Abstract
Upward long-distance mobile silencing has been shown to be phloem mediated in several different solanaceous species. We show that the Arabidopsis (Arabidopsis thaliana) seedling grafting system and a counterpart inducible system generate upwardly spreading long-distance silencing that travels not in the phloem but by template-dependent reiterated short-distance cell-to-cell spread through the cells of the central stele. Examining the movement of the silencing front revealed a largely unrecognized zone of tissue, below the apical meristem, that is resistant to the silencing signal and that may provide a gating or protective barrier against small RNA signals. Using a range of auxin and actin transport inhibitors revealed that, in this zone, alteration of vesicular transport together with cytoskeleton dynamics prevented or retarded the spread of the silencing signal. This suggests that small RNAs are transported from cell to cell via plasmodesmata rather than diffusing from their source in the phloem.
The coordination of growth and development in multicellular organisms relies on both local and long-distance communication between cells and tissues. Plants have specialized vascular tissue, the phloem and xylem, to transport nutrient, hormone, and signaling molecules to mediate the long-distance exchange of developmental and defense information. The phloem transports photoassimilates from source to various sink tissues and also transports macromolecules including mRNAs and small RNAs (Lough and Lucas, 2006; Atkins et al., 2011).
RNA interference (RNAi) is an important viral defense system found in plants and in some animals. It is guided by short interfering RNAs (siRNAs) and operates by directed RNA degradation (Cogoni and Macino, 2000; Waterhouse et al., 2001; Novina and Sharp, 2004). Posttranscriptional gene silencing is also mediated by the RNAi mechanism and can spread both locally (Himber et al., 2003; Dunoyer et al., 2005) and long distance to most parts of the plant (Palauqui et al., 1997; Voinnet and Baulcombe, 1997; Brosnan et al., 2007).
Long-distance silencing has been extensively studied in Nicotiana species by grafting experiments using silencing rootstocks, often generating small RNAs from synthetic hairpin RNA (hpRNA) transgenes, and scions containing target reporter genes (Mlotshwa et al., 2002; Kalantidis et al., 2008). However, for detailed molecular analysis of this process, there is neither the detailed genome sequence information nor the mutant stocks available in Nicotiana species that are available in Arabidopsis (Arabidopsis thaliana). When a graft-transmissible, rootstock-to-scion GFP silencing system was developed in Arabidopsis (Brosnan et al., 2007), this appeared to provide an excellent way to further study the components and mechanisms of mobile RNAi. Indeed, results from this system suggested that long-distance silencing required elements of both transcriptional and posttranscriptional silencing pathways, including Dicer-like3 (DCL3), RNA-dependent RNA polymerase2 (RDR2), RNA Polymerase IV, and RDR6, but that production of the signal did not require DCL2, -3, or -4. More recent work using a similar Arabidopsis grafting system, but examining shoot-to-root silencing (Dunoyer et al., 2010a; Molnar et al., 2010), concluded that one or more of the DCLs is needed for signal production. Dunoyer et al. (2010b) provided evidence that the mobile signal is composed of 21-nucleotide small RNAs, and Molnar et al. (2010) presented data showing that the mobile signal directing epigenetic modification in grafting experiments is made up of 24-nucleotide siRNAs.
In Nicotiana, the patterns of long-distance systemic silencing, the movement of some viruses, and the spreading of phloem-translocated dye are all quite similar, suggesting that a silencing signal moves through the phloem transport pathway (Roberts et al., 1997; Voinnet et al., 1998; Tournier et al., 2006). However, some virus-host combinations can show a “recovery” symptom (Wingard, 1928; Matthews, 1973) in which the lower leaves of an infected plant display virus symptoms but the leaves at the top of the plant appear healthy. Intriguingly, leaves at the interface can have their older, distal portions showing virus symptoms and their younger, proximal portions appearing healthy (see Fig. 7 in Wingard, 1928). This bizonal pattern is quite different from the vascular pattern that might be expected from an antiviral signal transported through phloem.
Figure 7.
Silencing progression in the hypocotyl of RtSS plants treated with Dex when 5 d old (time 0). The long arrow indicates the root-hypocotyl junction, where expression of both GUS and the inducible silencer ends. In median longitudinal sections (top panels, days 0–16), GFP silencing was detected 4 d after Dex treatment and gradually migrated upward from the base of the hypocotyl (bottom horizontal line), with the silencing front (top horizontal line) reaching the top of the hypocotyl 4 to 5 d later, at 8 to 9 d after Dex induction. By day 12 to 13, silencing had crossed the HEJ (dotted line), then it continued into higher shoot tissues. However, even 16 d after Dex treatment, when most shoot tissues were silenced, an isolated internal domain of tissue remained unsilenced (outlined), as did hypocotyl epidermal cells. AM, Axillary meristem; C, cortex; stem, floral bolt stem; V, vascular tissue; X, xylem. Asterisks indicate the base of the cotyledon petiole. The bottom four panels show cross-sections of hypocotyls (day 0–16) showing the central xylem strand (X; slightly less bright central zone at day 0) within the vascular cylinder (V), surrounded by two layers of cortical cells (C) and the outer layer of epidermal cells (E). The vascular cylinder shows strong GFP fluorescence at day 0 (all images are at the same exposure) but is completely silenced above the root-hypocotyl junction by day 12, while the cortex and epidermis still show GFP fluorescence. By day 16, the inner cortex shows some silencing, and free GFP can be seen in the two phloem strands (P). In RtSS hypocotyls without Dex (16 −Dex), the central tissue retains bright GFP fluorescence.
The Arabidopsis system developed by Brosnan et al. (2007) also gave a distinctly nonvascular pattern of silencing, including the production of bizonal patterned leaves, in the newly formed scions of grafted plants. To better understand how this silencing pattern is produced and to help resolve the apparently conflicting results about the components required to generate long-distance silencing, we examined the process in greater detail. To do this, we generated a system that relies on inducible root-specific production of the silencing signal rather than incurring the restrictions and possible complications of grafting. We refer to this transgenic system as root-to-shoot silencing (RtSS). Using this system, we demonstrate that long-range RtSS in Arabidopsis spreads largely by a series of cell-to-cell short-range mobile silencing events, rather than by transport through the phloem, and that the silencing front slows down at the transition zone between hypocotyl and epicotyl. Experiments using cellular trafficking inhibitors provided evidence suggesting that cells of this region act as a gating barrier for the transmission of the RtSS signals in Arabidopsis.
RESULTS
Graft-Transmissible mRNA Silencing in Arabidopsis
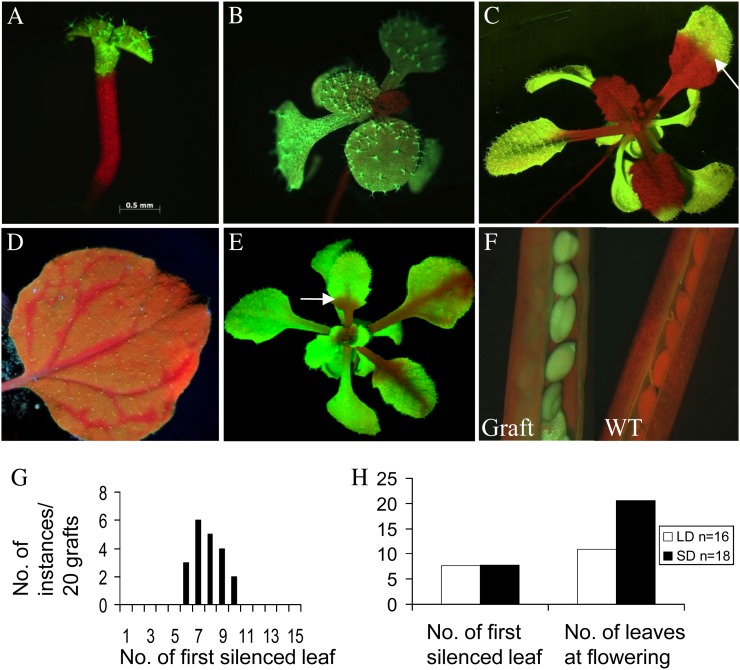
Brosnan et al. (2007) used graft-transmissible silencing of GFP in Arabidopsis to examine the induction, transport, and effector components of the silencing process. Silencing of GFP in the scion was observed only in newly formed leaves and not in older tissues. From this, it was concluded that long-distance signaling, as distinct from short-distance, cell-to-cell spread of transgene silencing, was causing the effect. Nevertheless, the silencing in the newly formed leaves was confined to proximal tissue that was meristematic at grafting rather than spreading to distal tissues in a vascular pattern, as seen in Nicotiana grafting experiments (Palauqui et al., 1997; Fusaro et al., 2006). Therefore, we examined the process in Arabidopsis in more detail. The grafting procedure, 35S-GFP reporter, and S1 (GF hairpin only [hpGF]) and S2 (GF hairpin plus 35S-GFP) GFP silencer lines were the same as those used by Brosnan et al. (2007). The only difference was that in our experiments, the grafted plantlets were maintained in petri dishes on Murashige and Skoog (MS) medium rather than being transferred to soil. From more than 50 grafts using hpGF rootstocks, the scions of nearly 70% of successful grafts (Fig. 1, A and B) showed conspicuous silencing in the basal portion of some rosette leaves, termed bizonal silencing (Fig. 1C). The pattern was very different from that of GFP silencing in expanding leaves of GFP-expressing Nicotiana benthamiana plants following agroinfiltration in lower leaves with a hpGF construct (Fig. 1D; Voinnet and Baulcombe, 1997). Of the remaining approximately 30% of grafts, most showed no silencing but some displayed a somewhat vascular silencing pattern (Fig. 1E).
Figure 1.
Graft-transmissible gene silencing in Arabidopsis. A, A graft between a 35S-GFP scion and 35S-hpGF rootstock 3 d after grafting. B, Graft as in A showing initial silencing 10 d after grafting. C, Bizonal silencing (arrow) in scion leaves 22 d after grafting. D, Vascular silencing pattern in GFP-expressing N. benthamiana plants infiltrated with a hpGF construct in the lower leaves. E, Some Arabidopsis grafts show initial vascular silencing followed by bizonal leaf silencing (arrow). F, GFP expression is recovered in the seeds of fully silenced scions (left). On the right is a wild-type Col-0 silique (WT). G, In 20 grafts of 35S-GFP on hpGF rootstock, the majority showed initial silencing on the seventh or eighth leaf. H, In both short-day (SD) and long-day (LD) conditions, initial silencing was seen in the seventh leaf.
From leaf counts on 20 successful grafts showing silencing, the first true leaf to display silencing ranged from leaf 6 to leaf 10 (Fig. 1G). Examination of scion apices immediately prior to and 6 d after grafting revealed that the transferred scion had three to five leaf primordia, which increased to five to eight leaves, including leaf primordia, 6 d later. Arabidopsis ecotype Columbia (Col-0) plants grown in long days undergo quicker transition to flowering than plants grown under short days, and this developmental transition has been shown to coincide with a decreased movement of symplastic tracer into the shoot apical meristem (Gisel et al., 2002). We asked whether this transition would also lead to changes in RNA-mediated gene-silencing movement. We monitored silencing in grafts grown under either long-day or short-day conditions. All grafts showed similar rates of silencing, and in this experiment, the seventh or eighth leaf was the first silenced organ in both conditions (Fig. 1H).
Two other features associated with long-distance silencing in Nicotiana are the ability of the silencing signal to self-perpetuate and that the silencing is lost in the next generation. To test whether these occur in Arabidopsis, 35S-GFP scions (10 per time point) were removed from their hpGF rootstocks 3, 5, 7, and 9 d post grafting and maintained on MS+Suc medium. Two scions from the 7-d-post-grafting and one from the 9-d-post-grafting time point developed silencing in the newly emerging leaves as the excised scions continued to grow on the medium, thus demonstrating that, once initiated in scion tissue, the silencing can self-perpetuate.
