Abstract
Disrupted in Schizophrenia-1 (DISC1) is a candidate gene for psychiatric disorders and has many roles during brain development. Common DISC1 polymorphisms (variants) are associated with neuropsychiatric phenotypes including altered cognition, brain structure and function; however, it is unknown how this occurs. Here we demonstrate using mouse, zebrafish and human model systems that DISC1 variants are loss of function in Wnt/GSK3β signaling and disrupt brain development. The DISC1 variants A83V, R264Q and L607F, but not S704C, do not activate Wnt signaling compared to wild type DISC1 resulting in decreased neural progenitor proliferation. In zebrafish, R264Q and L607F could not rescue DISC1 knockdown mediated aberrant brain development. Furthermore, human lymphoblast cell lines endogenously expressing R264Q displayed impaired Wnt signaling. Interestingly, S704C inhibited the migration of neurons in the developing neocortex. Our data demonstrate DISC1 variants impair Wnt signaling and brain development, and elucidate a possible mechanism for their role in neuropsychiatric phenotypes.
Keywords: DISC1 variants, Wnt signaling, GSK3β, neurogenesis, neuronal migration, human lymphoblast cells, DISC1 polymorphisms, schizophrenia, bipolar disorder
Introduction
There has recently been significant progress in understanding the genetic risk factors underlying psychiatric disease using genome-wide association studies and high throughput sequencing. These studies have determined at least two major risk factors for disease, common variants and rare genetic variants which comprise a significant proportion of risk. While rare genetic variants are highly penetrant and occur very infrequently, common variants occur frequently in the general population but confer modest risk. One example of a rare genetic cause of psychiatric disorders is Disrupted in Schizophrenia 1 (DISC1), which was first identified in a large Scottish pedigree displaying various psychiatric disorders including schizophrenia, bipolar disorder and major depression (Blackwood et al., 2001; Millar et al., 2000). Although studies to date indicate that other rare DISC1 variants conferring risk have yet to be identified, there is evidence that common genetic variation in DISC1 has significant impact on brain function and psychiatric disorders.
Recent studies have suggested that common variation in DISC1 is associated with different clinical and structural brain phenotypes in patients and healthy individuals. For example, individuals homozygous for the major Ser704Cys (S704C) allele display reduced hippocampal grey matter and functional engagement during cognitive tasks, and schizophrenia patients experienced increased positive symptoms (Callicott et al., 2005; DeRosse et al., 2007; Di Giorgio et al., 2008). In contrast, Hashimoto et al. demonstrated reduced grey matter volume in the cingulate cortex and decreased fractional anisotropy in prefrontal white matter of individuals carrying the minor allele for S704C (Hashimoto et al., 2006). Furthermore SS704 homozygous individuals showed greater activation of the dorsolateral prefrontal cortex during memory tasks compared to 704C individuals. These data suggest the S704C variant produces different functional effects depending upon which brain region is analyzed; although there is inconsistency, these studies suggest S704C has an impact on modulating human brain function.
In addition to the S704C variant, the common Leu607Phe (L607F) variant has also been implicated in altered brain function. Minor allele carriers of the L607F variant in both healthy individual and schizophrenia patients displayed reduced grey matter density in the superior frontal gyrus and anterior cingulate gyrus, and also experienced greater positive symptoms (Szeszko et al., 2008). Interestingly, a recent study from Rapoport and colleagues reported both S704C and L607F variants affect cortical thickness in developing children and adolescents (Raznahan et al., 2010). Here the authors determined complex interplay between S704C, L607F and cortical thickness, suggesting these variants functionally interact to regulate the structure of the developing brain. Together, these studies highlight that common DISC1 variants play an important role in regulating brain structure, function, and neuropsychiatric behavior. However it is still unclear how these polymorphisms lead to changes in brain structure and, moreover, whether they disrupt DISC1 signaling pathways that contribute to these effects.
Given the different brain structural and functional changes associated with common DISC1 polymorphisms, we hypothesized these effects were due to deleterious effects on brain development via disruption of specific signaling pathways. Various studies have uncovered a number of brain development events and signaling pathways that critically involve DISC1. For example, some of the processes DISC1 regulates includes neurogenesis, neuronal migration, axon/dendrite growth, synaptogenesis, adult neuron generation and synaptic integration, dopaminergic neuron function, and cell-cell adhesion (Enomoto et al., 2009; Hayashi-Takagi et al., 2010; Kamiya et al., 2005; Kamiya et al., 2006; Kim et al., 2009; Lipina et al., 2010b; Mao et al., 2009; Niwa et al., 2010; Pletnikov et al., 2007; Singh et al., 2010). This is further complicated by the number of DISC1-dependent pathways that have been identified to date, including signaling via Lis1/Ndel1, phosphodiesterase 4/cyclic adnosine monophosphate (cAMP), interaction with growth neuregulin, amyloid precursor protein (APP), Akt/Girdin signaling, Rac1, and our lab recently reported that DISC1 modulates canonical Wnt signaling via GSK3β and Dixdc1 (Enomoto et al., 2009; Jaaro-Peled et al., 2009; Kamiya et al., 2006; Kim et al., 2009; Mao et al., 2009; Millar et al., 2005; Shinoda et al., 2007; Young-Pearse et al., 2010). The finding that DISC1 directly inhibits GSK3β is interesting given that the common mood stabilizer drug lithium, and the schizophrenia risk gene Akt, also inhibit GSK3β which results in activation of canonical Wnt signaling, suggesting it may be an important target in psychiatric disease.
Here we present evidence that human DISC1 variants have a deleterious impact on two brain development events, neurogenesis and neuronal migration, via different signaling pathways. We demonstrate using mouse, zebrafish, and human model systems that the DISC1 variants Ala83Val (A83V), Arg264Gln (R264Q), and Leu607Phe (L607F) disrupt canonical Wnt/GSK3β signaling and neural progenitor cell proliferation. However, the Ser704Cys (S704C) variant does not affect Wnt signaling but does inhibit neuronal migration via a cytoskeleton signaling pathway. These results provide insight into the normal function of DISC1 genetic variation, and suggest that brain structural and functional phenotypes associated with DISC1 variants may arise due to their disruptive effects on specific signaling pathways during brain development.
Results
DISC1 variants inhibit Wnt signaling
To discover different human DISC1 variants, we performed deep-sequencing of all DISC1 exons (exome sequencing) on 717 individuals (166 bipolar disorder, 203 schizophrenia, and 369 control individuals). We initially identified common and rare DISC1 variants within this sample set and subsequently genotyped additional healthy individuals and patients as part of a larger study (~16,500 individuals). From the identified variants, we selected a subset of rare and common variants to determine their impact on canonical Wnt signaling (Table 1 and data not shown). We focused on the common variants R264Q, L607F and S704C specifically since they have been associated with brain structural and psychiatric behavioral phenotypes. We also tested the function of different rare DISC1 variants that were closer to the N-terminus since this is one of the regions of DISC1 that binds to GSK3β (data not shown) (Mao et al., 2009). We specifically focused on assaying the function of one rare variant for all experiments, A83V, in order to test its ability to bind GSK3β and modulate Wnt signaling and neural progenitor proliferation. Furthermore, although our initial DISC1 exome sequencing suggested some common and rare variants might be enriched in SZ or BPD populations, larger genotyping efforts revealed there was no statistically significant bias for variants associated with SZ or BPD.
Table 1. Results of genotyping DISC1 variants.
The table displays the number of healthy controls or bipolar (BD)/schizophrenia (SCZ) cases that are homozygous or heterozygous for the major or minor allele. The rare A83V variant was genotyped in a total of 655 individuals. For the common variants, a total of approximately 13,500 individuals were genotyped to obtain the imputed frequencies for R264Q and S704C, while L607F was directly genotyped in this sample.
| BD | SCZ | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Position | DBSNP | Exon | TYPE | ALLELES | CASES | CONTROLS | CASES | CONTROLS | Amino Acid |
| 229896375 | <NA> | 2 | Mtssense | T/C | 0/0/166 | 0/1/169 | 0/1/183 | 0/0/155 | A83V |
| 229896918 | rs3738401 | 2 | Missense | A/G | 356/1407/1459 | 251/1083/1135 | 379/1700/1795 | 352/1710/1794 | R264Q |
| 230020724 | rs6675281 | 9 | Missense | T/C | 102/1133/3333 | 60/714/2221 | 76/878/3015 | 123/1176/3436 | L607F |
| 230211221 | rs82l6l6 | 11 | Missense | T/A | 270/1278/1674 | 181/1016/1272 | 359/1587/1927 | 379/1588/1889 | S704C |
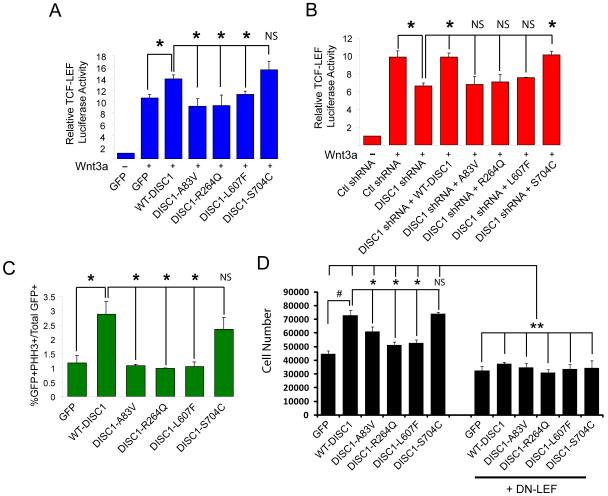
To determine if DISC1 variants regulate Wnt signaling, we performed in vitro assays to measure Wnt-induced T-Cell Factor/Lymphoid Enhancer Factor (TCF/LEF) transcriptional activity. We have previously reported that overexpressing mouse wild-type (WT)-mDISC1 can significantly potentiate 16 hour Wnt-induction of TCF/LEF activity in HEK293 cell lines (Mao et al., 2009). Using this result as our reference, we tested whether the human DISC1 variants also potentiated TCF/LEF activity similarly to WT-human DISC1 (WT-DISC1). Interestingly, we found that the majority of the rare DISC1 variants, including A83V, and the common R264Q and L607F variants unable to potentiate TCF/LEF activity compared to WT-DISC1, as they performed no different than GFP controls (Figure 1A, data not shown). However, the S704C common polymorphism was able to significantly potentiate activity, suggesting that it does not display loss of function activity. It is possible these results could arise due to differences in expression levels of the different hDISC1 variants. We therefore examined the expression levels of all GFP-tagged hDISC1 variants, however, we found there was no statistical difference (Figure S1A).
