Calcineurin B-like proteins (CBLs) function as membrane-anchored Ca2+ sensors that, when activated, recruit a specific set of Ser-Thr kinases, calcineurin B-like protein-interacting protein kinases (CIPKs), to their sites of action (for review, see Luan et al., 2009; Kudla et al., 2010). The CBL-CIPK network, and its roles in regulating ion transport and cellular homeostasis, have drawn considerable attention over the past decade. Arabidopsis (Arabidopsis thaliana) encodes 10 CBL and 26 CIPK proteins, many of which have been reported to show distinct and selective interactions among these complementary partners. This selectivity would allow for a complex interplay of different CBL-CIPK combinations that, in turn, could encode different stimuli through spatiotemporal regulation of downstream signaling cascades.
Several CBL-CIPK complexes have been identified that connect Ca2+ sensing with different physiological responses through a range of target proteins. Of these, the best known example is the salt overly-sensitive (SOS) pathway, which comprises the interaction of CBL4 (SOS3) with CIPK24 (SOS2). CBL4-CIPK24 binding recruits the kinase to the plasma membrane, where it activates the SOS1 H+/Na+ antiporter to drive Na+ export and reduce toxic sodium levels from the cytosol (Zhu et al., 1998). CBL-CIPK pairing plays a complementary role in K+ nutrition through the activation of the K+ channel AKT1, which mediates in K+ uptake by the roots: a forward-genetic screen for mutants sensitive to low potassium levels showed that loss of CIPK23 function impaired growth under K+-limiting conditions (Xu et al., 2006). In this case, direct interaction of the kinase with CBL1 or CBL9 recruited CIPK23 to the plasma membrane, where it phosphorylated AKT1 (Xu et al., 2006; Cheong et al., 2007).
To date, studies of CBL- and CIPK-dependent signaling have focused primarily on the interaction of the kinase with its target protein and on CIPK pairing with its cognate CBL protein(s). There is little known of the roles (if any) for the CBL proteins beyond their recruitment of the soluble CIPK proteins to one or another membrane surface. We ascribe this gap in knowledge first and foremost to difficulties associated with the yeast two-hybrid (Y2H) approach on which evidence of interaction is primarily based, for example, in the use of the C-terminal cytosolic domain of the channel in analysis of the AKT1-CIPK-CBL network (Li et al., 2006; Xu et al., 2006; Lee et al., 2007). Here, we draw attention to the consequences and often neglected limitations of the Y2H method in work with membrane proteins (for review, see Van Criekinge and Beyaert, 1999; Coates and Hall, 2003). Most important, Y2H methods necessitate nuclear localization of the interacting partners in order to activate reporter gene expression. Hence, membrane proteins need to be truncated to include only soluble domains that are small enough to pass through the nuclear pore. As a result, Y2H assays often are carried out after first eliminating large segments of the protein(s) of interest and, potentially, important interaction sites. Other methodical difficulties have frequently included the omission of data verifying protein expression, streaking of yeast rather than using exact dilution series, and the inherent flaw of most Y2H vector sets: the inability to control expression levels that could increase stringency and signal-to-noise ratios.
The mating-based split-ubiquitin system (SUS) in yeast offers a number of substantial advantages over the Y2H approach (Johnsson and Varshavsky, 1994; Stagljar et al., 1998; Grefen et al., 2009; Dünkler et al., 2012), and we commend it as the method of choice for work with integral membrane proteins and proteins that are membrane anchored. The SUS method enables the use of full-length membrane proteins, thus overcoming the most significant limitations of Y2H. SUS assays make use of the ubiquitin protein, split between two halves, with each half fused to one of the proteins of interest. The bait protein incorporates the C-terminal half of ubiquitin fused with a transcription factor, and the prey protein is fused to the N-terminal half of the ubiquitin, which is mutated (NubI to NubG) to prevent spontaneous association. Interaction of the bait and prey leads to reassembly of the ubiquitin moiety, its cleavage by ubiquitin-specific proteases, and release of the transcription factor, which then diffuses to the nucleus, where it activates reporter genes for auxotrophy selection and quantitative enzymatic assays (see Fig. 2C below). The bait protein construct is driven by the met25 promoter, which allows efficient control of its expression and simplifies testing for high stringency in interactions (Obrdlik et al., 2004; Grefen et al., 2007). Because the assay for binding relies on ubiquitin assembly at the cytosolic face of the membrane and release of a small, soluble transcription factor, this approach overcomes the need for the use of soluble protein domains.
