonary artery occlusion pressure; ROC: receiver operating characteristic; SvO2: mixed venous oxygen saturation.
Discussion
The main finding of our study is that the response to fluid challenge was independent of baseline SvO2 levels, with no correlation between baseline SvO2 levels and %DCI or DSVI% after fluid challenge. This is an important new finding. Moreover, high SvO2 levels did not correlate with a specific response to fluid challenge, as 13 (52%) of the 25 patients with SvO2 >70% responded to fluid challenge. SvO2 levels do not, therefore, predict responsiveness to fluid challenge in patients with severe sepsis.
The treatment of patients with severe sepsis and septic shock aims to restore and maintain hemodynamic parameters at levels that are able to sustain tissue and cellular perfusion. Fluid resuscitation plays a key role in this therapeutic strategy, and hemodynamic monitoring plays an important role in optimizing management. Low SvO2 is often associated with an inadequate CI, low arterial oxygen content or increased VO2 by the tissues and thus may suggest the need for an intervention to increase DO2 to the tissues. A main target in the early goal-directed therapy protocol in septic patients proposed by Rivers and co-workers [4] is to maintain SvO2 at least 70% and not to target a specific CI value. However, the patients in their study were in the emergency department before fluid resuscitation, and these patients had unexpectedly low ScvO2 values. ScvO2 is a measure of the oxygenation in blood coming from the upper body and cannot provide information about the global inadequacy between systemic oxygen delivery and tissue oxygen demands in such patients. As the pulmonary artery carries blood from all vascular beds, SvO2 better reflects the amount of oxygen left after passage through the tissues. However, evaluation of SvO2 may not provide a correct assessment of tissue oxygenation, particularly in the setting of septic shock, when oxygen extraction is altered. Nevertheless, SvO2 values must be interpreted within the context of the overall hemodynamic profile in septic patients [5,13]. SvO2 is still a global parameter and gives no specific information on regional tissue oxygenation, but neither does CI [14]. Importantly, a low SvO2 value is a warning sign of potential inadequacy of oxygen delivery for tissue demands, suggesting the need to increase oxygen delivery to the tissues with further fluids or transfusions or dobutamine administration. However, a normal or high SvO2 level does not necessarily indicate that oxygen metabolism is entirely normalized and does not exclude the presence of persisting tissue hypoxia. Microcirculatory shunting in sepsis can result in a normal SvO2 level despite local tissue dysoxia, and patients can develop multiple organ failure and die with supranormal SvO2 values [14].
Conversely, several patients may have responded to fluid administration, even though they had an adequate CI. Fluid responsiveness does not necessarily mean that fluid administration is mandatory. We cannot verify whether the increase in CI in the patients with a high SvO2 was associated with improved outcomes, as this was not the aim of the study. In our study, we chose a cutoff SvO2 value of 70% as suggested by Pinsky and Vincent [15], because our patients were at the late stage of sepsis, and not 65% as proposed by the Surviving Sepsis Campaign Guidelines, which referred to the early resuscitation phase [5]. Nevertheless, it is not clear which value should be considered as normal for septic patients in the ICU. In sepsis, DO2-VO2 relationships are altered and arterioventricular shunting may increase SvO2. Correction of hypovolemia with fluids and restoration of the distributed microcirculation is necessary, but SvO2 alone seems to be an inadequate parameter as a guide for therapy. Gattinoni et al. [16], in a study of surgical patients admitted to the ICU after developing organ failure, failed to show improved outcomes with therapies aimed at maintaining either DO2 or SvO2 at supranormal values. In a meta-analysis, Heyland et al. [17] demonstrated that therapy targeted at supraphysiologic end points (DO2 and VO2) was not associated with decreased mortality. Although it is impossible to establish an absolute "normal" value of SvO2, in most clinical situations, SvO2 levels ranging from 60% to 70% suggest that tissue DO2 is adequate. Usually, changes in SvO2 are more informative than absolute SvO2 values. The results of our study suggest that SvO2 and, in particular, high SvO2 levels cannot serve as predictors of fluid responsiveness in critically ill septic patients, as 52% of septic patients with SvO2 >70% responded to fluids by increasing their CI values >10%.