Grafted plants showing scion silencing were transferred to soil and allowed to flower and set seed. Although these plants showed complete GFP silencing throughout the rosette leaves, stems, flowers, and siliques, the newly formed seeds within the siliques displayed strong GFP expression (Fig. 1F). All seedlings germinated from these seeds had strong, ubiquitous GFP expression. This shows that the silencing had been lost and was not inherited by the next generation. Hence, apart from the nonvascular silencing pattern, the characteristics from these grafting experiments were consistent with the effects from a long-distance silencing signal. However, they are in contrast to the phloem-mediated source-to-sink transport through the hypocotyl that would be presumed to mainly flow from the scion to the rootstock. One possible explanation could be that the silencing signal moved against this phloem flow.
A GFP Signal Can Move from Sink to Source in Phloem
Phloem, as a component of the vascular system, generally transports photoassimilates from source cells and tissues to sink cells, which would be from shoot to root tissues in Arabidopsis seedling hypocotyls. It has been widely reviewed that proteins, including GFP (Imlau et al., 1999), RNAs, and gene-silencing signals, can move through the phloem (Ghoshroy et al., 1997; Kehr and Buhtz, 2008; Turgeon and Wolf, 2009). However, when wild-type scions were grafted onto rootstocks expressing GFP controlled by the AtSUC2 promoter, which is active only in the phloem companion cells (Fig. 2A; Stadler et al., 2005b), free GFP was translocated across the graft junction in the hypocotyl into scion tissue (Fig. 2, B–D). This suggests that, while slow, proteins can move in the phloem against the predominantly source-to-sink phloem flow. To further investigate the root-to-shoot signal transport without the plant stress and delay due to vascular reconnection of grafting, we developed a new system.
Figure 2.
Grafts between Arabidopsis C24 plants with either scion or rootstock expressing SUC2-GFP, producing fluorescence in the phloem companion cells. A, Graft between SUC2-GFP rootstock with C24 scion (dotted outline) after 3 d showing GFP in the two phloem strands of the rootstock hypocotyl but no GFP in the scion above the graft junction (dashed red line). B, After 18 d, GFP can be seen in phloem below the graft junction but not above the graft at low magnification. Xylem (X) has proliferated in the center of the hypocotyl. C and D, At higher magnification, GFP can be detected in the phloem (P) of the scion (C) and is concentrated in the phloem companion cells (CC; D). Sieve elements (SE) show blue fluorescence from aniline blue-stained callose. Red indicates chlorophyll autofluorescence.
A New RtSS System
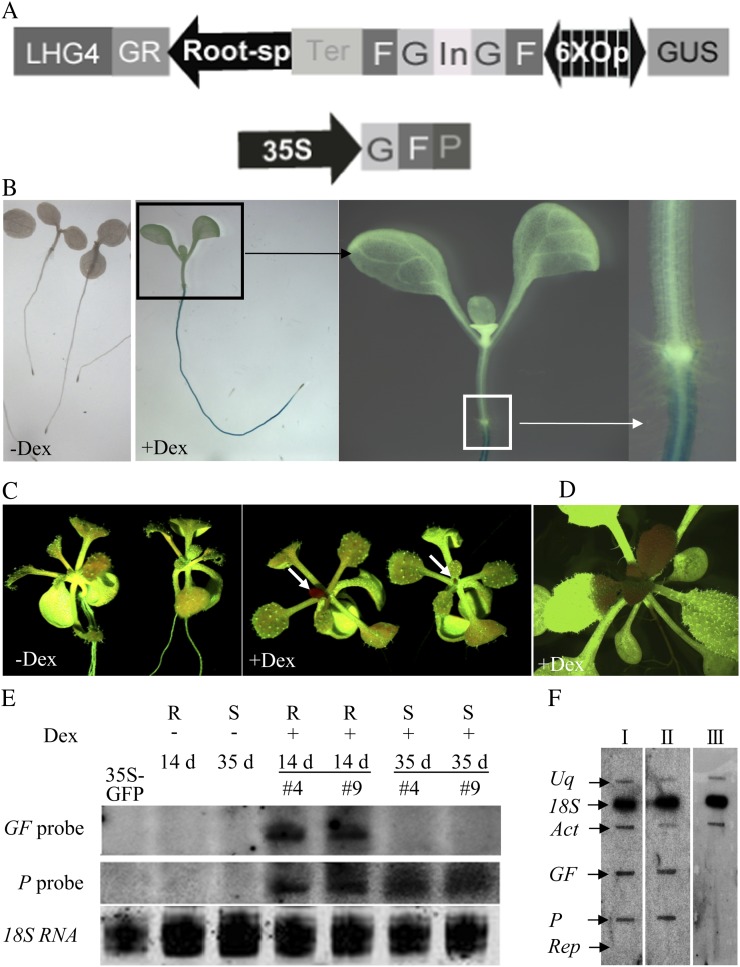
To establish a transgenic system that could mimic grafting experiments, we combined the dexamethasone (Dex)-inducible pOp/LhG4-GR system with a tissue-specific promoter to control the expression of hpRNA. Previous work has shown that the pOp/LhG4-GR system regulates very stringent transgene expression (Craft et al., 2005) and that this can be used for inducible RNAi (Wielopolska et al., 2005). We used the same GFP-expressing transgenic reporter line and the S1 GF hpRNA construct from Brosnan et al. (2007) but replaced the 35S promoter control of the hpRNA with the pOp/LhG4-GR system and regulated that with the root-specific promoter, TobRB7 (Yamamoto et al., 1991). With this construct, the transcriptional activator (LhG4-GR) is retained in the cell cytoplasm until Dex is added to displace cytoplasmic heat shock protein from the GR-binding site, allowing it to enter the nucleus. This activator binds to the 6xOP sequence and induces bidirectional transcription of GF hpRNA and the GUS gene (Fig. 3A). The hpRNA covering the first 400-bp fragment of the GFP gene is under the control of the pOp promoter, which is activated by LhG4 entering the nucleus (Fig. 3A). The construct was transformed into 35S-GFP-expressing plants and selected on hygromycin-containing medium.
Figure 3.
Phenotypic and molecular characterization of the RtSS Arabidopsis lines. A, Physical map of the inducible vector TobRb7-hpGF. In this vector, a root-specific promoter from tobacco, TobRb7, controls the expression of GR-LHG4, which in turn activates the expression of a silencing hairpin, hpGF, and a GUS reporter when Dex is present. B, Induced GUS expression in TobRB7-GRLHG4/Op-hpGF (RtSS) plants. There is no GUS expression in RtSS plants without Dex (−Dex); after Dex treatment, RtSS plants show GUS expression only in roots (+Dex). Enlarged views of shoot (black rectangle) and root-hypocotyl junction (white rectangle) show GUS expression confined below the junction. C, RtSS plants after 16 d of treatment with Dex showing GFP silencing in the shoots (+Dex) but no silencing without Dex (−Dex). Arrows indicate silencing in young leaves. D, A 22-d-old RtSS plant showing bizonal leaf silencing after Dex induction. E, Northern-blot analysis of siRNA production in the RtSS plants with and without Dex induction. Lanes #4 and #9 represent two separate RtSS lines. R, Root; S, shoot. F, Nuclear run-on assay showing that shoot silencing is posttranscriptional. I, Gel from a 41-d-old noninduced RtSS plant; II, gel from a completely silenced RtSS plant after 31 d of Dex treatment; III, gel from wild-type Col-0 Arabidopsis. Act, Actin; Uq, ubiquitin; Rep, E. coli replication protein.
Three independent hygromycin-resistant transformants (T1) showing no GFP silencing without Dex induction were propagated, and their seed (T2) was germinated on hygromycin selection medium. The seed from one line gave the 3:1 (resistance:sensitivity) segregation ratio indicative of a single-locus T-DNA insertion. This transgenic line was used in all subsequent experiments. The GUS expression in T2 seedlings germinated and maintained on 10 µm Dex-containing MS medium for 7 to 28 d could be seen in the mature root and was undetectable in the stem, leaf blade, veins, or shoot and root apical meristems, which was similar to the expression pattern described in transgenic tobacco (Nicotiana tabacum) plants containing the TobRB7-GUS construct (Yamamoto et al., 1991; Fig. 3B). No GUS activity was detected in any sister seedlings grown on MS medium without Dex (Fig. 3B). Since GUS expression was induced in roots, the hpGF RNA should also be produced in this tissue, and silencing observed in the shoot must come from a mobile silencing signal emanating from the root. In three replicate experiments, 20 RtSS seedlings were germinated and grown on Dex-containing medium for 14 d and the same number were grown on Dex-free medium. In all three experiments, all of the Dex-treated seedlings showed GFP silencing in their newly emerging leaves (Fig. 3C), whereas the ubiquitous GFP expression in the untreated plants was unaltered.
RtSS Mimics Four Features of Grafted Plants
In three experiments using a total of 120 RtSS plants treated with Dex at early stages (1- to 10-d-old plants), over 90% of them showed a bizonal silencing pattern in their rosette leaves (Fig. 3, C and D) that was indistinguishable from the patterns obtained after grafting (Fig. 1C). Similarly, a small proportion of plants displayed a vascular silencing pattern. A second feature shared by graft-silenced and RtSS-induced plants is the recovery of GFP expression in the next generation. Of seedlings germinated from seed set by Dex-treated RtSS plants displaying silencing throughout most of the rosette leaves and all of the floral bolt, 100% had high levels of GFP expression. This attribute has been retained in each of the seven generations of this RtSS line that has been tested.
At the molecular level, the graft-transmissible silencing in Arabidopsis is similar to that of induced silencing spread in N. benthamiana. When a silencing construct is targeted against one region of a reporter transgene, siRNAs are generated outside the targeted region of the gene’s transcript, most abundantly from the 3′ adjacent region (Vaistij et al., 2002). This process is termed transitivity (Sijen et al., 2001; Vaistij et al., 2002; Himber et al., 2003). In Arabidopsis grafts using GFP scions on hpGF rootstocks, siRNAs produced in the scion are overwhelmingly against the P region, which is in the 3′ direction from the GF target of the silencing hairpin (Brosnan et al., 2007). In our RtSS plants after exposure to Dex, both P- and GF-derived siRNA were detected in the roots; however, only P-derived siRNAs were detected in the silenced shoots (Fig. 3E), indicating this predominantly unidirectional transitivity of silencing. Brosnan et al. (2007) also showed that there was no detectable DNA methylation or histone modification in the promoter or coding regions of the target gene, implying that scion silencing caused by a mobile silencing signal occurred at the posttranscriptional level. However, two recent reports showed that 24-nucleotide siRNAs corresponding to the promoter region can cause graft-transmissible transcriptional gene silencing (Bai et al., 2011; Melnyk et al., 2011). To check whether the silencing in the RtSS system was transcriptional or posttranscriptional, we performed a nuclear run-on assay. In both Dex-induced and control noninduced RtSS plants, the nascent transcripts from GF and P fragments accumulated to a comparable level (Fig. 3F), and as expected, there was no transcript in the wild-type Col-0 Arabidopsis. These results clearly show that silencing induced by mobile signals operates at the posttranscriptional level, which is consistent with previous findings (Crété et al., 2001). From this combined evidence, we conclude that our RtSS system very closely mimics the effects produced by grafting hpRNA-expressing rootstocks onto hpRNA target-expressing scions.