Figure 2.
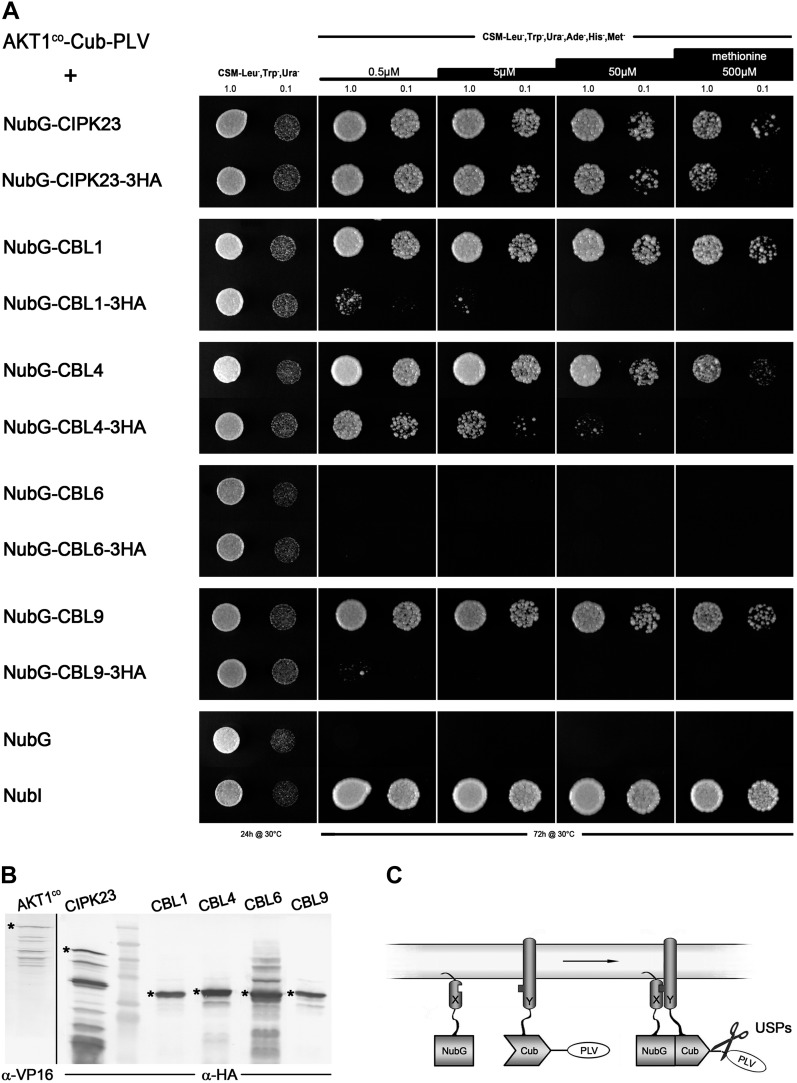
SUS analysis of full-length AKT1 with CIPK23 and exemplary CBL proteins. A, Growth assay of diploid yeast containing a Met-repressible bait construct, AKT1co-Cub-PLV, and different prey constructs. CBL and CIPK23 proteins were either N-terminally tagged (NubG; each top row) or N- and C-terminally tagged (NubG at the N terminus and triple-HA tag at the C terminus; each bottom row). Yeast were dropped at optical density values of 1.0 and 0.1 on vector-selective (CSM-Leu-,Trp-,Ura-) and interaction-selective (CSM-Leu-,Trp-,Ura-,Ade-,His-,Met-) media with increasing Met concentrations. Growth was monitored after 24 h for the vector-selective control plates and after 72 h for the actual interaction plates. As negative and positive controls, AKT1co-Cub-PLV-expressing yeast were mated with yeast containing only NubG or NubI (wild-type Nub) peptides. B, Western-blot analyses of all haploid yeast clones prior to mating, verifying the expression of both bait and prey (C-terminally HA-tagged) fusions. Asterisks mark the bands that correspond to the expected protein sizes of the respective fusion proteins: AKT1co-Cub-PLV = 150.5 kD; NubG-CIPK23-3xHA = 63.7 kD; NubG-CBL1-3xHA = 34.8 kD; NubG-CBL4-3xHA = 35.9 kD; NubG-CBL6-3xHA = 36.3 kD; NubG-CBL9-3xHA = 34.8 kD. C, Schematic depiction of the SUS assay demonstrating the cleavage of the PLV transcription factor construct upon reassembly of the ubiquitin halves. PLV, Protein A-LexA-VP16.
