EXECUTIVE SUMMARY
Objective
The Medical Advisory Secretariat undertook a review of the evidence on the effectiveness and cost-effectiveness of small bowel transplant in the treatment of intestinal failure.
Small Bowel Transplantation
Intestinal failure is the loss of absorptive capacity of the small intestine that results in an inability to meet the nutrient and fluid requirements of the body via the enteral route. Patients with intestinal failure usually receive nutrients intravenously, a procedure known as parenteral nutrition. However, long-term parenteral nutrition is associated with complications including liver failure and loss of venous access due to recurrent infections.
Small bowel transplant is the transplantation of a cadaveric intestinal allograft for the purpose of restoring intestinal function in patients with irreversible intestinal failure. The transplant may involve the small intestine alone (isolated small bowel ISB), the small intestine and the liver (SB-L) when there is irreversible liver failure, or multiple organs including the small bowel (multivisceral MV or cluster). Although living related donor transplant is being investigated at a limited number of centres, cadaveric donors have been used in most small bowel transplants.
The actual transplant procedure takes approximately 12-18 hours. After intestinal transplant, the patient is generally placed on prophylactic antibiotic medication and immunosuppressive regimen that, in the majority of cases, would include tacrolimus, corticosteroids and an induction agent. Close monitoring for infection and rejection are essential for early treatment.
Medical Advisory Secretariat Review
The Medical Advisory Secretariat undertook a review of 35 reports from 9 case series and 1 international registry. Sample size of the individual studies ranged from 9 to 155.
As of May 2001, 651 patients had received small bowel transplant procedures worldwide. According to information from the Canadian Organ Replacement Register, a total of 27 small bowel transplants were performed in Canada from 1988 to 2002.
Patient Outcomes
The experience in small bowel transplant is still limited. International data showed that during the last decade, patient survival and graft survival rates from SBT have improved, mainly because of improved immunosuppression therapy and earlier detection and treatment of infection and rejection. The Intestinal Transplant Registry reported 1-year actuarial patient survival rates of 69% for isolated small bowel transplant, 66% for small bowel-liver transplant, and 63% for multivisceral transplant, and a graft survival rate of 55% for ISB and 63% for SB-L and MV. The range of 1-year patient survival rates reported ranged from 33%-87%. Reported 1-year graft survival rates ranged from 46-71%.
Regression analysis performed by the International Transplant Registry in 1997 indicated that centres that have performed at least 10 small bowel transplants had better patient and graft survival rates than centres that performed less than 10 transplants. However, analysis of the data up to May 2001 suggests that the critical mass of 10 transplants no longer holds true for transplants after 1995, and that good results can be achieved at any multiorgan transplant program with moderate patient volumes.
The largest Centre reported an overall 1-year patient and graft survival rate of 72% and 64% respectively, and 5-year patient and graft survival of 48% and 40% respectively. The overall 1-year patient survival rate reported for Ontario pediatric small bowel transplants was 61% with the highest survival rate of 83% for ISB.
The majority (70% or higher) of surviving small bowel transplant recipients was able to wean from parenteral nutrition and meet all caloric needs enterally. Some may need enteral or parenteral supplementation during periods of illness. Growth and weight gain in children after ISB were reported by two studies while two other studies reported a decrease in growth velocity with no catch-up growth.
The quality of life after SBT was reported to be comparable to that of patients on home enteral nutrition. A study found that while the parents of pediatric SBT recipients reported significant limitations in the physical and psychological well being of the children compared with normal school children, the pediatric SBT recipients themselves reported a quality of life similar to other school children.
Survival was found to be better in transplants performed since 1991. Patient survival was associated with the type of organ transplanted with better survival in isolated small bowel recipients.
Adverse Events
Despite improvement in patient and graft survival rates, small bowel transplant is still associated with significant mortality and morbidity.
Infection with subsequent sepsis is the leading cause of death (51.3%). Bacterial, fungal and viral infections have all been reported. The most common viral infections are cytomegalorvirus (18-40%) and Epstein-Barr virus. The latter often led to ß-cell post-transplant lymphoproliferative disease.
Graft rejection is the second leading cause of death after SBT (10.4%) and is responsible for 57% of graft removal. Acute rejection rates ranged from 51% to 83% in the major programs. Most of the acute rejection episodes were mild and responded to steroids and OKT3. Antilymphocyte therapy was needed in up to 27% of patients. Isolated small bowel allograft and positive lymphocytotoxic cross-match were found to be risk factors for acute rejection.
Post-transplant lymphoproliferative disease occurred in 21% of SBT recipients and accounted for 7% of post-transplant mortality. The frequency was higher in pediatric recipients (31%) and in adults receiving composite visceral allografts (25%). The allograft itself is often involved in post-transplant lymphoproliferative disease. The reported incidence of host versus graft disease varied widely among centers (0% - 14%).
Surgical complications were reported to occur in 85% of SB-L transplants and 25% of ISB transplants. Reoperations were required in 45% - 66% of patients in a large series and the most common reason for reoperation was intra-abdominal abscess.
The median cost of intestinal transplant in the US was reported to be approximately $275,000US (approximately CDN$429,000) per case. A US study concluded that based on the US cost of home parenteral nutrition, small bowel transplant could be cost-effective by the second year after the transplant.
Conclusion
There is evidence that small bowel transplant can prolong the life of some patients with irreversible intestinal failure who can no longer continue to be managed by parenteral nutrition therapy. Both patient survival and graft survival rates have improved with time. However, small bowel transplant is still associated with significant mortality and morbidity. The outcomes are inferior to those of total parenteral nutrition. Evidence suggests that this procedure should only be used when total parenteral nutrition is no longer feasible.
Objective
The Medical Advisory Secretariat conducted a systematic review to evaluate the effectiveness and cost-effectiveness of small bowel transplant as a treatment for end-stage intestinal failure.
BACKGROUND
Clinical Need
Intestinal Failure
The small intestine is the major site for the absorption of nutrients. The minimum length of intestine necessary to maintain nutritional status has not been established, but is approximately 10-20 cm of small intestine with an ileocecal valve, and approximately 40 cm without an ileocecal valve. Function of the intestine is also dependent on the morphology of the intestinal mucosa and other factors such as gut motility, gut hormones, biliary and fat metabolism, and intestinal microflora.1
Intestinal failure is defined as the loss of absorptive capacity of the small intestine that results in an inability to meet the nutrient and fluid requirements of the body via the enteral route. Causes of intestinal failure include:
Massive intestinal resections such as short bowel syndrome.
Impaired absorptive function such as microvillus inclusion disease
Motility disorders such as pseudo-obstruction2
Loss of the intestine in adults is most commonly due to thrombotic disorders, Crohn’s disease and trauma and, in children, to volvulus, gastroschisis, necotizing enterocolitis and intestinal atresia. Most thrombotic disorders are due to protein C, S and antithrombin III deficiency, factor V/II mutation and development of lupus anticoagulant or anticardiolipin antibodies.3 (Appendix 1).
Intestinal failure prevents oral nutrition and may be associated with mortality and profound morbidity. Symptoms of intestinal failure include persistent diarrhea, dehydration, muscle wasting, poor growth, frequent infections, weight loss, and fatigue. These symptoms can significantly affect the quality of life of the patient.
The incidence of intestinal failure varies among countries. Prasad4 reported an incidence of 2.6 patients per million/year for the United Kingdom, 13.9 patients per million/year for Denmark, and 80 to 200 patients per million/year for the United States of America. There is no data on the incidence in Canada.
Treatment of Intestinal Failure
Surgical
Most of the non-transplant surgical options for intestinal failure, such as bowel lengthening, have been unsuccessful in improving the absorptive capacity of the residual bowel, and none are regarded as sufficiently safe and effective for routine use. Both Bueno et al5 and Kaufman6 emphasized that intestinal lengthening is useful for patients without liver disease who have almost been weaned from total parenteral nutrition, but is not effective for patients with short bowel and liver failure. Bueno et al5 studied the outcome of 27 infants and children who had previously undergone a longitudinal intestinal lengthening operation and found that the operation was not beneficial to infants and children with less than 50 cm of bowel pre-operatively. Only 33% of the patients subsequently tolerated more than 50% enteral feeding. Furthermore, the lengthening of the intestine failed to halt progression of liver disease when present at the time of the procedure.5
Total Parenteral Nutrition
The majority of patients with intestinal failure are managed by total parenteral nutrition (TPN) that delivers all the necessary nutrients intravenously, avoiding the need for absorption through the small bowel. TPN has allowed many patients to survive indefinitely with only a foot or two of small intestines. Long-term total parenteral nutrition using glucose as the chief energy source requires administration via a central venous catheter so that the hypertonic solution can be rapidly diluted in a high-flow system. TPN needs to be started slowly and monitored carefully through regular physical checks and laboratory tests.3
The preferred site for central venous infusion is the superior vena cava. Catheters made from silastic material or polyurethane are associated with lower complications than polyvinyl-chloride catheters.3
The following are major disadvantages of TPN.
Life-threatening complications that occur in approximately 15% of patients receiving home parenteral nutrition.
Risks of mechanical complications associated with the insertion of a central venous catheter, including pneumothorax, hemothorax from laceration of the subclavian artery or vein, brachial plexus injury and malposition of the catheter. Catheters may dislocate, develop leaks, or become detached from the hub and embolize into the heart or pulmonary artery.
Risks of metabolic complications including fluid overload causing congestive heart failure, glucose overload resulting in an osmotic diuresis, or electrolyte imbalance causing arrhythmia, cardiopulmonary dysfunction and neurologic symptoms. Catheter thrombosis may also occur, particularly if the catheter is used for withdrawing blood samples.
TPN associated cholestasis.
Access line infections which, if not treated appropriately, may result in sepsis.
The procedure is costly (approximately $54,900Cdn/year).
Impact on the patient’s life style and quality of life - TPN is cumbersome because of the requirement to infuse the intravenous feeding solutions for 8 to 12 hours every day.1
Prevalence of Intestinal failure in Canada and Ontario
There is presently no provincial or national registry for intestinal failure or for patients requiring home parenteral nutrition.
It is estimated that approximately 85 - 95 patients are presently enrolled in home parenteral nutrition (HPN) programs in Ontario. An expert in HPN indicated that the number of new HPN cases is about the same as the number of patients who leave the program or die, generally from underlying disease (verbal communication).
THE TECHNOLOGY
Small bowel transplant [SBT] is the transplantation of a cadaveric intestinal allograft for the purpose of restoring intestinal function in patients with irreversible intestinal failure.
There are three types of SBT procedures, depending on the need of the patients:
-
Isolated small bowel [ISB] transplantation
ISB is performed in patients with irreversible intestinal failure who are unable to continue TPN and any existing liver damage is reversible.
-
Combined small bowel-liver [SB-L] transplantation
Combined small bowel-liver transplantation is recommended for patients with irreversible failure of both the small bowel and the liver.
-
Multivisceral [MV] or cluster transplantation
Multivisceral grafts, which may include the stomach, duodenum, pancreas, jejuno-ileum, and liver, are given to patients with intestinal failure associated with involvement of other visceral structures. Underlying causes may include pancreatic failure, thromboses of the celiac axis and the superior mesenteric artery, or pseudo-obstruction affecting the entire gastrointestinal tract. In addition, some patients with low-grade, infiltrative malignancy such as Desmoid tumours may require multivisceral transplants.7
Procedure
Assessment
Patients are assessed to ensure that the selection criteria are met and to rule out potential contraindications to the transplant procedure.
Waiting for intestinal transplant
Management of complications and close monitoring are required for potential intestinal transplant candidates while waiting for a suitable donor. As a result of poor vascular access, deteriorating liver function and recurrent sepsis, about 52% of individuals who received intestinal transplant had to be hospitalized while waiting for a suitable donor.11
Donor selection and preparation
Cadaveric donors have been used in most intestinal transplantation, although isolated successful procedures have been performed with an intestinal segment from a living related donor.4
Intestinal grafts are obtained from blood-group-compatible donors. Selection criteria for donors include ABO identical, hemodynamic stability with no excessive inotropic requirements, and compatible size (preferably smaller than the recipient to enable comfortable placement of the graft within the peritoneal cavity). Negative lymphocytotoxic cross-match and cytomegalic virus [CMV] matching would be ideal and are being required in some programs.8
Intestinal decontamination is done in all donors with amphotericin B, an aminoglycoside, polimixin, and broad-spectrum antibiotics before and during procurement. In situ perfusion with University of Wisconsin preservation solution is performed with venous bed decompression. The graft is stored in ice for transport. Mean cold ischemia time, the time between organ procurement and implantation, is approximately 8 hours (range 2.8 - 14.8 hours). A cold ischemia time of less than 10 hours is recommended to avoid preservation injury.
Transplant Procedure
The proximal end of the intestinal graft is anastomosed to the native bowel. The donor colon is usually removed because it appears to increase the incidence of rejection and sepsis. The distal end of the small bowel graft is exteriorized as a stoma or anastomosed to the native colon. A loop ileostomy is created to divert the stool away from the intestinal anastomosis and provide access for biopsies. The ileostomy is closed after 3 to 6 months.
Different techniques are required for the vascular reconstruction of each type of intestinal transplant. With isolated SBT, the arterial supply is established by an end-to-side anastomosis to the infrarenal aorta; the superior mesenteric vein is anastomosed to the native vein or the inferior vena cava. The potential advantages of portal drainage include first-pass delivery of hepatotropic substances to the native liver, filtering of translocated organisms by the native liver, and possible protection from rejection.
Small bowel-liver grafts are removed en bloc, connected by the portal vein, superior mesenteric vein and an aortic segment containing the celiac artery and the superior mesenteric artery. The duodenum and the head of the pancreas are included with the graft to avoid the need for biliary reconstruction. The aortic segment is anastomosed to the infrarenal aorta of the recipient. The end of the native portal vein is anastomosed to the side of the donor portal vein or to the inferior vena cava. Similar techniques are used for multivisceral transplants.
According to an Ontario academic health science centre, the transplant procedure takes 12-18 hours depending on the type of intestinal transplant [written communication].
Post-transplant Care
Small bowel transplant recipients are initially cared for in the intensive care unit. Postoperative management of intestinal transplant recipients includes immunosuppression, anti-infective prophylaxis and graft assessment which will be discussed in greater detail in later sections.
After the initial 2 weeks, it is possible to initiate oral feeding with an isotonic dipeptide solution enriched with medium chain triglycerides and glutamine, changing to a gluten- and lactose-free diet within 2-4 weeks and eventually progressing to a normal diet.4
The mean lengths of stay in hospital for intestinal transplant was reported to be 59.5+/-56 days for isolated intestinal transplant, 81.8+/-79 days for intestine-liver transplant, and 83.5+/-56 days for multivisceral transplant.11
At one Ontario centre, patients are admitted to the ICU for approximately 14 days, then transferred to the Multi-Organ Transplant Unit for approximately 80 days, for a total average hospital length of stay of 100 days [Written communication].
Post Discharge Follow-up
After discharge, patients need regular follow-up at transplant clinics and close monitoring. They may need hospital readmission for treatment of complications associated with intestinal transplantation.
LITERATURE REVIEW
Objective
The objective of this review is to:
Determine the safety, effectiveness and cost-effectiveness of small bowel transplantation for individuals with chronic intestinal failure as compared to total parenteral nutrition.
Identify indications for small bowel transplantation.
Method
Inclusion Criteria
The studies included in the review met the following criteria:
English language journal articles reporting primary data on the effectiveness or cost effectiveness of isolated small bowel transplant or small bowel transplant in combination with transplantation of one or more of other organs (e.g. liver, stomach, duodenum, pancreas, or kidney). The data could be obtained in a clinical setting or from analysis of primary data maintained in registries or institutional databases..
The study design and methods are clearly described.
The studies are systematic reviews (1997 - 2003), randomized controlled studies, non-randomized controlled studies, cohort studies or case series with =/ > 20 transplants (published from 1999 to 2003) or Cost-effectiveness studies (published 1997 - 2003).
Canadian study irrespective of sample size.
The study is not superseded by a publication with the same purpose, by the same group or a later publication that included the data from centres involved in the same multicenter study. If multiple articles were published about one series, the most current report was selected (unless the articles address different endpoints).
