Abstract
Background
Specific antibody deficiency may predispose patients to recurrent respiratory tract infections. There is limited literature assessing specific antibody deficiency in chronic rhinosinusitis (CRS). This study evaluated the role of specific antibody deficiency in patients with CRS who have failed medical therapy.
Methods
We performed a retrospective chart review of patients with CRS who underwent functional endoscopic sinus surgery and had prior assessment for humoral immunodeficiency. Each patient’s record was reviewed for serum quantitative immunoglobulin G (IgG) and IgA and anti–Streptococcus pneumoniae antibody titers measured at baseline and 6 weeks postvaccination with the 23-valent unconjugated pneumococcal vaccine. Clinical characteristics, including asthma, atopy, and nasal polyps, were recorded.
Results
Of the 129 CRS patients who met inclusion criteria, 93 (72%) had low baseline antipneumococcal titers. Fifteen (11.6%) patients were diagnosed with specific antibody deficiency based on an inadequate response to the pneumococcal polysaccharide vaccine. The group of patients with specific antibody deficiency had significantly lower serum IgA levels when compared with those patients with normal preimmunization titers (138 ± 67.3 versus 330 ± 356; p < 0.05). Patients with specific antibody deficiency had a significantly lower number of preimmunization protective antipneumococcal titers when compared with vaccine responders (1.41 versus 2.72; p < 0.0005).
Conclusion
This retrospective study indicates that patients with medically refractory CRS may have a high prevalence of low preimmunization antipneumococcal titers and specific antibody deficiency. Furthermore, lower serum IgA levels identified in these specific antibody deficiency patients suggests that a prospective study to further characterize this relationship is warranted.
Chronic rhinosinusitis (CRS) affects >35 million Americans, and prevalence is rising. CRS is responsible for a significant burden on the health care system.1 Many patients fail to respond to medical therapy and require surgical treatment of this disease. CRS is classified into CRS with nasal polyps or without nasal polyps. Although the role of microorganisms in disease etiology or impact on disease severity is not clearly established, it is generally accepted that symptom exacerbations may be acutely triggered by viral, bacterial, or fungal infections. The most commonly recognized bacterial pathogens for acute sinusitis and chronic sinusitis are Streptococcus pneumoniae and other streptococcal species, Moraxella catarrhalis, Haemophilus influenzae, Staphylococcus aureus, anaerobes, and enteric Gram-negative rods. Patients with CRS refractory to medical therapy or those with recurrent sinus infections may warrant evaluation for immune deficiency.1
Specific antibody deficiency (SAD) is characterized by an impaired response to immunization with polysaccharide antigens in the presence of normal quantitative immunoglobulin levels. Sinopulmonary bacterial infections with pathogens including S. pneumoniae, M. catarrhalis, H. influenzae, and S. aureus are the most common manifestations of this syndrome.2 Patients with SAD are managed in a variety of ways including vaccination with conjugated vaccines when available, prophylactic antibiotics, and occasionally with i.v. immunoglobulin (IVIg). The prevalence of SAD in the general population is not known. SAD is recognized in 5–20% of children >2 years old who suffer from recurrent or severe infections.3–5 One study determined the prevalence of SAD among adults with recurrent community-acquired pneumonia to be ~8%.6 Prior studies have revealed dysfunction of T-cell subsets or common variable immunodeficiency as risk factors for CRS,7 and a recent retrospective study suggests a high prevalence of humoral immune dysfunction in patients with difficult-to-treat CRS.8 The aim of this study was to characterize the presence of SAD among patients with CRS that have failed medical therapy and required sinus surgery.