Figure 1. DISC1 variants inhibit Wnt signaling and cell proliferation.
(A) HEK293 cells were transfected with the TCF/LEF luciferase reporter together with GFP or the indicated DISC1 variant. Cells were then stimulated with either control or Wnt conditioned media (CM) for 16 hours and luciferase activity was determined. The graph represents relative Wnt-induced luciferase reporter activity. *p<0.05, NS=not significant. Error bars represent standard error of the mean (SEM). (B) P19 cells were transfected with the TCF/LEF luciferase reporter, control, or mouse DISC1 shRNA, together with the indicated hDISC1 variant and then treated as in (A). *p<0.05, NS=not significant. Error bars represent SEM. (C) N2A cells were transfected with the indicated constructs for 24 hours at which point cells were fixed and immunostained. The graph represents the percentage of transfected cells that were positive for phosphohistone H3 staining. *p<0.05, NS=not significant. Error bars represent standard SEM. (D) N2A cells were plated at equal densities and transfected with GFP, WT-DISC1 or the different DISC1 variants (Left panel) or together with DN-LEF (Right panel). Cells were fixed, staining with DAPI, and the number of cells were quantified 48 hours later and represented as a combined graph. Quantification was performed by taking 4 representative images from each condition in each experiment (n=3 experiments, #,*p<0.05, **p<0.005, mean ± SEM). See also Figure S1.
We further tested these DISC1 variants for their ability to rescue DISC1 shRNA-mediated inhibition of TCF/LEF reporter activity in mouse-derived P19 carcinoma cell lines. We first tested the level of knockdown of DISC1 mRNA transcript to confirm that transfection of multiple plasmids into N2A cells does indeed result in DISC1 downregulation (Supplementary Figure 1C). In this assay, WT human DISC1 completely rescues the reduction in TCF/LEF reporter activity after DISC1 knockdown (Figure 1B, data not shown). In line with the results from the previous assay, we found that all of the rare variants, including A83V, and the common R264Q and L607F DISC1 variants could not rescue this reduction. However, the S704C variant completely rescued DISC1 shRNA-mediated reduction in TCF/LEF luciferase reporter activity. These results were not due to differences in the expression levels of the hDISC1 variants (Figure S1B). Taken together, these experiments demonstrate DISC1 variants significantly inhibit Wnt signaling in vitro.
DISC1 variants inhibit cell proliferation
Wnt signaling is a well-known regulator of cell proliferation, particularly in neural progenitor cells (Shimizu et al., 2008; Wexler et al., 2007). Given that our results implicate DISC1 as a modulator of Wnt signaling, we tested the impact of the human DISC1 variants on the proliferation of N2A cells in vitro. Cells were transfected with the different DISC1 variant constructs and were then stained 48 hours later with phosphohistone-3 (PH3), which labels the nuclei of dividing cells. Using this assay, we found that overexpression of WT-DISC1 resulted in an almost 3-fold increase in the number PH3-positive cells (Figure 1C). When comparing the other DISC1 variants, we observed similar trends as the TCF/LEF reporter assays. The A83V, R264Q, and L607F variants did not significantly increase PH3 staining in transfected cells, while the S704C significantly increased PH3 staining similar to WT-DISC1 (Figure 1C). We next directly tested the impact of hDISC1 variants on the number of proliferating cells. N2A cells were plated vitro, transfected with GFP, WT-DISC1 or the different DISC1 variants, stimulated with Wnt3a and the number of cells were then counted after a 24 hour period. Here we found that WT-DISC1 expression caused a significant increase in cell number compared to GFP controls (Figure 1D). However, the A83V, R264Q and L607F variants did not significantly increase cell number, while the S704C variant increased the cell number similar to WT-DISC1 (Figure 1D). We then asked whether these effects required downstream activation of Wnt signaling. Using a similar experimental paradigm, N2A cells were co-transfected in the same manner but also with dominant-negative LEF and then stimulated with Wnt3a. We found that after 24 hours, there was a similar reduction of cell proliferation across all conditions. This demonstrates that Wnt-induced cell proliferation requires active LEF signaling. Importantly, we found the enhancement of Wnt-induced proliferation by WT-DISC1 or S704C was also significantly reduced upon co-expression of DN-LEF (Figure 1D). These data suggest that the effects of DISC1 and the variants on Wnt-induced cell proliferation do not occur when downstream Wnt signaling is inhibited. Taken together, these data strongly suggest the A83V, R264Q and L607F variants cannot stimulate cell division/proliferation compared to WT-DISC1 or the S704C variant.
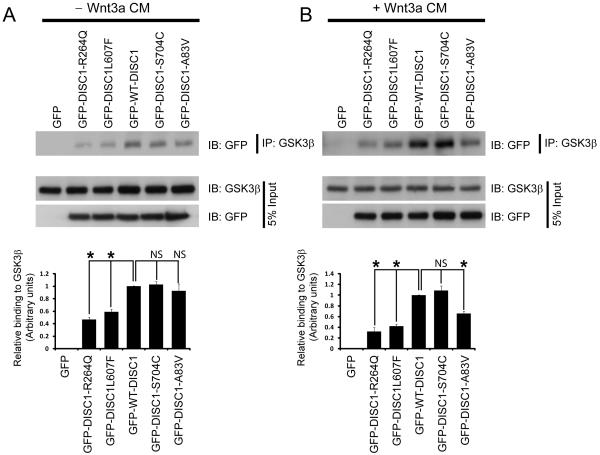
To address one potential molecular mechanism to explain these observations, we hypothesized that since DISC1 binds and inhibits GSK3β, the variants might have altered interaction with GSK3β. We overexpressed the different GFP-tagged DISC1 variants in HEK293 cells that were either stimulated or non-stimulated with Wnt3a. Cell lysates were immunoprecipitated with a GSK3β antibody (to immunoprecipitate endogenous GSK3β) and immunoblotted with GFP. We determined that in the absence of Wnt3a stimulation, the R264Q and L607F variants all significantly reduced binding to GSK3β in this assay (Figure 2A), potentially explaining why these variants have reduced signaling. However, the A83V variant did not show reduced binding to GSK3β (Figure 2B). Interestingly, after Wnt3a stimulation, we found that R264Q, L607F and the A83V variants had reduced binding to GSK3β (Figure 2B). Together these data suggest that the A83V, R264Q and L607F variants all have reduced binding to GSK3β, while S704C binds as well as WT-DISC1.
Figure 2. DISC1 variants have altered interaction with GSK3β.
(A) The interaction between DISC1 variants and GSK3β is disrupted. Top, Lysates of HEK293 cells were transfected with GFP-tagged DISC1 variants were immunoprecipitated with a GSK3β antibody and immunoblotted with a GFP antibody to detect DISC1 binding in the absence of Wnt3a stimulation. Bottom, graph showing quantification of the western blot (n=3, ns=not significant, mean ± SEM). (B) Top, the same experimental paradigm as in (A) was performed however cells were stimulated with Wnt3a for 16 hours prior to collection of cell lysates. Bottom, graph showing quantification of the western blot (n=3, ns=not significant, mean ± SEM).