Root Silencing Is Correlated with the Production of 21-Nucleotide Small RNAs
Since GF hpRNA was induced exclusively in the roots, we might expect silencing to occur first in this tissue. When the roots of RtSS plants were exposed to Dex for 1 or 2 d, the existing root tissue continued to show GFP fluorescence, although less brightly than in untreated plants (Fig. 4, A and B). However, by day 3 of the treatment, new lateral roots had emerged that were silenced for GFP expression (Fig. 4C, arrows). This silencing became more obvious over time (Fig. 4, D–G, arrows), until after 12 d of treatment, all of the roots were silenced (Fig. 4H). In small RNA northern blots, the 21-nucleotide GF-siRNA in roots gradually increased over the first 3 d of Dex treatment (Fig. 4I) as did the secondary siRNA (P region-derived siRNA), and after 12 d of treatment (Fig. 3E), both GF and P 21-nucleotide small RNAs (sRNAs) were readily detectable. This accumulation of siRNAs correlated with the initiation of silencing.
Figure 4.
Inducible GFP silencing in the roots of Dex-induced RtSS plants, and time course of production of 21-nucleotide siRNAs from the hpGF. A, A plant without Dex. B, Two days after Dex treatment. C, Initial silencing in the root can be seen 3 d after treatment. D to G, Silencing becomes more obvious with time. H, Roots are completely silenced by 12 d after Dex treatment. White arrows indicate the silenced roots. I, Small RNA northern-blot analysis of roots treated with Dex. WT, Wild-type Col-0 Arabidopsis. Roots of T1, T2, and T3 plants on MS medium without Dex (indicated by 0) were used as controls. Roots from the T3 generation treated with Dex showed increasing expression of 21-nucleotide (nt) siRNAs from the GF and P, with a peak 3 d after, and maintained at 12 d after induction.
Developmental Age Affects But Does Not Negate Mobile Silencing
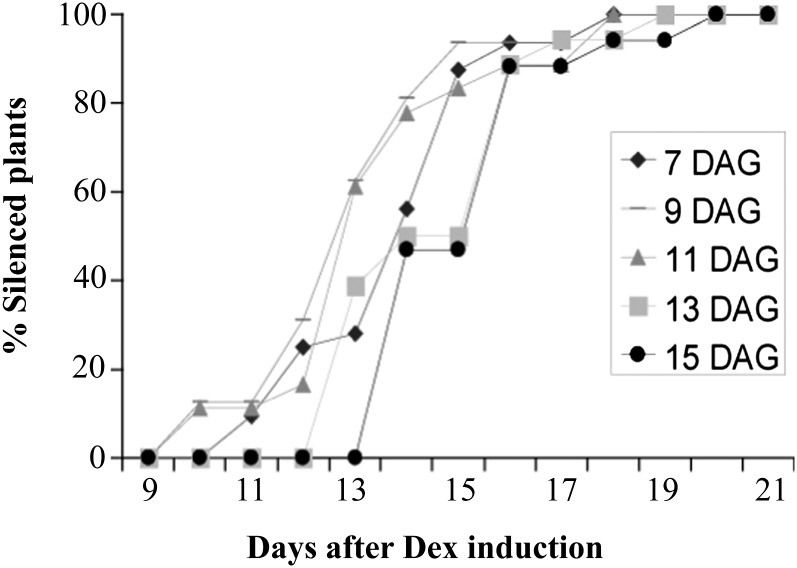
The trauma of cutting and the induction of healing and vascular reconnection that take place in establishing grafts may influence or mask the processes being examined in grafting experiments. In Arabidopsis, this is further confounded by the technical necessity of making grafts using very young seedlings. Our RtSS system removes this concern and this restriction. In tobacco, the age, and the developmental stage, of the tissues used in grafting experiments have been shown to have a major impact on the production of long-distance silencing (Crété et al., 2001). Similarly, the induction of long-distance silencing by agroinfiltration in N. benthamiana only operates efficiently in juvenile plants. To investigate the effect of timing on the production of RtSS in Arabidopsis, RtSS plants were treated with Dex at different ages ranging from 7 to 15 d after germination. This revealed that Arabidopsis was capable of producing mobile silencing at later developmental stages (Fig. 5). All of the plants in this experiment became silenced, irrespective of the time of Dex induction, taking between 12 and 22 d from Dex induction to the first signs of GFP silencing in the shoot. The last plant of each group showed silencing 5 to 8 d after the first plant in every case. However, the spatiotemporal distribution of silenced tissue in the plant was strongly affected by the timing of the treatment, with plants treated at a younger age developing more rapid and widespread silencing (Fig. 6A). Nevertheless, the first marked silencing in leaves of all plants was in the bizonal pattern (Fig. 6A).
Figure 5.
RtSS plants were treated with Dex at different ages (days after germination [DAG]), and the percentage showing silencing was recorded for the following 9 to 21 d.
Figure 6.
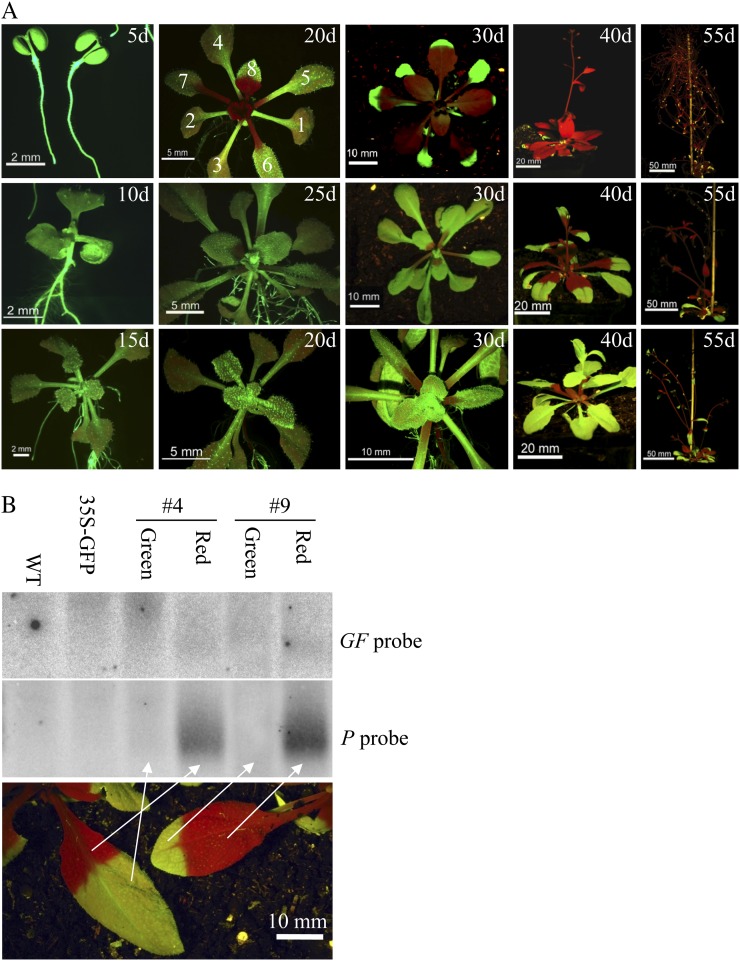
Spread of silencing in RtSS shoots when induced at different times after germination, and siRNA distribution in the silenced and unsilenced leaves. A, Plants were treated with Dex at 5, 10, or 15 d after germination. In 5-d-old plants, silencing appeared first in the petioles of the fifth, sixth, and seventh leaves after 20 d, with bizonal silencing in the eighth leaf. All lateral organs formed after the eighth leaf, and the floral bolt stem, were silenced. In 15-d-old plants treated with Dex, initial silencing was seen in rosette leaf petioles at 30 d, and the inflorescence meristem, floral bolt stem, and flowers escaped silencing. By 55 d, silencing had progressed through the bolt stem and flowers but was less complete than in plants treated earlier with Dex. Numbers 1 to 8 denote the order of leaf appearance. B, Small RNA detected in the silenced (red) and nonsilenced (green) segments of leaves from two different RtSS lines (lanes #4 and #9) 45 d after 3-d Dex induction of 10-d-old plants. WT, Wild type.
Bizonal Distribution of siRNA in the RtSS Leaf
We noticed that silencing could eventually spread through the whole plant (except for the first two or three leaves; Fig. 6A), so the origin of the bizonal silencing pattern in leaves of both Dex-induced RtSS plants and GFP scions on hpGF rootstocks was intriguing (Figs. 1C, 3D, and 6), especially if the silencing signals were phloem mobile. In plants induced with Dex at 5, 10, or 15 d after germination, the bizonal GFP silencing pattern was always observed in the basal petiole and leaf tissue of the first silenced leaf at 12 to 22 d after treatment.
This phenomenon prompted us to investigate how the silencing signal itself was distributed in the shoot. The silenced (red autofluorescence from chlorophyll) and nonsilenced (green fluorescence from GFP) parts were harvested separately, and total RNA from these samples was analyzed on a siRNA northern blot. As shown in Figure 6B, GF-derived siRNAs could not be detected in either the silenced or the nonsilenced part of the leaves, which agrees with our previous experiment analyzing siRNAs in grafts and RtSS plants (Fig. 3E). However, the P-derived secondary siRNAs could be detected only in the GFP-silenced tissue, not in the GFP-expressing leaf tissue (Fig. 6B). This bizonal distribution of P-derived siRNA coincided precisely with the silencing pattern.
A Slow Front of Cell-to-Cell Silencing Spreads through the Hypocotyl
The time for a molecule transported in the phloem to travel from a source to a sink tissue within a small, herbaceous plant typically ranges from a few minutes to several hours (Kiefer and Slusarenko, 2003; Windt et al., 2006). In the Arabidopsis grafting experiments, it generally took 7 to 10 d for the silencing initiator rootstock to induce the appearance of silencing in the shoot. This lag could be the time needed to reconnect phloem sieve elements, reestablish plasmodesmata between cells, load, move, and unload the signal, and then transform the signal through RNAi-associated cellular machinery into GFP silencing in the shoot tissues. However, with the RtSS system, which gives transcription and siRNA production within hours of Dex application (Fig. 4I; Supplemental Fig. S1) and has no necessity for reconnection or vascular repair, it took 12 to 22 d for silencing to appear in the shoot (Figs. 5 and 6A). This led us to further question the route taken by the signal. If the signal were spread via the phloem, we would expect cells adjacent to the two phloem strands in the hypocotyl to be the first to show GFP silencing.
We examined longitudinal sections of RtSS hypocotyls at different times after Dex treatment and observed the first GFP silencing in a region of cells at the base of the hypocotyl 4 d after Dex treatment. This region continued to expand shootward at a rate of 377 μm d−1 within the central tissue until it had reached the top of the hypocotyl (Fig. 7; Supplemental Fig. S2). The cortical cells were slower to silence, retaining GFP fluorescence up to 13 to 14 d after Dex induction and showing partial silencing by 16 d (Fig. 7). The epidermal cells, which are symplastically isolated from internal tissues (Duckett et al., 1994; Stadler et al., 2005a), were not silenced and continued to fluoresce. During the 14-d period of these experiments, the hypocotyl lengths did not increase (Gendreau et al., 1997).
As the silencing front moved through the hypocotyl-shoot junction or the hypocotyl-epicotyl junction (HEJ), the rate of movement slowed to 56 μm d−1 (Fig. 7; Supplemental Fig. S2). A central group of larger cells below the meristem was not silenced within the 16-d duration of this experiment (13 and 16 d in Fig. 7). Eventually, the silencing progressed around these central unsilenced cells and then was observed in the shoot meristem and leaf tissues.
RtSS Is Prevented if Not Bridged by Cells Providing a Silencing Amplification Template
We reasoned that if the apparent long-distance silencing arises from slow cell-to-cell spread, it would require an uninterrupted symplastic path of cells expressing GFP mRNA between the initiating cells in the rootstock and the visible receptor cells in the shoot. The GFP mRNA transcribed within each cell would provide the incoming siRNAs with a template to generate more siRNAs that could then invade adjacent cells and thus produce a reiterated silencing signal. Such a relay amplification mechanism has been proposed by Himber et al. (2003). To test this possibility, we needed to interrupt the path with cells producing no GFP mRNA and observe the effect on the spread of silencing. Grafting a section of wild-type Arabidopsis stem or hypocotyl between the initiator rootstock and the recipient shoot would accomplish this but would be technically challenging, so we took another approach.