METHODS
Patients
A retrospective chart review was performed after receiving approval from the Institutional Review Board of Northwestern University Feinberg School of Medicine. We identified adult patients who had failed medical therapy and underwent functional endoscopic surgery for the treatment of CRS by the Department of Otolaryngology, Northwestern University Feinberg School of Medicine, Chicago, between the years 2002 and 2010. All subjects met the criteria for CRS as defined by nationally recognized consensus statements.9,10 All subjects had rhinosinusitis symptoms for ≥12 weeks and had failed medical therapy, including at least 3 consecutive weeks of a broad-spectrum antibiotic and oral and/or intranasal corticosteroids. The presence of rhinosinusitis or bilateral nasal polyps was confirmed by office endoscopy and sinus CT scans. Patients were included in this study if they had had serum antipneumococcal antibody titers and quantitative immunoglobulin G (IgG; reference range, 750–1700 mg/dL) and IgA (reference range, 70–400 mg/dL) measured for immune evaluation of severe disease or recurrent infections. The decision to check these laboratory markers was made by the managing allergists on an individual patient basis. Exclusion criteria for this study included common variable immunodeficiency and/or known history of primary immunodeficiency, HIV, cystic fibrosis, or malignancy. All subjects had skin testing to pollens, dust mites, pets, molds, and cockroach using Hollister-Stier Canada (Toronto, Ontario, Canada) extracts. History of asthma was determined by prior physician diagnosis.
Evaluation of SAD
Serum antibody titers to 14 common pneumocccal bacterial serotypes (1, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 12F, 14, 18C, 19F, and 23F) were measured for each subject (Specialty Laboratories, Inc., Valencia, CA). A protective antibody titer for a serotype was defined as ≥1.3 μg/mL.11 Normal antibody levels were defined as protective titers against at least seven serotypes as suggested by an expert panel.12 Patients with normal preimmunization antibody levels are categorized as “normal baseline.” Patients with low preimmunization antibody levels (less than seven protective titers) were categorized as “low baseline,” immunized with Pneumovax (23-valent unconjugated pneumococcal polysaccharide vaccine; Merck, Whitehouse Station, NJ) and titers were rechecked 6 weeks later. An adequate response to vaccine was defined as an increase in postimmunization antipneumococcal antibody titers to ≥1.3 μg/mL for at least 7 of 14 serotypes;11,12 these patients were categorized as “responders.” Those patients who had fewer than seven protective postimmunization titers were categorized as “nonresponders” or patients with SAD.
Statistical Analysis
Patients were categorized into three groups based on antipneumococcal antibody titers as defined previously: (1) patients with seven or more protective preimmunization antibody titers (“normal baseline”); (2) patients with less than seven protective preimmunization antibody titers who responded adequately to vaccine (“responders”); and (3) SAD patients who did not respond adequately to vaccine (“non-responders”). These three categories of patients were compared with respect to the variables of age, sex, atopy, asthma, and nasal polyposis; total serum IgG; and total serum IgA. One-way analysis of variance and post hoc Tukey analysis tests were used to compare means of continuous variables and analogous chi-square tests were used to compare percentages for categorical variables. Within each group, the mean number of protective antibodies prevaccination was compared with the mean number of protective antibodies postvaccination via paired t-test. A value of p < 0.05 was considered statistically significant.
RESULTS
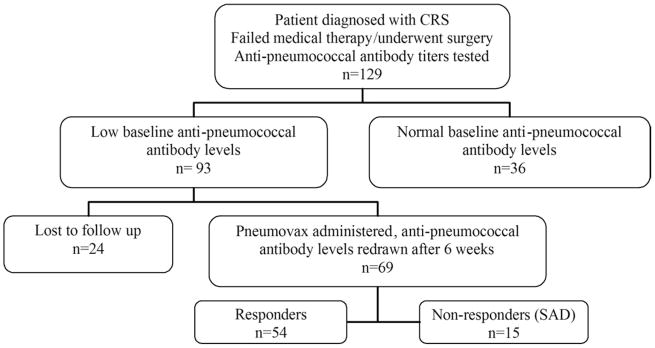
A total of 129 patients met inclusion criteria. Of these, 36 patients (28%) had normal baseline antipneumococcal antibody levels; 93 (72%) had low preimmunization antibody levels as previously defined. Of those with low baseline antibody levels, 69 patients underwent Pneumovax administration and had antipneumococcal antibody titers rechecked after 6 weeks. From this group, 54 (78%) had adequate response to the vaccine (“responders”) and 15 (22%) of these patients had inadequate response to vaccination (“nonresponders”; Fig. 1).
Figure 1.
Study design. SAD, specific antibody deficiency.