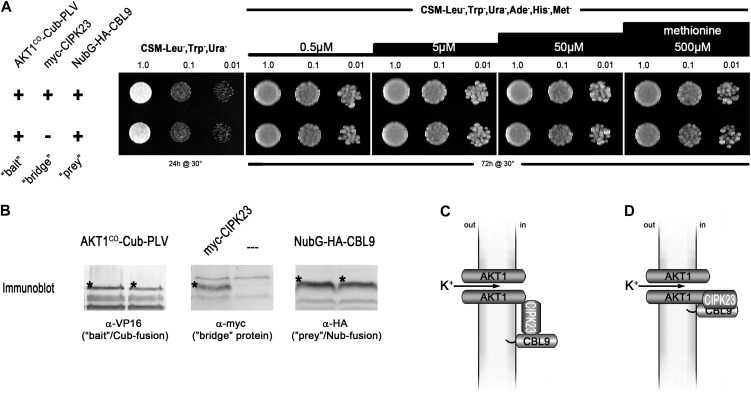
While developing a refinement of this method, the split-ubiquitin bridge (SUB) assay (Honsbein et al., 2009; Grefen, 2012), we examined a selection of interacting and noninteracting proteins. The concept behind the SUB approach is to detect multimeric interactions between proteins, two of which do not interact on their own but both of which will interact with a third, or “bridging,” protein (see Figs. 1 and 3). We generated vectors that allow constitutive, inducible, or repressible expression of bridge proteins in addition to the bait and prey fusion. As the first proof of concept, we demonstrated the ternary interaction of the AKT1 and KC1 K+ channel subunits with the vesicle-trafficking protein SYP121 (Honsbein et al., 2009) and have since sought other protein partners thought to form ternary interactions with which to test the SUB assay. The AKT1-CIPK23-CBL1/CBL9 complex, postulated from individual binary interaction analyses in Y2H assays, was an obvious model to choose. Interactions between CIPK23 and cytosolic parts of AKT1 on the one hand and between CIPK23 and either CBL1 or CBL9 on the other hand have been reported (Xu et al., 2006; Lee et al., 2007). Furthermore, several experimental approaches (electrophysiological recordings in oocytes, analysis of transfer-DNA insertion lines, and in vitro phosphorylation assays) provided convincing evidence of a functional relevance for these interactions (Li et al., 2006; Xu et al., 2006; Cheong et al., 2007; Lee et al., 2007). We coexpressed as bait the full-length AKT1 protein, optimized for codon usage in yeast, together with CBL9 as the prey, both with and without CIPK23 as the bridge protein. We assumed that bait and prey alone would not interact and expected yeast growth to be recovered on selective medium only when the CIPK23 bridge was included, thus indicating a ternary interaction. However, growth was recovered also in the absence of the kinase, indicating that CBL9 protein was able to interact directly with AKT1 and independent of CIPK23 (Fig. 1).
Figure 1.
SUB assay of full-length AKT1 with CIPK23 and CBL9. A, Growth assay of haploid yeast coexpressing the Met-repressible bait construct AKT1co-Cub-PLV (where “co” = codon optimized) and the prey construct NubG-2xHA-CBL9. A construct for a myc-tagged CIPK23 as bridge protein was included in the yeast in the top row but excluded in the yeast of the bottom row. Yeast were dropped at optical density values of 1.0, 0.1, and 0.01 on vector-selective (CSM-Leu-,Trp-,Ura-) and interaction-selective (CSM-Leu-,Trp-,Ura-,Ade-,His-,Met-) media with increasing Met concentrations. Growth was monitored after 24 h for the vector-selective control plates and after 72 h for the actual interaction plates. (Methodical details can be found in Grefen et al. [2009] and Grefen [2012].) B, Western-blot analyses of the two yeast clones, verifying the expression of bait, bridge, and prey fusions. Asterisks mark the bands that correspond to the expected protein sizes of the respective fusion proteins: AKT1co-Cub-PLV = 150.5 kD; myc-CIPK23 = 57.0 kD; NubG-2xHA-CBL9 = 32.1 kD. C, Schematic depiction of the anticipated tripartite interaction of CBL9-CIPK23 and AKT1 (according to Lee et al. [2007]). D, One possible alternative for the interaction that would accord with our observations. PLV, Protein A-LexA-VP16.