We made a Dex-inducible RtSS-hpGF construct controlled by the REA promoter that gives exclusively root tip expression in Arabidopsis (Fig. 8, A and B) and transformed it into wild-type Col-0 to generate REA-RtSS-hpGF-expressing plants. By crossing the REA-RtSS-hpGF line with 35S-GFP plants, we then generated REA-RtSS-hpGF-35S-GFP plants. The REA-RtSS-hpGF and REA-RtSS-hpGF-35S-GFP lines, together with the original TobRb7-RtSS line, were then used as rootstocks for grafting to 35S-GFP scions. In the Dex-induced REA-RtSS-hpGF rootstock, there is no bridge of GFP mRNA expression between the rootstock and the scion, and none of 27 grafts showed any sign of silencing throughout the life of the graft (Fig. 8C). However, all 30 grafts using the Dex-induced REA-RtSS-hpGF-35S-GFP as rootstock gave RtSS (Fig. 8D), although silencing took considerably longer to appear than in 35S-GFP/TobRb7-RtSS grafts (Fig. 8E) and was first detected at the base of the floral stem or in new branches originating from the rosette. These results show that the long-distance cell-to-cell silencing movement in Arabidopsis requires the overlapping expression of the mobile silencing signal and the target mRNA.
Figure 8.
Silencing was not transmitted through Arabidopsis tissue lacking the target gene. A, GUS expression in REA-RtSS-hpGF and GFP expression in REA-RtSS-hpGF-35S-GFP plants before Dex treatment. B, GUS expression in REA-RtSS-hpGF and GFP expression in REA-RtSS-hpGF-35S-GFP plants after Dex treatment. C, No GFP silencing was seen in the grafts of 35S-GFP scions on REA-hpGF rootstocks throughout the life of the plants. D, GFP silencing was seen first at the base of 35S-GFP scions grafted onto REA-RtSS-hpGF-35S-GFP rootstocks treated with Dex. The white rectangle indicates the enlarged base of the scion. E, Systemic silencing was observed in 35S-GFP scions grafted onto Dex-induced RtSS rootstocks.
A simple explanation for the mobile silencing-requiring uninterrupted root-to-shoot GFP transgene expression could be that GFP mRNAs are needed as a template from which siRNA-guided RNA-dependent RNA polymerase, RDR6 (Dalmay et al., 2000; Schwach et al., 2005), could generate more double-stranded RNA and hence facilitate the production of more siRNAs. The newly generated siRNAs would pass to adjacent cells to continue the process, and the long-distance spread of the signal would be a reiterative amplification process. To test this, the RtSS system was transferred into an rdr6 background. As predicted by the model, the induced RtSS in this genotype produced local silencing in the roots (Supplemental Fig. S3) but was unable to generate mobile GFP silencing.
A Central, Symplastically Isolated Zone under the Meristem
If mobile silencing depends on slow, cell-to-cell spread, we would expect the silencing front to move at a constant rate from root to shoot, rather than arresting at the HEJ (Fig. 7). Closer analysis revealed that the central tissue in this zone, below the shoot meristem, comprised large, loosely packed cells with reduced points of contact with each other (Fig. 9A). At the periphery of the zone, the cells were small and densely packed. The route of fewest cells across the HEJ to the meristem would be through the large central cells (route 1 in Fig. 9A). However, they, and the surrounding layer of small cells, remained unsilenced (Fig. 7, d 16), suggesting that the silencing front took a path to the meristem and leaf primordia that circumnavigated this zone (route 2 in Fig. 9A).
Figure 9.
Silencing route at the HEJ in Arabidopsis, and CF loading experiments. A, In route 1 (red arrows), the silencing moves through small tightly packed cells and then a zone with large loosely packed cells. In route 2 (yellow arrows), silencing moves around this zone to the meristem. The HEJ zone is enclosed by a dashed line. B, CFDA was loaded into the root of a 7-d-old wild-type Col-0 plant, and green CF fluorescence could be detected in the HEJ zone (dashed outline). C, In a 14-d-old wild-type Col-0 plant, the CF fluorescence was mainly detected around the edge of the HEJ. D, The internal HEJ tissue remained unsilenced in 16-d-old RtSS plants grown on medium containing Dex.
To investigate the symplastic connections from the hypocotyl through this central zone to the shoot apical meristem, we loaded the symplastic tracer dye 5(6)-carboxyfluorescein diacetate (CFDA) into the roots. The nonfluorescent CFDA enters living cells, and after the acetate groups are cleaved off by endogenous esterases, the green fluorescent carboxyfluorescein (CF) is trapped in the cell cytoplasm and moves cell to cell via plasmodesmata. In 7-d-old seedlings, CF in the cytoplasm of hypocotyl cells moved upward into cells of the central zone and was rapidly seen in the meristematic cells above (Fig. 9B). However, in 14-d-old seedlings, CF did not enter this tissue, suggesting closed plasmodesmata there (Fig. 9C), although the dye could move in the smaller cells around the central zone. Dex-induced RtSS plants are 13 to 15 d old when the silencing signal reaches the HEJ (Figs. 7, d 16, and 9D), at exactly the time when this zone prevents the entry of either CF or silencing signals. Since plasmodesmata appeared to be critical for signal transmission, we tested a number of inhibitors known to affect cell-to-cell transport.
Auxin Transport Inhibitors Repress the Spread of RtSS and Identify a Gating Barrier
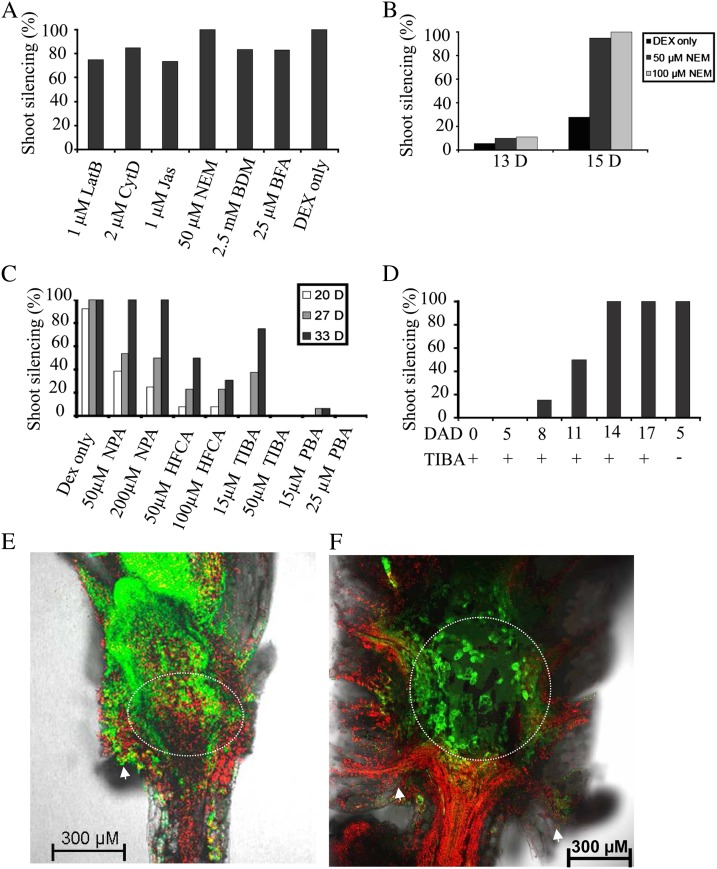
Targeting of plasmodesma protein components (Sagi et al., 2005; Thomas et al., 2008) and trafficking of some virus movement proteins through plasmodesmata depend on both a secretory pathway (Haupt et al., 2005; Ju et al., 2005; Genovés et al., 2010; Schoelz et al., 2011) and a component of cell cytoskeleton-directed transport (Ding et al., 1996; Harries et al., 2009; Su et al., 2010; Radford and White, 2011; White and Barton, 2011). To test whether these processes were involved in the spread of RNA silencing, we applied several chemical agents to Dex-induced plants at the highest concentrations known to be inhibitory without causing morbidity. We first tested inhibitors of cytoskeleton assembly or function, since the actin-myosin cytoskeleton is critical for cell-to-cell transport of viruses and other macromolecules in many cases (for review, see White and Barton, 2011). However, neither latrunculin B, an actin polymerization inhibitor, nor cytochalasin D, an actin filament disrupter, nor jasplakinolide, which can stabilize plant actin filaments, prevented the spread of silencing in Dex-treated RtSS plants (Fig. 10A). An inhibitor of myosin function, 2,3-butanedione monoxime (BDM), which binds the myosin head to actin filaments, also had no significant effect. In contrast, n-ethylmaleimide (NEM), which detaches myosin from actin, caused earlier silencing, such that almost 100% of plants showed silencing by 15 d in NEM-treated plants compared with less than 40% silencing in control or BDM-treated plants (Fig. 10, A and B).
Figure 10.
Effects of cytoskeleton and hormone inhibitors on RtSS. A, Percentage of systemic silencing 22 d after treatment with different inhibitors (27 d after Dex induction). B, NEM can slightly accelerate the rate of systemic silencing. C, Silencing on the shoot can be fully stopped by 50 µm TIBA and 25 µm PBA and partially inhibited by the lower concentration of 15 µm TIBA or 15 µm PBA. NPA at 50 or 200 µm also slowed shoot silencing. HFCA at 50 or 100 µm severely slowed shoot silencing. D, RtSS plants that were treated with Dex for longer times, such as 11 d after Dex (DAD), still developed systemic silencing when treated with 50 µm TIBA. CytD, Cytochalasin D; LatB, latrunculin B. E and F, Confocal images of longitudinal sections. E, A 32-d-old plant treated with 100 µm HFCA did not yet show shoot silencing but showed silencing in the meristematic HEJ zones. F, A 38-d-old RtSS plant treated with DEX for 5 d, then with TIBA, showed silencing excluded from the HEJ. Arrowheads indicate cut cotyledon petioles. The circle denotes the HEJ zone. Experiments were repeated two to three times, with at least 20 plants per treatment.
We then applied 25 µm brefeldin A (BFA), an inhibitor of vesicle trafficking in the secretory pathway, to 5-d-old Dex-treated RtSS plants in which the silencing front had just moved through the root-hypocotyl junction (Fig. 7). Although BFA-treated plants showed phenotypes such as stunting and agravitropism, the spread of cell-to-cell silencing was only slightly delayed (Fig. 10A), also suggesting that the silencing signal may not require the secretory pathway.
Both the secretory pathway and the actin cytoskeleton are altered by auxin transport inhibitors (Pétrasek et al., 2003; Dhonukshe et al., 2008), and one such inhibitor can partially complement the phenotypic defects caused by mutation in a component of the endogenous silencing pathway (Lu and Fedoroff, 2000). Therefore, we speculated that auxin transport, or transporters, may play a role in root-to-shoot movement of the silencing signal. Treating Dex-induced RtSS plants with the auxin transport inhibitor 2,3,5-triiodobenzoic acid (TIBA; 50 µm) or 2-(1-pyrenoyl) benzoic acid (PBA; 25 µm) prevented the spread of silencing from the root to the shoot for more than 33 d (Fig. 10C), whereas the Dex-induced control RtSS plants were fully silenced by day 27 (Fig. 10, A and C). Lowering the concentrations of TIBA and PBA to 15 µm reduced, but did not negate, this effect (Fig. 10C). The inhibitory effect of TIBA could be alleviated by transferring treated plants to TIBA-free medium (all of six plants transferred eventually showed shoot silencing). Two other auxin transport inhibitors, N-1-naphthylphthalamidic acid (NPA) and 9-hydroxy-9-fluorenecarboxylic acid (HFCA; Fig. 10C), also greatly retarded the movement of RtSS.