DISC1 variants impact neural progenitor proliferation in the developing cortex
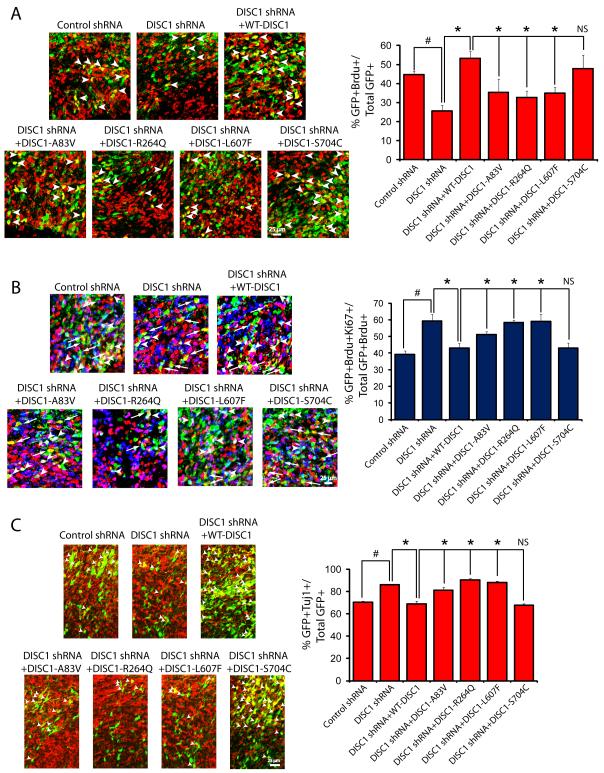
In order to test the significance of our in vitro data, we utilized in utero electroporation as an in vivo model to examine whether DISC1 variants regulated the proliferation of neural progenitor cells. We performed in utero electroporation on embryonic day 13 (E13) brains and analyzed brains at E16, a time period when neurogenesis is peaking. We tested the ability of WT-DISC1 or the different DISC1 variants to rescue the decrease in neural progenitor proliferation after DISC1 knockdown, which we previously reported (Mao et al., 2009). We co-transfected neural progenitor cells with Venus-GFP to visualize cells and plasmids expressing control or DISC1 shRNA, together with WT-DISC1 or the different DISC1 variants. To measure proliferation of neural stem cells, we performed a 24 hour pulse label of 5-bromo-2-deoxyuridine (BrdU) at E15. Here we found that expression of human WT-DISC1 was able to rescue the DISC1 shRNA-mediated decrease in the number of GFP/BrdU double positive cells, indicating that WT-DISC1 can rescue the neural progenitor proliferation defect caused by DISC1 downregulation (Figure 3A). When comparing the different DISC1 variants against WT-DISC1, we found that the A83V, R264Q and L607F variants could not rescue the number of double positive GFP/BrdU cells similar to WT-DISC1, suggesting these variants are loss of function when compared to WT-DISC1 (Figure 3A). However, similar to our previous experiments, the S704C variant was able to significantly rescue neural progenitor proliferation compared with WT-DISC1 (Figure 3A). These experiments suggest the DISC1 A83V, R264Q and L607F variants are not able to function similarly to WT-DISC1 in the regulation of neural progenitor proliferation.
Figure 3. DISC1 variants negatively impact neural progenitor proliferation and differentiation in vivo.
(A) DISC1 variants differentially rescue DISC1 shRNA-mediated inhibition of progenitor proliferation. Left, images of E16 embryonic mouse brain sections stained for Brdu and GFP after electroporation at E13 with the indicated constructs. Arrowheads indicate double positive cells (GFP+Brdu+). The scale bar represents 25 μm. Right, graph displaying quantification of the percentage of electroporated cells that were double positive for BrdU and GFP. *p<0.05, NS=not significant. Error bars represent mean ± standard error of the mean (SEM). (B) DISC1 variants have different abilities to rescue increased cell cycle exit due to DISC1 loss of function. Expression of DISC1 variants leads to increased premature cell cycle exit. E13 mouse brains were electroporated with constructs expressing DISC1 variants, BrdU (100 mg/kg) was administered at E15 and analyzed 24 hours later at E16. Left, confocal images of brain sections stained for GFP, Ki67 and BrdU. Arrowheads indicate GFP+BrdU+Ki67+ cells while arrows indicate GFP+BrdU+Ki67–cells. Right, graph showing quantification of the cell cycle exit index (n=3, #,*p<0.05, ns=not significant, mean ± SEM). The scale bar represents 25 μm. (C) DISC1 variants display different abilities to cause premature neural differentiation. Left, confocal images of E16 brain sections stained for Tuj1 after electroporation. Right, quantification of the percentage of GFP-Tuj1 double positive cells relative to total GFP positive cells is shown as a bar graph. (n=3 for each experiment, *p<0.05, ns=not significant, mean ± SEM). The scale bar represents 25 μm. See also Figure S2.
We then directly addressed if the changes in BrdU labeling were due to alterations in the numbers of progenitors exiting the cell cycle and prematurely differentiation. First, we performed the cell cycle exit assay in utero whereby electroporated brain sections were stained for GFP, BrdU, and Ki67. To assess the cell cycle exit index, we counted the percentage of GFP/BrdU double-positive cells that were negative for Ki67, Using this protocol, we found that overexpression of WT-DISC1 was able to rescue the increased cell cycle exit mediated by DISC1 shRNA (Figure 3B). Upon comparison to the DISC1 variants, we found that the A83V, R264 and L607F variants all were not able to rescue similar to WT-DISC1 and neural progenitor cells continued to prematurely exit the cell cycle. However, the S704C variant was able to rescue the DISC1-shRNA mediated increase in cell cycle exit (Figure 3B), in good agreement with the neural progenitor proliferation data in Figure 3A. We extended these experiments to determine whether the changes in cell cycle exit led to alterations in neuronal differentiation. Electroporated brain sections were co-stained with GFP and Tuj1 to visualize neurons. We determined that the increase in the number of double positive GFP/Tuj1 cells due to DISC1 shRNA was rescued when co-expressed with human WT-DISC1 (Figure 3C). In this assay, we observed that the A83V, R264Q and L607F variants all did not increase the number of double positive GFP/Tuj1 cells, while the S704C variant indeed functioned similar to WT-DISC1 and rescued similarly (Figure 3C). Together this data suggests that the A83V, R264Q and L607F DISC1 variants do not function similar to WT-DISC1 or S704C in the regulation of neural progenitor proliferation.
To determine whether the DISC1 variants possessed dominant negative activity in the presence of endogenous mouse DISC1, we performed the in utero electroporation and only overexpressed GFP, WT-DISC1, or the different variants. Staining for BrdU and GFP revealed that overexpression of human WT-DISC1 resulted in a significant increase in the percentage of cells double positive for GFP and BrdU demonstrating that WT-DISC1 expression alone increases the number of dividing neural progenitor cells (Figure S2A). Comparison to the DISC1 variants revealed that the S704C variant similarly increased the number of cycling cells as WT-DISC1. However, the A83V and L607F variant conditions revealed statistically similar numbers of GFP/BrdU-positive cells as GFP controls. Interestingly, the R264Q variant caused fewer numbers of GFP/BrdU double positive cells when expressed alone, suggesting itt may possess dominant negative activity and decreases progenitor proliferation (Figure S2A).
We extended analysis of these data to determine if the DISC1 variants affected cell cycle exit and neural differentiation in a dominant negative fashion. We found that expression of WT-DISC1 resulted in a significantly reduced the percentage of cells exiting the cell cycle and suppressed premature neural differentiation (Figure S2B,C). Comparison to the DISC1 variants revealed that the S704C variant caused no changes in the cell cycle index or neural differentiation (Figure S2B,C). However, the A83V and L607F variants resulted in a similar cell cycle exit index and degree of neural differentiation as GFP controls, demonstrating that they do not possess the same activity as WT-DISC1, but do not function in a dominant negative manner (Figure S2B,C). However, the R264Q variant alone significantly increased the percentage of cells exiting the cell cycle and increased premature neural differentiation compared to GFP control and WT-DISC1, suggesting it functions in a dominant negative manner in vivo.
The DISC1 R264Q and L607F variants impact zebrafish neural development
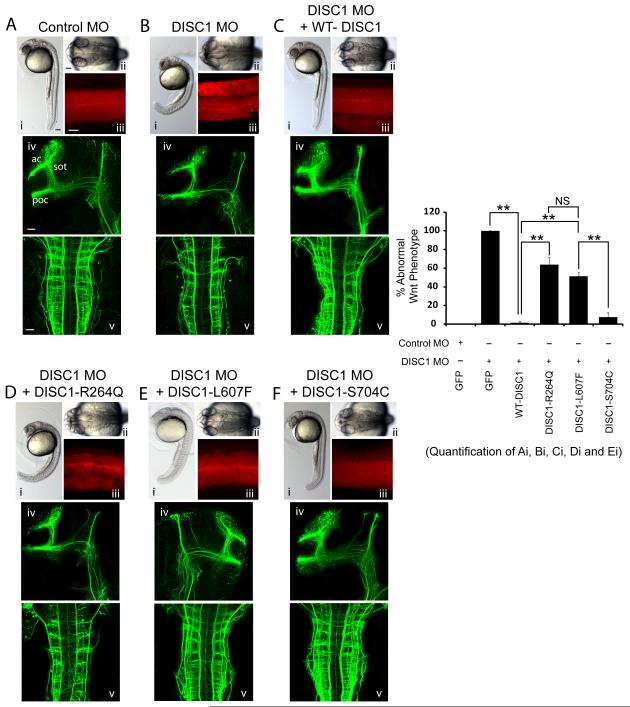
To further extend our results we utilized the developing zebrafish nervous system to test whether the DISC1 variants affect Wnt signaling and brain development. We took advantage of this system and compared the activities of WT-DISC1 versus the DISC1 variants in a series of neurodevelopmental assays. First, we reduced zDISC1 expression using antisense morpholino-modified oligonucleotides (MOs) into one/two cell state embryos which produces a very severe phenotype consisting of forebrain truncation (at 24 hours postfertilization) and abnormal brain and ventricle morphology (Figure 4A,Bii). Furthermore, reducing zDISC1 expression resulted in disorganization of the muscle segments and a bent tail (Figure 4A,B,i,iii), characteristic of a Wnt signaling defect (Lekven et al., 2001). Interestingly, downregulating zDISC1 expression also led to defects in the formation of the axon tracts as observed by immunostaining with acetylated tubulin. Specifically, the postoptic commissure (poc), anterior commissure (ac) and the supraoptic tract (sot) was either missing or strongly reduced (Figure 4A,B iv,v).
