Abstract
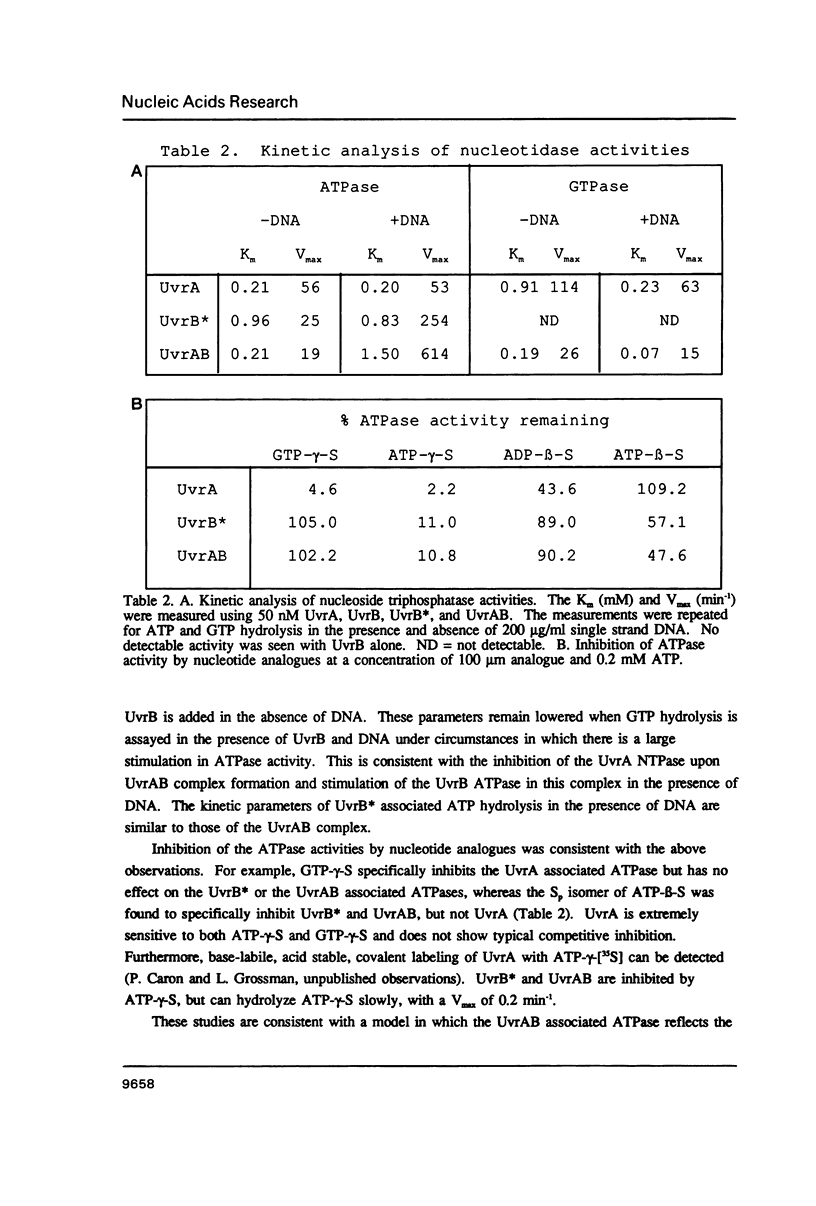
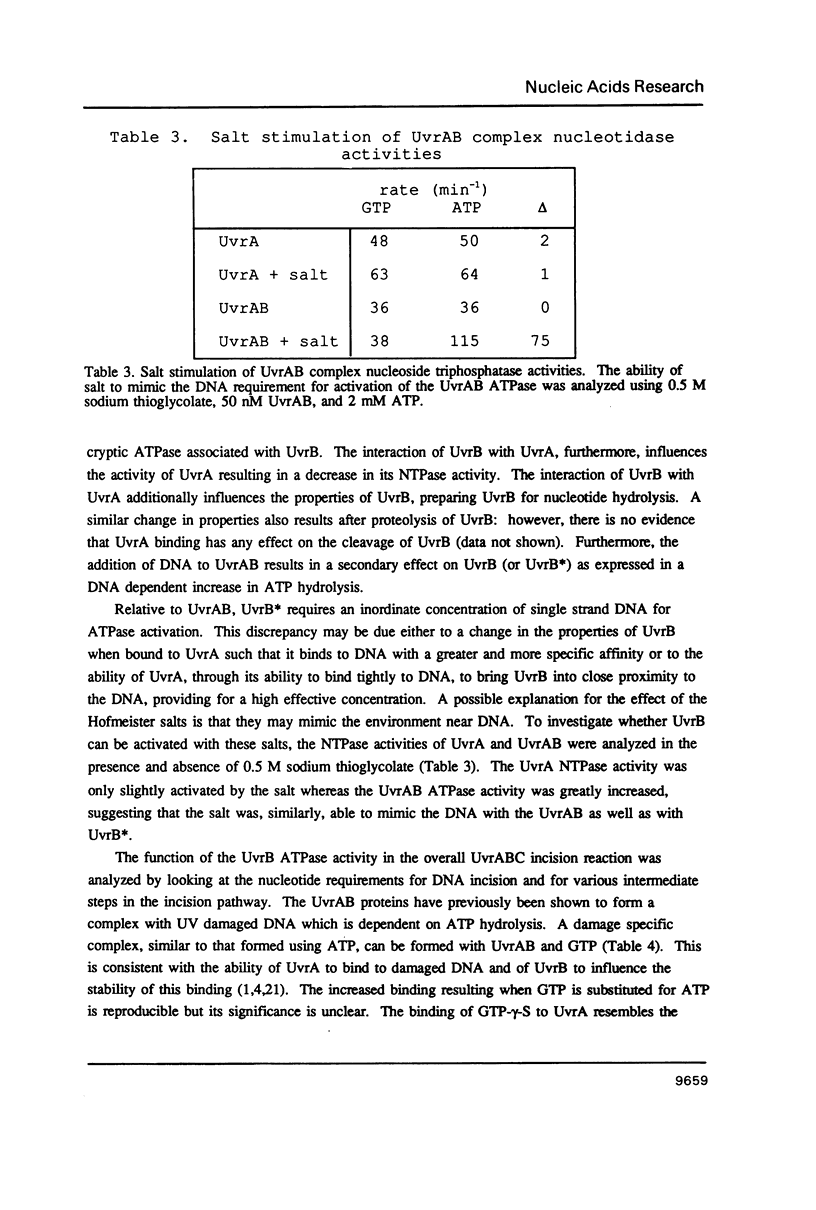
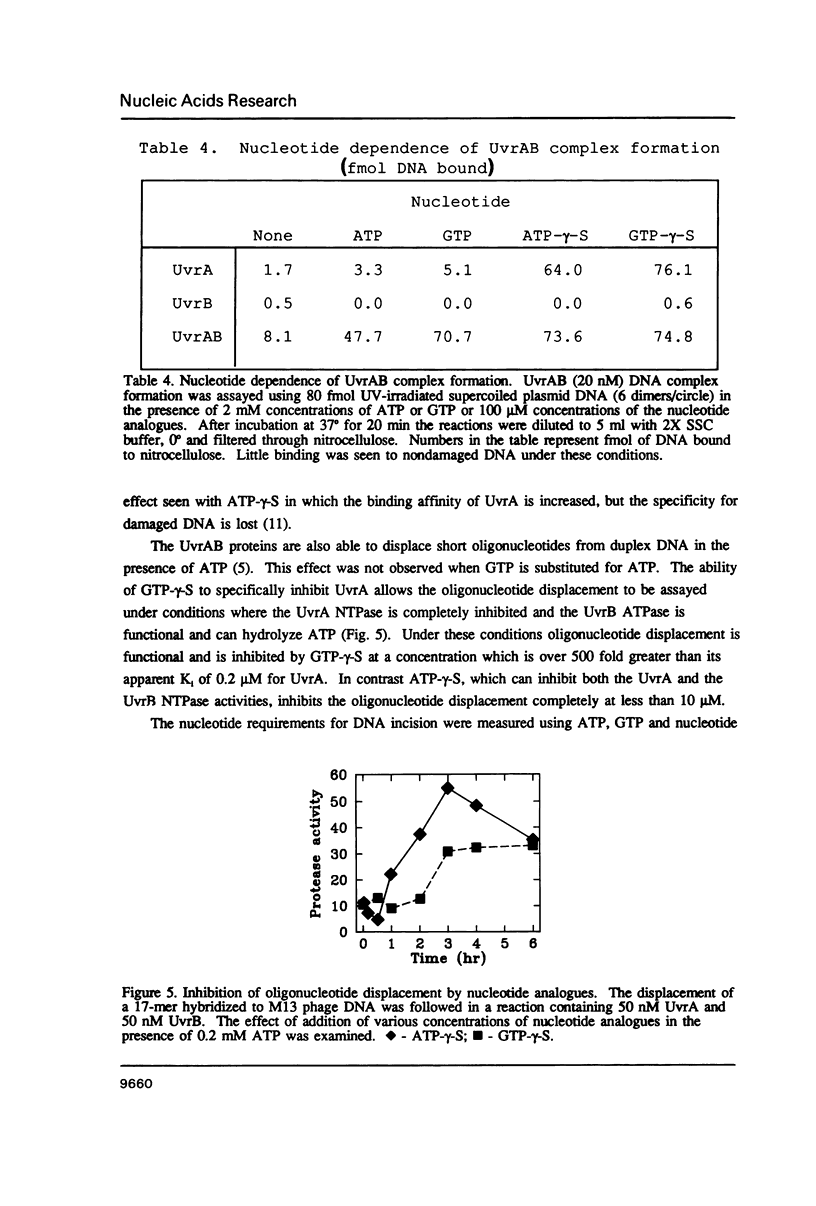
The incision of damaged DNA by the Escherichia coli UvrABC endonuclease requires ATP hydrolysis. Although the deduced sequence of the UvrB protein suggests a putative ATP binding site, no nucleoside triphosphatase activity is demonstrable with the purified UvrB protein. The UvrB protein is specifically proteolyzed in E. coli cell extracts to yield a 70 kD fragment, referred to as UvrB*, which has been purified and is shown to possess a single-strand DNA dependent ATPase activity. Substrate specificity and kinetic analyses of UvrB* catalyzed nucleotide hydrolysis indicate that the stimulation in DNA dependent ATPase activity following formation of the UvrAB complex results from the activation of the normally sequestered UvrB associated ATPase. Using nucleotide analogues, it can be shown that this activity is essential to the DNA incision reaction carried out by the UvrABC complex.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams A., Baron C., Schnebli H. P. The isolation of bacterial membrane ATPase and nectin. Methods Enzymol. 1974;32:428–439. doi: 10.1016/0076-6879(74)32042-3. [DOI] [PubMed] [Google Scholar]
- Alberts B. M., Barry J., Bedinger P., Formosa T., Jongeneel C. V., Kreuzer K. N. Studies on DNA replication in the bacteriophage T4 in vitro system. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):655–668. doi: 10.1101/sqb.1983.047.01.077. [DOI] [PubMed] [Google Scholar]