Patient:
(a) Human subjects of any age that have intestinal failure of any cause.
Intervention:
Small bowel transplantation in isolation or in combination with other organs.
Comparison:
Total parenteral nutrition
Endpoint measures:
Primary end-point: patient survival and graft survival, graft function as indicated by independence from parenteral nutrition, ability to meet nutrient needs through enteral route and growth in children. Secondary endpoints: complications Economic analysis data
The only alternative technology for treating intestinal failure is total parenteral nutrition.
Exclusion criteria
Non-systematic reviews, letters and editorials.
Animal studies, in-vitro studies or simulation studies
Studies that do not focus on the identified outcomes
Studies focusing on surgical procedures, diagnostic procedures and comparison of drug therapies for post transplantation complications.
Studies with less than 20 small bowel transplants (grafts) except Canadian studies
Search Strategy & Results
The Cochrane & International Agency for Health Technology Assessment (INAHTA) databases, MEDLINE, EMBASE and MEDSCAPE were searched using the following search terms: intestinal transplantation, small bowel transplant, and intestinal failure with the limitations listed above.
The search yielded two systematic reviews and 243 articles.
Level of Evidence Data Extraction
One researcher reviewed the abstract of each article and determined whether the article met the inclusion criteria. The full texts of eligible studies were reviewed to confirm eligibility and to assess the quality of the evidence. Levels of evidence were assigned according to a ranking system based on the hierarchy by Goodman [1985] (Table 1). An additional designation “g” was added for preliminary reports of studies that have been presented to international scientific meetings.
Table 1: Level of Evidence.
| Type of Study (Design) | Level of Evidence |
Number of Eligible Studies Analyzed |
|---|---|---|
| Large randomized controlled trial, Systematic reviews of RCTs | 1 | |
| Large randomized controlled trial unpublished but reported to an international scientific meeting | 1(g) | |
| Small randomized controlled trial | 2 | |
| Small randomized controlled trial unpublished but reported to an international scientific meeting | 2(g) | |
| Nonrandomized trial with contemporaneous controls | 3 a | |
| Nonrandomized trial with historical control | 3b | |
| Nonrandomized controlled trial unpublished but reported to an international scientific meeting | 3g | |
| Surveillance (database or register) | 4a | 1 |
| Case series, multi-site | 4b | |
| Case series, single-site | 4c | 34 |
| Case series unpublished but presented to an international scientific meeting | 4g | |
| TOTAL | 35 |
Of the 243 articles, 35 articles met the inclusion criteria and 208 articles were excluded for the following reasons:
Animal, in vitro or simulation studies
Less than 20 small bowel transplants (grafts)
Reviews, letters, case reports
Focused on drugs, surgical procedures or diagnostic procedures
More current report is available on the same study
In addition to the above articles, additional references were used for background information and were included in the bibliography list. These may include textbooks and review articles.
Limitation of Evidence
No controlled studies were found. The evidence presented was obtained solely from case series and an international registry. Since intestinal transplant has been used as a last-resort life-saving therapy, it is not likely that randomized controlled trials would be conducted on this procedure due to ethical concerns about randomization.
With the exception of three reports that have more than 100 subjects (accumulated over a decade), most of the reports were based on small samples (under 50). This is not likely to change significantly in the near future because the procedure is limited by the availability of cadaver organs.
The type of intestinal transplants performed, choice of immunosuppressive therapy and treatment for infection varied among transplant centres. This results in heterogeneity.
Outcomes were not reported in a standardized way. For example, actual patient survival rates were reported in some reports while other studies reported actuarial survival rates. Similarly, patient survival rates and graft survival rates were reported for different time periods by different studies. This made comparison among programs more difficult.
FINDINGS OF HEALTH TECHNOLOGY ASSESSMENTS
Full reports of the two HTAs are not available because they were not published reports. The following summary was found on the website of Centers for Medicare & Medicaid Services [http://www.cms.hhs.gov/coverage/8b3-g2.asp].
BlueCross/Blueshield HTA
In a 1996 assessment, the Blue Cross Blue Shield Technology Evaluation Center9 found that small bowel/liver combination transplants for adult and children, as well as small bowel transplants alone for children, meet their criteria for coverage. Small bowel transplant alone in adults did not meet their criteria.
In July 1999, the Technology Evaluation Center [TEC] conducted a further technology assessment of small bowel transplant in adults and multivisceral transplants in adult and children. The review was based on several case series. The largest data set analyzed long-term survival of 41 adults who received small bowel transplant alone and 30 patients receiving multivisceral transplant [MVT]. The findings of this HTA are summarized as follows:
Because of the small number of patients in each type of small bowel transplant, TEC could only reliably calculate the overall survival for the total number of patients undergoing these procedures.
Long-term graft survival rates for adult patients undergoing small bowel transplants alone range from 13-30%. It was not possible to predict which patients will survive longer on TPN versus small bowel transplantation. Both treatments caused substantial morbidity in survivors. Formal analysis of the quality of life between the treatments is not available.
Whether small bowel transplantation in adults improves health outcomes has not been demonstrated in the investigational setting.
Multivisceral transplantation in pediatric and adult patients has a similar survival at 33-50% at 5 years. Without this procedure, it is expected that these patients would face 100% mortality.
The results of multivisceral transplantation were derived from specialized treatment settings, using desperately ill patients. Similar results can be expected only in specialized centres that have equivalent training, experience and performance.
Based on the above findings, the Blue Cross/Blue Shield Technology Evaluation Center concluded that small bowel transplantation in adults does not meet its criteria. However, multivisceral transplantation in adults and pediatric patients meets the criteria.
Agency for Healthcare Research and Quality [AHRQ] HTA
The Center for Practice and Technology Assessment at AHRQ10 performed an assessment of intestinal transplantation in 2000. The assessment included a review of 211 full text reports on case series and national and international registries on both small bowel transplant and TPN.
AHRQ findings are summarized as follows:
The available data did not permit precise quantitative estimates of mortality rates for patients who are candidates for small bowel transplant either because of TPN failure or because of supposed high risk for TPN failure. The available data were not sufficient to determine the expected rates of other outcomes of interest.
In general, transplants have only been done on patients who have failed TPN. Based on available data, patient survival rates (adult and children) at 1, 3, and 5 years following SBT or related procedures range from 46% - 80%, 48% - 60%, and 48% - 55%, respectively.
Death is the expected outcome for patients failing TPN who do not receive a transplant.
Graft survival rates (adults and children at 1, 3, and 5 years following SBT or related procedures ranged from 50% - 90%, 36% -48% and 40% - 48%, respectively.
Survival rates at 1, 3, and 5 years for patients on long-term TPN were reported to be approximately 90%, 65 - 80%, and 60% respectively.
Criteria for identifying patients at “high risk” for TPN failure were not defined. Specific outcomes for this group of patients could not be determined.
The AHRQ assessment concluded that small bowel and related transplantation appear to be potentially life-saving options for patients who have failed TPN and would therefore face certain death. The data were not sufficient to determine whether the risks and benefits of small bowel transplant and related procedures may yield a net benefit to patients who can continue TPN, but are considered at high risk to fail TPN sometime in the future. In order to make this determination, well-done studies that compare small bowel transplant to continued TPN would need to be conducted in patients who meet an agreed upon definition of “high risk” for TPN failure.
The AHRQ assessment also concluded that data were not sufficient to determine whether young patients, who are known to require TPN for the rest of their lives without any chance of recovering intestinal function, should be provided the opportunity to receive a transplant prior to reaching the point of failing TPN.
EVIDENCE FROM REGISTRY AND TRANSPLANT PROGRAMS
International Intestinal Transplant Registry
The Intestinal Transplant Registry compiles and analyzes international data related to intestinal transplants performed since 1985. Although provision of information to the registry is voluntary, it has full participation from all intestinal transplant programs worldwide. A report is prepared every two years and one was last published in 1999. Some data up to May 2001 are available on the web site of the Registry. However, patient and graft survival rates are not available in the 2001 web-based report.11
As of May 2001, a total of 651 patients (56% male and 44% female) underwent 696 small bowel transplants at 55 centres. Pediatric intestinal transplants were performed in 38 centres and accounted for 61% of the total recipients.
Data on survival and complications were analyzed. In addition, Kaplan-Meier survival curves analysis using the Wilcoxon test and Cox Regression modeling was performed on the survival data. The outcomes of this analysis will be discussed in the following sections.
Intestinal transplant programs
Based on the 2001 report of the international Intestinal Transplant Registry, only 3 of the 55 centres have each performed more than 100 intestinal transplants (cumulative). Seven programs have published reports on 20 transplants or more.11
Two reports were published on small bowel transplants performed in Ontario. These reports were included even though the number of transplants is less than 20.
The seven programs and the Ontario programs together account for 79.3% (552/696) of the total small bowel transplant procedures performed worldwide. The following analysis will focus on the most current reports from these programs as well as on data from the international Intestinal Transplant Registry (Summary in Appendix 9). A brief description of each program and their outcomes are provided below.11
Indications and contraindications for Small Bowel Transplantation
(Appendices 3 & 4)
The 2001 Report of the Intestinal Transplant Registry11 showed that the most common indications for intestinal transplant in children were gastroschisis (21%), volvulus (18%), necrotizing enterocolitis (12%), pseudo-obstruction (9%) and intestinal atresia (7%).
Among adults, the most common indications were Ischemia (22%), Crohn’s disease (13%), trauma (12%), desmoid tumour (10%), short gut and volvulus (7% each).
Indications
Intestinal transplantation is usually performed under the following circumstances:12;15;16;22;23
TPN associated cholestasis
The most frequent indication for intestinal transplantation is progressive liver failure associated with parenteral nutrition therapy (TPN associated cholestasis). Sudan et al24 indicated that when evidence of cirrhosis on biopsy or when stigmata of portal hypertension (including hepatosplenomegaly, portal hypertensive gastropathy, gastroesophageal varices and dilated superficial abdominal veins) are present, a composite small bowel-liver allograft is needed.
Impending loss of central venous access
The other key indication for intestinal transplantation is impending loss of central venous access in TPN dependent patients, often in association with recurring sepsis. The consensus of the transplant community is that loss of half of the usual access sites is sufficient to warrant referral for SBT.25 The reason is that central venous access may be required for nutrition support for several months after SB transplant and for post-transplant care.
Intestinal psuedo-obstruction and obstruction
Patients with chronic idiopathic intestinal pseudo-obstruction may be appropriate candidates for transplantation because symptoms related to profound and refractory abdominal dilatation may unacceptably reduce quality of life even though central venous access and liver functions are satisfactory.26 Adult patients with unresectable low-grade mesenteric tumors causing obstruction were also offered transplantation.17
Gambarara et al27 reviewed the outcome of 74 patients who needed parenteral nutrition for at least five months. These patients were afflicted with intractable diarrhea [ID], short bowel syndrome [SBS] and chronic intestinal pseudo-obstruction [CIPO]. The results showed that only 24% of the entire series were potential candidates for small bowel transplant. These potential candidates included 38% of the patients with SBS, 20% of patients with ID and 12% of patients with CIPO.27
Contraindications to intestinal transplantation have changed over time, becoming fewer as advancements were made in the field and outcomes have improved. Currently, absolute contraindications include systemic malignancy, metastatic disease, AIDS, cardiopulmonary insufficiency and overwhelming sepsis. Relative contraindications are center-specific and generally include weight (infants weighing less than 5 kg), advanced age, and multiple previous abdominal surgical procedures.1
Types of Small Bowel Transplant Performed
Based on the 2001 report, small bowel transplants in the International Transplant Registry consisted of 42% isolated small bowel transplant, 44.4% small bowel-liver transplant and 13.6% multivisceral transplant. Children accounted for 61% of intestinal transplants. The types and distribution of small bowel transplant procedures performed varied from centre to centre (Table 2). At most centres, composite small bowel-liver transplant was the most common procedure. Multivisceral transplantation was a minority procedure, with the exception of the program at University of Miami School of Medicine, where multivisceral transplant accounted for 41% of all small bowel transplants.11
Table 2: Description of major intestinal transplant programs.
| Program | Time period | Patients | Total Grafts | Types of Grafts | ||
|---|---|---|---|---|---|---|
| %ISB | %SB-L | %MV | ||||
|
Pittsburgh (Abu-Elmagd, 2001)12 |
1990-2001 | 155 | 165 | 39% | 46% | 15% |
|
Miami (Pinna, 2000)13 |
1994-1999 | 69 | 77 | 56% | 31% | 43% |
|
Miami (Kato, 2002)14 |
1994-2001 | 111 | 120 | 32% | 27% | 41% |
|
Nebraska (Langnas, 02)15 |
1990-2001 | 106 | 117 | 37% | 63% | 0% |
|
New York (Fishbein, 2002)16 |
1998-2001 | 34 | 37 | 43% | 51.5% | 5.5% |
|
UCLA (Farmer, 2002)17 |
1991-2000 | 19 | 23 | 13% | 83% | 4% |
|
Paris (Goulet, 2002)18 |
1994-2001 | 36 | 37 | 35% | 65% | 0% |
|
UK (Beath, 2002)19;20 |
1993-2001 | 21 | 21 | 24% | 67% | 9% |
|
Ontario (LHSC) (Atkison, 1998) |
1993-1997 | 9 | 9 | 67% | 33% | 0% |
|
Ontario (Fecteau, 2001)21 |
1999-2000 | 13 | 13 | 46% | 31% | 23% |
| Total | 504 | 552 | ||||
| Intestinal Tx Registry May 2001 | 1985-May 2001 | 651 | 696 | 42% | 44.4% | 13.6% |
| ISB = Isolated small bowel | SB-L = Small bowel and Liver | MV = Multivisceral | ||||
The registry11 also showed different trends in small bowel transplants between adults and children. For adults, the most frequent procedure was isolated small bowel transplant (52%), followed by composite small bowel-liver transplant (25%) and multivisceral transplant (23%). Among children, the most common surgical procedure was composite small bowel-liver transplant (57%) followed by isolated small bowel transplant (35.5%). Multivisceral transplant only made up 7.5% of all pediatric intestinal transplant procedures.
Immunosuppression Therapy
The immunosuppression therapies used in intestinal transplant programs are summarized in Appendix 2. These include the following:
Immunosuppression drugs
Cyclosporin was used as the main immunosuppression agent in early recipients but was replaced by tacrolimus in 199015. The use of tacrolimus as the primary immunosuppression agent is considered one of the most significant advances in the management of small bowel transplant recipients in the past decade.4
Most centres manage patients with a triple regimen for baseline immunosuppression including tacrolimus (a calcineurin inhibitor), variable-dose corticosteroid, and an adjunctive antiproliferative agent (either azathioprine or mycophenolate mofetil [MMF].12;18. The Intestinal Transplant Registry showed that of the 335 surviving transplant recipients as of May 2001, 97% were receiving tacrolimus, and 92% receiving prednisone. Nephrotoxicity was reported in 7 of 13 children in the Ontario program. The condition improved with decreasing doses of tacrolimus.21
The adjunctive induction agent(s) used varied among the programs and may include anti-thymocyte globulin, basiliximab15;16, cyclophosphamide and anti-CD3 monoclonal antibodies (OKT3)12. Interleukin-2 receptor antagonists such as Dalizumab (Zenapax) have been used by an increasing number of programs, including the programs at Pittsburgh, UCLA and Miami.15;17;28
Another adjunctive agent being studied is sirolimus (rapamycin)12. Florman et al29 compared 21 intestinal transplant recipients who received daclizumab in addition to tacrolimus and steroid with 16 transplants who received sirolimus in addition to the primary immunosuppressive regimen. The results showed that patients on sirolimus had fewer early rejections compared with those not receiving sirolimus (17% vs 68% in the first 30 days) and had less severe rejection episodes. The difference in mortality or morbidity between the two groups was not statistically significant.29
Bone Marrow Infusion
Abu-Elmagd et al12 reported that in addition to immunosuppression drugs, bone marrow cells recovered from the thoracolumbar vertebrae of the intestinal donors were infused intravenously into the organ recipients at the time of surgery in all but 60 recipients who were considered prospective contemporaneous controls.