Figure 4. The DISC1 R264Q and L607F variants are unable to rescue abnormal brain development in zebrafish due to DISC1 loss of function.
Embryos were injected with Control MO or Disc1 MO, together with mRNA encoding DISC1 variant proteins or GFP, and assayed at 30hpf in 3 independent experiments. 3.5ng morpholino and 200pg mRNA was injected/embryo. (Ai-Fi) Lateral images of the whole embryo. (Bii-Fii) images of the dorsal view of the head; (Aiii-Fiii) Somites stained for phalloidin (actin filaments). (Aiv,v- Fiv,v) Neurons and axon tracts stained for acetylated tubulin. ac: anterior commissure; poc: postoptic commissure; sot: supraoptic tract. z, Quantification of the percentage of embryos displaying a curved tail in each condition that is similar to DISC1-dependent Wnt signaling. **p<0.005, ns=not significant. Error bars represent mean ± SEM. All scale bars represent 50 μm.
Using this system, we examined whether DISC1 variants produced detrimental phenotypes in the development of the zebrafish nervous system. To do this, we first tested whether expression of human WT-DISC1 rescued the zDISC1 MO-induced phenotypes. Indeed, expression of WT-DISC1 rescued the abnormal tail brain ventricle, tail and muscle segment structures and deficits in all axon tracts (Figure 4A-C), demonstrating that the human and zDISC1 genes have conserved functions. We then compared the ability of three different DISC1 variants (R264Q L607F and S704C) to rescue the zDISC1 MO phenotypes. Interestingly, we found expression of the R264Q variant was not able to rescue the tail structural defect (Figure 4D Moreover, we found the L607F variant had an intermediate phenotype, and partially rescued measured phenotypes, however, it did not significantly rescue compared to WT-DISC1 (Figure 4E). The S704C was able to fully rescue the zDISC1 MO phenotypes, suggesting it functions similarly to WT-DISC1 in the developing zebrafish nervous system (Figure 4F). Furthermore, the S704C variant also rescued the zDISC1 MO-induced axon tract phenotype and restored axon tracts to WT-DISC1 levels. However, the R264Q variant did not rescue the zDISC1 MO-mediated axon morphologies, while the L607F variant again had an intermediate phenotype (Figure 4D-F). Taken together, these data suggest that R264Q and L607F reduce Wnt-dependent brain developmental phenotypes in the zebrafish, while the S704C variant behaves similar to WT-DISC1. This data is in good agreement with our previous data obtained using mouse in vitro and in vivo systems (refer to Figures 1-3).
The R264Q DISC1 variant endogenously inhibits Wnt signaling in human lymphoblast cell lines
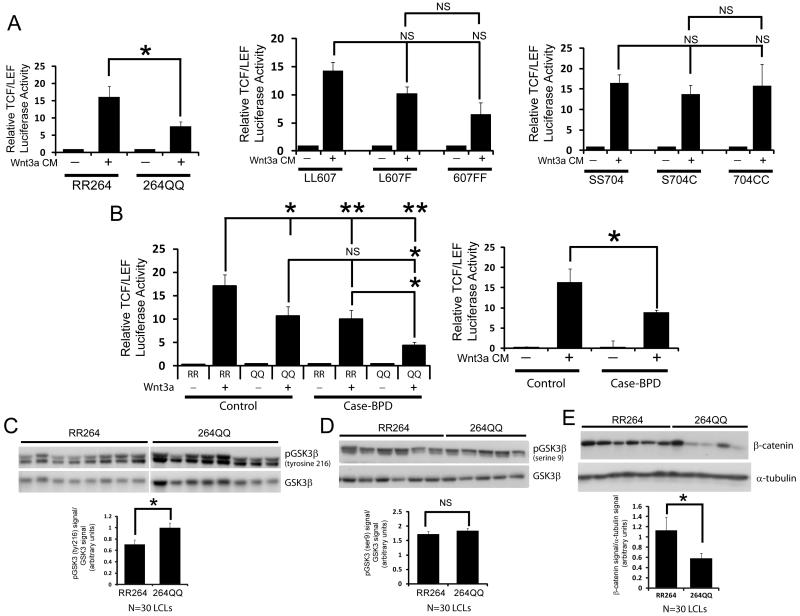
Our data suggests that the three out of the four DISC1 variants produce Wnt signaling defects and brain developmental abnormalities (A83V, R264Q, and L607F), while the S704C variant does not. One caveat to these experiments is that we are measuring these parameters usin non-human systems and overexpressed DISC1 protein, and therefore it is unknown whether these data translate to human cells under physiological conditions. To determine whether DISC1 variants affect Wnt signaling in human cells, we obtained human lymphoblast cell lines to determine the impact of endogenously expressed DISC1 variants on Wnt signaling, since these human cells could be genotyped for DISC1 to determine what variants they expressed. We focused on the R264Q variant since it produced the most robust and consistent inhibition of Wnt signaling in our studies. Furthermore, we could obtain a large number of human lymphoblast cell lines (LCLs) from both healthy individuals and bipolar disorder patients that were homozygous for RR264 (major allele) or 264QQ (minor allele). We then infected these LCLs with a lentivirus encoding the Wnt-TCF/LEF luciferase reporter gene to determine the level of Wnt activity. We found that the LCLs carrying the homozygous RR264 variant had significantly higher Wnt-stimulated TCF/LEF reporter activity compared to homozygous 264QQ LCLs (Figure 5A, left panel). Additionally, when these LCLs were segregated based on the L607F variant, we found there was a trend towards decreased TCF/LEF reporter activity in cells that were heterozygous for L607F or homozygous 607FF (Figure 5A, middle panel). The lack of significance is likely due to the low number of 607FF expressing LCLs in our cell lines (only 2 lines out of the 64 in total), however this will be addressed with the analysis of additional L607F lines. Lastly, when the LCLs are segregated based on the S704C variant, comparing the SS704, S704C and 704CC expressing cells revealed there was no statistical difference in Wnt-induced TCF/LEF luciferase reporter activity between the alleles (Figure 5A, right panel). These data strongly demonstrate that in human cells the R264Q, but not the S704C common variant, reduces Wnt signaling.
Figure 5. Human lymphoblast cell lines endogenously expressing the DISC1 R264Q variant have reduced Wnt signaling.
(A) Cells were infected with a lentivirus encoding a Wnt-TCF/LEF luciferase reporter for 24 hours and were then stimulated with control or Wnt conditioned media for 16 hours followed by quantification of luciferase activity. The data is presented as the relative level of Wnt-luciferase activity in human lymphoblast cell lines that were homozygous for either the major R or minor Q allele at residue 264 (left graph), the L607F variant (middle graph) or the S704C variant (right graph). (N=64 cell lines total in 5 independent experiments; *p<0.05, ns=not significant, mean ± SEM). (B) The same data displayed in (A) but segregated by healthy control vs. bipolar disorder (case) (right graph) or co-segregated by genotype for R264Q (left graph). (N=64 cell lines total in 5 independent experiments; *p<0.05, ns=not significant, mean ± SEM). (C-E) Top, lysates of LCLs were probed for phosphorylated (tyrosine 216) GSK3β (C), pGSK3β (serine 9) (D) or total β-catenin (E). Bottom, graph showing quantification of the levels of the indicated protein. (N=30 cell lines total in 3 independent experiments; *p<0.05, ns=not significant, mean ± SEM). See also Figure S3.
Given that Wnt signaling may play a role in psychiatric disorders, we asked whether Wnt signaling was overall diminished in LCLs obtained from bipolar patients compared to healthy controls. Interestingly, when we re-analyzed our data and segregated based on case or control, we found a significant decreased in Wnt-stimulated TCF/LEF activity in bipolar patient LCLs, in a genotype-sensitive manner (Figure 5B, both panels). This data suggests that Wnt signaling may indeed be impaired in these patients, and lithium treatment may help to increase this impairment. As a control, we ascertained whether the differences in Wnt signaling in the LCLs could be due to overall changes in the receptors for Wnt, such as the Frizzled and LRP genes. We selected a subset of Frizzled isoforms (Fzd 3, 4 and 5) and LRP6 to determine whether if their expression levels differed in LCLs. We found that when comparing either RR264 vs. 264QQ LCLs or control vs. bipolar case LCLs, there was no significant different in Wnt receptor levels (Figure S3A). This data suggests that the differences in Wnt signaling in the LCLs expressing the R264Q variants is not due to changes in upstream Wnt receptor signaling.