To assess whether TIBA blocked the spread of silencing throughout the shoot or only affected its movement across the HEJ, we exposed RtSS plants to Dex at different time points and then transferred them to 50 µm TIBA. If the silencing front had not crossed the HEJ before the addition of TIBA (before day 11), silencing stopped at this junction (Fig. 10, E and F). If the front had passed the HEJ before the addition of TIBA, it continued unabated into the shoot tissues (Fig. 10D). These results suggest that the HEJ is a gating zone for RNA signals in Arabidopsis and that it operates, by an as yet unknown mechanism, using auxin transport machinery that facilitates the passage of sRNAs through plasmodesmata.
DISCUSSION
Transport Route of Systemic Silencing in Arabidopsis
It has been widely accepted that a mobile silencing signal initiated in the rootstock of grafted tobacco plants or in Agrobacterium tumefaciens-infiltrated N. benthamiana leaves is transported through the phloem to induce silencing in a vascular-associated pattern in the leaves of the scion or in newly initiated leaves, respectively (Palauqui et al., 1997; Roberts et al., 1997; Voinnet and Baulcombe, 1997; Voinnet et al., 1998; Citovsky and Zambryski, 2000; Tournier et al., 2006; Kehr and Buhtz, 2008). Recently, Molnar et al. (2010) suggested that GFP silencing in Arabidopsis was more efficient in the shoot-to-root direction and that its spread was via the phloem in the source-to-sink direction. Silencing and GFP protein can move rapidly from shoot to root (Ghoshroy et al., 1997; Imlau et al., 1999; Kehr and Buhtz, 2008; Turgeon and Wolf, 2009), but we also observed effective silencing and GFP movement from root to shoot (Figs. 1, A–C, and 2).
In this study and in previous work (Brosnan et al., 2007) using grafted Arabidopsis, silencing initiated in the rootstock induced silencing in newly emerging, but not mature, leaves of the scion. Although this appeared to provide an excellent system with which to study long-distance signal transport and subsequent silencing in remote tissues, it gave a very different silencing pattern in vegetative tissues from that seen in N. benthamiana. In either grafted or RtSS plants, if the silencing signal from the hairpin silencer is a small RNA or a protein-RNA complex, it should be generated within, or have the capacity to move into, root phloem. However, the net photoassimilate flow in the Arabidopsis seedling is from shoot to root (especially through the hypocotyl), and like the GFP from roots (Fig. 2), silencing signal molecules may move only a short distance upward through the phloem. We observed that the silencing moved upward from cell to cell in the vascular parenchyma and cortical tissues to generate the pattern previously interpreted to indicate long-distance phloem-mediated transport.
On this upward journey, silencing also spreads throughout young leaf primordia and may subsequently advance slowly as a front down the length of the leaves (Fig. 11) at a rate of about five to seven cells per day. If a petiole and leaf are already well developed when the silencing reaches its stem-petiole junction, the advancing front has a considerable distance to travel before appearing in the leaf blade and must traverse older, less permeable plasmodesmata (Oparka et al., 1999; Burch-Smith et al., 2011). This pattern is very similar to the upward movement of free GFP from the shoot apical meristem (Kim et al., 2005b), which also displays a bizonal pattern, giving green fluorescence in the basal portion of young leaves but no fluorescence in the older, apical portion. It thus gives the outward appearance of being unsilenced for a long time (e.g. the oldest leaves in Figs. 1, B and C, and 6A). However, when the front reaches young leaves and leaf primordia, it moves more rapidly, being aided by division and expansion of the petiole and leaf cells (e.g. the bizonally silenced leaves in Figs. 1C and 6A).
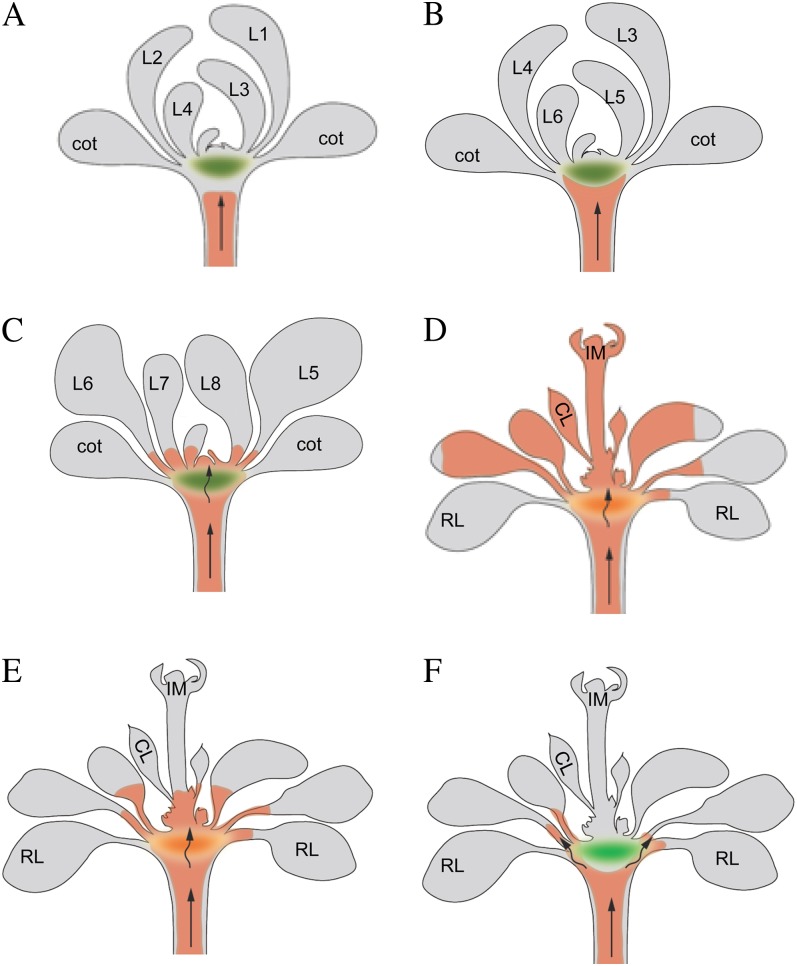
Figure 11.
A simplified model of RtSS. A, A silencing front moves through the hypocotyl cell to cell involving a signal-amplifying mechanism from root to shoot. The red color denotes the silencing front. The green ellipse denotes a tissue domain within the cotyledon node zone. B, When the silencing front reaches the HEJ, just below this tissue domain, the rate of silencing slows. C and D, Once the front gets through/around this barrier (C), the silencing signal moves to the meristem and causes the silencing of all subsequent lateral organs (D). The silencing front can also move into the petioles of older existing leaves and penetrates the lower parts of younger leaves with primary (more open) plasmodesmata. Leaf expansion reveals bizonal silencing in these leaves (D). E, If the silencing signal is generated later, the silencing front can also move through the barrier zone, but it cannot cause silencing of the inflorescence meristem due to the lower rate of cell-to-cell movement. F, The silencing front can be stopped at the barrier zone by the actin stabilizer TIBA, but cell-to-cell movement in other cell types or tissues cannot be stopped. CL, Cauline leaf; cot, cotyledon; IM, inflorescence meristem; L1 to L8, leaf numbers 1 to 8; RL, rosette leaf.
When the front reaches the shoot apex, all subsequent tissues produced will be silenced (apical leaves in Figs. 1B and 6A). The architecture of 1- to 2-week-old Arabidopsis plants is such that the distance between the apical meristem and the hypocotyl-epicotyl transition zone is very small. This allows the silencing, traveling cell to cell, to reach the apex and produce silencing throughout newly emerging leaves, sometimes even before the bizonal silencing is obvious in the slightly older leaves (Fig. 11). The timing of silencing induction, the growing conditions for the plants, and where the silencing signals are initiated will have a dramatic effect on the silencing pattern, as they alter the relationship between the position of the silencing front and the developmental architecture of the plant (Figs. 6A, 8D, and 11). Such developmental features include the symplastically isolated outer tissues of the hypocotyl and the developmental junction between the hypocotyl and epicotyl.
Because the front of silencing did not enter the epidermal cells of the hypocotyl, the silencing of its internal tissues could not be detected beneath the strong GFP expression in the outer cell layers by observation of intact tissues using a fluorescence dissecting microscope. This slow front of cell-to-cell silencing in the internal tissues provides a mechanism for long-distance spread with a long time period between induction at the base of the hypocotyl and distal silencing, and if the silencing signal is unable to enter and move through the phloem, it provides the explanation for the nonvascular pattern of distal silencing. It is also consistent with the slightly earlier appearance of shoot silencing in grafting experiments, as the silencing front would be initiated from the top of the hypocotyl, where the graft junction is usually made.
Symplastic Domains Restrict Silencing Spread
Cell-to-cell information transfer via plasmodesmata in plants is often confined to specific tissue domains termed symplastic domains (Ding et al., 2003; Ding and Itaya, 2007). Within a domain, symplastic signaling molecules appear to move freely, but at domain boundaries, their symplastic transport is either blocked completely or is only one way, either into or out of the domain. The regulated traffic across domain boundaries is one mechanism to define and coordinate plant development (Ding et al., 2003; Roberts and Oparka, 2003; Ding and Itaya, 2007). One well-known domain boundary exists between the epidermal and internal tissues of the Arabidopsis hypocotyl, such that even a small fluorescent tracer, such as CF, is unable to move from the epidermis into the underlying cortical tissues (Duckett et al., 1994). This boundary can be seen in our RtSS plants, in which the central part of the hypocotyl is progressively silenced while the epidermis retains GFP fluorescence (Fig. 7). However, the epidermis is not completely or permanently isolated, as the GFP expression in this tissue is eventually silenced.
Our results reveal the existence of a second symplastic boundary at the HEJ, where the rate of silencing spread was slowed (Fig. 7). This region is similar to the symplastic subdomain at the HEJ described by Kim et al. (2005a, 2005b) and was shown to prevent the shootward spread of silencing signals in Arabidopsis embryos by Kobayashi and Zambryski (2007). The central cells of this region are large, loosely packed, and have reduced points of contact with each other (Fig. 9A). At the periphery of the zone, the cells are small and densely packed. The route of fewest cells across the HEJ to the meristem is through the large central cells (route 1 in Fig. 9A). However, they, and the surrounding layer of small cells, remained unsilenced (Figs. 7 and 9D), and the silencing front took a path to the meristem that circumnavigated that zone (route 2 in Fig. 9A). This deviated route may partially account for the silencing front’s apparent retardation, although progress was only 15% of the speed through the hypocotyl. As discussed below, the spread of silencing from cell to cell requires the expression of the target GFP mRNA to fuel the amplification of the silencing signal. If the cells of the HEJ were transcriptionally inactive, this could prevent the silencing front from passing through the tissue; however, strong GFP expression was observed in these cells, negating this explanation. Another potential mechanism preventing the silencing penetrating these cells is that they are isolated by plasmodesmata closure, since in 14-d-old RtSS plants, the small tracer dye CF could not enter these cells. Although CF was able to enter this tissue zone in 7-d-old plants, silencing signals were excluded even in young embryos (Kobayashi and Zambryski, 2007), suggesting that the silencing signal is either too large, or lacks the required signal sequence, to traverse the connecting plasmodesmata. Interestingly, there is an additional symplastic boundary to dye transport just below the L3 layer of the shoot apical meristem, seen especially well in inflorescence meristems (Gisel et al., 1999), but this boundary appeared to have little effect on silencing spread. The front of silencing migrated around the HEJ zone and then silenced not only the L1 to L3 layers of the shoot apical meristem but also several additional internal cell layers (Fig. 9, A and D). Indeed, even when the floral bolt stem and inflorescence meristem were clearly silenced (Fig. 7, day 16), these HEJ tissues remained unsilenced.