- Arikan E., Kulkarni M. S., Thomas D. C., Sancar A. Sequences of the E. coli uvrB gene and protein. Nucleic Acids Res. 1986 Mar 25;14(6):2637–2650. doi: 10.1093/nar/14.6.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backendorf C., Spaink H., Barbeiro A. P., van de Putte P. Structure of the uvrB gene of Escherichia coli. Homology with other DNA repair enzymes and characterization of the uvrB5 mutation. Nucleic Acids Res. 1986 Apr 11;14(7):2877–2890. doi: 10.1093/nar/14.7.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas E. E., Biswas S. B., Bishop J. E. The dnaB protein of Escherichia coli: mechanism of nucleotide binding, hydrolysis, and modulation by dnaC protein. Biochemistry. 1986 Nov 18;25(23):7368–7374. doi: 10.1021/bi00371a019. [DOI] [PubMed] [Google Scholar]
- Biswas S. B., Biswas E. E. Regulation of dnaB function in DNA replication in Escherichia coli by dnaC and lambda P gene products. J Biol Chem. 1987 Jun 5;262(16):7831–7838. [PubMed] [Google Scholar]
- Caron P. R., Grossman L. Incision of damaged versus nondamaged DNA by the Escherichia coli UvrABC proteins. Nucleic Acids Res. 1988 Aug 25;16(16):7855–7865. doi: 10.1093/nar/16.16.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins K. D., Washabaugh M. W. The Hofmeister effect and the behaviour of water at interfaces. Q Rev Biophys. 1985 Nov;18(4):323–422. doi: 10.1017/s0033583500005369. [DOI] [PubMed] [Google Scholar]
- Eckstein F., Goody R. S. Synthesis and properties of diastereoisomers of adenosine 5'-(O-1-thiotriphosphate) and adenosine 5'-(O-2-thiotriphosphate). Biochemistry. 1976 Apr 20;15(8):1685–1691. doi: 10.1021/bi00653a015. [DOI] [PubMed] [Google Scholar]
- Hinton D. M., Nossal N. G. Bacteriophage T4 DNA primase-helicase. Characterization of oligomer synthesis by T4 61 protein alone and in conjunction with T4 41 protein. J Biol Chem. 1987 Aug 5;262(22):10873–10878. [PubMed] [Google Scholar]