There was no significant difference in other baseline variables between responders and nonresponders, except for CVP and PAOP, which were lower in the responders. Furthermore, the ROC analysis showed poor sensitivity and specificity for CVP and PAOP, indicating that targeting volume therapy to these filling pressure values should be discouraged. Our results are in agreement with those found in other clinical studies that showed that CVP and PAOP were not reliable predictors of volume responsiveness in sepsis [3,18-20]. Some authors have demonstrated that filling pressures have a low predictive value for estimating fluid responsiveness during mechanical ventilation in septic patients and suggested that using them to guide fluid therapy can lead to inappropriate therapeutic decisions [21,22]. In a recent systematic review of the literature, researchers found a lack of agreement on hemodynamic goals for the management of sepsis and proposed that this lack of consistency may contribute to heterogeneity in treatment effects in clinical trials of novel sepsis therapies [23]. It is likely that many variables need to be assessed together at the bedside to enable the most effective treatment of patients.
The present study has some limitations. First, we do not have information on the exact time of onset of severe sepsis; it would have been interesting to differentiate patients with early and late sepsis. Furthermore, this retrospective, observational study was conducted with a relatively small sample size and a highly selected cohort in that only patients who required a pulmonary artery catheter were included. Nevertheless, it represents a useful pilot study for further prospective investigations with larger numbers of patients.
Conclusions
Although SvO2 monitoring has been shown to be a useful tool to evaluate the balance between oxygen consumption and supply, SvO2 levels before fluid challenge are poor predictors of fluid responsiveness in patients with severe sepsis. The presence of a normal or high SvO2 does not necessarily exclude the need for further fluid administration.
Key messages
• Evaluation of SvO2 in patients with sepsis cannot provide information about the reason for the inadequacy between oxygen delivery and demand.
• The presence of a normal or high SvO2 level does not necessarily indicate that oxygen metabolism is normalized.
• The presence of a normal or high SvO2 level does not exclude the need for the administration of further fluids.
• SvO2 levels are poor indicators of fluid responsiveness in septic patients.
Abbreviations
AUC: area under the curve; CI: cardiac index; CVP: central venous pressure; DO2: oxygen delivery; HR: heart rate; MAP: mean arterial pressure; PAOP: pulmonary artery occluded pressure; PEEP: positive end-expiratory pressure; ROC: receiver operating characteristic; ScvO2: central venous oxygen saturation; SOFA: Sequential Organ Failure Assessment; SVI: stroke volume index; SvO2: mixed venous oxygen saturation; VO2: oxygen uptake.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
DV participated in the design of the study, collected the data, performed the statistical analysis and drafted the manuscript. CP and SS helped collect the data. DDB and JLV participated in the design of the study and helped revise the manuscript. All authors read and approved the final manuscript.
Contributor Information
Dimitrios Velissaris, Email: dimitrisvelissaris@yahoo.com.
Charalampos Pierrakos, Email: charalampos_p@hotmail.com.
Sabino Scolletta, Email: scolletta@unisi.it.
Daniel De Backer, Email: ddebacke@ulb.ac.be.
Jean Louis Vincent, Email: jlvincen@ulb.ac.be.