In the course of these analyses, we noted also a difference in the interaction readout of yeast growth subject to the presence of a triple-hemagglutinin (HA) tag masking the C terminus of the CBL proteins (Fig. 2A): while the C-terminally tagged CBL1 and CBL9 proteins did not interact with AKT1, removing the triple-HA tag was sufficient to recover yeast growth. Interestingly, CBL4 also rescued growth, suggesting that it does interact with AKT1, although CBL4 has been reported not to interact with CIPK23 (Xu et al., 2006; Lee et al., 2007). In this case, adding the C-terminal triple-HA tag did not abolish interaction completely. By contrast, we observed no rescue of growth with CBL6, regardless of whether the C terminus was masked, indicating selectivity among the CBL proteins for binding with AKT1. CIPK23 interaction with AKT1 was not strongly affected by C-terminal tagging, although we detected a slight reduction of growth compared with the control under high stringency (500 µm Met added; Fig. 2A). These observations served to underscore in our minds the sensitivity of CIPK and CBL proteins to modest changes that are likely to mask domains important for their interactions.
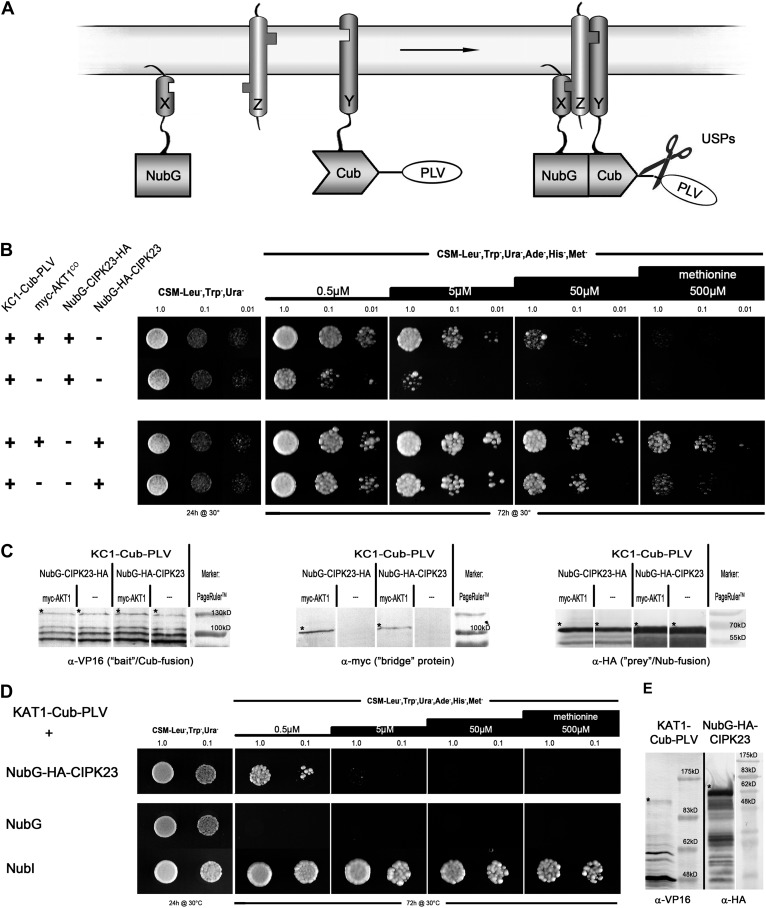
In the past, structure-function analysis of the AKT1-CIPK23 interaction using the Y2H approach led to the assumption that the C-terminal ankyrin domain of AKT1 and the kinase domain of CIPK23 are the minimal motifs needed for interaction (Lee et al., 2007). The important regions in CIPK23 for interaction with the corresponding CBL proteins is localized to the C-terminal domain of the kinase; by contrast, most of the CBL protein is needed for binding, only a small stretch at the N terminus being expendable (Kim et al., 2000). Again, this structure-function analysis is based on Y2H screens with N-terminally tagged proteins and truncated fusions. It would be interesting to see whether masking of the C termini would hinder interaction (Stellberger et al., 2010) and whether use of the SUS assay with CBL proteins anchored to the membrane could affect their selectivity for CIPK proteins. With these thoughts in mind, we set out to test whether the SUB assay could be used in a different approach, namely, testing a trimeric interaction between KC1-AKT1 and CIPK23. As a truncated KC1 was reported not to interact with CIPK23 (Li et al., 2006), both proteins could be used as bait and prey and their interaction could be facilitated through AKT1 as the bridging protein.