- Hinton D. M., Silver L. L., Nossal N. G. Bacteriophage T4 DNA replication protein 41. Cloning of the gene and purification of the expressed protein. J Biol Chem. 1985 Oct 15;260(23):12851–12857. [PubMed] [Google Scholar]
- Husain I., Van Houten B., Thomas D. C., Sancar A. Sequences of Escherichia coli uvrA gene and protein reveal two potential ATP binding sites. J Biol Chem. 1986 Apr 15;261(11):4895–4901. [PubMed] [Google Scholar]
- Kobori J. A., Kornberg A. The Escherichia coli dnaC gene product. III. Properties of the dnaB-dnaC protein complex. J Biol Chem. 1982 Nov 25;257(22):13770–13775. [PubMed] [Google Scholar]
- LeBowitz J. H., McMacken R. The Escherichia coli dnaB replication protein is a DNA helicase. J Biol Chem. 1986 Apr 5;261(10):4738–4748. [PubMed] [Google Scholar]
- McGraw R. A., 3rd Dideoxy DNA sequencing with end-labeled oligonucleotide primers. Anal Biochem. 1984 Dec;143(2):298–303. doi: 10.1016/0003-2697(84)90666-3. [DOI] [PubMed] [Google Scholar]
- Oh E. Y., Grossman L. Helicase properties of the Escherichia coli UvrAB protein complex. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3638–3642. doi: 10.1073/pnas.84.11.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh B. F., Cox M. M. High salt activation of recA protein ATPase in the absence of DNA. J Biol Chem. 1988 Jan 5;263(1):76–83. [PubMed] [Google Scholar]
- Sancar A., Rupp W. D. A novel repair enzyme: UVRABC excision nuclease of Escherichia coli cuts a DNA strand on both sides of the damaged region. Cell. 1983 May;33(1):249–260. doi: 10.1016/0092-8674(83)90354-9. [DOI] [PubMed] [Google Scholar]