Low Dose Ex Vivo Graft irradiation
Recent experimental data suggested that low-dose graft irradiation when combined with donor specific bone marrow transfusions may have a beneficial effect on graft outcome. In an attempt to prevent Graft Versus Host Disease, ex vivo intestinal allograft irradiation was also tested at the Pittsburgh program. The intestine of 11 allografts for 10 primary recipients was irradiated with a single dose of 750 cGy prior to the transplant.12
Monitoring of Rejection
Most programs monitor and diagnose rejection in the intestinal allograft by twice weekly serial surveillance endoscopy and endoscopy guided multiple mucosal biopsies. General endoscopic signs of rejection include mucosal edema, erthema, friability and focal ulceration. Signs of severe rejection are a granular mucosal pattern with diffuse ulceration, mucosal sloughing and absence of peristalsis. Chronic rejection is diagnosed on the basis of histologic examination of full-thickness enteric specimens.12 The criteria for diagnoses of acute and chronic rejection have been standardized by Demetris AJ et al [http://tpis.upmc.edu].
Patient Survival and Graft Survival after Small Bowel Transplant
International Outcome
The Intestinal Transplant Registry provided an analysis of the pooled international data. The Registry provided the following survival data for international intestinal transplants:30
Table 3: International One-year and Three-year Survival Rates and Graft Survival Rates.
| 1 year KM survival rates ISB/SB-L/MV |
3 year KM survival rates (2001) ISB/SB-L/MV |
|
|---|---|---|
| Patient survival | 69%/66%/63% (1997) Overall 1 year = 63% (2001) (for transplants after Feb 1995) |
Overall 3 year = 51% (for transplants after Feb 1995) |
| Graft survival | 55%/63%/63% (1997) Overall 1 year = 58% (2001) For transplants after Feb 1995 |
Overall 3 year = 45% (2001) (For transplants after Feb 1995) 5 year >45%/43%/30% (2001) |
As of May 31, 2001, 335 of the 651 patients that have received some form of small bowel transplant are still alive.
A presentation at the 9th Keio University International Symposium on transplantation reported that the data from the registry showed a one-year patient survival of 63% with a graft survival rate of 58% for transplants performed after February 1995. The 3-year patient survival rate is 51% with a graft survival rate of 45%.31
More experienced programs have reported higher patient and graft survival rates than the overall rates reported by the Intestinal Transplant Registry. The patient survival rates of the major programs are described below.
University of Pittsburgh
University of Pittsburgh has the largest intestinal transplant program.14 A total of 165 intestinal transplants were performed in 155 patients (54% pediatric) during the period of 1990 - 20017. These included 39% isolated small bowel transplants, 46% composite small bowel-liver transplants and 15% multivisceral transplants. Abu-Elmagd et al14 reported actuarial patient survival rates as follows:
Table 4: Patient Survival and Graft Survival Rates - University of Pittsburg.
| Actuarial survival rate | 1 year | 5 year | 10 year |
|---|---|---|---|
| 1990-2001 | |||
| KM Patient survival for entire cohort (155 patients) | 75% | 54% | 42% |
| KM Graft survival | ? | ? | |
| Patient survival since 1994 (93 patients) | 78% | 63% (p=0.03) | |
| Survival of patients treated with daclizumab (48) | 86% (p=0.3) | ||
| Survival of bone marrow augmented patients (38) | 79% | 74% | |
| 1990-1998 | |||
| Overall cumulative patient survival (109 patients) | 72% | 48% | |
| Overall cumulative graft survival (115 grafts) | 64% | 40% |
Analysis of patient survival by the type of transplant initially showed that isolated small bowel grafts restored alimentary function at the highest rate during all follow-up periods, and were associated with better patient survival than composite transplants.32 More recent analysis reported that combined small bowel-liver transplant showed the best survival prognosis beyond 5 years.12 At the time of the latest report, 53.6% of the patients are still alive. Patient and graft survival rates and mean ICU lengths of stay have improved since 1994. Complete discontinuation of TPN occurred at a mean of 20 days post-transplant after 1994 compared to a mean of 42 days before 1994. Because of the complexity of the cases and of the treatment strategies, the reasons for the improvements were considered indeterminate.
An earlier study showed that the actuarial 1-year, 3-year and 5-year survival of children who underwent intestinal transplantation at this centre were 72%, 55%, and 55% respectively. For comparison, the 1-year and 2-year survival of children who were on the transplant waiting list but did not undergo transplantation were 30% and 22% respectively.21
University of Miami
During the period of August 1994 to September 1999, the University of Miami program performed 77 transplants in 69 patients, 61% of whom were children. Pinna et al13 analyzed patient survival according to three different time periods: Period I (August 1994-June 1995), Period II (July 1995 - December 1997) and Period III (January 1998 - September 1999). Three different induction agents (cyclophosphamide, OKT3, MMF) were tested in Period I; mycophenolate mofetil was used as the induction agent Period II and daclizumab used in period III. Patient and graft survival rates for the three periods were reported as follows:
Table 5: Patient Survival and Graft Survival at University of Miami Hospital.
| Two-year survival rates | Period I mixed |
Period 2 MMF |
Period 3 daclizumab |
|
|---|---|---|---|---|
| ISB | Patient survival | 50% | 50% | 90% |
| Graft Survival | 0% | 50% | 80% | |
| SB-L | Patient survival | 40% | 33% | 58% |
| Graft survival | 40% | 30% | 48% | |
| MV | Patient survival | 30% | 30% | 70% |
| Graft survival | 27% | 27% | 60% | |
The results showed improved patient survival and graft survival using induction therapy with daclizumab with implementation of close rejection surveillance. Delayed feeding and inclusion of the pancreas in the combined liver and intestine allograft were thought to have played a role in this improvement.13
In a 2002 report, Kato14 compared the survival rates of 110 patients transplanted between 1994 and 2001 who received different induction agents including Campath-1H. The results are summarized below.
Table 6: Comparison of Patient Survival Rates at Miami University Hospital.
| Patient survival rates | 6 months | 1 year |
|---|---|---|
| Period I (6/94-12/97, cyclophosphamide, OK3, MMF) n=44 | 53% | 48% |
| Period II (6/98-01/00, daclizumab) n=53 | 79% | 66% |
| Period III (01/00-08/01, Campath-1H in adults) n=23 | 89% | ongoing |
Period III showed the highest overall 6-month rate and the overall one-year survival rate for Period II is significantly higher than that of Period I. The overall one-year survival rate is not yet available for Period III. Univariate analysis showed that transplantation in the most recent period (p=0.037) and the presence of concomitant liver failure (p=0.015) were both statistically significant predictors of outcome (multivariate analysis p=0.031 and 0.052).14
Kato14 concluded that the results of intestinal transplantation have continued to improve due to technical innovation and advances in postoperative management. Patients who were free of liver failure at the time of transplantation appeared to have a better outcome. No graft survival data were provided.
University of Nebraska
At the University of Nebraska, 117 intestinal transplants were performed in 106 adults and children in the period of 1990 - 2001.15 The majority (89%) of the transplants were performed in children. Of the 117 allografts, 37% were isolated small bowel transplants and 63% combined small bowel-liver transplants. The patient and graft survival rate for 97 primary recipients treated with tacrolimus were:
Table 7: Patient and Graft Survival at University of Nebraska.
| Overall | ISB | SB-L | |
|---|---|---|---|
| 2 year patient survival rate | 70% | 82% | 60% |
| 2 year graft survival rate | 67% |
For 5 patients transplanted under cyclosporine immunity, one patient survived at 9.5 years. Of the 16 re-transplant patients, 6 (38%) survived. The longest survival with a functioning isolated SBT graft is 7 years and the longest survival with a SB-L graft is 10 years. The main cause of death in both groups was sepsis. The cause for graft loss for the ISB group included severe rejection, ischemic necrosis, chronic rejection, arterial thrombosis and portal vein thrombosis.15
Langnas et al15 concluded that the short-term survival for recipients of both isolated small bowel transplant and combined small bowel-liver transplant is satisfactory but the long-term results are still unknown.
Iyer (Nebraska) reported that 9 of 46 children that received ISB or SB-L transplant died, yielding a survival rate of 80%.33
Mount Sinai Medical Centre, New York
The Mount Sinai Medical Centre in New York has performed 37 grafts in 34 patients, 68% of whom are children.16 The majority of the transplants were isolated small bowel (43%) or combined small bowel-liver (51.5%) transplants with only 5.5% multivisceral transplants. Eight patients died of rejection (2), adenovirus infection (2), fungal sepsis (1), PTLD (1) and surgical complication (2). The patient and graft survival rates were:
Table 8: Patient and Graft Survival Rates at Mount Sinai Medical Centre, New York.
| Actuarial 1 year survival rate | Overall | ISB | Muli-organ |
|---|---|---|---|
| Patient survival | 74% | 87% | 63% |
| Graft survival | 64% | 64% | 58% |
There was no difference in patient and graft survival between adults and children.16
University of Los Angeles
Between 1991 and July 2001, 23 transplants were performed in 19 patients. The majority of patients received combined small bowel-liver transplant as most of these patients had advanced liver disease resulting from TPN. Sepsis accounted for 63% of all patient deaths. Patient and graft survival after intestinal transplant were:
Table 9: Patient and Graft Survival at the University of Los Angeles.
| Survival rates | 1 year | 3 year |
|---|---|---|
| Overall patient survival rate | 67% | 60% |
| Overall graft survival rate | 56% | 45% |
| Overall patient survival rate for 16 patients transplanted since 1995 (Difference insignificant) | 73% | 64% |
| Overall graft survival rate since 1995 | 57% | 50% |
Isolated small bowel transplant showed better patient survival rates than small bowel-liver transplant or multivisceral transplant.17
Hopital Necker-Enfants Malades, Paris
Hopital Necker-Enfants Malades reported a series with 37 intestinal transplants in 36 children with a median age of 5 years (range 2.5 - 15 years). The transplants included 13 isolated small bowel allografts and 24 combined small bowel-liver allografts. Goulet18 reported the following survival rates:
Table 10: Actuarial Patient and Graft Survival Rates at Hopital Necker-Enfants Malades.
| Actuarial Survival Rates | 6 months | 1 year | 3 year | |
|---|---|---|---|---|
| ISB - | Patient survival rate | 77% | 77% | 77% |
| Graft survival rate | 61.5% | 46% | 31% | |
| SB-L | Patient survival rate | 74% | 74% | 74% |
| Graft survival rate | 71% | 71% | 71% | |
Birmingham, UK
The Birmingham Children’s Hospital reported a series of intestinal transplants in 21 patients with a mean age of 30 months (range 6-127 months). These procedures included 5 isolated small bowel, 14 combined small bowel-liver, 2 multivisceral transplants). Four patients died of acute respiratory distress and multiorgan failure within 6 weeks of transplant in intensive care and 17 patients were followed-up for a mean of 27 months (range 3-56 months). Excluding patients with less than 12 months follow-up, the reported survival rates were:
Table 11: Patient and Graft Survival Rates at Birmingham Children’s Hospital.
| Survival rates | 1 year | 2 year |
|---|---|---|
| Overall patient survival rate | 61% | 50% |
| Graft survival: 81% survived to be weaned from parenteral nutrition. |
The main cause of late mortality was Epstein-Barr virus infection and the onset of post-transplant lymphoproliferative disease.19
Ontario Programs
Fecteau et al21 analyzed the outcomes of 13 pediatric patients who underwent intestinal transplant in Ontario (11 at the Children’s Hospital of Western Ontario, LHSC, and 2 at Hospital for Sick Children, Toronto). Of this cohort, 6 patients received small bowel transplant alone, 4 patients a combined small bowel-liver transplant, and 3 patients a multivisceral transplant (1 including a kidney). The average age at the time of transplant was 4.9 years (median 3.8 years, range 5 months - 12 years).
Table 12: Patient and Graft Survival Rates in Ontario.
| Actual 1-year survival rates | Overall | ISB | SB-L | MV |
|---|---|---|---|---|
| 1 year patient survival rate | 61% | 83% | 50% | 33% |
| (5/6) | (2/4) | (1/3) | ||
| 1 year graft survival rate | 53% | - | - | - |
Two patients with functioning graft died of cardiac arrest, one presumably caused by tacrolimus cardiac toxicity, and the other of liver necrosis postpercuatneous liver biopsy. Fifty-eight percent of patients underwent reoperations for removal of graft, re-exploration, bleeding, liver necrosis and bowel perforation, rerouting of gastroenterostomy, gastrotomy tube insertion and renal stent insertion.16
Summary
Table 13: Patient survival rates of the intestinal transplant programs are summarized below.
| Overall Patient survival rates | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Registry Actuarial |
Pittsburgh Actuarial |
Nebraska | Miami | New York Actuarial |
UCLA | Paris Actuarial |
UK | Ontario | |
| 1 year | 63-69% | 72% | 74% | 67% | 74-77% | 61% | 61% | ||
| 2 year | 70% | 30-90% | 50% | ||||||
| 3 year | 60% | 74-77% | |||||||
| 5 year | 48% | ||||||||
A detailed summary of patient survival rates is provided in Appendix 5.
The international 1-year actuarial patient survival rates ranged from 63% to 69% depending on the type of intestinal transplant. Higher survival rates have been reported for transplants performed since 1994 and for those performed at larger programs such as the Pittsburgh program (Overall, 72% at 1year & 48% at 5 years). Both the Pittsburgh and Miami program reported better survival for patients who received daclizumab as an induction agent12;13. The Pittsburgh program reported a 1-year survival rate of 86% for patients who received daclizumab. Isolated small bowel transplant showed the highest patient survival rates with 1-year rate reaching 87%16 and 2-year rate reaching 82%.15
Graft Survival
Table 14: The graft survival rates of the intestinal transplant programs are summarized below.
| Overall Graft survival rates | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Registry Actuarial |
Pittsburgh Actuarial |
Nebraska | Miami | New York Actuarial |
UCLA Death-censored |
Paris Actuarial |
UK | Ontario | |
| 1 year | 55-63% | 64% | 64% | 56% | 46-71% | ? | 53% | ||
| 2 year | 67% ISB | 0-80% | |||||||
| 3 year | 45% | ||||||||
| 5 year | 30-45% | 40% | 31-71% | ||||||
Detailed summary on graft survival is provided in Appendix 6.
In 1997, the Intestinal Transplant Registry reported that the 1-year actuarial graft survival rates for isolated small bowel, small bowel -liver and multivisceral transplants were 55%, 63% and 63%.30
The 2001 report showed that the actuarial graft survival rates of the total international experience at 5 years post-transplantation were:
45% for isolated small intestine transplants;
43% for combined intestine-liver transplants; and
Nearly 30% for multivisceral transplantation.
This report also indicated that for patients who have survived six months or more after transplant, approximately 70% have full functioning grafts.
A total of 121 (17.4%) grafts were removed. The causes of graft removal were rejection (57%), thrombosis/ischemia/bleeding (20.7%), Sepsis (6.6%), lymphoma (1.6%) and other causes (14%) [May 2001, Intestinal Transplant Registry].
Statistical analysis showed that for transplants performed before 1995, graft survival was significantly better for programs that had performed at least ten transplants compared to programs that had performed less than 10 transplants. It was reported that this no longer holds true for transplants performed after 1995 [D. Grant, e-mail communication]. Analysis also showed that graft survival was significantly better for transplants after 1995.
In 2002, the largest program of 155 patients reported improved graft survival since 1994. The overall 1 year and 5 year actuarial graft survival rates were 64% and 40% respectively. The highest 1 year actuarial graft survival rate of 82% was reported for 48 patients who received daclizumab as an adjunctive induction agent and 68% among patients who received bone marrow infusion. Overall, Forty-nine percent (49%) of the recipients had a functional graft after a mean follow-up of 43+/-40 months with unrestricted oral diet. The longest survival functional grafts were a combined small-liver graft (129 months) and a multivisceral graft (114 months).12
The pediatric program in France (36 patients) reported actuarial 1 year and 3 year patient survival rate of 46% and 31% respectively for isolated small bowel transplant and 71% for small bowel-liver transplants.18
Fecteau21 reported a 1-year survival rate of 53% for 13 Ontario children.