Figure 3 shows the results of this SUB assay. Again, the outcomes differ, depending on the orientation of the tag (starting from the bottom row of yeast drops and working up in Fig. 3B): KC1 grew with the Nub-2xHA-CIPK23 prey, despite the absence of AKT1; in the presence of AKT1, growth was maintained even under high stringency (increased Met levels), suggesting that a previously unrecognized interaction between CIPK23 and KC1 is enhanced when AKT1 is present. When CIPK23 was tagged on both termini, the basal interaction with KC1 was reduced; again, adding AKT1 enhanced growth, albeit to less of an extent than with the untagged CIPK23. It is possible that the higher expression of the untagged CIPK23 could explain the generally higher level of interaction under more stringent conditions, but in both cases it is clear that AKT1 enhanced the interactions. Clearly, these observations call for further study and validation through independent, biochemical methods. Nonetheless, KC1 and AKT1 subunits normally assemble as heterotetramers to form functional channels in vivo (Duby et al., 2008; Honsbein et al., 2009; Grefen et al., 2010); therefore, it seems likely that CIPK23 should associate with both.
Figure 3.
SUB assay of full-length KC1 with AKT1 and CIPK23. A, Schematic depiction of a SUB assay. Two proteins, X and Y, do not interact, but addition of a third protein, Z, that interacts with both X and Y facilitates the binding and reassembly of ubiquitin. B, Growth assay of haploid yeast containing a Met-repressible bait construct, KC1-Cub-PLV, and different bridge or prey constructs. Each top line shows interaction with AKT1co being present compared with each second line, which excludes the expression of a bridge protein. The top row uses CIPK23 that was tagged N terminally with NubG and C terminally with a triple-HA tag, whereas the bottom row contains CIPK23 tagged with an N-terminal NubG-2xHA. Yeast were dropped at optical density values of 1.0, 0.1, and 0.01 on vector-selective (CSM-Leu-,Trp-,Ura-) and interaction-selective (CSM-Leu-,Trp-,Ura-,Ade-,His-,Met-) media with increasing Met concentrations. Growth was monitored after 24 h for the vector-selective control plates and after 72 h for the actual interaction plates. C, Western-blot analyses of all haploid yeast clones, verifying the expression of bait, bridge, and prey fusions. Asterisks mark the bands that correspond to the expected protein sizes of the respective fusion proteins: KC1-Cub-PLV = 129.1 kD; myc-AKT1co = 102.3 kD; NubG-CIPK23-3xHA = 63.7 kD; NubG-2xHA-CIPK23 = 61.1 kD. D, Growth assay of diploid yeast using KAT1-Cub-PLV as bait to exclude promiscuity of the NubG-2xHA-CIPK23 construct. Increasing Met levels demonstrate that KAT1 fails to interact with CIPK23. E, Western-blot analysis of haploid KAT1-Cub-PLV and NubG-2xHA-CIPK23, verifying their expression. Asterisks mark the corresponding bands: KAT1-Cub-PLV = 131.8 kD; NubG-2xHA-CIPK23 = 61.1 kD. PLV, Protein A-LexA-VP16.
We hope that these findings will stimulate debate and would urge a revisiting of past conclusions about CBL-CIPK signaling and specificity drawn from Y2H assays. In particular, it will be of interest to know the functional consequences of the direct interaction of the CBL proteins with AKT1. Could these interactions affect trafficking of the channel, or might they alter the activity of the channel? This would surely add to our understanding of Ca2+ sensing and its integration of cellular responses associated with CBL and CIPK protein binding.