- Sancar G. B., Sancar A., Rupp W. D. Sequences of the E. coli uvrC gene and protein. Nucleic Acids Res. 1984 Jun 11;12(11):4593–4608. doi: 10.1093/nar/12.11.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeberg E., Steinum A. L. Purification and properties of the uvrA protein from Escherichia coli. Proc Natl Acad Sci U S A. 1982 Feb;79(4):988–992. doi: 10.1073/pnas.79.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strike P., Rupp W. D. Cross-linking studies with the uvrA and uvrB proteins of E. coli. Mutat Res. 1985 Jan-Mar;145(1-2):43–48. doi: 10.1016/0167-8817(85)90038-0. [DOI] [PubMed] [Google Scholar]
- Teo I. A. Proteolytic processing of the Ada protein that repairs DNA O6-methylguanine residues in E. coli. Mutat Res. 1987 Mar;183(2):123–127. doi: 10.1016/0167-8817(87)90054-x. [DOI] [PubMed] [Google Scholar]
- Thomas D. C., Levy M., Sancar A. Amplification and purification of UvrA, UvrB, and UvrC proteins of Escherichia coli. J Biol Chem. 1985 Aug 15;260(17):9875–9883. [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung A. T., Mattes W. B., Grossman L. Protein complexes formed during the incision reaction catalyzed by the Escherichia coli UvrABC endonuclease. Nucleic Acids Res. 1986 Mar 25;14(6):2567–2582. doi: 10.1093/nar/14.6.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung A. T., Mattes W. B., Oh E. Y., Grossman L. Enzymatic properties of purified Escherichia coli uvrABC proteins. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6157–6161. doi: 10.1073/pnas.80.20.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung A. T., Mattes W. B., Oh E. Y., Yoakum G. H., Grossman L. The purification of the Escherichia coli UvrABC incision system. Nucleic Acids Res. 1986 Nov 11;14(21):8535–8556. doi: 10.1093/nar/14.21.8535. [DOI] [PMC free article] [PubMed] [Google Scholar]