Improvements in graft survival rates have been observed over the years. The program in Miami with 111 patients reported that the 2-year graft survival rates for isolated small bowel transplant, small bowel-liver and multivisceral transplants were 0%, 40% and 27% for the earliest period compared to 80%, 48% and 60% for the most recent period.13
Graft Function
It was reported that following small bowel transplant, patients were initially managed using parenteral nutrition with enteral support initiated early after transplant. Enteral nutrition was initiated with return of allograft function through a surgically placed tube in the allograft jejunum using elemental formulas. TPN was discontinued once all caloric needs were met using the enteral route.17;34
About 71% to 100% of small bowel transplant recipients were able to wean from parenteral nutrition at 20-39 days after the transplant procedure.24;35 Most patients were able to meet all caloric needs enterally, although a small percentage of patients reported requiring supplementation of intravenous fluid and calories.
Growth and Development
Sudan et al24 reported that growth appeared normal in 50% of 27 children with greater than 1 year graft survival. An additional 11% maintained pre-transplant growth at <10th percentile and 15% demonstrated catch-up growth. Sixty-three percent (63%) of children required special education services, speech therapy or physical therapy. Eighty-four percent (84%) of children have been able to return to preschool, day care, or school at the appropriate level for their development and many report participation in extracurricular activities including sports, dance, and scouts. Seventy-five percent (75%) of adult returned to work or school and all reported participation in leisure activities27.
Goulet18 reported in a series of 36 children that all transplant recipients who were weaned from parenteral nutrition gained weight and recovered normal growth velocity.18
In contrast, Iyer et al33 reported that despite meeting caloric needs enterally, the children in his study showed significant inhibition of linear growth with no evidence of catch-up growth within a 2-year period. Consistent with Iyer’s finding, Nucci36 studied 24 children after small bowel transplant and found a positive trend in Z scores for weight and height only in 39% and 22% of the patients respectively, demonstrating a decrease in growth velocity over time.
Adverse Events
Infections and Re-operations
Despite the use of prophylactic anti-microbial agents and alprostadil, infection with uncontrolled sepsis is still the leading cause of morbidity and mortality following intestinal transplant.15 Intestinal transplant recipients are predisposed to severe bacterial, viral and fungal infections because of the high levels of immunosuppression, presence of pre-existing infection, and the potential for bacterial translocation from the graft during periods of stress such as ischemia, reperfusion and rejection. The Intestinal Transplant Registry identified sepsis as the leading cause of post-intestinal transplant deaths worldwide (51.3%). This is five times as high as the mortality rate due to rejection, the second most common cause of death.
Bacterial & fungal infections are the most common infection and an incidence of 93% was reported in a series of 106 patients.15 Farmer et al reported in a series of 19 patients an infection rate of 2.6 episodes per patient with 66% being bacterial and 16% fungal.17 The most common organisms identified were pseudomonas and candida. Fecteau21 reported 15% bacterial infection and 7% fungal infection among 13 children in Ontario. Fungal infection was also reported at 25% in another series.15
Translocation of microorganisms from the gastrointestinal tract has been demonstrated in human studies and has been suggested to be the mechanism responsible for the high rate of infection occurring after small bowel transplantation. Cicalese et al37 studied the correlation of bacteria translocation and various factors in 50 pediatric small bowel transplant recipients with a mean follow-up of 30+/-10 months (range: 20 days - 6 years). Blood, stool, liver biopsies and peritoneal fluid were collected and cultured using standard microbiologic techniques as part of follow-up or when infection was clinically suspected. A bacteria translocation episode is determined when microorganisms are found simultaneously in blood or liver biopsy and feces. Approximately 4,000 cultures were evaluated in the study. The results showed that acute rejection was not accompanied by an increased bacteria translocation rate. The inclusion of colon in the allograft significantly increased fecal bacterial count (100% cultures vs 84.6% cultures >1x106 CFU) and bacterial translocation rate (75% of patients vs 33% of patients) compared to transplants without the colon. Bacteria translocation was observed in a significantly higher number when cold ischemia time is longer than 9 hours compared to cold ischemia time of less than 9 hours (76% vs 20.8%, p = 0.002).
Viral Infections:
Cytomegalovirus [CMV] is the most common viral infections in intestinal transplant recipients.
Langnas reported that 15% of 106 recipients developed CMV infection involving the small bowel, lung and blood that did not result in any graft loss.15 In a pediatric series of 36 patients, CMV was diagnosed in 17% of the patients and all resolved18. Fishbein described that 11% of 36 intestinal transplant recipients had symptomatic CMV infection.16 Beath reported CMV in 19% (4 patients) with intestinal perforation and one death.19 Tissue invasive CMV infection was observed in 2 of 19 patients in a small series.17 The 1997 report of the Intestinal Transplant Registry showed that CMV infection rates for ISB, SB-L and MV transplants were 24%, 18% and 40% respectively.
Ebstein-Barr Virus Infection [EBV]
Ebstein-Barr viral (EBV) infection is one of the most serious consequences of immunosuppressive management in transplantation. EBV often leads to ß-cell post-transplant lymphoproliferative disease [PTLD], a quasi-neoplastic and potentially life-threatening disorder.
Diverse incidences of EBV infections have been reported. Goulet reported that 11% of a pediatric series developed EBV infection.18 Beath reported that EBV viraemia was diagnosed in 48% in the UK pediatric series.19
Adenovirus has been reported in 41% of a large series that resulted in two deaths15 and in 30% of all children in a smaller series, all of which happened within a 10-month period16. Both adenoviral and cryptosporidium infections generally have benign outcomes.
Strategies for Controlling CMV and EBV Infections
Gancyclovir has been used as a prophylactic anti-viral agent programs.15;16 Farmer et al described the intravenous administration of gancyclovir for 100 days following intestinal transplant. A dose of 5 mg/kg was administered twice per day for 14 days, followed by 6 mg/kg IV daily. Thereafter, patients were converted to oral acelovir at a dose of 40 mg/kg per day until 2 years after transplant.38 As an adjunct to ganciclovir, CMV-specific hyperimmune globulin has also been added to the early postoperative antiviral prophylaxis for high-risk patients.12
Over the last 5 years, surveillance for CMV & EBV has improved with the development of more sensitive polymerase chain reaction [PCR] assays of peripheral blood to detect virus-specific DNA, allowing for preemptive treatment prior to the development of the infection. The PP65 antigenemia test has been used for early detection of CMV reactivation or de novo and has improved the preemptive treatment of this virus12. The surveillance protocol used by Farmer et al included PCR assay weekly during hospitalization, monthly during the first outpatient year, and every 4 months thereafter. Positive PCR prompted preemptive therapy with gancyclovir. Persistent viremia or intolerance to therapy was treated with CMV IV immunoglobulin. Patients also underwent periodic physical examination, as well as endoscopy and biopsy. The presence of unexplained fever or suspicious findings prompted an aggressive evaluation that included viral culture, radiographic imaging and biopsy.38
Farmer et al reported that prophylaxis prevented viral disease throughout the duration of therapy. More than 50% of patients did not have evidence of CMV and EBV viremia or disease after intestinal transplant. They further reported that frequent monitoring with virus-specific PCR demonstrated viremia in 46% of IT recipients. However, CMV and EBV diseases occurred in only 15.4% and 7.7% of recipients respectively. Farmer et al concluded that excellent control of CMV and EBV disease can be obtained using a protocol of long-term prophylaxis, monitoring and preemptive therapy.38
In addition, more effective antiviral agents against CMV are now available. Attempts are being made to avoid mismatching the serologic status of recipients and donor, although some programs are less restrictive with respect to CMV serologic matching between donor and recipient.
Graft Rejection
Rejection remains the most formidable barrier to successful intestinal transplant because it occurs frequently, precipitates opportunistic infection and contributes to graft loss. The Intestinal Transplant Registry reported in 2001 that rejection was the second leading cause of death (10.4%) after intestinal transplant and that 57% of the 121 grafts removed were due to rejection. Graft loss due to rejection occurred more frequently among pediatric recipients (65.3%) than among adults (44.9%). In 1997, the Registry reported that 56% of all patients had at least one or two rejection episodes, particularly in the first year after transplant.30 Graft rejection rates of the programs are summarized in Table
Table 14: Graft Rejection Rates.
| Program | Rejection Rates | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abu-Elmagd 200212 (Pittsburgh) | Before daclizumab 85%; current 67% (first 30 days post-op) Chronic rejection in 11% of grafts Refractory rejection - primary cause of failure in 19% of 165 grafts Cumulative risk of rejection significantly greater in ISB |
||||||||||||||||||||||||||||||
| Pinna 2000 (Miami) | Rejection rate:
|
||||||||||||||||||||||||||||||
| Langnas 2002 (Nebraska)15 | ISB: 77% of 36 had at least 1 rejection episode at a median time of 12 days SB-L: 60% of 61 at a median time of 16 days; overall 65/97 (67%) Antilymphocyte therapy required in 17/60 SB-L 7/36 (19.4%) ISB Explantation needed in 7/36 ISB -Baxilixmab induction in 24 pts resulted in significant reduction in mean number of rejection episodes. |
||||||||||||||||||||||||||||||
| Fishbein 2002 (Mt Sinai)16 | 51% of cases within 30 days of transplant Recurrent rejection developed in 16% of the cases Antilymphocyte therapy required in 27% of patients. 3 grafts lost due to exfoliative rejection. |
||||||||||||||||||||||||||||||
| Farmer 200217 (UCLA) | Acute rejection in 83% of grafts with >30 day follow-up 1.4 episode per graft 88% of the rejection episodes responded to steroids or OK3 12% required enterectomy |
||||||||||||||||||||||||||||||
| Goulet 200218 (Paris) Peds. | 42% of patients 75% successfully treated by increasing tacrolimus dosages 3 pts (8%) received antilymphoglobulins |
||||||||||||||||||||||||||||||
| Beath 200219;20 (UK) Ped | Mild to moderate in 71% of patients. Late mild rejection in 29% of pts | ||||||||||||||||||||||||||||||
| Fecteau 2001 (ON) Ped | 66% experienced rejection 3 had mucosal denudation of the graft; 1 responded to antithymocyte globulin, 2 died. All other rejections responded to steroid therapy. |
||||||||||||||||||||||||||||||
| International Registry 2001 | A total of 57% of grafts removal was due to rejection (65.3% among pediatrics and 44.9% among adults) |
The five major programs reported acute rejection rates of 51% - 83% with a median of about 67%.12;12;14-16;34 Pediatric programs reported rejection rates of 42% to 71%.18;19 The rejection rate among the 13 children in the Ontario program was 66%16.
Risk factors identified for acute rejection were use of small bowel grafts alone when compared with composite grafts, and positive lymphocytotoxic cross-match. The incidence of rejection in the liver of composite grafts is less than half the incidence in the intestine.39
Most of the rejections were mild to moderate and occurred within 30 days post-transplant. However Beath reported late mild rejection in 29% of patients.19;20 The Pittsburgh programs reported that its rejection rate improved from 85% to 67% after the use of daclizumab and that the cumulative risk of rejection is significantly greater in ISB12. The Nebraska program reported that baxilixmab induction in 24 patients resulted in significant reduction in the mean number of rejection episodes.15
The ileum is the most commonly involved site. However, graft involvement can be patchy and involvement of proximal small bowel without affecting the ileum occurred in 20-25% of rejection episodes.40
Acute rejection was usually treated with a steroid bolus and increasing the daily tacrolimus dose to achieve higher trough levels. OKT3 or thymoglobulin was reserved for the treatment of steroid-resistant and severe rejections. They were more often needed for intestine-only grafts.41 The majority of the rejection episodes (70-88%) responded to steroid and OKT3. Antilymphocyte therapy was needed in as many as 27% of the patients in one series.16 About 12% - 25% of rejections were reported to be refractory to these treatments, resulting in enterectomy (12-20%) or death (33% of all deaths after small bowel transplant internationally).11
Chronic rejection has been well documented and contributes to graft loss, especially in isolated small bowel grafts. Chronic rejection occurs as a consequence of persistent episodes of refractory acute rejections and manifests clinically with persistent abdominal pain and intestinal dysmotility. Abu-Elmagd et al reported a chronic rejection rate of 11% among 155 patients, the largest cohort of intestinal transplant recipients.12
Cumulated risk of graft loss from acute and chronic rejection of isolated small bowel allografts was significantly greater than that of small bowel-liver and multivisceral transplants (p=0.00001) and the risk is not significantly reduced by the use of daclizumab.12
Over immunosuppression increases the risk of post-transplant lymphoproliferative disease and death caused by severe infections. The delicate balance required to manage rejection with augmented immunosuppression while controlling infection with decreased immunosuppression is critical for patient and graft survival.
Post-transplant Lymphoproliferative Disease [PTLD]
Post-transplant lymphoproliferative disease [PTLD] is a significant cause of morbidity and mortality in intestinal transplantation, with an overall incidence that is higher than that observed in other types of solid organ transplantation. PTLD comprises a range of disorders, from nonspecific viral illness or self-limiting mononucleosis and ultimately to lymphoma.
Nalesnik et al compared rates of PTLD among 73 pediatric and 54 adult intestinal transplant recipients in the Pittsburgh program.42 These included 48 isolated small bowel transplants and 79 composite small bowel transplants including liver. Overall, 21% of the patients developed PTLD. The rate of PTLD is significantly higher in pediatric recipients (30%) compared to adult recipients (9%) (p=0.004). The only significant association between PTLD frequency and type of transplant appeared in adult patients with multivisceral versus isolated small bowel transplants (25% vs 3.4%, p=0.05). There was no significant difference in PTLD frequency on the basis of EBV seropositivity versus seronegativity at the time of transplant for either children or adults. The overall frequency of PTLD was significantly higher in the first half of the study than the second half (41% versus 16%, p=0.01). The reduction in PTLD frequency in the second half of the study coincided temporally with both the introduction of genomic EBV surveillance in peripheral blood and with the institution of supplemental donor bone marrow infusion to enhance microchimerism. Bowel involvement by PTLD was observed in 78% of the patients. The 2-year actuarial survival for PTLD patients was 37%35.
Rates of PTLD reported by other series included 29%20 and 11%16;18. The risk of PTLD was proportional to the intensity of immunosuppressive therapy. However, some studies showed that there was no significant difference in the incidence of PTLD based on EBV seronegativity. Conventional treatment of PTLD includes reduction of immunosuppressive therapy and treatment with ganciclovir. Non-responders and those patients developing significant rejection because of reduced immunosuppressive therapy receive chemotherapy.3
Surgical Complications and Reoperations
Goulet reported that surgical complications occurred in 85% of small bowel-liver transplants and 25% of isolated small bowel transplants.18
In the Nebraska program of 106 recipients, reoperations were required in 45% of the ISB transplants and 66% of the SB-L transplants. The most common reason for reoperation was intra-abdominal abscess.15
Graft-versus-host Disease
Graft versus host disease (GVHD) is a condition in which the donor’s immune cells in the transplanted organ make antibodies against the recipient’s tissues and attack vital organs. The conditions may be acute or chronic, mild or severe. Severe cases can often be life-threatening.
To detect graft-versus-host disease [GVHD], biopsy samples of recipient skin and native gastrointestinal tract were examined histopathologically and immunocytochemically. This allowed the identification of donor leukocytes with the in situ hybridization technique using Y-chromosome-specific probe or the immuno- histologic staining of donor-specific HLA antigens. Other GVHD target organs also were closely observed and biopsy samples were taken when indicated.12
The incidence of GVHD following intestinal transplant ranged from 0% to 14%.
Pittsburgh programs - GVHD was clinically observed in 8.4% of patients and was histologically documented in 4.5% of patients. It was fatal in 1 L-SB patient.12
Miami - GVHD had an overall incidence of 14% during the study period. Incidence of GVHD in the different groups was 11% in period 1, 20% in period 2, and 9% in period 3.13
Ontario - GVHD of the skin and rectum was observed in one (8%) of the patients who received an ABO compatible graft.6
No GVHD was mentioned in the following programs: Nebraska, Birmingham, New York, Paris & UCLA. This is thought to be the result of bidirectional migration of donor and recipient leukocytes, leading to the recipient becoming a genetic composite of cells of the donor and self.