Auxin and other flavonoids are known to accumulate in the upper part of the Arabidopsis hypocotyl (Murphy et al., 2000; Peer et al., 2001), which may alter hormone transport or other cell functions. Four inhibitors of auxin transport, TIBA, PBA, NPA, and HFCA, were assayed for their effects on the spread of silencing through the HEJ, and all of them either arrested or retarded the spread. These compounds are also described as inhibitors of vesicle transport (Geldner et al., 2001; Dhonukshe et al., 2008), and it has been recently reported that microRNAs are transported in mammals in secretory vesicles (Kosaka et al., 2010). One possibility is that sRNAs may move from cell to cell in plants by regulated vesicular transport; however, the well-known vesicle transport inhibitor BFA had little effect on silencing spread. We note that NPA and HFCA were only effective when applied at the highest concentrations, but TIBA and PBA blocked spread at moderate concentrations. Dhonukshe et al. (2008) showed that TIBA and PBA affected actin dynamics by stabilizing actin filaments, whereas NPA appeared to have little or no effect on actin dynamics (Geldner et al., 2001; Petrásek et al., 2003). Furthermore, TIBA and NPA had opposite effects on the growth of hyl1 mutants, suggesting that their modes of action are genetically separable (Lu and Fedoroff, 2000). This raises the tantalizing possibility that the combination of a functional actin cytoskeleton and localized vesicle transport is required for cell-to-cell movement of the silencing signal. Perhaps TIBA and PBA are the most effective inhibitors of silencing spread through the HEJ because they act on both actin stabilization and vesicle motility.
Bizonal Silencing in Grafts and RtSS Reflects Source-Sink Transitions
A striking feature of both the grafting and RtSS system was the production of bizonal silencing in the first silenced leaves followed by the silencing of all subsequent leaf primordia. We suggest that this indicates a limit to the movement of signal through older plasmodesmata in the leaf tips (Oparka et al., 1999; Burch-Smith et al., 2011). Cytoplasmic GFP can move throughout young leaf primordia, but even in very young leaves, it will move from a site of synthesis within or just below the shoot apical meristem only into the lower part of the leaf blade (Kim et al., 2005b). This pattern of GFP movement exactly parallels the pattern of GFP silencing we observed. As petioles elongate and leaf blades enlarge, cytoplasmic GFP is restricted to the veins (Kim et al., 2005b), and we very occasionally observed vascular-pattern silencing, although its absence is further evidence that the silencing signal generally moved cell to cell rather than through the phloem.
This bizonal pattern in rosette leaves is also very reminiscent of the pattern seen in plants showing the “recovery” phenotype observed in some plant virus/host combinations for close to a century (Wingard, 1928). In such situations, the plant appears ubiquitously infected by the virus but then produces new leaves with a bizonal pattern of virus symptoms only in the apical portion of the leaf, followed by leaves and tissues that are completely symptomless. A similar recovery from symptoms also commonly occurs when a virus infects a transgenic plant expressing a transgene derived from a fragment of the virus (Moore et al., 2001).
Our results and the transgene-mediated viral recovery seem closely related. In the latter case, the transgenic plants express mRNA containing virus-derived sequences in every cell, and once initiated by virus infection, the signal and silencing can move around the plant by both cell-to-cell and phloem-mediated transport. We suggest that once the antiviral silencing signal reaches the apical meristem, it can spread cell to cell to the limits of plasmodesma permeability. The spread is fueled by RDR-mediated secondary siRNA production from the viral transgene. The meristematic cells are dividing to generate new leaves, and because they now contain siRNAs amplified from transgene mRNA, the new tissue is protected from invasion by the virus. The same principles can be applied to “natural” virus recovery, but they require critically balanced conditions. The template for secondary siRNA production is the viral RNA, so the recovery phenotype is perpetuated by virus replication and secondary siRNA production achieving a balance in the peripheral meristem cells, so that new tissue is generated from cells with amplified siRNA levels sufficient to keep the viral replication at a subliminal level.
Signal Amplification Is Essential for Transmission of Cell-to-Cell Silencing
Previous work analyzing graft-transmitted silencing in Nicotiana (Palauqui et al., 1997) and Arabidopsis (Brosnan et al., 2007) concluded that transmission of the silencing signal did not require the hpRNA and the target mRNA to be expressed in the same tissue. In Nicotiana, a 30-cm-long wild-type intergraft between a silenced rootstock and a target-expressing scion did not interfere with transmission of the signal (Palauqui et al., 1997). However, we show here that in Arabidopsis, separation of the REA-RtSS-hpGF and the 35S-GFP target within a single plant prevented silencing in the target scion (Fig. 8). This raises the question: how is the signal transmitted in grafted Arabidopsis? We suggest that there is a direct exchange of genes and cell components at the graft junction, as seen in tobacco (Stegemann and Bock, 2009). Tissue from the graft junction between tobacco scions expressing nuclear and cytoplasmic yellow fluorescent protein and rootstocks expressing chloroplastic GFP was excised and cultured on selection medium containing antibiotics that would have eliminated tissue expressing only a single transgene (Stegemann and Bock, 2009). The surviving callus tissue contained both cytoplasmic yellow fluorescent protein and chloroplastic GFP, indicating an exchange of transgenes at the junction where the two tissues reconnected. A similar exchange, not only of transgenes but also of proteins and RNA, may also occur between cells at the graft junctions between Arabidopsis rootstocks and scions. This would explain some of the contradictory results on the identity of the silencing signal molecules. For example, grafting experiments using a rootstock expressing a hpGF RNA, but no GFP mRNA, in a dcl2,3,4 defective background were able to induce silencing in scions containing the 35S-GFP transgene (Brosnan et al., 2007). This was interpreted to mean that the siRNAs made by DCL2, -3, or -4 were unnecessary for silencing and, therefore, were not the signal. However, by sharing cell contents at the graft junction, DCLs from the scion cell fusion partner have access to hpGF-RNA from the rootstock cell partner, enabling the production of siRNA. Furthermore, the siRNAs have access to GFP mRNA from which they could amplify secondary siRNAs to fuel the cell-to-cell spread of silencing through the hypocotyl to the apex and then generate the usual silencing pattern. This is consistent with our demonstration that the RtSS system cannot function in an rdr6 background. With this scenario, the results do not negate the suggestion that siRNAs are a long-distance silencing signal (Dunoyer et al., 2010b; Molnar et al., 2010).
In conclusion, we have shown that the Arabidopsis seedling grafting system using a GFP reporter scion with a hpRNA silencing-initiating rootstock, and a counterpart inducible system, generate long-distance silencing that operates by reiterated short-distance cell-to-cell movement. This contrasts with the situation in Nicotiana species, in which long-distance silencing of transgenes, such as GFP, is clearly phloem mediated. Nevertheless, the Arabidopsis systems recapitulate the bizonal leaf pattern seen in viral recovery symptoms and provide a mechanism for the symptom generation. They also provide a model for the challenges faced by viruses that infect plants via the roots, such as those vectored by nematodes and soil-borne fungi. Examining the movement of the silencing front revealed that there is a previously unrecognized zone of tissue, below the apical meristem, that is resistant to the silencing signal and that may play some part in providing a gating or protective barrier against signals and/or viruses. Intriguingly, auxin transport inhibitors that also modify cytoskeleton dynamics prevented the spread of the silencing signal around this zone, suggesting that sRNA transport from cell to cell may be actively gated by plasmodesmata rather than spread by unregulated diffusion.
MATERIALS AND METHODS
Plasmid Construct and Arabidopsis Transformation
We used the binary vector pH-top as the backbone for the specific expression of RNAi (Craft et al., 2005). Briefly, the LHG4, GR, and tml terminator fragments were amplified from pOp-off2 with primers containing restriction enzyme sites (LhG4-2F1, 5′-AAAGGTACCCGGGAGGATCCTTGGAGAGGACAGACGTCGAAGATC-3′; LhG4-1F1, 5′-CAGACGTCGAAGATCATGAAACCGGTAACGTTATACGACGTCGCTGAAT-3′; LhG4R1, 5′-AAAAGATCTAGCTTCTGAATAAGCCCTCGTAATATATTTTCATGAAG-3′; Tml-terF1, 5′-AAAGTCGACAGCGGCGCGCCATCCTGCAGGATCTTTCCGCATAATTCCC-3′; Tml-terR2, 5′-AAAGGTACCTGCCGTACGGTCCCTAGGGATCGTGGTGATATTAAAGAGAGTTA-3′; BamHIGR-partLhF2, 5′-AAAGGATCCATTTCATTTGGAGAGGACACGCTGACATCCCAATTCCGGG-3′; GR-partLhF1, 5′-TGACATCCCAATTCCGGGCGGAATGGCTAGTGAAGCTCGAAAAACAAAG-3′; GR-partLhR1, 5′-CAAGCTCGAGGTCGCGACACCGATCAGCAAGCTTTGTTTACCAGCCAGC-3′) and sequentially cloned into pH-top to form the intermediate vector, pGRLOP. A fragment of the first 400 nucleotides of GFP was amplified with the following primers: attB1-ASC-FhR1 (5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTGGCGCGCCCCTCCTTGAAGT-3′) and attB2-GhF1 (5′-GGGACCACTTTGTACAAGAAAGCTGGGTATGGTGAGCAAGGGCGAGGA-3′). This fragment was introduced into pDONR201 using BP clonase reaction (Gateway Cloning, Invitrogen), followed by a LR clonase reaction (Gateway Cloning, Invitrogen) with pOpoff2 (Wielopolska et al., 2005), then a 1.9-kb AscI fragment from the plasmid above containing the hpGF and a pyruvate dehydrogenase kinase intron was cloned into the AscI site of pGRLOP to form the pGRLOP-hpGF plasmid. The TobRB7 fragment was amplified using the following primers (RobTob7-proF1, 5′-TGACCTAGGGTCCTACACAATGTGAATTTG-3′; RobTob7-proR1, 5′-AGTCGTACGTAGTTCTCACTAGAAAAATGC-3′), then it was cloned into pGRLOP-hpGF to form the final construct, pTob-GRLOP-hpGF.
For the REA-hpGF construct, the REA fragment was amplified using the following primers (1rootspF1, 5′-AAACCTAGGTGCAGAGGTAGATATGGGTC-3′; 1rootspR1, 5′-TTTCGTACGACAGGTTATGGAGTTTAGGG-3′). The amplified fragment was inserted into pGRLOP-hpGF with the partial fragment of the Rubisco small subunit promoter to form pREA-hpGF.
These constructs were then cotransformed with pSoup vector into Agrobacterium tumefaciens GV3101. The wild-type Col-0 Arabidopsis (Arabidopsis thaliana) and previously used 35S-GFP Arabidopsis (Brosnan et al., 2007) plants were transformed with Agrobacterium containing pTob-GRLOP-hpGF and pREA-hpGF, respectively, using the floral dipping method. Transformed plants were selected on medium containing 15 mg L−1 hygromycin and screened by observing GFP fluorescence. Plants with autonomous silencing were discarded, and only plants with GFP fluorescence maintained through their entire life were selected for further study. These plants displayed inducible silencing.
Grafting and Locally Induced Systemic Silencing
To investigate the mobility of GFP from rootstock into scion tissue, we grafted Arabidopsis C24 wild-type scions onto SUC2-GFP rootstocks (Stadler et al., 2005b). In all subsequent graft-mediated silencing experiments, plants containing the 35S-GFP construct (Brosnan et al., 2007) were used as the scion and RtSS, 35S-hpGF, or the S1 silencer plants described previously (Brosnan et al., 2007) were used as the rootstock. In all cases, the grafting procedure was as described in detail by Brosnan et al. (2007). Longitudinally sectioned grafts were examined using a Leica SP2 confocal laser scanning microscope. Systemic GFP silencing in Nicotiana benthamiana was induced by agroinfiltration of the lower leaves of 21-d-old GFP-expressing (N. benthamiana transgenic line 16c) plants with Agrobacterium, containing a 35S:GFP construct, essentially as described by Voinnet et al. (1998).