We next performed biochemical experiments using the human cell lines to determine the influence of the R264Q variant on the state of Wnt signaling. We first asked whether DISC1 R264Q regulated GSK3β activation by measuring phosphorylation of the tyrosine 216 (Y216) and the serine 9 (S9) residues. Interestingly we found that LCLs that were homozygous for DISC1 RR264 had significantly lower levels of phosphorylated Y216-GSK3β compared to LCLs homozygous for DISC1 264QQ (Figure 5C), suggesting GSK3β is more active in LCLs homozygous for 264QQ. However, quantification of the level of serine 9 phosphorylation revealed this remained unchanged (Figure 5D). This data suggests that the R264Q variant regulates GSK3β activation by modulating phosphorylation of Y216, and indicates the reduced Wnt signaling in LCLs expressing the 264QQ variant may be due to increased activation of GSK3β. Finally, we determined whether the R264Q variant regulates the overall levels of β-catenin in human LCLs. We found that after examining a large number of cell lines, cells expressing the RR264 variant indeed had significantly higher levels of β-catenin, consistent with their reduced activation of GSK3β and elevated Wnt signaling (Figure 5E).
Lastly, we examined whether the DISC1 variants affected another signaling pathway in addition to Wnt signaling. We specifically focused on cyclic adenosine monophosphate (cAMP) signaling since one of the best studied functions of DISC1 is to interact with the phosphodiesterase family (PDE4B) to regulate cAMP levels (Bradshaw et al., 2011; Millar et al., 2005). To determine whether the DISC1 variants affect cAMP levels in cells, we overexpressed GFP, WT-DISC1 or the different variants in N2A cells and measured cAMP levels. We determined that the overexpression of WT-DISC1 led to a small increase in cAMP levels, however it was not statistically significant (Figure S3B). Evaluation of the different DISC1 variants in this assay further revealed no difference in cAMP levels compared to GFP controls. Therefore our data suggests that the DISC1 variants do not specifically regulate cAMP levels in a dominant-negative manner. Taken together, the analysis of human LCLs demonstrates that the R264Q variant directly regulates Wnt signaling by regulating the activation of Wnt signaling proteins.
The S704C DISC1 variant impairs neuronal migration in the developing cortex
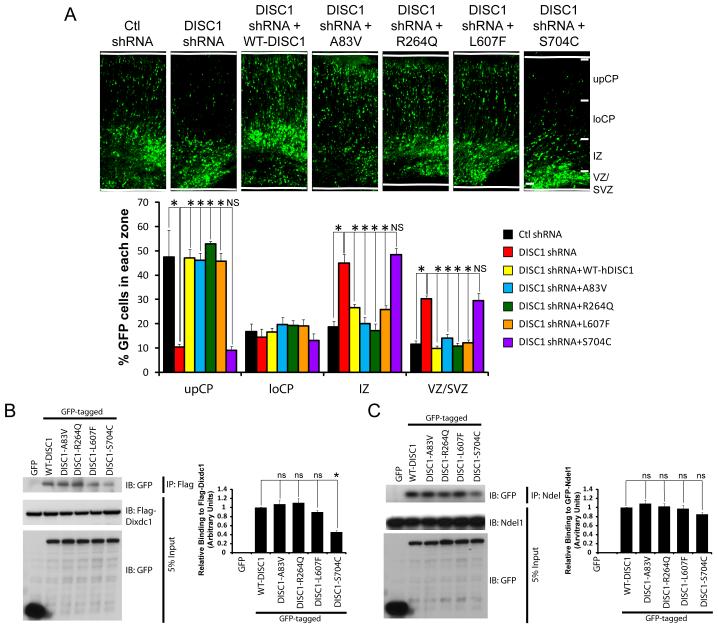
Since all of our studies suggest the S704C variant does not affect Wnt signaling or neural progenitor proliferation, we hypothesized this variant might regulate another Wnt-independent neurodevelopmental event. Since this variant lies in the C-terminus of DISC1 which interacts with the neuronal migration genes Ndel1 and Dix domain containing 1 (Dixdc1), we asked whether it alters the migration the newborn neurons. Using in utero electroporation, we tested the ability of WT-DISC1 versus the DISC1 variants to rescue the neuronal migration defect caused by downregulation of DISC1. We found that first, expression of human WT-DISC1 rescues the GFP positive cells that are normally arrested in the intermediate and subventricular zones due to DISC1 downregulation, restoring their migration to the upper layers of the cortex, similar to control shRNA (Figure 6A). We then tested the different DISC1 variants in this paradigm, and found that the A83V, R264Q and L607F variants functioned similar to WT-DISC1 and also restored the migration of arrested GFP positive cells to the upper cortical layers (Figure 6A). However, we determined that the S704C variant was not able to completely restore migration, since there a significant number of GFP cells still remaining in the VZ/SVZ and IZ compared to the other conditions, suggesting this C-terminal variant is required for neuronal migration (Figure 6A).
Figure 6. The S704C DISC1 variant inhibits neuronal migration during embryonic brain development.
(A) DISC1 variants have differential abilities to rescue DISC1 shRNA-mediated inhibition of neuronal migration. E15 brains were co-electroporated with either control or DISC1 shRNA together with WT-DISC1 or the indicated DISC1 variants and GFP for visualization. Brains were analyzed at E19 and cell positioning was determined. Top, confocal images depicting the distribution of GFP-positive cells. Bottom, graph showing quantification of GFP-positive cells in the subdivisions (n=4, *p<0.05, NS=not significant, mean ± SEM). upCP; upper cortical plate, loCP; lower cortical plate, IZ; intermediate zone, VZ/SVZ; ventricular zone/subventricular zone. The scale bar represents 50 μm. (B) The interaction between S704C is diminished with Dixdc1. Left, Western blot showing HEK293 cells that were transfected with flag-tagged Dixdc1 together with GFP or the indicated DISC1 variants, immunoprecipated with flag antibodies and immunoblotted with a GFP antibody. Right, graph showing quantification of the western blot demonstrating S704C has significantly reduced binding to Dixdc1. (n=3, *p<0.05, mean ± SEM). (C) The interaction between DISC1 variants and Ndel1 is not significantly decreased. Lysates of HEK293 cells were transfected with GFP-tagged DISC1 variants and GFP-tagged Ndel1 were immunoprecipitated with Ndel1 antibody, and immunoblotted with a GFP antibody. Right, graph showing quantification of the western blot (n=3, ns=not significant, mean ± SEM).
Given that we found the S704C variant affects neuronal migration, we asked whether overexpression of this variant alone could cause a neuronal migration defect in a dominant-negative fashion. We overexpressed the different DISC1 variants and found that only the S704C variant disrupted neuronal migration compared to the other DISC1 variants, demonstrating S704C has consistent effects on migration using two different experimental paradigms (Figure S4). To determine the mechanism by which the S704C variant inhibited migration, we hypothesized this variant might have disrupted interaction with Ndel1 and/or Dixdc1, and therefore tested the ability of all the variants to bind these molecules. Interestingly, we found that there was reduced binding between the S704C variant and Dixdc1, but not Ndel1 (Figure 6B and C), whereas all other DISC1 variants all had equal interaction with Ndel1 and Dixdc1. Together these data argue that the S704C variant disrupts neuronal migration via reduced binding to Dixdc1, but has no effect on Wnt signaling.
Discussion
In this study, we examined the functional impact of common and rare DISC1 variants on neuronal development using three model systems; mouse, zebrafish, and human cells. We demonstrate that these DISC1 variants have distinct roles in regulating Wnt-dependent and - independent pathways during cortical development. Specifically, we found that the rare DISC1 A83V, and common R264Q and L607F variants had reduced interaction with GSK3β, resulting in an inhibition of Wnt signaling and diminished neural progenitor cell proliferation. However, the DISC1 C-terminal S704C variant did not affect Wnt signaling, but did have reduced binding to Dixdc1 that resulted in inhibited neuronal migration. Of particular note, we show that human lymphoblast cells lines that endogenously express DISC1 variants show reduced Wnt signaling via reporter and biochemical assays. Taken together, all the variants studied ultimately impacted brain development, suggesting a mechanism underlying the association of DISC1 variants to psychiatric phenotypes and human brain structural differences.
The use of multiple model systems to validate the function of genetic variants
In our effort to understand the function of DISC1 genetic variation in Wnt signaling and brain development, we chose to use an experimental approach that included model systems spanning three species (mouse, zebrafish, and human cells). The use of multiple model systems is important given that recent human genetic findings revealed common polymorphisms in psychiatric risk genes, but their functional impact is unknown. Additionally, re-sequencing efforts are now underway to determine if candidate psychiatric risk genes discovered by GWAS will uncover rare and deleterious mutations. These studies will uncover numerous genetic changes that will need to be validated in. An example of such an approach was recently shown using the zebrafish and in vitro cell systems to validate genetic variants in fourteen different genes associated with Bardet-Biedl Syndrome (BBS) (Zaghloul et al., 2010). In this study, the authors used physiologically relevant assays such as measures of gastrulation, body and brain size, and in vitro protein localization in cells to clearly demonstrate that both rare and common genetic variants in BBS candidate genes are loss of function and contribute to pathology. This study provides evidence that using multiple model systems will provide an effective means to understanding the functional impact of risk gene variants.
Genotype to phenotype: How do genetic changes in DISC1 lead to distinct cell signaling and neuronal phenotypes?