Quality of Life [QOL]
Intestinal transplant can pose challenges to patients. For approximately 6-12 months post-transplantation, care routines for patients may include 7-15 daily medications, tube-feedings, IV fluids and maintenance of the gastrostomy tube, jejunostomy tube, ostomy and other catheters.
Sudan et al35 conducted a study of 31 intestinal transplant recipients (27 pediatric and 4 adults) with greater than 1 year graft survival. The patients included 13 isolated small bowel transplants and 18 combined small bowel-liver transplants. The patients were contacted by telephone or seen at an outpatient clinic and completed a questionnaire for evaluation of, among other things, long-term QOL. QOL was assessed on the basis of hospitalization/illness, presence of ostomy, and number of bowel movements per day. The results showed that the mean number of hospital admissions after initial hospital discharge was 2.3+/-1.1. Approximately 50% required admission in the year preceding this evaluation. This translates to one readmission every 1-2 years.35
The most common reasons for readmission were infection (43%), surgical procedures (14%) and acute rejection (14%). Twenty-eight of the 31 recipients (90%) were free of ileostomy and the median time to ostomy closure was 10 months (range 4 - 36 months). The average number of stools per day was reported to be three.35
In a follow-up study, Sudan et al43 conducted a QOL study of 33 pediatric small bowel transplant recipients with intact intestinal allografts and greater than 1 year follow-up after transplant. The recipients and their parents completed parent and child forms of the Child Health Questionnaire [CHQ]. The questionnaire assessed 14 domains including global health, physical functioning, limitations to role or social functions, bodily pain, behaviour, emotion, mental health, self-esteem and parental impact. Compared with the normative population sample, the response of the parents revealed markedly lower function in intestinal transplant recipients in six domains including physical function, role limitations due to physical problems, general health perception, emotional and time impact on parents, and negative effect on family activities. However, the pediatric recipients reported no statistically significant difference in any domain compared with the sample norm. There was an overall trend toward parental assessments being lower than that of the pediatric intestinal transplant recipients. In a comparison of pediatric intestinal transplant recipients to patients with end-stage renal disease, there was a general trend toward lower scores in many domains but only the “mental health” domain achieved significance. Parents of intestinal transplant recipients reported significant limitations in the physical and psychological well being of their children compared with normal school children. The intestinal transplant recipients themselves reported a quality of life similar to other school children. The researchers indicated that this discrepancy might be related to the young age of many of the children at the time of transplantation, and the relatively long period of time after transplantation of most of the recipients who responded.
Rovera et al44 compared quality of life in a group of patients receiving home parenteral nutrition [HPN] to a group that had undergone intestinal transplantation. A significant improvement in quality of life was noted in the transplantation group compared to their pre-transplantation status when ill and receiving HPN. Of greater importance, when comparing transplantation patients with a group of patients who were stable and receiving HPN, the quality of life was similar between the two groups in spite of frequent complications in the early post-transplant period.44
Living Donor Segmental Transplantation
While most of the small bowel transplants involve whole small bowels obtained from cadaveric donors, a small number of centres have been experimenting with segmental small bowel grafts obtained from living, related donors. In living related segmental transplants, a segment of 180 to 200 cm of ileum is harvested preserving 15 cm of the terminal ileum in the donor. Early reports suggest that living related segmental transplants might reduce the risk of acute rejection and infection compared to cadaveric intestinal transplants. These improvements may have resulted from better tissue matching, reduction in cold ischemia time, better donor bowel preparation and optimization of recipient medical condition.45 Cicalese et al45 also estimated that the procedure may be cost-effective from the first year post-transplant. However, the number of such transplants was too small for definitive conclusions. Moreover, many questions remain concerning risks to donor safety, including surgical complications of bowel resection and potential long-term impairment of intestinal absorption. Other issues include optimum drainage system, choice of segment of the intestine for transplantation and timing and closure of the ostomy.
Comparison between Small Bowel Transplant and Home Parenteral Nutrition
The closest technology for comparison with small bowel transplant is home parenteral nutrition [HPN]. Comparison between HPN patients and recipients of small bowel transplantation is not straight forward because, to date, small bowel transplant has only been performed on patients with life threatening complications.46 There has been no study that directly compares the two technologies.
The literature showed that HPN is currently the treatment of choice for patients with irreversible intestinal failure and is associated with an excellent prognosis for patients with benign disease.
An extensive 2001 review by Buchman47 showed that survival rates for patients with benign gastrointestinal disorders were 91% at 1 year, 70% at three years, and 62% at five years. Rehospitalization and therapy-related complications accounted for 5% of deaths in the United States and 11% in France.
Similar results were found in a multicenter European survey of home parenteral nutrition outcome in 1993 and in 1997. This survey reported a 96% survival rate for patients with Crohn’s disease during their first year of home parenteral nutrition. In the United Kingdom, the 1-year survival rate of patients requiring HPN is 96% and 3-year survival is 85%.48
Scolapiao et al49 at the Mayo Clinic reported on a retrospective review of 225 patients treated with HPN between 1975 and 1995. The study showed that most deaths during treatment with HPN were the result of the primary disease, and that HPN-related deaths are uncommon. Survival of HPN-related patients is best predicted on the basis of primary disease and age at initiation of HPN (probability of 5-year survival: overall 60%, 92% for inflammatory bowel disease, 60% for ischemic bowel, 54% for radiation enteritis, 48% for motility disorder, and 38% for cancer43.
The above outcomes showed that HPN is associated with an excellent prognosis for patients with benign disease. Patient survival rates are superior to the survival rates of small bowel transplants even at experienced centres (Pittsburgh: actuarial 1year 75%, 5 year 54% and 10 year 42%).
Studies by Cameron45 and Sudan43 both showed that the quality of life following small bowel transplantation is at least equivalent to that of patients on HPN and seems better than their QOL before transplantation.
ECONOMIC ANALYSIS
Literature Review
Langnas 200115
The Nebraska series reported that the median cost of intestinal transplantation was $275,000 (approximately CDN$429,000) with a range of US$77,000 to US$1,800,000 (approximately CDN$120,100 - $2,808,000). No detail breakdowns were provided.
Abu-Elmagd 199950
The Pittsburgh series reported 1999 average costs of intestinal transplantation as follows:
| 1990-1994 | 1995-1999 | |
|---|---|---|
| Isolated small bowl transplant - | US$203,111 | US$132,285 |
| Combined liver-intestine transplant | US$252,453 | US$214,716 |
| Multivisceral transplant | US$284,452 | US$219,098 |
Based on Medicare data, the average yearly cost of TPN in 1992 was more than $150,000 per patient not including cost of frequent hospitalization, medical equipment and nursing care. The total dollars spent on TPN are increasing every year because of the yearly increase in TPN cost and the cumulative increase of home and hospital bound TPN population. Based on these data, Abu-Elmagd et al concluded that intestinal transplantation would become cost-effective by the second year after transplantation.
Summary of Findings
Effectiveness
All small bowel transplants were performed on individuals with intestinal failure who could no longer tolerate total parenteral nutrition or for whom total parenteral nutrition was no longer feasible
Most of the small bowel transplants have been performed using whole small bowel grafts obtained from cadaveric donors. A small number of centres are experimenting with living related segmental small bowel transplants.
As of May 2001, 651 patients had received 696 small bowel transplants worldwide.
International data showed that patient survival and graft survival have improved since 1995 due to more effective immunosuppression agents, particularly tacrolimus, better prevention and treatment of infection and improved surgical procedures.
The Intestinal Transplant Registry showed that the overall actuarial 1-year patient survival after small bowel transplant ranged from 63-69%. Large intestinal transplant programs have reported higher survival rates (e.g. Pittsburgh Program: 1- year overall actuarial patient survival rates of 61- 75% and 5- year rate of 54%. Nebraska program: 82% at 2 years). Small programs have reported 1-year survival rates as low as 30% for multivisceral transplant.
The international 1-year and 5 year actuarial graft survival rates were reported to be 55% - 63% and 30% - >45% respectively. Major transplant centres reported 1-year actuarial graft survival rate of 58% - 73% and 2-year rate of 67%. The longest surviving functioning graft is a composite small bowel-liver graft with a survival period of 10 years.
Survival at 1, 3 and 5 years on HPN is reported to be 91%, 70% and 62% respectively.
Regression analysis performed in 1997 on the data from the Intestinal Transplant showed that Centres that have performed at least 10 transplants have better patient and graft survival than Centre that have performed less than 10 transplants. Recent analysis of transplants performed from 1995 to May 2001 showed that this no longer holds true for transplants performed after 1995, and that good results can be achieved at any multiorgan transplant program with moderate patient volumes [Dr. D. Grant, e-mail communication].
Analysis of 13 pediatric small bowel transplants in Ontario showed an overall 1-year actual patient survival rate of 61% (ISB 83%, SB-L 50%, MV 33%) and an overall 1-year actual graft survival rate of 53%.
Overall, 71% to 100% of surviving small bowel transplant recipients were able to wean from parenteral nutrition at 20 to39 days after transplant. Most patients were able to meet all caloric needs enterally, with enteral or parenteral supplementation during periods of illness.
While weight gain and normal growth after small bowel transplant were reported by two pediatric series, two other series reported significant inhibition of linear growth and a decrease in growth velocity with no evidence of catch-up growth.
Infection with subsequent sepsis was the most common cause of mortality following small bowel transplant, accounting for 51.3% of all post-transplant deaths.
Bacterial, fungal and viral infections have been reported. The most common reported viral infections were cytomegalovirus (18% - 40% of patients) and Epstein-Barr virus infections. The latter often led to beta-cell post-transplant lymphoproliferative disease.
Recent reports demonstrated that improved control of CMV and EBV disease could be obtained using a protocol of long-term prophylaxis of gancyclovir, surveillance of peripheral blood for CMV/EBV specific DNA, and preemptive therapy with gancyclovir when signs of viral DNA activity were detected.
Graft rejection was the second leading cause of death (10.4%) and was responsible for 57% of graft removal. Acute rejection rates were reported to range from 51% to 83% in the major programs with a median of 67%. Pediatric programs reported rejection rates of 42% -71%. Risk factors for acute rejection were use of small bowel graft alone and positive lymphocytotoxic cross-match.
Most of the acute rejections were mild to moderate and occurred within 30 days post-transplant. About 70% to 88% responded to steroid and OKT3. Antilymphocyte therapy was needed in up to 27% if patients. About 12%-25% of rejection episodes were refractory to these treatments and resulted in enterectomy or death (10.4% of all deaths reported to Intestinal Transplant Registry).
Post-transplant lymphoproliferative disease was a significant cause of mortality (7%) and morbidity. The overall frequency of PTLD is 21%. Pediatric small bowel recipients and adults receiving composite visceral allografts are at higher risks for PTLD (31% and 25% respectively). The allograft bowel itself is involved in PTLD in a high percentage of cases.
The incidence of graft versus host disease ranged from 0% to 14%.
Reoperations were reported to be required in 45% - 66% of patients in a large series and the most common reason for reoperation was intra-abdominal abscess.
Children who underwent intestinal transplantation had significantly better survival rates than children on the transplant waiting list who did not undergo transplantation.
A study showed that pediatric transplant recipients reported a quality of life similar to other school children while their parents reported markedly lower function in intestinal transplant recipients in six domains including physical function, role limitation, and negative impact on family activities. Another study showed significant improvement in quality of life in the transplant group compared to their pre-transplantation status. The quality of life was reported to be similar to that of patients with intestinal failure who were stable on home enteral nutrition.
Between 1988 and 2002, 27 Canadians received intestinal transplantation; twenty-one (21) of these were performed in Ontario (0-3 transplant/year). One hospital in Western Ontario performed 76% of the intestinal transplants in the province.
Canadian Organ Replacement Registry informed MOHLTC that Ontario is the only province performing intestinal transplantation.
Economic Analysis Literature
The median cost of intestinal transplant in the US was reported to be approximately US$275,000 per case.
A US study concluded that based on the US cost of home parenteral nutrition, small bowel transplant could be cost-effective by the second year after the transplant.
Conclusion
Intestinal transplant is a high cost, high acuity, and low volume procedure. For patients who can no longer continue home enteral nutrition due to life-threatening complications associated with this therapy, small bowel transplant offers the only viable alternative. The majority of patients who survived small bowel transplant were able to discontinue total parenteral nutrition and receive the required nutrients enterally. Limited evidence suggests that the quality of life of these patients is equivalent to that of patients on home parenteral nutrition.
Although much improvement in patient outcomes has been observed since 1995 and better outcomes were reported at more experienced centres, small bowel transplant is still associated with significant patient mortality (overall 37-45% in the 1st year) and morbidity as a result of infection, rejection and post-transplant lymphoproliferative disease. The procedure is also associated with high rates (45%-66%) of re-operations after transplant. These outcomes are inferior to those reported for home enteral nutrition. For the above reasons, home enteral nutrition remains the treatment of choice for individuals with intestinal failure who can tolerate this treatment.
There has been no head-to-head comparison between small bowel transplant and home enteral nutrition because small bowel transplant has only been performed in patients with intestinal failure who could no longer continue total parenteral nutrition. There is no evidence to support small bowel transplantation in patients with intestinal failure who can be treated with parenteral nutrition.
Appendix
Appendix 1: Causes of Intestinal Failure
| Age Group | Causes of Intestinal Failure |
|---|---|
| Neonates and infants Children Adults |
Gastroschisis Intestinal atresia Intractable diarrhea of infancy Microvillus inclusion disease Necrotizing enterocolitis Ompahlocele Autoimmune enteritis Intestinal polyposis Idiopathic pseudo-obstruction Visceral myopathy or neuropathy Volvulus Total intestinal Hirschsprung disease Crohn’s disease Gut infarction secondary to thrombosis or embolism Neoplasms (especially desmoid tumors) Radiation injury Trauma resulting in an unreconstructable gastrointesinal tract. |
Ghanekar A, Grant D., 200151
Appendix 2: Immunosuppression therapy used by the intestinal transplant centres
Nebraska
In the Nebraska program, immunosuppression consisted of cyclosporine in the first five patients and tacrolimus for the remaining patients. Eleven patients received concomitant sirolimus therapy and 26 patients received induction therapy with basiliximab. [Langnas 2002]
Pittsburgh
All recipients were treated primarily with tacrolimus and steroids. Postaglandin E1 was infused intravenously during the first post-op week to all but the first eight recipients. A 4-week course of 2-3 mg/kg/day cyclophosphamide was added to the treatment of 24 recipients of primary allografts and replaced for the following 2-3 months by mycophenolate mofetil or azathioprine.
Daclizumab (a humanized IgG1 monoclonal antibody directed at the alpha subunit of the human interleukin -2 receptor) was used as a third agent for recipients of 48 primary allografts and 2 retransplants. In addition to adjunct daclizumab, rapamycin was used as a fourth drug for the first 3-6 months in the last 18 patients.
Rejection episodes were treated with a steroid and a 5-day dose taper, with adjustment of the daily tacrolimus dose to achieve higher trough levels. OKT3 or thymoglobulin was used throughout to treat steroid-resistant and severe rejection episodes.
Bone-marrow augmentation: donor bone marrow cells were infused intravenously into the recipients at the time of surgery (not available for 60 intestinal recipients who were considered to be prospective contemporaneous controls).
Ex Vivo Graft irradiation: A clinical trial of low-dose ex vivo intestinal allograft irradiation was initiated in adults. The intestine of 11 allografts (6 ISB, 1 SM-L, 4 MV) for 10 primary recipients was irradiated with a single dose of 750 cGy.
UCLA
Immunosuppressive regimens varied during the study period. The current regimen is tacrolimus-based and includes induction with an interleukin-2 receptor antagonist and maintenance with mycophenolate mofetil, steroids and daclizumab. Endoscopic graft surveillance was undertaken at predetermined intervals as well as when clinically indicated. Acute cellular rejection was diagnosed using standard histopathologic and clinical criteria and was treated with pulsed steroids as the first line of therapy. Mucromonab-CD3 was given for steroid-resistant episodes.
Acute rejection episodes were treated with either steroids or OKT3 (some required enterectomy).