Statistical analysis of the comparison of patient characteristics among patients in the normal baseline, responder, and nonresponder groups is shown in Table 1. There was a tendency toward higher female prevalence and diagnosis of asthma in the group with normal baseline values compared with the low baseline groups; this was not statistically significant. No correlation with atopy or nasal polyposis was identified.
Table 1.
Comparisons of clinical characteristics among the three patient groups studied: Normal preimmunization antipneumococcal antibody titers (“normal baseline”); those with low preimmunization antipneumococcal antibody titers and adequate response to vaccine (“responders”); and those with low baseline antipneumococcal antibody titers and inadequate response to vaccine (“nonresponders”)
| Normal Baseline (n = 69) | Low Baseline
|
p Value | ||
|---|---|---|---|---|
| Responders (n = 54) | Nonresponders (n = 15) | |||
| Age, yr (mean ± SD) | 50 ± 10.4 | 48.4 ± 12 | 45.4 ± 11.8 | NS |
| Female | 27 (75%) | 31 (57%) | 8 (53%) | NS |
| Polyps | 15 (42%) | 25 (46%) | 7 (47%) | NS |
| Asthma | 27 (75%) | 37 (69%) | 8 (53%) | NS |
| Atopy | 25 (69%) | 40 (74%) | 11 (73%) | NS |
NS = nonsignificant.
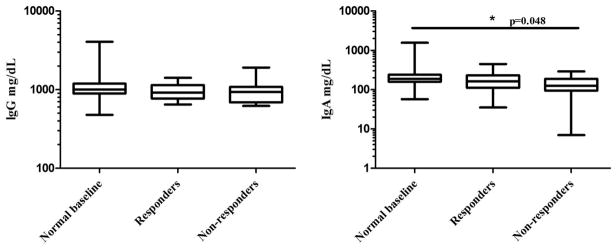
Although serum IgG levels did not differ significantly among the three groups, the level of serum IgA was significantly lower in nonresponders versus patients with normal baseline (mean and SD, 138 ± 67.3 versus 330 ± 356; p = 0.048; Fig. 2). The nonresponder group had a significantly lower mean number of protective baseline antibody titers against pneumococcal serotypes when compared with responders (1.41 versus 2.72; p < 0.005), and had a smaller increase in the mean number of protective titers postimmunization when compared with responders (2.82 versus 7.28; p < 0.0001; Fig. 3).
Figure 2.
Comparison of serum immunoglobulin G (IgG) and IgA among the three patient groups. The serum IgA levels were significantly lower in the nonresponder group when compared with the normal baseline group (138 ± 67.3 versus 330 ± 356; p = 0.048). The serum IgG levels did not differ significantly. Box plots represent 25th–75th percentile range with middle bar depicting median value and whiskers reflecting range of data.
Figure 3.
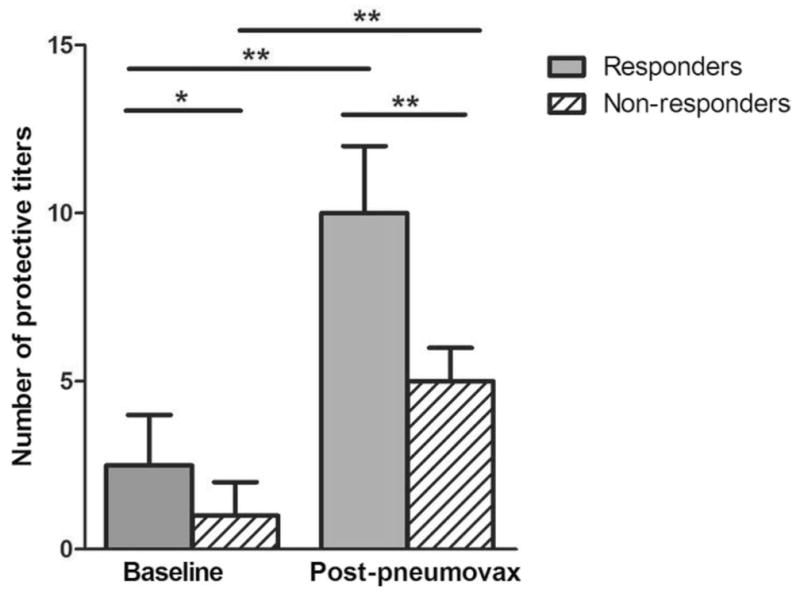
Vaccine-induced change in number of protective antipneumococcal titers for the responder and nonresponder groups. The mean number of baseline titers for the responders was significantly higher than that of the nonresponders (2.72 versus 1.41; p < 0.005). Additionally, the mean change in number of protective postvaccination titers was significantly higher for the responders compared with nonresponders (7.28 versus 2.82; p < 0.0001). Data are expressed as median and interquartile boxes (*p < 0.005; **p < 0.0001).