Some variants are common in the general population but, what then determines whether someone carrying the common variant will develop psychiatric disease? In the case of DISC1, as healthy individuals also carry the minor alleles of the variants that decrease Wnt signaling and cause aberrant brain development, it is possible that DISC1 variants interact with SNPs in other genes that are protective. Furthermore, it is likely that different common and rare DISC1 variants in each individual genetically interact with SNPs in other Wnt-related genes to determine the overall impact on Wnt signaling. Common DISC1 variants affecting baseline Wnt signaling may therefore predispose individuals to reduced Wnt signaling as a sort of “first hit”, while the presence of other concomitant risk alleles that also reduce Wnt signaling would serve as a the “second hit”, and would be sufficient for the onset of psychiatric disease. Recently, Kleinman and colleagues demonstrated that individuals carrying the L607F variant had increased DISC1 transcripts lacking exons 3 and/or 7/8, which are very close to or within the GSK3β binding domain (Nakata et al., 2009). Therefore, it is possible that common DISC1 variants not only reduce Wnt signaling through the decreased inhibition of GSK3β, but also through decreased overall levels of DISC1 transcripts. However although the L607F DISC1 variant demonstrated reduced binding to GSK3β and caused decreased Wnt signaling and progenitor proliferation, this SNP does not lie directly in the mapped GSK3β binding region. One explanation may be that the L607F phenotype results from a tertiary change in DISC1 conformation that reduces the efficiency of GSK3β binding.
Our data suggest that the DISC1 SNPs are divided into two categories: those that affect Wnt signaling (A83V, R264Q, and L607F) and the S704C variant, which does not affect the Wnt pathway. A recent study implicating the R264Q variant in schizophrenia suggests that treatment-resistant schizophrenia, whereby patients do not respond to typical antipsychotic medication, are likely to carry the minor allele of the R264Q variant (Mouaffak et al., 2010). This is very interesting, as these findings suggest that this SNP may regulate the efficacy of antipsychotic treatment. One explanation for this finding is that, since antipsychotics partially activate GSK3β and Wnt signaling (Freyberg et al., 2010; Sutton et al., 2007), therefore in patients with the minor 264Q DISC1 allele, the DISC1-mediated inhibition of GSK3β may be faulty, thus reducing Wnt activation in response to antipsychotic treatment. This concept is supported by mice possessing a mutation in DISC1 exon 2 (L100P) which display reduced neurogenesis and aberrant cortical development, reduced dendrite branching and schizophrenia behavioral phenotypes (Clapcote et al., 2007; Lee et al., 2011; Lipina et al., 2010a). Interestingly, DISC1 (L100P) in these mice display reduced binding to GSK3β. These findings support the conclusion that mutations in DISC1 which disrupt binding to GSK3β inhibit Wnt signaling, leading to impaired cortical development and psychiatric behavioral phenotypes. However, we cannot exclude the possibility that these DISC1 variants, in particular R264Q, may impact binding to other proteins that may also be required in regulating DISC1 in Wnt signaling.
Our human LCL data demonstrates that patient cell lines harboring the R264Q variant (homozygous 264QQ) show a markedly reduced TCF/LEF activity compared to the RR264 cell lines. In addition to this data, we provide biochemical evidence that the activation of GSK3β and overall levels of β-catenin, both of which result in reduced Wnt signaling. This data together further implicates a role for Wnt signaling in psychiatric disease, and also suggests that patients with bipolar disorder have impaired Wnt signaling, which might be alleviated by lithium treatment, since lithium increases Wnt signaling. However, this DISC1 variant alone is not sufficient to cause disease, and additional genetic factors would likely contribute to further impacting Wnt signaling disease progression.
In addition to DISC1 SNPs that affect Wnt signaling, we found that the common S704C variant affects a cytoskeletal pathway by altering DISC1 binding to Dixdc1, thereby disrupting neuronal migration. Interestingly, this variant has also been linked to the mitogen activated protein kinase (MAPK) signaling pathway, where the minor DISC1 704C homozygous mutation causes reduced ERK activation (Hashimoto et al., 2006). As the MAPK pathway is a well-known regulator of neuronal morphology, it is likely that the S704C variant affects neuronal migration and morphology via Dixdc1/Ndel1 and ERK signaling, and suggests the possibility that these pathways converge to regulate the cytoskeleton in a DISC1-dependent fashion.
Wnt Signaling, DISC1, and Psychiatric Disorders
Our current and previous work (Mao et al., 2009) suggest a prominent role for DISC1 in regulating the Wnt signaling pathway during brain development. Given that our data suggests that specific human DISC1 variants affect Wnt signaling and brain development, this pathway may indeed play a significant role in psychiatric disorders. This is particularly interesting given that a common mood stabilizer Lithium, is a known GSK3β inhibitor (Beaulieu et al., 2008; Harwood, 2005), and can directly activate Wnt-TCF/LEF gene transcription (Stambolic et al., 1996). Furthermore, some of the recently identified genetic susceptibility factors also fall within the Wnt signaling pathway, directly and indirectly. For example, the Akt gene, which can directly regulate GSK3β activity and downstream TCF/LEF signaling, is itself a schizophrenia risk gene and has been shown to be downregulated in postmortem brains of schizophrenia patients (Emamian et al., 2004). In addition, one of the critical-domain genes in the schizophrenia risk-associated 1q21.1 copy number variation (CNV) is B-cell lymphoma 9 (Bcl9), which is required for shuttling and keeping β-catenin within the nucleus in response to Wnt stimulation (Consortium, 2008; Kramps et al., 2002; Stefansson et al., 2008). Interestingly, a recent study from the Nestler laboratory has shown that Wnt/GSK3β signaling is important in a mouse model of depression (Wilkinson et al., 2011). This collective data suggest that Wnt/GSK3β signaling is important in mediating mood and psychiatric disorders, and may represent at least one pathogenic signaling pathway.
In conclusion, our data demonstrates that rare and common DISC1 variants impact Wnt signaling in different model systems to ultimately impair brain development. These data provide a framework from which to explain previously-reported associations between common DISC1 variants and human brain structural changes and psychiatric phenotypes. Given that future studies will begin to provide sequencing data for genes that regulate Wnt signaling and brain development, it will be critical to understand how DISC1 variants interact with these genes and how these interactions influence risk for psychiatric disorders.
Materials and Methods
Cell Culture
Human embryonic kidney 293T cells (HEK293T), mouse P19 carcinoma, and mouse neuroblastoma (N2A) cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% FBS, penicillin/streptomycin and L-glutamine. Human Lymphoblastoid cell lines (transformed via Epstein-Barr virus (EBV)) were obtained from the NIMH Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) and generated through the STEP Genetic Repository for Participants. Cell lines were maintained in RPMI media containing 15% FBS and penicillin/streptomycin. The methods for the STEP-BD study and a description of the patients have previously been described (Perlis et al., 2006; Perlis et al., 2009; Sachs et al., 2003).
Reagents
Antibodies
The following primary antibodies were used in this study: rabbit anti-phosphorylated Y216 GSK3 (Abcam), rabbit-anti-Ndel1 antibody (Sasaki et al., 2000), mouse and rabbit anti-GFP antibodies (Invitrogen); mouse anti-FLAG antibody, rabbit anti-Ki67 antibody (Neomarkers), mouse Tuj-1 antibody (BABCO), mouse anti-BrdU antibody (DakoCytomation), chicken anti-GFP antibody (Aves Labs), mouse anti-acetylated alpha tubulin antibody (Sigma), mouse anti-neurofilament RM044 antibody (Zymed) and Phalloidin antibody (Invitrogen). Wnt3a and control conditioned media was produced using Wnt3a-expressing and control L-cells (ATCC). Wnt3a conditional medium was produced according to the ATCC protocol. Purified Wnt3a was obtained from R&D Systems.
Plasmids
The sequences for shRNAs are as follows: control shRNA: 5′-CGGCTGAAACAAGAGTTGG-3′, DISC1 shRNA-1: 5′-GGCAAACACTGTGAAGT GC-3′ (Kamiya et al., 2005). Full length human GSK3β and mouse Dixdc1 were amplified by PCR and subcloned into the FLAG expression vectors. Super 8XTOPFLASH (which contains 8 copies of the TCF/LEF binding site), provided by Dr. R. Moon (University of Washington, WA) and a Renilla-Luc-TK reporter (pRL-TK, Promega) were used for testing TCF transcriptional activity. pCAGIG-Venus was provided by Dr. Zhigang Xie (Boston University, MA).
Animals and In utero electroporation
Swiss Webster pregnant female mice were purchased from Taconic for in utero electroporation experiments as described previously (Sanada and Tsai, 2005). E13 or E15 embryonic brains were injected with either GFP, GFP-tagged human WT DISC1, or GFP-tagged DISC1 variants (final concentration 2.4 μg/μl) together with an enhanced GFP (EGFP)-expressing plasmid (pCAGIG-enhanced YFP; final concentration 0.8 μg/μl). For all cell cycle exit experiments, E13 electroporated mice were intraperitoneally (i.p.) injected with BrdU (100 mg/kg) at E15, sacrificed and processed 24 hr later.