Miami
Induction agents: Period I tested cyclophosphamide, OKT3 and MMF. Period II- Daclizumab was used exclusively. Period III- Campath-1H was used in adult patients and daclizumab for children.
Tacrolimus and corticosteroids were used as baseline immunosuppression in all cases. The CMV-positive grafts were used regardless of recipient serology in periods II and III while it was only used in emergent cases in period I.
Protocol graft surveillance examinations with a zoom endoscope was introduced in period II and III whereas endoscopies were only performed as needed during period I.14
Appendix 3: Indications for small bowel transplant in children*
Indications in Children.
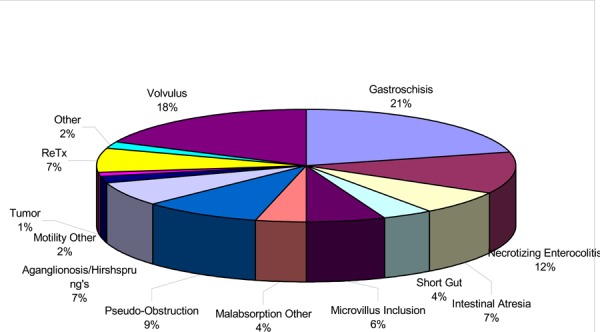
Reprinted from Intestinal Transplant Registry December 2001 data, with permission from Dr. David Grant, Director of the Intestinal Transplant Rgistry. Retrieved from http://www.intestinaltransplant.org/ITR_Reports/Report_2001/Report2001_slide0002.htm
Appendix 4: Indication for small bowel transplant in adults*
Indications in Adults.
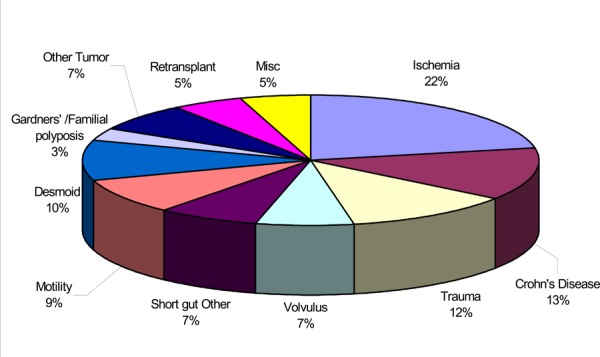
Reprinted from Intestinal Transplant Registry December 2001 data, with permission from Dr. David Grant, Director of the Intestinal Transplant Registry. Retrieved from http://www.intestinaltransplant.org/ITR_Reports/Report_2001/Report2001_slide0002.htm
Appendix 5: Summary of patient survival data rates
| Program | No. of Patients | Patient Survival | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | ISB | SB-L | MV | ||||||
| Pittsburgh Abu-Elmagd 200112 | 155 | Actuarial
|
|||||||
| Miami Kato 200214 | 110 |
|
|||||||
| Nebraska Langnas 200215 | 106 | 2 yr 70% | 2 yr 82% | 2 yr 60% | |||||
| New York Fishbein 200216 | 34 | Actuarial 1 yr 74% |
Actuarial 1 yr 87% |
Actuarial 1 yr 63% |
|||||
| UCLA Farmer 200217 | 19 |
|
|||||||
| Paris Goulet 200218 (Ped) |
36 | Actuarial 6 mos, 1 yr, 3 yr 77% |
Actuarial 6 mos, 1 yr, 3 yr 74% |
||||||
| UK (Ped)20 Beath 2002 |
21 | 1 yr 61% 2 yr 50% |
|||||||
| Ontario (Feateau 01) (Ped)21 | 13 | Actual 1 yr 61% |
Actual 1 yr 83% |
Actual 1 yr 50% |
Actual 1 yr 33% |
||||
| Intestinal Tx Registry Grant 1999 | 651 | 1 year 63% 3 year 51% As of May 2001 335/651 patients survived Survival rate 51.5% |
Mar 1995 - 1997 1 yr 69% |
Mar 1995 - 1997 1 yr 66% |
Mar 1995 - 1997 1 yr 63% |
||||
Appendix 6: Summary of graft survival rates following intestinal transplant
| Program | No. of Patients | Graft Survival | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | ISB | SB-L | MV | ||||||||||||||||||||
| Pittsburgh12 | 155 | 49% graft survival at a mean length of 43 months | Longest surviving L-SB 129 months |
Longest surviving MV graft 114 months | |||||||||||||||||||
| Miami14 | 110 | Graft survival @ 2 yrs
|
@ 2 yrs for Multiorgan
|
@ 2 yrs for MV
|
|||||||||||||||||||
| Nebraska15 | 106 | 2 year 67% longest surviving functional graft ISB 7 years |
Longest surviving functional graft 10 years | N/A | |||||||||||||||||||
| New York16 | 34 | Actuarial 1 year graft survival 64% | Actuarial 1 year 64% | Actuarial 1 year Multiorgan 58% |
|||||||||||||||||||
| UCLA17 (Death censored graft survival) |
19 |
|
|||||||||||||||||||||
| Paris18 (pediatric) |
36 | Actuarial 6 months 61.5%
|
Actuarial 6 months, 1 year & 3 year 71% |
N/A | |||||||||||||||||||
| UK (pediatric)20 | 21 | 81% survived to be weaned from parenteral nutrition | |||||||||||||||||||||
| Ontario (Feateau 01)21 (Ped) | 13 | 1 year actual survival 53% | |||||||||||||||||||||
| Intestinal Transplant Registry | 651 | 1 year 58% 3 year 45% Of those who survived more than 6 months after transplant, 70% - full gut function 15% - partial gut function 15% - has graft removed |
(transplants after 1995) 1 yr K-M survival 55% 5 yr K-M >45% |
(transplants after 1995) 1 yr K-M survival 63% 5 yr K-M 43% |
(transplants after 1995) 1 yr K-M survival 63% 5 yr K-M nearly 30% |
||||||||||||||||||
Appendix 7: Summary of Reports on Small Bowel Transplant
| Study & Type | Sample size Patient characteristics. | Type of Tx & Protocol | Patient Survival | Graft Survival/weaned from TPN/Hospital stay | Adverse events/complications |
|---|---|---|---|---|---|
|
Abu-Elmagd et al /Clinical Intestinal Transplantation: A decade of experience at a single centre, /Annals of Surgery 2001; 234(3) 404 (U of Pittsburgh Med Centre) Study period = 1990 - Feb. 2001 (1 yr interruption Case series |
N = 155 primary recipients (71 adults mean age 38+/-11 years; 84 children mean age 4.9+/-4.8 years) -165 allografts Indications: Short gut syndrome - 82% Dysmotility syndrome - 9% Intestinal neoplasm - 6% Enterocyte failure 3% |
Adults: 54% ISB 46% L/SB or MV Tx Children: 27% ISB, 73% L-SB or MV Tx Overall: 39% ISB, 46% L-SB, 15% Immunosuppresant: tacrolimus & steroids, IV Prostaglandin E1, Adjunct Cyclophosphamide or daclizumab (IgG1 antibody) and rapamycin. -Donor bone marrow augmentation in 39 pts -11 grafts irradiated -Most common reason for the composite allografts was liver failure induced by TPN. |
Overall actuarial Survival rate: 1 yr - 75% 5 yrs - 54% 10 yrs - 42% Treated with daclizumab, 1 yr actuarial survial:86% (P=0.3) Graft survival 82% (P=0.2) L-SB Tx best survival prognosis beyond 5 yrs. -83 (53.6%) still alive Total deaths = 72 (47%) Cause: Infection: 18% Rejection: 5% PTLD: 6% Technical: 6% Others: 12% |
49% (76)of recipients had functional graft after a mean follow-up of 43+/-40 moths, unrestricted oral diet. 7 (8%) of the surviving recipients returned to TPN Longest surviving functional graft: 1 L-SB Tx 129 months 1 MV Tx: 114 months Pt. & graft survival rates improved since 1994, reasons indeterminate. Mean ICU stay: before 1994: 29+/-44 days, after 1994: 16+/-23 days Due to improved speed of recovery & allograft absorptive functions. 1995-2001 complete D/C from TPN after a median of 20 days (42 days median before 1995) |
Rejection: First 30 post-op days: 67% - 85%(before daclizumab) Refractory rejection -primary cause of failure of 19% of the 165 grafts (ISB 37%, L-SB 8% and MV 8%) -Cumulated risk of loss from acute and chronic rejection of ISB allografts significantly greater than that of composite L-SB & MV Txs (P=0.00001) and risk not significantly reduced by use of daclizumab. -chronic rejection in 11% of grafts. Graft versus Host Disease Clinically observed in 8.4% histologically documented in 4.5%. Fatal in 1 L-SB recipient with preexisting IGA deficiency. PTLD: in 19% pts (before 1994 33%, after 1994 15%), fatality before 1994: 44%, after 1994 8%) 1 yr cumulative risk with adjunct donor bone marrow: 7%, control 20%. |
ISB- Isolated small bowel Tx; SB-L - Small bowel-Liver Tx; MV- Multivisceral transplant.
| Study & Type | Sample size Patient characteristics. | Type of Tx & Protocol | Patient Survival | Graft Survival/weaned from TPN/Hospital stay | Adverse events/complications |
|---|---|---|---|---|---|
| Nucci A et al. Long-term nutritional outcome after pediatric intestinal transplantation. J Ped Surg 2002;37:460-63. Children’s Hospital of Pittsburgh, U of Pittsburgh Med Centre Registry |
N=24 pediatric pts 82% male median age 3.2 yrs (8.5 months -17.4 yrs) 75% diagnosed as surgical short bowel syndrome. All dependent on TPN @ time of transplant 44% receiving some form of enteral nutrition before Tx. |
ISB = 8 L-SB = 13 MV = 3 |
Cumulative survival rate 1-yr = 91.3% 2 yrs = 86.2% Cumulative survival rage for those weaned from TPN 1-yr = 100% 1-yr = 94.1% |
87% weaned from TPN to an AA or peptide-based enteral formula or solid food within 3 months. 1 or more food allergies developed in 4% of pts (milk allergy common to all, egg allergy in 50%) Anthropometric & lab data Positive trend in Z-scores for weight & height observed in only 39% & 22% of pts respectively. Comparison of baseline & 1 yr height/length showed statistical significance (p=0.01) indicating a decrease in growth velocity over time. -Positive correlation observed between albumin level & linear growth velocity. |
Mild to moderate eosinophilic gastroenteritis was diagnosed in 48% of patients as early as 1 month post-Tx. Rejection & infection 1 pt had evidence of mild acute rejection and 1 had concurrent EBV infection. Remaining pts showed no evidence of rejection or infection. |
| Kato T et al Intestinal Transplantation at the University of Miami. Transpl Proc. 2002;34:868. June 1994 - August 2001 Retrospective analysis University of Miami School of Medicine |
N = 111 patients (120 allografts) Divided into 3 periods: Period I June 1994 - Dec 1997 = 44 pts Period II June 1998 - Jan 2000 = 53 pts Period III Jan 2001 - August 2001 = 23 pts 47 (39%) adults and 61% children Indications for Tx in Children Gastroschisis 24% Necrotizing enterocolitis 24% Volvulus 14% Intestinal atresia 13% Hirschsprung’s disease 6% |
ISB = 38 grafts L-SB = 33 grafts MV = 49 grafts Technical modifications Systemic drainage of bowel graft (16), inclusion of pancreaticoduodenal complex in L-SB tx, reduced size graft, separate partial liver & partial intestinal grafts (4) MV tx without liver (6) For period II & III Daclizumab was used exclusively in period II and Campath-1H used in adults in period III. Tacrolimus and corticosteriods were used as baseline immunosuppression in all cases. CMV positive grafts were used regardless of recipient serology in period II & III |
Overall 6 months survival Period I = 53% Period II = 79% Period III = 89% Overall 1 year survival Period I = 48% Period II = 66% Period III = ongoing Univariate analysis and multivariate analyses showed that patient age, graft types, period and presence of concomitant liver failure were not significant predictors of outcome. Transplantation in recent period (p=0.037) and the presence of concomitant liver failure (p=0.015) were both statistically significant. (multivariate analysis p =0.031 &0.052) |
Conclusion: The results of intestinal transplantation have continued to improve due to technical innovations and advances in postoperative management. Patients who were free of liver failure at the time of transplantation appeared to have a better outcome. |
Indications for adults: Mesenteric thrombosis 33% Desmoid tumor & Gardner’s syndrome 17%, Crohn’s disease 11% Trauma 15% Chronic pseudo obstruction 11% Volvulus 4% Others 9% |
| Megacystis microcolon & psuedoobstructi on 11% Microvillus inclusion disease 5% Others 3% |
Graft surveillance examination with a zoom endoscope was introduced in periods II & III but only as need in period I. |
| Study & Type | Sample size Patient characteristics. | Type of Tx & Protocol | Patient Survival | Graft Survival/weaned from TPN/Hospital stay | Adverse events/complications |
|---|---|---|---|---|---|
| Langnas A et al Intestinal Transplantation at the University of Nebraska:1990-2001. Transpl Proc. 2002;34:958-60. U of Nebraska Oct 1990-Mar 2001 Case series |
N=106 patients 117 grafts. Adults = 13 grafts Pediatrics =104 grafts 7 had previous Tx Mean age: Pediatrics ISB 6 yrs L-SB 2.8 yrs Adult: ISB 35 yrs L-SB 69 yrs Causes ISB: most common Midgut volvulus, pseudo obstruction & massive gut resection L-SB: most common-Midgut volvulus, Gastroschisis & intestinal atresia. |
37 ISB Tx pts (43 grafts0 69 L-SB Tx pts (74 grafts) -New surgical technique to preserve hilum of the liver -11 pts underwent abdominal evisceration & preserve proximal stomach & distal colon. -18 pts used portal vein for venous outflow -15 used the inferior vena cava Post-op management: -Prophylactic antimicrobial agents & alprostadil. -Immunosuppression : cyclosporin in first 5 pts, others received tacrolimus. 11 also got concomitant sirolimus & 26 pts had induction therapy with basiliximab. |
2 year patient survival: -Overall primary pts with tacrolimus=70% -ISB = 82% L-SB = 60% -Longest survival ISB with fully functional allograft = 7 yrs. -Longest surviving L-SB recipient = 10 yrs. -Main cause of death in both groups was sepsis. |
2 yr graft survival for ISB =67% Causes of loss of graft: ISB (18/43) rejection (5), ischemic necrosis (2), chronic rejection (1), arterial thrombosis (1), portal vein thrombosis (1) -3 pts died with functioning graft Average time to discontinuing TPN ISB = 39 days L-SB = 31 days. Median length of stay for the entire group = 64 days (range 5 - 599 days) Median cost of intestinal transplantation $275,000 (range $77,000 - $1,800,000) Conclusion: -Effective life-saving procedure for select patients -Surgical techniques refined & standardized. -Short-term survival satisfactory but long-term still unknown Rejection & sepsis still major obstacle to success. -Cost & LOS still too great |
Rejection Among 61 primary L-SB pts with tacrolimus: 60% had at least 1 episode of rejection with median time to first rejection of 16 days. Among 36 ISB recipients with tacrolimus: 77% had rejection episodes with median time of 12 days. -Antilymphocyte therapy was required to treat rejection in 17/61 L-SB pts & 7/36 ISB pts. -Explantation used to treat rejection in 7/36 ISB pts. -Induction in 24 pts resulted in significant reduction in mean number of rejection episodes & fungal infection. Infection -Bacterial infection in 93% of pts -Fungal infection in 25% of pts. -CMV infection in 16/106 pts (small bowel 10, lung 4). -No graft loss due to CMV. Reoperation in 45% of ISB & 66% of L-SB pts mostly because of intra-abdominal abscess. Post-transplant lymphoproliferative disease: -in 9% of patients median time to diagnosis 4 months -5/10 disease free, functional graft -2/10 required explantation of graft -3/10 died. |
| Study & Type | Sample size Patient characteristics. | Type of Tx & Protocol | Patient Survival | Graft Survival/weaned from TPN/Hospital stay | Adverse events/complications |
|---|---|---|---|---|---|
| Sudan DL et al. Assessment of Function, growth & development, & long-term quality of life after small bowel transplant. Transpl proc 2001;32:1211-12 University of Nebraska Medical Centre |
N=31 Patients alive with >1 yr graft survival (27 pediatrics, 4 adults, 16 males, 15 females) ISB 13 pts L-SB 18 patients -Mean length of follow-up = 39+/-20 months. |
-pts contacted by phone or seen in outpatient clinic. 31 completed standard questionnaire -Intestinal function evaluated on basis of need for IV fluids, tube feeding PN & medications to alter gut motility. Growth considered normal if maintained growth curve on standard charts, maintained preTx height/age curve in the < 10th percentile or exhibit catch-up growth. Development: assessed based on activities currently performed relative to age related expectations, school attendance & need for special service (speech, education, PT) To assess QOL, pts were asked about hospitalization/illnesses, presence of an ostomy and number of bowel movements per day. |
Intestinal function -IV fluids required in 2/3 of pts @ discharge. -Median time to D/C IV fluids = 7 months. -Required during periods of illness, 3 ongoing needs. -65% pts reported meeting nutritional needs by oral intake. -Supplementary tube feeding required for re rejection episodes(2), poor weight/inadequate growth (3) & food aversion (9). -Overall, 35% of pats reported temporary PN needed at a median of 2 yrs post transplant for bacterial or viral enteritis and sepsis, but D/C after recovery from serious illness. At time of assessment, 2/31 pts receiving PN. -50% of pts required Imodium regularly to decrease the number of BMs. Growth50% appears normal 11% maintained pre-transplant growth. 15 showed catch-up growth |