References
- Anning PB, Finney SJ, Singh S, Winlove CP, Evans TW. Fluids reverse the early lipopolysaccharide-induced albumin leakage in rodent mesenteric venules. Intensive Care Med. 2004;30:1944–1949. doi: 10.1007/s00134-004-2385-3. [DOI] [PubMed] [Google Scholar]
- Haupt MT, Gilbert EM, Carlson RW. Fluid loading increases oxygen consumption in septic patients with lactic acidosis. Am Rev Respir Dis. 1985;131:912–916. doi: 10.1164/arrd.1985.131.6.912. [DOI] [PubMed] [Google Scholar]
- Tavernier B, Makhotine O, Lebuffe G, Dupont J, Scherpereel P. Systolic pressure variation as a guide to fluid therapy in patients with sepsis-induced hypotension. Anesthesiology. 1998;89:1313–1321. doi: 10.1097/00000542-199812000-00007. [DOI] [PubMed] [Google Scholar]
- Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;34:17–60. doi: 10.1007/s00134-007-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002;121:2000–2008. doi: 10.1378/chest.121.6.2000. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Gerlach H. Fluid resuscitation in severe sepsis and septic shock: an evidence-based review. Crit Care Med. 2004;32:S451–S454. doi: 10.1097/01.CCM.0000142984.44321.A4. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Weil MH. Fluid challenge revisited. Crit Care Med. 2006;34:1333–1337. doi: 10.1097/01.CCM.0000214677.76535.A5. [DOI] [PubMed] [Google Scholar]
- Birman H, Haq A, Hew E, Aberman A. Continuous monitoring of mixed venous oxygen saturation in hemodynamically unstable patients. Chest. 1984;86:753–756. doi: 10.1378/chest.86.5.753. [DOI] [PubMed] [Google Scholar]
- Casserly B, Read R, Levy MM. Hemodynamic monitoring in sepsis. Crit Care Clin. 2009;25:803–823. doi: 10.1016/j.ccc.2009.08.006. ix. [DOI] [PubMed] [Google Scholar]
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29:530–538. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on "sepsis-related problems" of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26:1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- Cariou A, Monchi M, Dhainaut JF. Continuous cardiac output and mixed venous oxygen saturation monitoring. J Crit Care. 1998;13:198–213. doi: 10.1016/S0883-9441(98)90006-5. [DOI] [PubMed] [Google Scholar]
- Zanotti Cavazzoni SL, Dellinger RP. Hemodynamic optimization of sepsis-induced tissue hypoperfusion. Crit Care. 2006;10(Suppl 3):S2. doi: 10.1186/cc4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsky MR, Vincent JL. Let us use the pulmonary artery catheter correctly and only when we need it. Crit Care Med. 2005;33:1119–1122. doi: 10.1097/01.CCM.0000163238.64905.56. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Brazzi L, Pelosi P, Latini R, Tognoni G, Pesenti A, Fumagalli R. A trial of goal-oriented hemodynamic therapy in critically ill patients. SvO2 Collaborative Group. N Engl J Med. 1995;333:1025–1032. doi: 10.1056/NEJM199510193331601. [DOI] [PubMed] [Google Scholar]
- Heyland DK, Cook DJ, King D, Kernerman P, Brun-Buisson C. Maximizing oxygen delivery in critically ill patients: a methodologic appraisal of the evidence. Crit Care Med. 1996;24:517–524. doi: 10.1097/00003246-199603000-00025. [DOI] [PubMed] [Google Scholar]
- Osman D, Ridel C, Ray P, Monnet X, Anguel N, Richard C, Teboul JL. Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med. 2007;35:64–68. doi: 10.1097/01.CCM.0000249851.94101.4F. [DOI] [PubMed] [Google Scholar]
- Schneider AJ, Teule GJ, Groeneveld AB, Nauta J, Heidendal GA, Thijs LG. Biventricular performance during volume loading in patients with early septic shock, with emphasis on the right ventricle: a combined hemodynamic and radionuclide study. Am Heart J. 1988;116:103–112. doi: 10.1016/0002-8703(88)90256-6. [DOI] [PubMed] [Google Scholar]
- Packman MI, Rackow EC. Optimum left heart filling pressure during fluid resuscitation of patients with hypovolemic and septic shock. Crit Care Med. 1983;11:165–169. doi: 10.1097/00003246-198303000-00003. [DOI] [PubMed] [Google Scholar]
- Perel A. Bench-to-bedside review: the initial hemodynamic resuscitation of the septic patient according to Surviving Sepsis Campaign guidelines-does one size fit all? Crit Care. 2008;12:223. doi: 10.1186/cc6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendjelid K, Romand JA. Fluid responsiveness in mechanically ventilated patients: a review of indices used in intensive care. Intensive Care Med. 2003;29:352–360. doi: 10.1007/s00134-002-1615-9. [DOI] [PubMed] [Google Scholar]
- Sevransky JE, Nour S, Susla GM, Needham DM, Hollenberg S, Pronovost P. Hemodynamic goals in randomized clinical trials in patients with sepsis: a systematic review of the literature. Crit Care. 2007;11:R67. doi: 10.1186/cc5948. [DOI] [PMC free article] [PubMed] [Google Scholar]