Zebrafish Experiments
Antisense morpholino oligonucleotides (MO) (Gene Tools, LLC) were injected into embryos at the 1-2 cell stage. 1 nL was injected into the single cell of each embryo with a MO concentration of 3.5ng for CMO and 3.5ng for Disc1MO. For RNA injections, DISC1 variant coding sequences were obtained by digestion from pEGF constructs using EcoRI/BamHI sites and then subcloned into pCS2HA plasmid. The amount of mRNA injected was 200 pg for human WT-DISC1 and DISC1 variants. Whole-mount immunostaining was carried out using mouse anti-acetylated alpha tubulin (Sigma, 1:1000) and phalloidin texas red (ph) (Molecular Probes, 1:100). Goat anti-mouse Alexa Fluor 488 (Molecular Probes, 1:500) was used as a secondary (Molecular Probes, 1:100).
Immunocytochemistry
Embryonic brains were fixed in 4% paraformaldehyde and cryoprotected using 30% sucrose overnight. Brains were cryosectioned at 14 μm on a Leica cryostat. Brain sections were rehydrated in PBS and blocked for 1 hour in PBS (containing 10% Donkey serum with 0.3% Triton-X), followed by incubation with the appropriate antibody overnight at 4 degrees. The next day, slides were washed 3 times with PBS and incubated with the appropriate secondary antibody for 2 hours at room temperature, washed an additional 3 times in PBS and mounted using Prolong Gold antifade (Invitrogen). For brains injected with Brdu, slides were treated with 4N HCl for 2 hours prior to blocking.
Luciferase, Cell Proliferation and cAMP Assays
P19 and 293T cells at 1×105 cells/well density were plated into 24-well plates and each well was transfected with 0.8 μg of cDNA plasmid together with 50 ng of Super8XTOPFLASH and 10 ng of pRL-TK using Lipofectamine 2000 (Invitrogen). 24 hours after transfection, transfected cells were stimulated with Wnt3a-conditioned medium (Wnt3a CM) for 16 hours and TCF reporter activity was measured using the Dual-Luciferase Assay System (Promega). For all rescue experiments, 0.6 μg of the pCMV-WT-DISC1 or DISC1 variants, were co-transfected with 0.2 μg of mouse DISC1 shRNA expressing plasmid, together with 50 ng of Super8XTOPFLASH and 10 ng of pRL-TK using Lipofectamine 2000 (Invitrogen). Transfected cells were treated with Wnt3a conditioned media and TCF reporter activity was detected 16 hours later. For human lymphoblast cell experiments, cells were plated at 105 cells/well in a 96-well plate in media containing a lentivirus encoding Super8XTOPFLASH (pBAR) and pRL-TK (SL9) (provided by Dr. Randall T. Moon). Wnt3a or control conditioned media was added 24 hours later, and TCF reporter activity was determined 16 hours after the addition of conditioned media.
For the N2A cell proliferation assays, cells were plated at 25,000 cells per well, transfected with the appropriate plasmids, and counted manually (4 representative fields per condition per experiment) 48 hours later using DAPI stain. For cAMP assays, cells were treated similar to the N2A proliferation assays, however after 48 hours, cells were collected and cAMP levels were measured using a commercial kit (Promega).
Biochemical Experiments
Immunoprecipation and western blot experiments were performed as described in (Mao et al., 2009).
Image and Statistical Analysis
All images were acquired using a confocal Zeiss LSM 510 microscope. Images were further analyzed with Adobe Photoshop and ImageJ v1.42q. Statistical analysis was performed with the student’s t test. All bar graphs are plotted as mean ±SEM.
Supplementary Material
Highlights.
-
-
zebrafish, mouse and human model systems used to assay DISC1 genetic variants
-
-
DISC1 variants inhibit Wnt signaling and neurogenesis via altered GSK3β interaction
-
-
human lymphoblast cells expressing human DISC1 variants have reduced Wnt signaling
-
-
DISC1 S704C variant reduces neuronal migration
Acknowledgements
We would like to thank Tsai lab members and Stanley Center members for helpful discussions. We would like to give a special thanks to Janice Kranz, Kimberly Chambert, Doug Ruderfer, Doug Barker, Jennifer Moran, and Edward M. Scolnick for their intellectual input and logistical support. L.-H. T. is an investigator of the Howard Hughes Medical Institute and the director of the neurobiology program at the Stanley Center for Psychiatric Research. K.K.S. is a recipient of the Human Frontiers Science Program Long-term fellowship and an NSERC postdoctoral fellowship. Y.M. is a recipient of the National Alliance for Research on Schizophrenia and Depression Young Investigator Award. This work was partially supported by a National Institute of Mental Health grant (MH091115) and a grant from the Stanley Center for Psychiatric Research to L.-H.T.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no competing financial interests.
References
- Beaulieu JM, Marion S, Rodriguiz RM, Medvedev IO, Sotnikova TD, Ghisi V, Wetsel WC, Lefkowitz RJ, Gainetdinov RR, Caron MG. A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell. 2008;132:125–136. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
- Blackwood DH, Fordyce A, Walker MT, St Clair DM, Porteous DJ, Muir WJ. Schizophrenia and affective disorders--cosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: clinical and P300 findings in a family. Am J Hum Genet. 2001;69:428–433. doi: 10.1086/321969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw NJ, Soares DC, Carlyle BC, Ogawa F, Davidson-Smith H, Christie S, Mackie S, Thomson PA, Porteous DJ, Millar JK. PKA Phosphorylation of NDE1 Is DISC1/PDE4 Dependent and Modulates Its Interaction with LIS1 and NDEL1. J Neurosci. 2011;31:9043–9054. doi: 10.1523/JNEUROSCI.5410-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callicott JH, Straub RE, Pezawas L, Egan MF, Mattay VS, Hariri AR, Verchinski BA, Meyer-Lindenberg A, Balkissoon R, Kolachana B, et al. Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc Natl Acad Sci U S A. 2005;102:8627–8632. doi: 10.1073/pnas.0500515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapcote SJ, Lipina TV, Millar JK, Mackie S, Christie S, Ogawa F, Lerch JP, Trimble K, Uchiyama M, Sakuraba Y, et al. Behavioral phenotypes of Disc1 missense mutations in mice. Neuron. 2007;54:387–402. doi: 10.1016/j.neuron.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Consortium, I.S. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455:237–241. doi: 10.1038/nature07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRosse P, Hodgkinson CA, Lencz T, Burdick KE, Kane JM, Goldman D, Malhotra AK. Disrupted in schizophrenia 1 genotype and positive symptoms in schizophrenia. Biol Psychiatry. 2007;61:1208–1210. doi: 10.1016/j.biopsych.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Di Giorgio A, Blasi G, Sambataro F, Rampino A, Papazacharias A, Gambi F, Romano R, Caforio G, Rizzo M, Latorre V, et al. Association of the SerCys DISC1 polymorphism with human hippocampal formation gray matter and function during memory encoding. Eur J Neurosci. 2008;28:2129–2136. doi: 10.1111/j.1460-9568.2008.06482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet. 2004;36:131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- Enomoto A, Asai N, Namba T, Wang Y, Kato T, Tanaka M, Tatsumi H, Taya S, Tsuboi D, Kuroda K, et al. Roles of disrupted-in-schizophrenia 1-interacting protein girdin in postnatal development of the dentate gyrus. Neuron. 2009;63:774–787. doi: 10.1016/j.neuron.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Freyberg Z, Ferrando SJ, Javitch JA. Roles of the Akt/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. Am J Psychiatry. 2010;167:388–396. doi: 10.1176/appi.ajp.2009.08121873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood AJ. Lithium and bipolar mood disorder: the inositol-depletion hypothesis revisited. Mol Psychiatry. 2005;10:117–126. doi: 10.1038/sj.mp.4001618. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Numakawa T, Ohnishi T, Kumamaru E, Yagasaki Y, Ishimoto T, Mori T, Nemoto K, Adachi N, Izumi A, et al. Impact of the DISC1 Ser704Cys polymorphism on risk for major depression, brain morphology and ERK signaling. Hum Mol Genet. 2006;15:3024–3033. doi: 10.1093/hmg/ddl244. [DOI] [PubMed] [Google Scholar]
- Hayashi-Takagi A, Takaki M, Graziane N, Seshadri S, Murdoch H, Dunlop AJ, Makino Y, Seshadri AJ, Ishizuka K, Srivastava DP, et al. Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci. 13:327–332. doi: 10.1038/nn.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi-Takagi A, Takaki M, Graziane N, Seshadri S, Murdoch H, Dunlop AJ, Makino Y, Seshadri AJ, Ishizuka K, Srivastava DP, et al. Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci. 2010;13:327–332. doi: 10.1038/nn.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A. Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through neuregulin-1-ErbB4 and DISC1. Trends Neurosci. 2009;32:485–495. doi: 10.1016/j.tins.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya A, Kubo K, Tomoda T, Takaki M, Youn R, Ozeki Y, Sawamura N, Park U, Kudo C, Okawa M, et al. A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat Cell Biol. 2005;7:1167–1178. doi: 10.1038/ncb1328. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Tomoda T, Chang J, Takaki M, Zhan C, Morita M, Cascio MB, Elashvili S, Koizumi H, Takanezawa Y, et al. DISC1-NDEL1/NUDEL protein interaction, an essential component for neurite outgrowth, is modulated by genetic variations of DISC1. Hum Mol Genet. 2006;15:3313–3323. doi: 10.1093/hmg/ddl407. [DOI] [PubMed] [Google Scholar]