Development 84% of children able to return to preschool, daycare or school at appropriate level for their development -many report participation in extracurricular activities -63% of children required special education services. -75% of adults returned to work or school and all reported participation in leisure activities. Quality of life Mean number of hospital admission after discharge 2.3+/-1.1. 50% required hospital in the year before the study. -Most common reason for hospital admission were: Infection (43%) Surgical procedures (14%) Acute rejection (14%) -28/31 (90%) free of ileostomy -Median time to ostomy closure was 10 months (4-36 months) Average number of stools per day = 3 |
Summary The majority of intestinal transplant recipients maintained good graft function reflected by the oral intake of nutrition without the need for IV fluids and long-term PN. 75% of children grew normally or achieve catch-up growth and most were able to return to their age-appropriate activities, although most require some educational or developmental services in order to achieve this. -Intestinal transplant recipients, however, require hospital readmission at an average of once every 1-2 years for infection, surgical procedures and/or acute rejection. |
| Study & Type | Sample size Patient characteristics. | Type of Tx & Protocol | Patient Survival | Graft Survival/weaned from TPN/Hospital stay | Adverse events/complications |
|---|---|---|---|---|---|
| Goulet O et al Intestinal Transplantation in Children: Paris Experience. Transpl Proc 2002:34(5): 1887-88. November 1994 - May 2001 Hopital Necker-Enfants Malades, Paris, France. |
N = 36 children (37 grafts) (12 girls) Median age 5 years (range 2.5-15 years) Median time on TPN = 4.5 years Indications: -Intractable diarrhea 14 -Short bowel S 11 -Hirschsprung’s 7 pseudo-obstruction 4 |
ISB 13 L-SB 24 IV tacrolimus first 2 days, then converted to oral tacrolimus for 5 months. -methylprednisolone -Azathioprine All received IV antibiotics until intestinal transit recovery and total bowel decontamination for 1 month and in case of acute graft rejection. -Quantitative EBVPCR was performed weekly in the peripheral blood. -Oral & enteral feeding using gastrostomy tube started from the end of the first post-op week. -acyclovir or gancyclovir also included in the first 3 months. |
Actuarial survival: 6 months, 1 year and 3 years ISB = 77% L-SB 6 months = 74% |
Actuarial Graft survival ISB 6 months 61.5% 1 year 46% 3 years 31% L-SB 6 months, 1year & 3 years = 71% (4 pts died within first 2 months & 1 pt had retransplantation after 4 months) -Actuarial survival after liver-small bowel tx significantly higher than after isolated SB transplant. Gut function -Feeding introduced at a median of 9 days post-op 95%(18/19) weaned from PN 3-30 weeks after tx. -All TPN-weaned recipients gained weight and have recovered normal growth velocity (evidence of normal graft function. |
Rejection: -Occurred 20 times in 15 patients (15/36) 42% of patients -16 (75% successfully treated by increasing tacrolimus dosages). 3 pts received antilymphoglobulins -Acute liver rejection 6 times in 6 patients, successfully treated. -Surgical complications occurred in 85% of L-SB tx and 25% in ISB tx. -4 L-SB recipients had EBV associated PTLD. - 2L-SB recipients presented with lymphoma @ 3 & 18 months post-op. CMV disease in 6 patients - resolved. |
| Fishbein et al Intestinal and Multiorgan Transplantation: The Mount Sinai Experience. Transpl Proc 2002;34(3):891-92. The Mount Sinai Medical Center, NY. Study period: Nov 1998 - Sept 2001. Case series |
N = 34 pts (37 transplants) 23 children 11 adults (78 adults, 62 children assessed, Tx recommended in 79/140, 49 had major liver disease) Eligibility Criteria: Irreversible intestinal failure & life-threatening complications of parenteral nutrition; adult pts with low-grade mesenteric tumors causing obstruction. Etiology: Short gut 102/140 Motility or secretory disorders: 23, Mesenteric tumors: 9 Others: 6. |
ISB Tx = (14 pts) 16/37 grafts L-SB Tx = 19/37 grafts MV Tx = 2/37 grafts. Immunosuppression: tacrolimus, sirolimus first 30 days, Basiliximab & steroids. -target levels for tacrolimus & sirolimus. -IITx (-) cytotoxic cross-match pre-transplant (T-cell) Prophylaxis: Ganciclovir IV x 2 wks then CMV PCR Cytogam: 12 weeks, CMV (-) blood, standard antibiotics. |
Actuarial 1 year pt survival Overall = 74% ISB Tx = 87% Multiorgan Tx = 63% 8 pts died (rejection -2, adenovirus infection -2, fungal sepsis - 1, PTLD - 1, surgical complications - 2. Patient & graft survival higher for isolated intestinal transplants than for multiorgan transplant (p=0.06) No difference between patient & graft survival for children vs adults. Conclusion: Emphasis should be placed on earlier referral of patients before end-stage liver disease develops. Viral infection in children, rejection & surgical complications continue to complicate intestinal Tx. |
Actuarial 1 year graft survival Overall = 64% ISB Tx = 73% Multiorgan Tx = 58% Causes of graft loss: Thrombosis (2) Noncompliance (1) Rejection & PTLD (1) Chronic allograft enteropathy (1) |
Rejection: -Rejection within 30 days of transplantation = 51% of cases. -Recurrent rejection developed in 16% of cases. -Treatment with antilymphocyte antibody (OKT3 or thymoglobulin) required in 27% (10/37). - 2 ISB grafts & 1 L-SB graft lost due to exfoliative rejection. Infection -Symptomatic CMV infection developed in 11% of patients. -Adenovirus infection occurred in 30% (7/23) of children all within a 10 month period. -PTLD seen in 11% (4/34) cases, all involved the intestinal allograft. |
| Study & Type | Sample size Patient characteristics. | Type of Tx & Protocol | Patient Survival | Graft Survival/weaned from TPN/Hospital stay | Adverse events/complications |
|---|---|---|---|---|---|
| Lorman S et al Improved results in small bowel transplantation using sirolimus. Transpl Proc 2002; 34:2002 Mount Sinai Medical Centre, NY. 1998 - Double cohort on sirolimus |
With Sirolimus (N = 16) 4 adults (mean age 41.2+/-10 yrs), 12 children (mean age 2.6+/-2.5 yrs). Without sirolimus (N = 21): 9 adults (mean age 39.8+/-11.2 yrs) 9 children (mean age 1.4+/-2.4 yrs) |
With sirolimus ISB = 11 L-SB = 9 MV = 1 Without sirolimus ISB 7 L-SB = 8 MV N = 1 Immunosuppression Immediately post-transplant -All received tacrolimus and steroids -First 17 pts used daclizumab -last 17 pts used sirolimus and basiliximab Monitoring Ileal biopsy twice weekly for first 6 weeks, once weekly for next 6 weeks then monthly. Jujenal biopsy if ileal biopsy is negative but suspicion remains high. |
Actuarial 1 year survival With sirolimus = 79% Without sirolimus =70% (p=0.78) |
Actuarial 1-year graft survival: With sirolimus 80% Without sirolimus 56% (p=0.18) Conclusion Addition of sirolimus to primary immunosuppression regimen resulted in fewer early rejections and less severe rejection episodes with no cases of exfoliative rejection. Results achieved without increased morbidity and trend towards improved 1 yr patient and graft survival. More experience & larger numbers needed. |
Rejection First 30 days Without sirolimus: 68% With Sirolimus: 17% (P=0.002) First 90 days Without sirolimus 85% With sirolimus 31% The first rejection more severe in those not receiving sirolimus (p=0.002). Infection Symptomatic viral infection CMV, EBV, adenovirus, difference between 2 groups not significant. With sirolimus 4 deaths Without sirolimus 6 deaths Reoperation First 30 days incidence Without sirolimus 98.5% With sirolimus 60% (p=0.07) Higher tissue healing in sirolimus group but not significant, no impact on the overall reoperation rate. |
| Beath SCV et al Induction therapy for small bowel transplant Recipients: Early experience in Birmingham, UK. Transpl Proc 2002; 34(5):1892-1893. April 1993 - Dec 2001 Birminghan Children’s Hosp., UK Case series. |
N = 21 children -Mean age 30 -Mean wt 11.9 kg -45% hospitalized -Chronic intestinal failure + life-threatening complications secondary to PN. -Indications: Short bowel S (11), Pseudo obstruction (6), mucosal disorders (4) |
ISB - 5 pts L-SB 14 pts MV 2 Protocol Oral tacrolimus starting post-op day 1 to achieve 12-hour trough levels of 20-25 ng/mL. All patients also receive hydrocortisone & azathioprine orally for fist 4 weeks. -Maintenance immune suppression comprised tacrolimus (12-hr trough level of 10-15 ng/mL) and prednisione 0.5-1.0 mg/kg per day. |
4 (19%) children died of multi-organ failure & respiratory distress syndrome within 6 weeks of transplant. Excluding 3 pts with <12 months follow-up) 1 year survival = 61% (11/18) 2-year survival = 50% (9/18). Cause of late mortality: -Epstein-Barr virus infection & PTLD |
Gut function -17/21 survived to be independent of PN -15/21 (71.4%) became independent of PN and recovered to be discharged home at a mean of 8 weeks after transplant. Note This group of pediatric patients appears to have greater tendency towards malignant transformation of EBV primary infection. The maintenance of immune suppression regimen of low-dose prednisolone & tacrolimus alone may have exacerbated the effect of EBV in B lymphocytes because tacrolimus has minimal effect in B cells and will prevent a cytotoxic t-cell response to EBV-infection in B lymphocytes |
Small bowel allograft rejection 15 (mild to moderate) CMV disease in 4 of 6 children given CMV positive grafts within 8 weeks (1 graft loss) Intestinal perforation in 4 patients (resulted in death in 1 pt) Neutropenia in 8/17 patients (2-12 weeks post-transplant) - probably drug related. Late complications (8 - 52 wks) Mild rejection (6 patients) Neutropenia (10 pts) Severe gastroenteritis requiring temporary PN (9 pts) Broviac line infection (4 pts) Chronic rejection (4 pts) Intestinal perforation/obstruction (2 pts) EBV viraemia (10 pts) PTLD (6 pts) Conclusion Induction with tacrolimus, azathioprine & hydrocortisone was successful in inducing tolerance & good graft function after small bowel transplantation. Results suggest that maintenance immune suppression of tacrolimus and prednisolone alone should be avoided in EBV, naïve recipients & antiproliferative agents such as mycophenolate or rapamycin be added. |
| Study & Type | Sample size Patient characteristics. | Type of Tx & Protocol | Patient Survival | Graft Survival/weaned from TPN/Hospital stay | Adverse events/complications |
|---|---|---|---|---|---|
|
Farmer DG et al Outcome after intestinal transplantation: Results from one Center’s 9-year experience. Archives of Surgery 2001;136(9):102 7. UCLA school of Med Study period: 1991 - Dec 2000 Case series - retrospective analysis |
N = 17 patients & 21 grafts Indications for SB tx: intestinal failure assoc with 1 or more life-threatening complications including liver disease, loss of venous access sites, frequent central venous catheter infections and major fluid & electrolyte imbalance assoc with proximal gastrointestinal stomas or fistulas. 12/17 children mean age 13.2 yrs mean wt: 36.7 kg received TPN 68% of lifetime. -41% hospital bound |
ISB = 2/17 L-SB = 14/17 MV = 1/17 Most pts have advanced liver disease withTPN as primary cause. Secondary: hepatitis etc. |
1 year 63% 3 year 55% Cause of deaths: Sepsis 63% |
Death censored graft survival 1 year 73% 3 year 55% Most common cause of loss of graft: acute & chronic rejections, primary non-function and hepatic artery thrombosis. 3 died with functioning grafts. Enteral tube feeding initiated 9.6+/-4.3 days after Tx. Independence from TPN achieved in 81.3% of the 16 grafts surviving more than 30 days. At 1 year, pts demonstrated a 20%+/-14% weight gain above pre-Tx Serum prealbumin level 23.5+/-7.3mg/dL (reference 19.0 - 38.4 mg/dL). |
Rejection: 16 grafts functioning more than 30 days Mean time to first ACR 40+/-26 days. No graft lost to rejection before post-op day 30. Total of 20 ACR episodes yielding an incidence of 1.5 +/-1.1 episodes per graft. 70% of ACR responded to adjustment in primary immunosuppression regimen or addition of pulsed tapered steroids. Viral infection 13 pts received preemptive & prophylactic therapies, Viremia 30.7% incidence, tissue-invasive infection (incidence 15.4%) All treated & no pt or graft lost to CMV disease. |
|
Farmer DG et al Outcome after intestinal transplantation: A single-center experience over a decade. Transpl Proceed 2002;34(3):896-97. Dumont UCLA Transplant Center. Study period: Nov. 1991 - Jul 2001 Case series retrospective analysis |
N = 19 (23 grafts) Median age 8.6 years (10 months -49 years) -Primary cause of intestinal failure was trauma, jejunoileal atresia, necrotizing enterocolistis, gastroschisis, inflammatory bowel disease, volvulus, microvilli inclusion disease, pseudo-obstruction and demoid tumor. |
ISB grafts = 3/19 pts (3 grafts L-SB grafts = 15/19 pts (19 grafts) Pancreas + SB = 1/19 pts (1 graft) -Immunotherapy included tacrolimus, steroids, mycophenolate mofetil and daclizumab. -Surveillance endoscopy & biopsy were preformed per protocol & clinically indicated. -Prophylaxis using ganciclovir against CMV & EBV -Pts transplanted after 1995 were treated preemptively for CMV & EBV based on detection of peripheral blood viral DNA using polymperase chain reaction. -All pts were initially managed using PN with enteral support initiated early after transplant. - Median follow-up: 12 months (0.1 - 75.3 months) |
Overall 1-year Pt Survival Rate = 67% 3 year pt survival rate = 60% For 16 pts transplanted since 1995: 1 year pt survival rate =73% 3 year pt survival rate = 64% Conclusion Substantial improvement in survival outcomes; Improved immunosuppression markedly reduced incidence of AR. Aggressive prophylactic & preemptive therapy against CMV & EBV reduced impact of viruses. Significant incidence of other post-op infections contributes to patient mortality & morbidity. |
Overall 1 year graft survival rate =56% 3-year graft survival rate = 45% For 16 pts transplanted since 1995: 1 year graft survival = 57% 3yr graft survival = 50% (P = insignificant) Gut function Enteral feeding was successfully initiated using elemental formulas at a median of 8 days after transplant. PN was completely discontinued at a median of 35.4days (14-516 days) 80% of all grafted patients achieved independence from PN. |
Rejection Incidence of acute rejection in 19 grafts with >30 days follow-up = 1.4+/-1.1 episodes per graft. 88% of the episodes were rescued with either steroids or OKT3 12% of the episodes required enterectomy. Infection 2 episodes of tissue-invasive CMV disease and 1 episode of EBV transformed lymphocytes that did not progress to lymphoma. No patient or graft was lost to infection by these viruses. Other major infection episodes =2.6 episodes per patient including 66% of bacterial and 16% of fungal etiology. The most common organisms identified were Psuedomonas and candida species. |
Liver disease can occur during extended use of TPN even when the majority of nutrition is taken enterally.
