Abstract
Objectives
The objectives of the study were to determine the prevalence of chronic rhinosinusitis (CRS) overall and its 2 phenotypic variants, CRS with and without polyposis (NP), in patients with chronic inflammatory comorbidities including autoimmune disorders, inflammatory bowel disease, and atopic dermatitis. These findings were compared with data in patients with asthma. Patients with hypertension were also used as a reference group to estimate the incidence of CRS in a group with regular medical follow-up.
Study design
A retrospective, cross-sectional query of a large tertiary care electronic medical record database was performed.
Results
Electronic medical record database prevalence of CRS in patients with hypertension was 4.4%. The prevalence of CRS was 18% in asthma (P < .0001), 7% in atopic dermatitis, 3.5% in inflammatory bowel disease, and ranged from 1.4% to 5.9% in autoimmune disorders. The frequency of CRS patients exhibiting the NP phenotype was similarly low in patients with autoimmune disease and hypertension, but was significantly greater in patients with asthma (P < .0001), inflammatory bowel disease (P = .033), and atopic dermatitis (P = .049),
Conclusions
These findings suggest similar prevalence of overall CRS in patients with autoimmune disease and inflammatory bowel disease, and background rates as estimated by observations in hypertension patients. Inflammatory bowel disease and atopic dermatitis patients with CRS exhibit some skewing toward the NP phenotype, as do asthmatics, where this association is well known.
1. Introduction
Chronic rhinosinusitis (CRS) is a heterogeneous disorder with multiple phenotypic presentations. Broadly, the disease is often subcategorized as either CRS with nasal polyps (CRSwNP) or CRS without nasal polyps (CRSsNP). Although this may reflect an oversimplification of a heterogeneous disorder, the distinction is convenient for clinical purposes; and pathophysiologic differences between these entities have been described. Specifically, CRSwNP, at least in Western populations, is characterized by a skewed T-helper 2 (Th2) responses and tissue eosinophilia; CRSsNP is more likely to exhibit T-helper 1 (Th1) responses and a predominantly neutrophilic inflammation [1].
The significant concordance between CRS and asthma is well known, and studies have demonstrated as high as 70% of asthmatics to have symptoms of CRS [2]. Chronic rhinosinusitis also appears to be more frequent, and severe, in those with severe asthma [2]. Furthermore, this association may be of even greater significance for patients with the CRSwNP phenotype [3]; and previous studies from our institution revealed that the presence of comorbid asthma was the most significant factor associated with the presence of NP in CRS patients [4]. Taken together, these observations suggest that Th2-mediated disorders affecting the airway tend to coexist, in support of a unified airway concept [5].
Other investigators have examined the hypothesis that the presence of comorbid autoimmune mediated conditions may also impact the prevalence of CRS subtypes [6]. Specifically, disorders associated with a bias toward Th1 immunity (eg, multiple sclerosis, psoriasis, rheumatoid arthritis) have been proposed to skew comorbidities away from manifestations conventionally attributed to Th2-mediated pathways [7]. Given the presence of a Th1 autoimmune disorder, this hypothesis would predict a higher prevalence of CRSsNP and a lower prevalence of CRSwNP than observed in the general population. We sought to test this hypothesis by investigation of a large tertiary care electronic medical record (EMR) database to determine the frequency CRS overall and its 2 phenotypic variants (CRSsNP and CRSwNP) in patients with various autoimmune disorders.
Unlike rheumatologic conditions, CRS affects a barrier membrane that separates host tissues from the external environment. This has led to the hypothesis that defects in this intrinsic epithelial barrier may predispose patients to CRS [8]. Similar mechanisms have been proposed for inflammatory bowel disease [9] and atopic dermatitis [10], as these disorders also affect tissues at the interface between the host and the external environment. Thus, we also sought to determine among patients in the Northwestern EMR the frequency of CRS overall and its 2 phenotypic variants (CRSsNP and CRSwNP) in patients with inflammatory bowel disease and atopic dermatitis.
The prevalence of CRS and its phenotypic variants was determined and compared among patients with autoimmune conditions, inflammatory bowel disease, atopic dermatitis, and asthma, and was compared with a reference group of patients with hypertension.