Evaluation of the nonresponders as a group revealed that none of these patients had protective antibody concentrations against more than three serotypes at baseline. One of these patients has benign lymphadenopathy, one patient has allergic bronchopulmonary aspergillosis and Samter’s triad, one patient was receiving antibiotic prophylaxis for recurrent infection due to SAD, and only one patient required IVIg replacement for management of disease. Recurrent pneumonia was not identified as a comorbidity for any of the subjects.
DISCUSSION
Patients with CRS may have a high frequency of comorbid SAD, which may be an unrecognized contributor to the prevalence and severity of illness and failure of medical treatment for this disease. However, the diagnosis of SAD can be challenging. The range of normal baseline antibody levels against S. pneumoniae serotypes in healthy adults has been documented,13–15 but what constitutes a protective level of antibody against each serotype and whether that protective titer is different for each serotype remains controversial.16 Additionally, there is no universal definition of adequate response to pneumococcal vaccination for diagnosis of SAD despite meta-analysis and specificity studies.17,18 Previous studies have described the range of postvaccination antibody levels in healthy individuals, revealing achievement of normal antibody titers (defined by antibody titer of >1.0 μg/mL or twofold increase in titer) against most serotypes in adults,19,20 as well as a normal response (defined by serotype-specific antibody levels using alternate multiplexed bead assay) in >90% of normal patients.14 Although some experts recommend requiring a fourfold increase in individual antibody levels over preimmunization titers, this loses validity with preimmunization titer levels in the low–normal range.21 Consensus of the American Academy of Allergy, Asthma, and Immunology and American College of Allergy, Asthma, and Immunology as outlined in the practice parameters12 defines an adequate response as antibody concentration of ≥1.3 μg/mL or a fourfold increase over baseline in ~50–70% of pneumococcal serotypes that are evaluated. For the purposes of this study, a conservative cutoff of ≥1.3 μg/mL of antibody concentration against each serotype tested and a requirement of protective titers against only 50% of the serotypes to rule out SAD was chosen to minimize mis-diagnosis of SAD.
The patient population examined in this study represents a highly selected subgroup of CRS patients who require surgical intervention for their CRS and whose clinical characteristics prompted evaluation for immunodeficiency by their physician and, therefore, may not represent the CRS population in general. However, within this selected population there were only 28% of patients with normal pre-immunization antipneumococcal titers, suggesting that there was already evidence for impaired responsiveness to streptococci or that their exposure history was not normal. Of those patients with low baseline titers, there was only a 78% response rate to polysaccharide vaccination, which, despite a conservative cutoff value, is much lower than expected based on the available population pre- and postvaccination data.13,14,19,20 From the group of 129 patients, 15 (11.6%) have been diagnosed with SAD by the strict criteria we have used, a prevalence that is higher than that previously reported in adults with recurrent pneumonia.6 Although the prevalence of SAD is lower in our study patients than in those reported by Alqudah et al.,8 41% of those previously described subjects who did not respond to pneumococcal vaccination also suffered from recurrent pneumonia; in contrast, chart review did not reveal a history of life-threatening pneumococcal infection or invasive pneumonia in any of our patients. It should be noted that the patients diagnosed with SAD, despite their severe sinus disease, were rarely treated with IVIg. Although the purpose of this study was not to evaluate the therapeutic effect of the pneumococcal polysaccharide vaccine in this subgroup, this is an intended area of future study.