Dex Induction, GUS Staining, and Fluorescence Microscopy
For Dex treatment, seeds were germinated on 10 µm Dex-containing MS medium, or plants growing on vertical plates were transferred to 10 µm Dex-containing medium and grown vertically. Dex-treated RtSS plants were either maintained on agar medium or transferred to soil (where they were drenched with Dex solution) to observe GFP silencing. For GUS staining, 7-d-old plants were immersed in the GUS staining buffer (50 mm sodium phosphate buffer, pH 7.0, 1 mm EDTA, 0.5 mm potassium ferricyanide, 0.5 mm potassium ferrocyanide, 0.1% Triton X-100, and 1 mm 5-bromo-4-chloro-3-indolyl-β-glucuronic acid) at 37°C overnight, destained by rinsing in phosphate buffer, and then stored in 70% ethanol.
For GFP fluorescence, the plants were screened using a NightSea torch (BlueStar), and individual plants were examined using a Leica MZFLIII fluorescence dissecting microscope equipped with an Axiocam digital camera or photographed with a Nikon D2 camera using UV illumination with appropriate filters. For more detailed analysis, longitudinal or transverse sections were examined on a Zeiss Axioimager fluorescence microscope or on a Leica SP2 confocal laser scanning microscope.
RNA Isolation and Northern Blots
Total RNA from shoots or roots was extracted using TRI reagent (Sigma) according to the manufacturer’s procedure. About 25 µg of total RNA from each sample was separated on a 17% denaturing polyacrylamide gel and then blotted onto a Hybond-N+ membrane using a Bio-Rad electroblotting apparatus. The blotted membrane was then UV cross-linked and baked for 2 h at 80°C. Hybridization analyses were essentially performed as described previously (Fusaro et al., 2006).
Nuclear Run-On Assays
DNA probe fragments including the target regions (GF and P regions of the GFP gene), positive controls (18S, ubiquitin, and actin), and a negative control (Escherichia coli replication protein) were amplified, cleaned, and fixed on a Hybond-N+ membrane using a Bio-Rad blotter. Nuclear run-on analyses were carried out as described previously (Meng and Lemaux, 2003).
Tracer Analysis
Five- and 12-d-old Col-0 plants were grown on MS medium and then transferred to fresh agar with the central part of their root system placed on sterile Parafilm until they were 7 and 14 d old, respectively. Before application, a fresh working solution of 2 µm CFDA (Sigma) in distilled water was prepared from a 1 mm stock solution in dimethyl sulfoxide (DMSO). The CFDA solution was then applied to the part of the root system lying on the Parafilm in the 7- and 14-d-old plants. The roots were crushed with a pair of forceps to allow CFDA to enter into the internal tissues. The resulting CF fluorescence was monitored with a fluorescence dissecting microscope until CF was detected in the shoot. Those plants with CF fluorescence in the shoots were dissected to examine the fluorescence in the shoot apex and the upper part of the hypocotyl with the confocal microscope.
Inhibitor Experiments
Five-day-old plants germinated on Dex-containing medium or normal MS medium were transferred to medium containing the inhibitors or equal amounts of solvents (controls). Additional controls included transfer of Dex-untreated plants to MS medium. BFA (Sigma-Aldrich), TIBA (Sigma-Aldrich), NPA (Sigma-Aldrich), and HFCA (Sigma-Aldrich) were diluted from 100 mm stocks in DMSO. PBA (OlChemIm) was dissolved in DMSO at a stock concentration of 30 mm; 2 µm cytochalasin D (Sigma-Aldrich; 10 mg mL−1 stock in DMSO), 1 µm latrunculin B (Sigma-Aldrich; 2 mm stock in DMSO), 1 µm jasplakinolide (Calbiochem; 1 mm stock in DMSO), 2.5 mm BDM (500 mm stock solution, freshly dissolved in water), and 50 µm NEM (50 mm stock, freshly dissolved in water) were made to their final dilutions on MS agar plates. The concentrations used inhibited plant growth and were at the high end of the concentrations applied to Arabidopsis for BFA (Baskin and Bivens, 1995), TIBA (Dhonukshe et al., 2008), NPA (Okada et al., 1991), HFCA (Okada et al., 1991), PBA (Dhonukshe et al., 2008), cytochalasin D (Collings et al., 2006), latrunculin B (Collings et al., 2006), jasplakinolide (Dhonukshe et al., 2008), BDM (Baskin and Bivens, 1995; Paves and Truve, 2007), and NEM (Paves and Truve, 2007).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Dex-inducible GUS expression in RtSS.
Supplemental Figure S2. The migration rate of silencing front.
Supplemental Figure S3. The role of RDR6 in the root-to-shoot silencing transmission (D. Liang, R.G. White, and P.M. Waterhouse, unpublished data).
Supplementary Material
Acknowledgments
We thank Chris Helliwell and Ming-Bo Wang for many discussions and Carl Davies for help in taking photographs. We also thank Adriana Fusaro for the N. benthamiana experiment and Bethany Clark, Ebony Perkins, Anna Wielopolska, and Judith Gaudron for technical support.
Glossary
- RNAi
RNA interference
- siRNA
short interfering RNA
- hpRNA
hairpin RNA
- RtSS
root-to-shoot silencing
- MS
Murashige and Skoog
- Col-0
ecotype Columbia
- Dex
dexamethasone
- HEJ
hypocotyl-epicotyl junction
- CFDA
5,FAM diacetate
- CF
carboxyfluorescein
- BDM
2,3-butanedione monoxime
- NEM
n-ethylmaleimide
- BFA
brefeldin A
- TIBA
2,3,5-triiodobenzoic acid
- PBA
2-(1-pyrenoyl) benzoic acid
- NPA
N-1-naphthylphthalamidic acid
- HFCA
9-hydroxy-9-fluorenecarboxylic acid
- DMSO
dimethyl sulfoxide
References
- Atkins CA, Smith PM, Rodriguez-Medina C. (2011) Macromolecules in phloem exudates: a review. Protoplasma 248: 165–172 [DOI] [PubMed] [Google Scholar]
- Bai S, Kasai A, Yamada K, Li T, Harada T. (2011) A mobile signal transported over a long distance induces systemic transcriptional gene silencing in a grafted partner. J Exp Bot 62: 4561–4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin TI, Bivens NJ. (1995) Stimulation of radial expansion in Arabidopsis roots by inhibitors of actomyosin and vesicle secretion but not by various inhibitors of metabolism. Planta 197: 514–521 [DOI] [PubMed] [Google Scholar]
- Brosnan CA, Mitter N, Christie M, Smith NA, Waterhouse PM, Carroll BJ. (2007) Nuclear gene silencing directs reception of long-distance mRNA silencing in Arabidopsis. Proc Natl Acad Sci USA 104: 14741–14746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch-Smith TM, Stonebloom S, Xu M, Zambryski PC. (2011) Plasmodesmata during development: re-examination of the importance of primary, secondary, and branched plasmodesmata structure versus function. Protoplasma 248: 61–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citovsky V, Zambryski P. (2000) Systemic transport of RNA in plants. Trends Plant Sci 5: 52–54 [DOI] [PubMed] [Google Scholar]
- Cogoni C, Macino G. (2000) Post-transcriptional gene silencing across kingdoms. Curr Opin Genet Dev 10: 638–643 [DOI] [PubMed] [Google Scholar]
- Collings DA, Lill AW, Himmelspach R, Wasteneys GO. (2006) Hypersensitivity to cytoskeletal antagonists demonstrates microtubule-microfilament cross-talk in the control of root elongation in Arabidopsis thaliana. New Phytol 170: 275–290 [DOI] [PubMed] [Google Scholar]
- Craft J, Samalova M, Baroux C, Townley H, Martinez A, Jepson I, Tsiantis M, Moore I. (2005) New pOp/LhG4 vectors for stringent glucocorticoid-dependent transgene expression in Arabidopsis. Plant J 41: 899–918 [DOI] [PubMed] [Google Scholar]
- Crété P, Leuenberger S, Iglesias VA, Suarez V, Schöb H, Holtorf H, van Eeden S, Meins F. (2001) Graft transmission of induced and spontaneous post-transcriptional silencing of chitinase genes. Plant J 28: 493–501 [DOI] [PubMed] [Google Scholar]
- Dalmay T, Hamilton A, Rudd S, Angell S, Baulcombe DC. (2000) An RNA-dependent RNA polymerase gene in Arabidopsis is required for posttranscriptional gene silencing mediated by a transgene but not by a virus. Cell 101: 543–553 [DOI] [PubMed] [Google Scholar]
- Dhonukshe P, Grigoriev I, Fischer R, Tominaga M, Robinson DG, Hasek J, Paciorek T, Petrásek J, Seifertová D, Tejos R, et al. (2008) Auxin transport inhibitors impair vesicle motility and actin cytoskeleton dynamics in diverse eukaryotes. Proc Natl Acad Sci USA 105: 4489–4494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B, Itaya A. (2007) Control of directional macromolecular trafficking across specific cellular boundaries: a key to integrative plant biology. J Integr Plant Biol 49: 1227–1234 [Google Scholar]
- Ding B, Itaya A, Qi Y. (2003) Symplasmic protein and RNA traffic: regulatory points and regulatory factors. Curr Opin Plant Biol 6: 596–602 [DOI] [PubMed] [Google Scholar]
- Ding B, Kwon M-O, Warnberg L. (1996) Evidence that actin filaments are involved in controlling the permeability of plasmodesmata in tobacco mesophyll. Plant J 10: 157–164 [Google Scholar]
- Duckett CM, Oparka KJ, Prior DAM, Dolan L, Roberts K. (1994) Dye-coupling in the root epidermis of Arabidopsis is progressively reduced during development. Development 120: 3247–3255 [Google Scholar]
- Dunoyer P, Brosnan CA, Schott G, Wang Y, Jay F, Alioua A, Himber C, Voinnet O. (2010a) An endogenous, systemic RNAi pathway in plants. EMBO J 29: 1699–1712 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dunoyer P, Himber C, Voinnet O. (2005) DICER-LIKE 4 is required for RNA interference and produces the 21-nucleotide small interfering RNA component of the plant cell-to-cell silencing signal. Nat Genet 37: 1356–1360 [DOI] [PubMed] [Google Scholar]
- Dunoyer P, Schott G, Himber C, Meyer D, Takeda A, Carrington JC, Voinnet O. (2010b) Small RNA duplexes function as mobile silencing signals between plant cells. Science 328: 912–916 [DOI] [PubMed] [Google Scholar]
- Fusaro AF, Matthew L, Smith NA, Curtin SJ, Dedic-Hagan J, Ellacott GA, Watson JM, Wang MB, Brosnan C, Carroll BJ, et al. (2006) RNA interference-inducing hairpin RNAs in plants act through the viral defence pathway. EMBO Rep 7: 1168–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner N, Friml J, Stierhof YD, Jürgens G, Palme K. (2001) Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413: 425–428 [DOI] [PubMed] [Google Scholar]
- Gendreau E, Traas J, Desnos T, Grandjean O, Caboche M, Höfte H. (1997) Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol 114: 295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovés A, Navarro JA, Pallás V. (2010) The intra- and intercellular movement of Melon necrotic spot virus (MNSV) depends on an active secretory pathway. Mol Plant Microbe Interact 23: 263–272 [DOI] [PubMed] [Google Scholar]
- Ghoshroy S, Lartey R, Sheng J, Citovsky V. (1997) Transport of proteins and nucleic acids through plasmodesmata. Annu Rev Plant Physiol Plant Mol Biol 48: 27–50 [DOI] [PubMed] [Google Scholar]
- Gisel A, Barella S, Hempel FD, Zambryski PC. (1999) Temporal and spatial regulation of symplastic trafficking during development in Arabidopsis thaliana apices. Development 126: 1879–1889 [DOI] [PubMed] [Google Scholar]