- Kim JY, Duan X, Liu CY, Jang MH, Guo JU, Pow-anpongkul N, Kang E, Song H, Ming GL. DISC1 regulates new neuron development in the adult brain via modulation of AKT-mTOR signaling through KIAA1212. Neuron. 2009;63:761–773. doi: 10.1016/j.neuron.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramps T, Peter O, Brunner E, Nellen D, Froesch B, Chatterjee S, Murone M, Zullig S, Basler K. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell. 2002;109:47–60. doi: 10.1016/s0092-8674(02)00679-7. [DOI] [PubMed] [Google Scholar]
- Lee FH, Fadel MP, Preston-Maher K, Cordes SP, Clapcote SJ, Price DJ, Roder JC, Wong AH. Disc1 point mutations in mice affect development of the cerebral cortex. J Neurosci. 2011;31:3197–3206. doi: 10.1523/JNEUROSCI.4219-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekven AC, Thorpe CJ, Waxman JS, Moon RT. Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev Cell. 2001;1:103–114. doi: 10.1016/s1534-5807(01)00007-7. [DOI] [PubMed] [Google Scholar]
- Lipina TV, Kaidanovich-Beilin O, Patel S, Wang M, Clapcote SJ, Liu F, Woodgett JR, Roder JC. Genetic and pharmacological evidence for schizophrenia-related Disc1 interaction with GSK-3. Synapse. 2010a doi: 10.1002/syn.20839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipina TV, Niwa M, Jaaro-Peled H, Fletcher PJ, Seeman P, Sawa A, Roder JC. Enhanced dopamine function in DISC1-L100P mutant mice: implications for schizophrenia. Genes Brain Behav. 2010b doi: 10.1111/j.1601-183X.2010.00615.x. [DOI] [PubMed] [Google Scholar]
- Mao Y, Ge X, Frank CL, Madison JM, Koehler AN, Doud MK, Tassa C, Berry EM, Soda T, Singh KK, et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136:1017–1031. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JK, Pickard BS, Mackie S, James R, Christie S, Buchanan SR, Malloy MP, Chubb JE, Huston E, Baillie GS, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–1191. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA, Devon RS, St Clair DM, Muir WJ, Blackwood DH, et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet. 2000;9:1415–1423. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- Mouaffak F, Kebir O, Chayet M, Tordjman S, Vacheron MN, Millet B, Jaafari N, Bellon A, Olie JP, Krebs MO. Association of Disrupted in Schizophrenia 1 (DISC1) missense variants with ultra-resistant schizophrenia. Pharmacogenomics J. 2010 doi: 10.1038/tpj.2010.40. [DOI] [PubMed] [Google Scholar]
- Nakata K, Lipska BK, Hyde TM, Ye T, Newburn EN, Morita Y, Vakkalanka R, Barenboim M, Sei Y, Weinberger DR, et al. DISC1 splice variants are upregulated in schizophrenia and associated with risk polymorphisms. Proc Natl Acad Sci U S A. 2009;106:15873–15878. doi: 10.1073/pnas.0903413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa M, Kamiya A, Murai R, Kubo K, Gruber AJ, Tomita K, Lu L, Tomisato S, Jaaro-Peled H, Seshadri S, et al. Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron. 65:480–489. doi: 10.1016/j.neuron.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa M, Kamiya A, Murai R, Kubo K, Gruber AJ, Tomita K, Lu L, Tomisato S, Jaaro-Peled H, Seshadri S, et al. Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron. 2010;65:480–489. doi: 10.1016/j.neuron.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis RH, Ostacher MJ, Patel JK, Marangell LB, Zhang H, Wisniewski SR, Ketter TA, Miklowitz DJ, Otto MW, Gyulai L, et al. Predictors of recurrence in bipolar disorder: primary outcomes from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Am J Psychiatry. 2006;163:217–224. doi: 10.1176/appi.ajp.163.2.217. [DOI] [PubMed] [Google Scholar]
- Perlis RH, Smoller JW, Ferreira MA, McQuillin A, Bass N, Lawrence J, Sachs GS, Nimgaonkar V, Scolnick EM, Gurling H, et al. A genomewide association study of response to lithium for prevention of recurrence in bipolar disorder. Am J Psychiatry. 2009;166:718–725. doi: 10.1176/appi.ajp.2009.08111633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletnikov MV, Xu Y, Ovanesov MV, Kamiya A, Sawa A, Ross CA. PC12 cell model of inducible expression of mutant DISC1: new evidence for a dominant-negative mechanism of abnormal neuronal differentiation. Neurosci Res. 2007;58:234–244. doi: 10.1016/j.neures.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Lee Y, Long R, Greenstein D, Clasen L, Addington A, Rapoport JL, Giedd JN. Common functional polymorphisms of DISC1 and cortical maturation in typically developing children and adolescents. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs GS, Thase ME, Otto MW, Bauer M, Miklowitz D, Wisniewski SR, Lavori P, Lebowitz B, Rudorfer M, Frank E, et al. Rationale, design, and methods of the systematic treatment enhancement program for bipolar disorder (STEP-BD) Biol Psychiatry. 2003;53:1028–1042. doi: 10.1016/s0006-3223(03)00165-3. [DOI] [PubMed] [Google Scholar]
- Sanada K, Tsai LH. G protein betagamma subunits and AGS3 control spindle orientation and asymmetric cell fate of cerebral cortical progenitors. Cell. 2005;122:119–131. doi: 10.1016/j.cell.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Shionoya A, Ishida M, Gambello MJ, Yingling J, Wynshaw-Boris A, Hirotsune S. A LIS1/NUDEL/cytoplasmic dynein heavy chain complex in the developing and adult nervous system. Neuron. 2000;28:681–696. doi: 10.1016/s0896-6273(00)00146-x. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Kagawa T, Inoue T, Nonaka A, Takada S, Aburatani H, Taga T. Stabilized beta-catenin functions through TCF/LEF proteins and the Notch/RBP-Jkappa complex to promote proliferation and suppress differentiation of neural precursor cells. Mol Cell Biol. 2008;28:7427–7441. doi: 10.1128/MCB.01962-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda T, Taya S, Tsuboi D, Hikita T, Matsuzawa R, Kuroda S, Iwamatsu A, Kaibuchi K. DISC1 regulates neurotrophin-induced axon elongation via interaction with Grb2. J Neurosci. 2007;27:4–14. doi: 10.1523/JNEUROSCI.3825-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KK, Ge X, Mao Y, Drane L, Meletis K, Samuels BA, Tsai LH. Dixdc1 is a critical regulator of DISC1 and embryonic cortical development. Neuron. 67:33–48. doi: 10.1016/j.neuron.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KK, Ge X, Mao Y, Drane L, Meletis K, Samuels BA, Tsai LH. Dixdc1 is a critical regulator of DISC1 and embryonic cortical development. Neuron. 2010;67:33–48. doi: 10.1016/j.neuron.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6:1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Rujescu D, Cichon S, Pietilainen OP, Ingason A, Steinberg S, Fossdal R, Sigurdsson E, Sigmundsson T, Buizer-Voskamp JE, et al. Large recurrent microdeletions associated with schizophrenia. Nature. 2008;455:232–236. doi: 10.1038/nature07229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton LP, Honardoust D, Mouyal J, Rajakumar N, Rushlow WJ. Activation of the canonical Wnt pathway by the antipsychotics haloperidol and clozapine involves dishevelled-3. J Neurochem. 2007;102:153–169. doi: 10.1111/j.1471-4159.2007.04527.x. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Hodgkinson CA, Robinson DG, Derosse P, Bilder RM, Lencz T, Burdick KE, Napolitano B, Betensky JD, Kane JM, et al. DISC1 is associated with prefrontal cortical gray matter and positive symptoms in schizophrenia. Biol Psychol. 2008;79:103–110. doi: 10.1016/j.biopsycho.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler EM, Geschwind DH, Palmer TD. Lithium regulates adult hippocampal progenitor development through canonical Wnt pathway activation. Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4002093. [DOI] [PubMed] [Google Scholar]
- Young-Pearse TL, Suth S, Luth ES, Sawa A, Selkoe DJ. Biochemical and functional interaction of disrupted-in-schizophrenia 1 and amyloid precursor protein regulates neuronal migration during mammalian cortical development. J Neurosci. 30:10431–10440. doi: 10.1523/JNEUROSCI.1445-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Pearse TL, Suth S, Luth ES, Sawa A, Selkoe DJ. Biochemical and functional interaction of disrupted-in-schizophrenia 1 and amyloid precursor protein regulates neuronal migration during mammalian cortical development. J Neurosci. 2010;30:10431–10440. doi: 10.1523/JNEUROSCI.1445-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghloul NA, Liu Y, Gerdes JM, Gascue C, Oh EC, Leitch CC, Bromberg Y, Binkley J, Leibel RL, Sidow A, et al. Functional analyses of variants reveal a significant role for dominant negative and common alleles in oligogenic Bardet-Biedl syndrome. Proc Natl Acad Sci U S A. 2010;107:10602–10607. doi: 10.1073/pnas.1000219107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson WB, Dias C, Magida J, Mazei-Robison M, Lobo MK, et al. A Novel role of the WNT-Dishevelled-GSKβ signaling cascade in the mouse nucleus accumbens I na social defeat model of depression. J Neurosci. 2011;31:9084–9092. doi: 10.1523/JNEUROSCI.0039-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.