Glossary
- Y2H
yeast two-hybrid
- SUS
split-ubiquitin system
- SUB
split-ubiquitin bridge
- HA
hemagglutinin
References
- Cheong YH, Pandey GK, Grant JJ, Batistic O, Li L, Kim BG, Lee SC, Kudla J, Luan S. (2007) Two calcineurin B-like calcium sensors, interacting with protein kinase CIPK23, regulate leaf transpiration and root potassium uptake in Arabidopsis. Plant J 52: 223–239 [DOI] [PubMed] [Google Scholar]
- Coates PJ, Hall PA. (2003) The yeast two-hybrid system for identifying protein-protein interactions. J Pathol 199: 4–7 [DOI] [PubMed] [Google Scholar]
- Duby G, Hosy E, Fizames C, Alcon C, Costa A, Sentenac H, Thibaud JB. (2008) AtKC1, a conditionally targeted Shaker-type subunit, regulates the activity of plant K+ channels. Plant J 53: 115–123 [DOI] [PubMed] [Google Scholar]
- Dünkler A, Müller J, Johnsson N. (2012) Detecting protein-protein interactions with the split-ubiquitin sensor. Methods Mol Biol 786: 115–130 [DOI] [PubMed] [Google Scholar]
- Grefen C. (2012) The split ubiquitin system for the analysis of three-component interactions. In JJ Sanchez-Serrano, J Salinas, eds. Methods in Molecular Biology: Arabidopsis Protocols, Ed 3. Humana Press, New York (in press) [Google Scholar]
- Grefen C, Chen Z, Honsbein A, Donald N, Hills A, Blatt MR. (2010) A novel motif essential for SNARE interaction with the K+ channel KC1 and channel gating in Arabidopsis. Plant Cell 22: 3076–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefen C, Lalonde S, Obrdlik P. (2007) Split-ubiquitin system for identifying protein-protein interactions in membrane and full-length proteins. Curr Protoc Neurosci Chapter 5: Unit 5.27 [DOI] [PubMed] [Google Scholar]
- Grefen C, Obrdlik P, Harter K. (2009) The determination of protein-protein interactions by the mating-based split-ubiquitin system (mbSUS). Methods Mol Biol 479: 217–233 [DOI] [PubMed] [Google Scholar]
- Honsbein A, Sokolovski S, Grefen C, Campanoni P, Pratelli R, Paneque M, Chen Z, Johansson I, Blatt MR. (2009) A tripartite SNARE-K+ channel complex mediates in channel-dependent K+ nutrition in Arabidopsis. Plant Cell 21: 2859–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson N, Varshavsky A. (1994) Split ubiquitin as a sensor of protein interactions in vivo. Proc Natl Acad Sci USA 91: 10340–10344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KN, Cheong YH, Gupta R, Luan S. (2000) Interaction specificity of Arabidopsis calcineurin B-like calcium sensors and their target kinases. Plant Physiol 124: 1844–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudla J, Batistic O, Hashimoto K. (2010) Calcium signals: the lead currency of plant information processing. Plant Cell 22: 541–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Lan WZ, Kim BG, Li L, Cheong YH, Pandey GK, Lu G, Buchanan BB, Luan S. (2007) A protein phosphorylation/dephosphorylation network regulates a plant potassium channel. Proc Natl Acad Sci USA 104: 15959–15964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Kim BG, Cheong YH, Pandey GK, Luan S. (2006) A Ca2+ signaling pathway regulates a K+ channel for low-K response in Arabidopsis. Proc Natl Acad Sci USA 103: 12625–12630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan S, Lan W, Chul Lee S. (2009) Potassium nutrition, sodium toxicity, and calcium signaling: connections through the CBL-CIPK network. Curr Opin Plant Biol 12: 339–346 [DOI] [PubMed] [Google Scholar]
- Obrdlik P, El-Bakkoury M, Hamacher T, Cappellaro C, Vilarino C, Fleischer C, Ellerbrok H, Kamuzinzi R, Ledent V, Blaudez D, et al. (2004) K+ channel interactions detected by a genetic system optimized for systematic studies of membrane protein interactions. Proc Natl Acad Sci USA 101: 12242–12247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagljar I, Korostensky C, Johnsson N, te Heesen S. (1998) A genetic system based on split-ubiquitin for the analysis of interactions between membrane proteins in vivo. Proc Natl Acad Sci USA 95: 5187–5192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellberger T, Häuser R, Baiker A, Pothineni VR, Haas J, Uetz P. (2010) Improving the yeast two-hybrid system with permutated fusions proteins: the Varicella zoster virus interactome. Proteome Sci 8: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Criekinge W, Beyaert R. (1999) Yeast two-hybrid: state of the art. Biol Proced Online 2: 1–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Li HD, Chen LQ, Wang Y, Liu LL, He L, Wu WH. (2006) A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 125: 1347–1360 [DOI] [PubMed] [Google Scholar]
- Zhu JK, Liu J, Xiong L. (1998) Genetic analysis of salt tolerance in Arabidopsis: evidence for a critical role of potassium nutrition. Plant Cell 10: 1181–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]