- Gisel A, Hempel FD, Barella S, Zambryski P. (2002) Leaf-to-shoot apex movement of symplastic tracer is restricted coincident with flowering in Arabidopsis. Proc Natl Acad Sci USA 99: 1713–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries PA, Park J-W, Sasaki N, Ballard KD, Maule AJ, Nelson RS. (2009) Differing requirements for actin and myosin by plant viruses for sustained intercellular movement. Proc Natl Acad Sci USA 106: 17594–17599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt S, Cowan GH, Ziegler A, Roberts AG, Oparka KJ, Torrance L. (2005) Two plant-viral movement proteins traffic in the endocytic recycling pathway. Plant Cell 17: 164–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himber C, Dunoyer P, Moissiard G, Ritzenthaler C, Voinnet O. (2003) Transitivity-dependent and -independent cell-to-cell movement of RNA silencing. EMBO J 22: 4523–4533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlau A, Truernit E, Sauer N. (1999) Cell-to-cell and long-distance trafficking of the green fluorescent protein in the phloem and symplastic unloading of the protein into sink tissues. Plant Cell 11: 309–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju H-J, Samuels TD, Wang Y-S, Blancaflor E, Payton M, Mitra R, Krishnamurthy K, Nelson RS, Verchot-Lubicz J. (2005) The Potato virus X TGBp2 movement protein associates with endoplasmic reticulum-derived vesicles during virus infection. Plant Physiol 138: 1877–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalantidis K, Schumacher HT, Alexiadis T, Helm JM. (2008) RNA silencing movement in plants. Biol Cell 100: 13–26 [DOI] [PubMed] [Google Scholar]
- Kehr J, Buhtz A. (2008) Long distance transport and movement of RNA through the phloem. J Exp Bot 59: 85–92 [DOI] [PubMed] [Google Scholar]
- Kiefer IW, Slusarenko AJ. (2003) The pattern of systemic acquired resistance induction within the Arabidopsis rosette in relation to the pattern of translocation. Plant Physiol 132: 840–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Cho E, Crawford K, Hempel FD, Zambryski PC. (2005a) Cell-to-cell movement of GFP during embryogenesis and early seedling development in Arabidopsis. Proc Natl Acad Sci USA 102: 2227–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Kobayashi K, Cho E, Zambryski PC. (2005b) Subdomains for transport via plasmodesmata corresponding to the apical-basal axis are established during Arabidopsis embryogenesis. Proc Natl Acad Sci USA 102: 11945–11950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Zambryski P. (2007) RNA silencing and its cell-to-cell spread during Arabidopsis embryogenesis. Plant J 50: 597–604 [DOI] [PubMed] [Google Scholar]
- Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. (2010) Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 285: 17442–17452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lough TJ, Lucas WJ. (2006) Integrative plant biology: role of phloem long-distance macromolecular trafficking. Annu Rev Plant Biol 57: 203–232 [DOI] [PubMed] [Google Scholar]
- Lu C, Fedoroff N. (2000) A mutation in the Arabidopsis HYL1 gene encoding a dsRNA binding protein affects responses to abscisic acid, auxin, and cytokinin. Plant Cell 12: 2351–2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews REF. (1973) Induction of disease by viruses, with special reference to turnip yellow mosaic virus. Annu Rev Phytopathol 11: 147–168 [Google Scholar]
- Melnyk CW, Molnar A, Bassett A, Baulcombe DC. (2011) Mobile 24 nt small RNAs direct transcriptional gene silencing in the root meristems of Arabidopsis thaliana. Curr Biol 21: 1678–1683 [DOI] [PubMed] [Google Scholar]
- Meng L, Lemaux P. (2003) A simple and rapid method for nuclear run-on transcription assays in plants. Plant Mol Biol Rep 21: 65–71 [Google Scholar]
- Mlotshwa S, Voinnet O, Mette MF, Matzke M, Vaucheret H, Ding SW, Pruss G, Vance VB. (2002) RNA silencing and the mobile silencing signal. Plant Cell (Suppl) 14: S289–S301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar A, Melnyk CW, Bassett A, Hardcastle TJ, Dunn R, Baulcombe DC. (2010) Small silencing RNAs in plants are mobile and direct epigenetic modification in recipient cells. Science 328: 872–875 [DOI] [PubMed] [Google Scholar]
- Moore CJ, Sutherland PW, Forster RL, Gardner RC, MacDiarmid RM. (2001) Dark green islands in plant virus infection are the result of posttranscriptional gene silencing. Mol Plant Microbe Interact 14: 939–946 [DOI] [PubMed] [Google Scholar]
- Murphy A, Peer WA, Taiz L. (2000) Regulation of auxin transport by aminopeptidases and endogenous flavonoids. Planta 211: 315–324 [DOI] [PubMed] [Google Scholar]
- Novina CD, Sharp PA. (2004) The RNAi revolution. Nature 430: 161–164 [DOI] [PubMed] [Google Scholar]
- Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y. (1991) Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 3: 677–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oparka KJ, Roberts AG, Boevink P, Santa Cruz S, Roberts I, Pradel KS, Imlau A, Kotlizky G, Sauer N, Epel B. (1999) Simple, but not branched, plasmodesmata allow the nonspecific trafficking of proteins in developing tobacco leaves. Cell 97: 743–754 [DOI] [PubMed] [Google Scholar]
- Palauqui JC, Elmayan T, Pollien JM, Vaucheret H. (1997) Systemic acquired silencing: transgene-specific post-transcriptional silencing is transmitted by grafting from silenced stocks to non-silenced scions. EMBO J 16: 4738–4745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paves H, Truve E. (2007) Myosin inhibitors block accumulation movement of chloroplasts in Arabidopsis thaliana leaf cells. Protoplasma 230: 165–169 [DOI] [PubMed] [Google Scholar]
- Peer WA, Brown DE, Tague BW, Muday GK, Taiz L, Murphy AS. (2001) Flavonoid accumulation patterns of transparent testa mutants of Arabidopsis. Plant Physiol 126: 536–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrásek J, Cerná A, Schwarzerová K, Elčkner M, Morris DA, Zazímalová E. (2003) Do phytotropins inhibit auxin efflux by impairing vesicle traffic? Plant Physiol 131: 254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford JE, White RG. (2011) Inhibitors of myosin, but not actin, alter transport through Tradescantia plasmodesmata. Protoplasma 248: 205–216 [DOI] [PubMed] [Google Scholar]
- Roberts AG, Cruz SS, Roberts IM, Prior D, Turgeon R, Oparka KJ. (1997) Phloem unloading in sink leaves of Nicotiana benthamiana: comparison of a fluorescent solute with a fluorescent virus. Plant Cell 9: 1381–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AG, Oparka KJ. (2003) Plasmodesmata and the control of symplastic transport. Plant Cell Environ 26: 103–124 [Google Scholar]
- Sagi G, Katz A, Guenoune-Gelbart D, Epel BL. (2005) Class 1 reversibly glycosylated polypeptides are plasmodesmal-associated proteins delivered to plasmodesmata via the Golgi apparatus. Plant Cell 17: 1788–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoelz JE, Harries PA, Nelson RS. (2011) Intracellular transport of plant viruses: finding the door out of the cell. Mol Plant 4: 813–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwach F, Vaistij FE, Jones L, Baulcombe DC. (2005) An RNA-dependent RNA polymerase prevents meristem invasion by Potato virus X and is required for the activity but not the production of a systemic silencing signal. Plant Physiol 138: 1842–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijen T, Fleenor J, Simmer F, Thijssen KL, Parrish S, Timmons L, Plasterk RH, Fire A. (2001) On the role of RNA amplification in dsRNA-triggered gene silencing. Cell 107: 465–476 [DOI] [PubMed] [Google Scholar]
- Stadler R, Lauterbach C, Sauer N. (2005a) Cell-to-cell movement of green fluorescent protein reveals post-phloem transport in the outer integument and identifies symplastic domains in Arabidopsis seeds and embryos. Plant Physiol 139: 701–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler R, Wright KM, Lauterbach C, Amon G, Gahrtz M, Feuerstein A, Oparka KJ, Sauer N. (2005b) Expression of GFP-fusions in Arabidopsis companion cells reveals non-specific protein trafficking into sieve elements and identifies a novel post-phloem domain in roots. Plant J 41: 319–331 [DOI] [PubMed] [Google Scholar]
- Stegemann S, Bock R. (2009) Exchange of genetic material between cells in plant tissue grafts. Science 324: 649–651 [DOI] [PubMed] [Google Scholar]
- Su S, Liu Z, Chen C, Zhang Y, Wang X, Zhu L, Miao L, Wang X-C, Yuan M. (2010) Cucumber mosaic virus movement protein severs actin filaments to increase the plasmodesmal size exclusion limit in tobacco. Plant Cell 22: 1373–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CL, Bayer EM, Ritzenthaler C, Fernandez-Calvino L, Maule AJ. (2008) Specific targeting of a plasmodesmal protein affecting cell-to-cell communication. PLoS Biol 6: e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier B, Tabler M, Kalantidis K. (2006) Phloem flow strongly influences the systemic spread of silencing in GFP Nicotiana benthamiana plants. Plant J 47: 383–394 [DOI] [PubMed] [Google Scholar]
- Turgeon R, Wolf S. (2009) Phloem transport: cellular pathways and molecular trafficking. Annu Rev Plant Biol 60: 207–221 [DOI] [PubMed] [Google Scholar]
- Vaistij FE, Jones L, Baulcombe DC. (2002) Spreading of RNA targeting and DNA methylation in RNA silencing requires transcription of the target gene and a putative RNA-dependent RNA polymerase. Plant Cell 14: 857–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voinnet O, Baulcombe DC. (1997) Systemic signalling in gene silencing. Nature 389: 553. [DOI] [PubMed] [Google Scholar]
- Voinnet O, Vain P, Angell S, Baulcombe DC. (1998) Systemic spread of sequence-specific transgene RNA degradation in plants is initiated by localized introduction of ectopic promoterless DNA. Cell 95: 177–187 [DOI] [PubMed] [Google Scholar]
- Waterhouse PM, Wang M-B, Lough T. (2001) Gene silencing as an adaptive defence against viruses. Nature 411: 834–842 [DOI] [PubMed] [Google Scholar]
- White RG, Barton DA. (2011) The cytoskeleton in plasmodesmata: a role in intercellular transport? J Exp Bot 62: 5249–5266 [DOI] [PubMed] [Google Scholar]
- Wielopolska A, Townley H, Moore I, Waterhouse P, Helliwell C. (2005) A high-throughput inducible RNAi vector for plants. Plant Biotechnol J 3: 583–590 [DOI] [PubMed] [Google Scholar]
- Windt CW, Vergeldt FJ, de Jager PA, van As H. (2006) MRI of long-distance water transport: a comparison of the phloem and xylem flow characteristics and dynamics in poplar, castor bean, tomato and tobacco. Plant Cell Environ 29: 1715–1729 [DOI] [PubMed] [Google Scholar]
- Wingard SA. (1928) Hosts and symptoms of ring spot, a virus disease of plants. J Agric Res 37: 127–153 [Google Scholar]
- Yamamoto YT, Taylor CG, Acedo GN, Cheng CL, Conkling MA. (1991) Characterization of cis-acting sequences regulating root-specific gene expression in tobacco. Plant Cell 3: 371–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.