2. Methods
The EPIC EMR database of the Northwestern Medical Faculty Foundation, Chicago, IL, has enrolled a total of 1 970 695 patients between December 1, 1998, and December 1, 2008. Fields in the database are established to capture comorbid conditions in an ongoing problem list. These data are typically entered by the primary care physician before the referral but can also be entered at the time of the otolaryngology visit (eg, self-referred patient). This database was queried by the International Classification of Diseases, Ninth Revision (ICD-9) code. For patients within each disease category, a query was run to determine the frequency of patients also diagnosed with CRSsNP (ICD-9 code in the 473 series, but no 471 code) or with CRSwNP (ICD-9 code 471 with or without a 473 series code). Because all analyses were conducted on aggregated deidentified data, this study was approved by the institutional review board at the Northwestern University Feinberg School of Medicine and was deemed exempt from patient consent and periodic review. The relative proportion of CRS patients manifesting the NP phenotype was also determined for each of the selected diseases. These findings were compared statistically by χ2 analysis, and P < .05 was considered significant.
3. Results
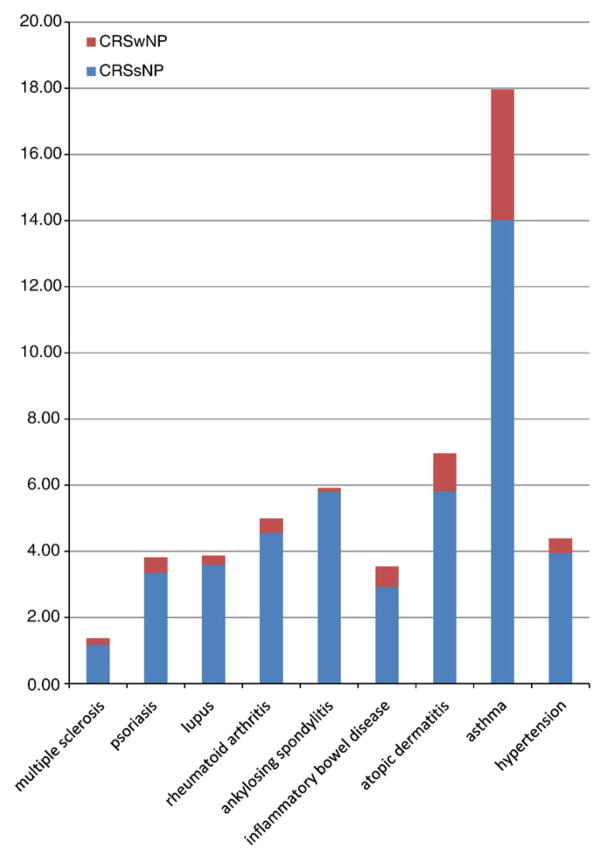
Patients with hypertension were used as a control group, to which patients with chronic inflammatory conditions were compared. The prevalence of any form of CRS in patients with autoimmune disorders (multiple sclerosis, psoriasis, lupus, rheumatoid arthritis, anyklosing spondylitis) ranged from 1.4% to 5.9% (Fig. 1). Among the groups of patients with autoimmune conditions, the frequency of CRS was significantly greater in those with either rheumatoid arthritis or ankylosing spondylitis than in patients with lupus, psoriasis, or multiple sclerosis (P < .0001). The prevalence of CRS in autoimmune patients overall (3.9%) was similar to that observed in hypertension patients (4.4%). Patients with inflammatory bowel disease also exhibited CRS at a similar frequency (3.5%). In contrast, the prevalence of CRS in patients with either atopic dermatitis (7.0%) or asthma (18%) was significantly greater than that observed in patients with hypertension (P < .0001 for both) (Table 1).
Fig. 1.
Each bar reflects total percentage of patients with CRS. Bars are fractioned to stratify each group by NP status.
Table 1.
Prevalence of CRS and its subtypes, by disease state
| Disease | n | Prevalence of either form of CRS (%) | Prevalence of CRSsNP (%) | Prevalence of CRSwNP (%) |
|---|---|---|---|---|
| Multiple sclerosis | 1533 | 1.4 | 1.2 | 0.20 |
| Psoriasis | 4009 | 3.8 | 3.3 | 0.47 |
| Lupus | 2788 | 3.9 | 3.6 | 0.29 |
| Rheumatoid arthritis | 2721 | 5.0* | 4.6 | 0.44 |
| Ankylosing spondylitis | 777 | 5.9* | 5.9 | 0.13 |
| Inflammatory bowel disease | 2259 | 3.5 | 2.9 | 0.62 |
| Atopic dermatitis | 1307 | 7.0† | 5.8 | 1.15 |
| Asthma | 11481 | 18.0† | 14.0 | 3.95 |
| Hypertension | 32319 | 4.4 | 4.0 | 0.44 |
Among the autoimmune conditions, prevalence (percentage) of CRS was greater in patients with rheumatoid arthritis or ankylosing spondylitis than those with lupus, psoriasis, or multiple sclerosis (*P < .0001). The prevalence of CRS was significantly greater in asthmatics and atopic dermatitis patients than those with hypertension (†P < .0001).