The role of the epithelial barrier and interaction between the innate and adaptive immune systems in the pathogenesis of CRS are areas of active research. Recently, we published studies suggesting impairment in innate immunity in patients with CRS.22 The patients with low baseline titers but adequate response to vaccine (responders) may have normal adaptive or systemic immune function but impaired local innate responsiveness. Because we excluded patients with pre-existing primary immunodeficiency, we expect the range of measured quantitative serum immunoglobulins to be within normal values. However, we were able to detect subtle but statistically significant immunologic differences among the groups. Although the clinical significance of lower serum IgA levels in the nonresponder patients when compared with the other groups is unclear, the presence of lower serum IgA levels and lower number of protective baseline antipneumococcal titers in vaccine nonresponders may indicate failure of both the innate and the adaptive immune systems to provide an adequate mucosal immunity in these patients. These results suggest that further studies on the relevance of SAD to the pathogenesis, diagnosis, and treatment of severe CRS are likely to be highly worthwhile. Although the outcome of sinus surgery in medically refractory CRS patients with immune dysfunction or autoimmunity has been shown to be similar to that of control CRS patients in a case–control study,23 it is not known whether low baseline antipneumococcal antibody titers or SAD contribute to the severity of disease or need for surgery.
Potential weaknesses of this study include the limitations inherent in retrospective chart review and the selection bias of a tertiary care referral center patient population. However, this is the largest study of its kind to specifically examine SAD in medically refractory CRS without confounding factors of other recurrent infections. The prevalence of SAD may correlate with disease severity and should be evaluated in CRS patients with medically refractory disease.
Acknowledgments
Supported by NIH/NIAID RO1 HL 078860, AI 072570, R37 HL 068546, and the Ernest S. Bazley Trust
Footnotes
Approval through the Institutional Review Board of Northwestern University Feinberg School of Medicine
Presented at the meeting of the American Academy of Allergy Asthma and Immunology, San Francisco, CA, March 18–22, 2011
The authors have no conflicts to declare pertaining to this article
References
- 1.Anand VK. Epidemiology and economic impact of rhinosinusitis. Ann Otol Rhinol Laryngol Suppl. 2004;193:3–5. doi: 10.1177/00034894041130s502. [DOI] [PubMed] [Google Scholar]
- 2.Cheng YK, Decker PA, O’Byrne MM, et al. Clinical and laboratory characteristics of 75 patients with specific polysaccharide antibody deficiency syndrome. Ann Allergy Asthma Immunol. 2006;97:306–311. doi: 10.1016/S1081-1206(10)60794-6. [DOI] [PubMed] [Google Scholar]
- 3.Hidalgo H, Moore C, Leiva L, et al. Preimmunization and postimmunization pneumococcal antibody titers in children with recurrent infections. Ann Allergy Asthma Immunol. 1996;76:341–346. doi: 10.1016/S1081-1206(10)60035-X. [DOI] [PubMed] [Google Scholar]
- 4.Epstein MM, Gruskay F. Selective deficiency in pneumococcal antibody response in children with recurrent infections. Ann Allergy Asthma Immunol. 1995;75:125–131. [PubMed] [Google Scholar]
- 5.Javier FC, Moore CM, Sorensen RU. Distribution of primary immunodeficiency diseases diagnosed in a pediatric tertiary hospital. Ann Allergy Asthma Immunol. 2000;84:25–30. doi: 10.1016/S1081-1206(10)62736-6. [DOI] [PubMed] [Google Scholar]
- 6.Ekdahl K, Braconier JH, Svanborg C. Immunoglobulin deficiencies and impaired immune response to polysaccharide antigens in adult patients with recurrent community-acquired pneumonia. Scand J Infect Dis. 1997;29:401–407. doi: 10.3109/00365549709011838. [DOI] [PubMed] [Google Scholar]
- 7.Chee L, Graham SM, Carothers DG, et al. Immune dysfunction in refractory sinusitis in a tertiary care setting. Laryngoscope. 2001;111:233–235. doi: 10.1097/00005537-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Alqudah M, Graham SM, Ballas ZK. High prevalence of humoral immunodeficiency patients with refractory chronic rhinosinusitis. Am J Rhinol Allergy. 2010;24:409–412. doi: 10.2500/ajra.2010.24.3532. [DOI] [PubMed] [Google Scholar]
- 9.Benninger MS, Ferguson BJ, Hadley JA, et al. Adult chronic rhinosinusitis: Definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngology. 2003;129:S1–S32. doi: 10.1016/s0194-5998(03)01397-4. [DOI] [PubMed] [Google Scholar]
- 10.Meltzer E, Hamilos DA, Hadley JA, et al. Rhinosinusitis: Establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004;114:S115–S212. doi: 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landesman SH, Schiffman G. Assessment of the antibody response to pneumococcal vaccine in high-risk populations. Rev Infect Dis. 1981;3(suppl):S184–S197. doi: 10.1093/clinids/3.supplement_1.s184. [DOI] [PubMed] [Google Scholar]
- 12.Bonilla FA, Bernstein IL, Khan DA, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. Ann Allergy Asthma Immunol. 2005;94:S26–S29. doi: 10.1016/s1081-1206(10)61142-8. [DOI] [PubMed] [Google Scholar]
- 13.Borgers H, Jeruissen A, Flamaing J, et al. Elderly subjects do not show impaired pneumococcal capsular polysaccharide serotype-specific antibody responses as assessed by a multiplexed bead assay. Clin Immunol. 2010;135:501–502. doi: 10.1016/j.clim.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Borgers H, Moens L, Picard C, et al. Laboratory diagnosis of specific antibody deficiency to pneumococcal capsular polysaccharide antigens by multiplexed bead assay. Clin Immunol. 2010;134:198–205. doi: 10.1016/j.clim.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Mayo Clinic Mayo Medical Laboratories. Reference values, Unit Code 83640: Streptococcus pneumoniae IgG Antibody, 23 serotypes, serum. [last accessed May 2011]; Available online at www.mayomedicallaboratories.com.
- 16.Jeurissen A, Moens L, Raes M, et al. Laboratory diagnosis of specific antibody deficiency to pneumococcal capsular polysaccharide antigens. Clin Chem. 2007;53:505–510. doi: 10.1373/clinchem.2006.080051. [DOI] [PubMed] [Google Scholar]
- 17.Go ES, Ballas ZK. Anti-pneumococcal antibody response in normal subjects: A meta-analysis. J Allergy Clin Immunol. 1996;98:205–215. doi: 10.1016/s0091-6749(96)70244-0. [DOI] [PubMed] [Google Scholar]
- 18.Kamchaisatian W, Wanwatsuntikul W, Sleasman JW, et al. Validation of current joint American Academy of Allergy, Asthma & Immunology and American College of Allergy, Asthma and Immunology guidelines for antibody response to the 23-valent pneumococcal vaccine using a population of HIV infected children. J Allergy Clin Immunol. 2006;118:1336–1341. doi: 10.1016/j.jaci.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 19.Musher DM, Luchi MJ, Watson DA, et al. Pneumococcal polysaccharide vaccine in young adults and older bronchitics: Determination of IgG responses by ELISA and the effect of adsorption of serum with non-type-specific cell wall polysaccharide. J Infect Dis. 1990;161:728–735. doi: 10.1093/infdis/161.4.728. [DOI] [PubMed] [Google Scholar]
- 20.Musher DM, Manoff SB, Liss C, et al. Safety and antibody response, including antibody persistence of 5 years, after primary vaccination or revaccination with pneumococcal polysaccharide vaccine in middle-aged and older adults. J Infect Dis. 2010;201:516–524. doi: 10.1086/649839. [DOI] [PubMed] [Google Scholar]
- 21.Hare ND, Smith BJ, Ballas ZK. Antibody response to pneumococcal vaccination as a function of preimmunization titer. J Allergy Clin Immunol. 2009;123:195–200. doi: 10.1016/j.jaci.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tieu DD, Peters AT, Carter RT, et al. Evidence for diminished levels of epithelial psoriasin and calprotectin in chronic rhinosinusitis. J Allergy Clin Immunol. 2010;125:667–675. doi: 10.1016/j.jaci.2009.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khalid AN, Mace JC, Smith TL. Outcomes of sinus surgery in ambulatory patients with immune dysfunction. Am J Rhinol Allergy. 2010;24:230–233. doi: 10.2500/ajra.2010.24.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]