The proportion of the NP phenotype was then examined for CRS patients within each disease category (Table 2). Among the CRS patients with various types of autoimmune conditions, there was a seemingly higher proportion of NP in patients with multiple sclerosis and psoriasis, but this did not achieve significance (P = .23); and the overall frequency of NP phenotype in autoimmune patients with CRS (9.2%) was similar to that of hypertension patients with CRS (10%). In contrast, patients with CRS comorbid with inflammatory bowel disease (17.5% with NP), atopic dermatitis (16.5%), and asthma (22%) were significantly more likely to manifest NP (P = .033, P = .049, and P < .0001, respectively, see parentheses).
Table 2.
Proportion of CRS patients expressing NP phenotype
| Group | n | % also manifesting NP |
|---|---|---|
| Multiple sclerosis patients with CRS | 21 | 14.3 |
| Psoriasis patients with CRS | 153 | 12.4 |
| Lupus patients with CRS | 108 | 7.4 |
| Rheumatoid arthritis patients with CRS | 136 | 8.8 |
| Ankylosing spondylitis patients with CRS | 46 | 2.3 |
| Inflammatory bowel disease patients with CRS | 80 | 17.5† |
| Atopic dermatitis patients with CRS | 91 | 16.5‡ |
| Asthma patients with CRS | 2063 | 22.0* |
| Hypertension patients with CRS | 1420 | 10.0 |
The proportion (percentage) of CRS patients exhibiting the NP phenotype is significantly greater in asthmatics than any of the autoimmune conditions (*P < .0001). Chronic rhinosinusitis patients with inflammatory bowel disease (†P =.033)and atopic dermatitis (‡P = .049)were also significantly more likely to manifest NP than were hypertension patients. Differences were not statistically significant among patients with different autoimmune disorders (P = .23) or between autoimmune patients and those with hypertension.
4. Discussion
The background prevalence of CRS in our patient population was estimated using patients with hypertension, as this cohort manifests a chronic condition for which regular follow-up is warranted. These patients carried a diagnosis of CRS in 4.4% of cases. In contrast, the frequency of CRS in the United States has been estimated at more than 10%, depending on the methodology chosen [11]. This is less than that observed in our patients with hypertension; however, these published estimates were derived from patient surveys and are thus likely overestimated by self-diagnosis. In the present series, the diagnosis of CRS was established during a physician-patient encounter, potentially eliminating some of this bias. Nevertheless, chronic inflammatory conditions typically manifest with a relapsing and remitting course where long-term follow-up is the norm. Accurate documentation of comorbid conditions such as CRS is much more probable in this setting. Thus, the present methodology does permit comparisons and analysis of these patients who are followed on a long-term basis. In the present study, the prevalence of CRS in patients with autoimmune disease and inflammatory bowel disease was similar to that of CRS in patients with hypertension. This suggests that neither autoimmune disorders nor inflammatory bowel disease alters the expected prevalence of comorbid CRS. On the other hand, atopic dermatitis and asthma are disease groups in which we observed a much greater overall prevalence of CRS, a least suggesting the hypothesis of related causation.
CRSsNP is generally associated with Th1 inflammation, whereas CRSwNP demonstrates a Th2 skewing. It is theoretically possible that some chronic inflammatory disorders may be associated with alterations in the expected incidence of these T-lymphocyte subsets, while leaving the overall incidence of CRS unchanged. For some of the autoimmune disorders studied herein, for example, including multiple sclerosis, psoriasis, and rheumatoid arthritis, a Th1-predominant or Th17-inflammatory pathway is theorized [7]. In contrast, a clear Th1 vs Th2 bias is not well demonstrated for other rheumatologic conditions, such as lupus and ankylosing spondylitis. A study by Rashid et al [6] suggested that autoimmune conditions may skew airway inflammatory disease away from Th2 pathways and would thus be associated with a decreased frequency of CRS. However, that study examined a limited sample of patients with autoimmune disorders (n = 1026 over a year period) and did not stratify CRS patients by NP status. We observed no significant differences regarding the prevalence of NP between subsets of autoimmune patients. In addition, the prevalence of NP phenotype in CRS patients with autoimmune comorbidities was similar to that of background estimates, derived from data in hypertensive CRS patients. These observations cannot prove a clear shift toward Th1 presentation of CRS in autoimmune patients, but does suggest tendency toward Th2 phenotype in CRS patients with inflammatory bowel disease, atopic dermatitis, or asthma. It is likely that underlying Th1/Th2 status is only one of several factors that influence phenotypic presentation of comorbid CRS.
Previous findings from our laboratory have demonstrated deficiencies in expression of genes involved in epithelial barrier maintenance and repair in tissues from CRS patients [12]. Among the comorbidities studied herein, inflammatory bowel disease, atopic dermatitis, and asthma are distinct in that the pathophysiology is thought to involve abnormalities of the innate immune system and the mechanical epithelial barrier [9,10,13]. This is thought to promote inflammation secondary to augmentation of antigenic uptake, increased local sensitization, and ongoing antigenic exposure. Interestingly, patients with CRS comorbid with inflammatory bowel disease, atopic dermatitis, and asthma all exhibited significant trends toward the phenotype with NP. This observation raises the hypothesis that innate barrier dysfunction may not only predispose patients to those conditions, but may also influence the presentation of comorbid CRS, particularly the polypoid form, which has also been shown to reflect abnormalities of epithelial barrier function [8,12].
The present study lends insight into how pathophysiologic mechanisms proposed for other chronic inflammatory disorders may also be relevant to defining the etiology of CRS. Further clinical and laboratory investigations are warranted to explore the various overlapping or counterbalancing immunologic mechanisms that may affect the prevalence and phenotypic pattern of CRS.
Footnotes
Conflicts of interest: none. Financial support: none.
Presented at the Annual Meeting of the American Academy of Otolaryngology–Head and Neck Surgery, October 6, 2009, San Diego, CA.
References
- 1.Van Zele T, Claeys S, Gevaert P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61:1280–9. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 2.Bresciani M, Paradis L, Des Roches A, et al. Rhinosinusitis in severe asthma. J Allergy Clin Immunol. 2001;107:73–80. doi: 10.1067/mai.2001.111593. [DOI] [PubMed] [Google Scholar]
- 3.Hamilos DL. Chronic rhinosinusitis patterns of illness. Clin Allergy Immunol. 2007;20:1–13. [PubMed] [Google Scholar]
- 4.Pearlman AN, Chandra RK, Chang D, et al. Relationships between severity of chronic rhinosinusitis and nasal polyposis, asthma, and atopy. Am J Rhinol Allergy. 2009;23:145–8. doi: 10.2500/ajra.2009.23.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipworth BJ, White PS. Allergic inflammation in the unified airway: start with the nose. Thorax. 2000;55:878–81. doi: 10.1136/thorax.55.10.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rashid RM, Miller A, Scianna JM, et al. Chronic rhinosinusitis and psoriasis: do mutually exclusive systemic Th1 and Th2 disease patterns exist? Acta Otolaryngol. 2007;127:780–3. doi: 10.1080/00016480601002054. [DOI] [PubMed] [Google Scholar]
- 7.van Roon JA, Bijlsma JW. Th2 mediated regulation in RA and the spondyloarthropathies. Ann Rheum Dis. 2002;61:951–4. doi: 10.1136/ard.61.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schleimer RP, Kato A, Peters A, et al. Epithelium, inflammation, and immunity in the upper airways of humans: studies in chronic rhinosinusitis. Proc Am Thorac Soc. 2009;6:288–94. doi: 10.1513/pats.200808-088RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mankertz J, Schulzke JD. Altered permeability in inflammatory bowel disease: pathophysiology and clinical implications. Curr Opin Gastroenterol. 2007;23:379–83. doi: 10.1097/MOG.0b013e32816aa392. [DOI] [PubMed] [Google Scholar]
- 10.Strid J, Strobel S. Skin barrier dysfunction and systemic sensitization to allergens through the skin. Curr Drug Targets Inflamm Allergy. 2005;4:531–41. doi: 10.2174/156801005774322199. [DOI] [PubMed] [Google Scholar]
- 11.Smith WM, Davidson TM, Murphy C. Regional variations in chronic rhinosinusitis, 2003–2006. Otolaryngol Head Neck Surg. 2009;141:347–52. doi: 10.1016/j.otohns.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richer SL, Truong-Tran AQ, Conley DB, et al. Epithelial genes in chronic rhinosinusitis with and without nasal polyps. Am J Rhinol. 2008;22:228–34. doi: 10.2500/ajr.2008.22.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holgate ST. Has the time come to rethink the pathogenesis of asthma? Curr Opin Allergy Clin Immunol. 2010;10:48–53. doi: 10.1097/ACI.0b013e3283347be5. [DOI] [PubMed] [Google Scholar]