| Study & Type | Sample size Patient characteristics. | Type of Tx & Protocol | Patient Survival | Graft Survival/weaned from TPN/Hospital stay | Adverse events/complications |
|---|---|---|---|---|---|
| Fecteau A et al Early referral is essential for successful pediatric small bowel transplantation: The Canadian Experience. J Ped Surg 2001;36(5): 681-84. Jan 1993-Dec 1999 Children’s Hosp for W. Ont. & Hosp. For Sick Children Toronto. Canada Case series |
N = 13 pediatric patients 11 pts@ CHWO 2 pts @ HSC Median age = 3.8 years (5 mos - 12 yrs) ISB pts on average older than L-SB tx pts and MV Tx pts. Indications: Psuedo obstruction = 3 Mucosal abnormality = 4 Byler’s disease = 1 Gastroschisis = 3 Atresia =2 |
ISB = 6 pts L-SB = 4 pts MV Tx = 3 pts |
1-year actual survival Overall =61% ISB = 83% (5/6) L-SB = 50% (2/4) MV = 33% (1/3) |
1-year actual graft survival Overall = 53% Graft function All survivors have resumed a normal diet; 2 pts needed occasional tube feeding to supplement caloric intake. Independence from TPN was achieved in 5 weeks on average. |
Rejection 66% of pts experienced rejection 3 had mucosal denudation of the graft; 1 responded to antithymocyte globulin, 2 died. -All other rejections responded to steroids. Graft Versus Host Disease GVHD of the skin and rectum was observed in one of the patients who received the ABO compatible graft Infection 2 pts had bacterial and 1 had fungal septicemia. EBV induced lymphoproliferative disease occurred in 1 pt & resolved with decreasing dose of immunosuppression. Renal tubular acidosis seen in most patients & treated with bicarbonate. Nephrotoxicity seen in 7 patients, improved with decreasing levels of tacrolimus. |
| International Transplant Registry December 2001 Report (Web site) All transplants between April 1985 - May 31, 2001 |
-55 centres -Total patient 651 56% male -Total # of transplant 696 (61% pediatric) -Pre-transplant: 52.1% hospitalized |
ISB = 291 grafts (42%) L-SB = 310 grafts (44%) MV = 95 grafts (14%) Maintenance of immunosuppression for patients alive: Tacrolimus 97% Prednisone 92% Rapamycin 16% Azathioprine 12% Mycophenolate Mofetil 8% Daclizumab 5% Cyclosporin 2% Others 1% |
Current survivors 335 Factors affecting graft and patient survival: Centre size >/= 10 pts vs <10 pts (pts transplanted) Pre-transplant status Era of the transplant affect only graft survival not patient survival. Major causes of death Sepsis 162/316 (51.3%) Rejection 33/316 (10.4%) Technical 23/316 (7.3%) Lymphoma 22/316 (7% Respiratory causes 21/316 (6.6%) |
Total graft removed 121 (17.4%) Reason for graft removal: -Rejection 57% (65% in pediatric, 44.9% adults) -Thrombosis/ischemia bleeding 20.7% (28% adults, 15.3% pediatrics) Sepsis 6.6% Lymphoma 1.6% (0% in adults, 2.7% pediatrics) Others 14%. Graft function For transplants survived >6 months: Full graft function about 70% Partial graft function 15% Graft removed 15% |
Suggested Citation
This report should be cited as follows:
Medical Advisory Secretariat. Small bowel transplant: an evidence-based analysis. Ontario Health Technology Assessment Series 2003; 3(1)
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to MASinfo@moh.gov.on.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: www.health.gov.on.ca/ohtas.
Print copies can be obtained by contacting MASinfo@moh.gov.on.ca.
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All Medical Advisory Secretariat analyses are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Contact Information
The Medical Advisory Secretariat
Ministry of Health and Long-Term Care
20 Dundas Street West, 10th floor
Toronto, Ontario
CANADA
M5G 2N6
Email: MASinfo@moh.gov.on.ca
Telephone: 416-314-1092
ISSN 1915-7398 (Online)
ISBN 978-1-4249-7267-8 (PDF)
About the Medical Advisory Secretariat
The Medical Advisory Secretariat is part of the Ontario Ministry of Health and Long-Term Care. The mandate of the Medical Advisory Secretariat is to provide evidence-based policy advice on the coordinated uptake of health services and new health technologies in Ontario to the Ministry of Health and Long-Term Care and to the healthcare system. The aim is to ensure that residents of Ontario have access to the best available new health technologies that will improve patient outcomes.
The Medical Advisory Secretariat also provides a secretariat function and evidence-based health technology policy analysis for review by the Ontario Health Technology Advisory Committee (OHTAC).
The Medical Advisory Secretariat conducts systematic reviews of scientific evidence and consultations with experts in the health care services community to produce the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, the Medical Advisory Secretariat systematically reviews available scientific literature, collaborates with partners across relevant government branches, and consults with clinical and other external experts and manufacturers, and solicits any necessary advice to gather information. The Medical Advisory Secretariat makes every effort to ensure that all relevant research, nationally and internationally, is included in the systematic literature reviews conducted.
The information gathered is the foundation of the evidence to determine if a technology is effective and safe for use in a particular clinical population or setting. Information is collected to understand how a new technology fits within current practice and treatment alternatives. Details of the technology’s diffusion into current practice and input from practicing medical experts and industry add important information to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist policy makers to make timely and relevant decisions to optimize patient outcomes.
If you are aware of any current additional evidence to inform an existing evidence-based analysis, please contact the Medical Advisory Secretariat: MASInfo@moh.gov.on.ca. The public consultation process is also available to individuals wishing to comment on an analysis prior to publication. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Disclaimer
This evidence-based analysis was prepared by the Medical Advisory Secretariat, Ontario Ministry of Health and Long-Term Care, for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation, and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data, and information provided by experts and applicants to the Medical Advisory Secretariat to inform the analysis. While every effort has been made to reflect all scientific research available, this document may not fully do so. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of publication. This analysis may be superseded by an updated publication on the same topic. Please check the Medical Advisory Secretariat Website for a list of all evidence-based analyses: http://www.health.gov.on.ca/ohtas.
ABBREVIATIONS AND GLOSSARY
- CMV
Cytomegalovirus
- EBV
Epstein-Barr virus
- GVHD
Graft-versus-host disease
- SBT
Small bowel transplant
- ISB Tx
Isolated small bowel transplant
- SB-L Tx
Composite small bowel-liver transplant
- MV Tx
Multivisceral transplant
- HPN
Home enteral nutrition
- PTLD
Post transplant lymphoproliferative disease
- OKT3
Ortho-Kung-T cell 3 (anti-T cell monoclonal anti bodies) (Mabs)
- TPN
Total parenteral nutrition
- Bacterial translocation
The passage of viable bacteria from the intestinal lumen of the graft, through the intestinal epithelium into the lamina propria, and consequently to the mesenteric lymph nodes, peritoneal cavity and other previously sterile tissues and organs.
- Chronic intestinal pseudo-obstruction
Includes a heterogeneous group of disorders that are characterized by intestinal obstruction in the absence of an anatomic obstruction and are caused by ineffective intestinal contractions. It may involve the entire intestinal tract or selectively involve the colon and/or other hollow viscera.
- Cold ischemia time
The time between harvesting, transportation and implantation of an allograft during which the allograft is infused with preserving solution and stored at cold temperature.
- Desmoid tumour
Fibrous or fibroid tumour
- Gastroschisis
An abnormality (defect or hole) in the abdominal wall that allows the abdominal contents to protrude outside the body. There is no peritoneal covering over the bowel or other contents.
- Hirshsprung disease
A disease caused by failure of migration of the enteric ganglia derived from neural crest cells. This migration normally occurs from cranial to caudal and explains why the anus is always involved in Hirshsprung’s disease. As the lack of nerve connection moves proximally, progressively more colon is involved.
- Intestinal Atresia
Congenital obstruction of the intestine at any level, most commonly of the ileum, due to lack of continuity of the lumen.
- Microvillus inclusion disease (congenital mircovillus atrophy)
A congenital disorder that is characterized by the electron microscopy finding of disruption of the enterocytes’ microvilli and the appearance of characteristic inclusion vacuoles whose inner surface is lined by typical microvilli.
- Necrotizing enterocolitis
An inflammation of the bowel mucosa with the formation of pseudomembranous plaques overlying an area of superficial ulceration, and the passage of the pseudomembranous material in the feces. A disorder that presents in premature infants and is associated with early initiation of hyperosmotic feedings, vascular insufficiency, hypoxia, and early colonization of the gut with gram-negative bacteria.
- Volvulus
Intestinal obstruction that is due to knotting and twisting of the bowel.
Bibliography
- 1.Gerber D. Intestinal Transplantation. Medscape. March 23. 2002.
- 2.Ghanekar A, Grant D. Small bowel transplantation. Curr. Opin. Crit Care. 2001;7:133–7. doi: 10.1097/00075198-200104000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Braunwald E FAKDHSLDJJ. Harrison’s Principles of Internal Medicine, Part 5, p.473. McGraw-Hill Medical Publishing. 15th. 2001.
- 4.Prasad KR PS. Small bowel transplantation. Current Opinion in Gastroenterology. 2000;2004;16(2):126–133. doi: 10.1097/00001574-200003000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Bueno J, Guiterrez J, Mazariegos GV, Abu-Elmagd K, Madariaga J, Ohwada S, et al. Analysis of patients with longitudinal intestinal lengthening procedure referred for intestinal transplantation. Journal of Pediatric Surgery. 2001;36:178–83. doi: 10.1053/jpsu.2001.20047. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman SS. Small bowel transplantation: selection criteria, operative techniques, advances in specific immunosuppression, prognosis. [Review] [18 refs] Current Opinion in Pediatrics. 2001;13:425–8. doi: 10.1097/00008480-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Ghobrial RM, Farmer DG, Amersi F, Busuttil RW. Advances in pediatric liver and intestingal transplantation. American Journal of Surgery. 2000;180:328–34. doi: 10.1016/s0002-9610(00)00550-x. [DOI] [PubMed] [Google Scholar]
- 8.Kosmach Park B. Intestinal Transplantation. From Organ Transplantation: Concepts, Issues, Practice and Outcomes. Medscape. Jun 17, 2002.
- 9.Technology Assessment of Small Bowel Transplants in Adults and Multivisceral Transplants in Adults and Children, corpauthor. Blue Cross Blue Shield Technology Evaluation Center. Jul, 1999. [PubMed]
- 10.Handelsman H ZD. Small bowel transplantation. AHRQ Technology Assessment (Unpublished) 2000.
- 11.The Intestinal Transplant Registry, corpauthor. Report 2001. 2003. [http://www.intestinaltransplant.org/ITR_Reports/Report_2001/Report2001_slide0002.htm. ]
- 12.Abu-Elmagd K, Reyes J, Bond G, Mazariegos G, Wu T, Murase N, et al. Clinical intestinal transplantation: A decade of experience at a single center. Annals of Surgery. 2001;234:404–17. doi: 10.1097/00000658-200109000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinna AD, Weppler D, Nery J, Ruiz P, Kato T, Khan F, et al. Intestinal transplantation at the University of Miami - Five years of experience. Transplantation Proceedings. 2000;32:1226–7. doi: 10.1016/s0041-1345(00)01199-4. [DOI] [PubMed] [Google Scholar]
- 14.Kato T, Nishida S, Mittal N, Levi D, Nery J, Madariaga J, et al. Intestinal transplantation at the University of Miami. Transplantation Proceedings. 2002;34(868) doi: 10.1016/s0041-1345(02)02646-5. [DOI] [PubMed] [Google Scholar]
- 15.Langnas A, Chinnakotla S, Sudan D, Horslen S, McCashland T, Schafer D, et al. Intestinal transplantation at the University of Nebraska Medical Center: 1990 to 2001. Transplantation Proceedings. 2002;34:958–60. doi: 10.1016/s0041-1345(02)02716-1. [DOI] [PubMed] [Google Scholar]
- 16.Fishbein T, Kaufman S, Schiano T, Gondolesi G, Florman S, Tschernia A, et al. Intestinal and multiorgan transplantation: The Mount Sinai experience. Transplantation Proceedings. 2002;34:891–2. doi: 10.1016/s0041-1345(02)02655-6. [DOI] [PubMed] [Google Scholar]
- 17.Farmer DG, McDiarmid SV, Yersiz H, Cortina G, Vargas J, Maxfield AJ, et al. Outcomes after intestinal transplantation: A single-center experience over a decade. Transplantation Proceedings. 2002;34:896–7. doi: 10.1016/s0041-1345(02)02657-x. [DOI] [PubMed] [Google Scholar]
- 18.Goulet O, Lacaille F, Colomb V, Jan D, Canioni D, Cezard JP, et al. Intestinal transplantation in children: Paris experience. Transplantation Proceedings. 2002;34:1887–8. doi: 10.1016/s0041-1345(02)03110-x. [DOI] [PubMed] [Google Scholar]
- 19.Beath SV, Protheroe SP, Brook GA, Kelly DA, McKiernan PJ, Murphy MS, et al. Early experience of paediatric intestinal transplantation in the United Kingdom, 1993 to 1999. Transplantation Proceedings. 2000;32(1225) doi: 10.1016/s0041-1345(00)01198-2. [DOI] [PubMed] [Google Scholar]
- 20.Beath SV, De Ville dG, Kelly DA, McKiernan PJ, Buckels JAC, Mirza D, et al. Induction therapy for small bowel transplant recipients: Early experience in Birmingham, UK. Transplantation Proceedings. 2002;34:1892–3. doi: 10.1016/s0041-1345(02)03112-3. [DOI] [PubMed] [Google Scholar]
- 21.Fecteau A, Atkinson P, Grant D. Early referral is essential for successful pediatric small bowel transplantation: The Canadian experience. Journal of Pediatric Surgery. 2001;36:681–4. doi: 10.1053/jpsu.2001.22936. [DOI] [PubMed] [Google Scholar]
- 22.Atkison P, Williams S, Wall W, Grant D. Results of pediatric small bowel transplantation in Canada. Transplantation Proceedings. 1998;30:2521–2. doi: 10.1016/s0041-1345(98)00708-8. [DOI] [PubMed] [Google Scholar]
- 23.Beath SV, Brook GA, Kelly DA, Buckels JA, Mayer AD. Demand for pediatric small bowel transplantation in the United Kingdom. Transplantation Proceedings. 1998;30:2531–2. doi: 10.1016/s0041-1345(98)00713-1. [DOI] [PubMed] [Google Scholar]
- 24.Sudan DL, Kaufman SS, Shaw BW, Jr, Fox IJ, McCashland TM, Schafer DF, et al. Isolated intestinal transplantation for intestinal failure. American Journal of Gastroenterology. 2000;95:1506–15. doi: 10.1111/j.1572-0241.2000.02088.x. [DOI] [PubMed] [Google Scholar]
- 25.Langnas AN. VIth International Small Bowel Transplant Symposium. Transplantation Proceedings. 2000;32(1189) doi: 10.1016/s0041-1345(00)01178-7. [DOI] [PubMed] [Google Scholar]
- 26.Iyer K, Kaufman S, Sudan D, Horslen S, Shaw B, Fox I, et al. Long-term results of intestinal transplantation for pseudo-obstruction in children. Journal of Pediatric Surgery. 2001;36:174–7. doi: 10.1053/jpsu.2001.20046. [DOI] [PubMed] [Google Scholar]
- 27.Gambarara M, Knafelz D, Diamanti A, Ferretti F, Papadatou B, Sabbi T, et al. Indication for small bowel transplant in patients affected by chronic intestinal pseudo-obstruction. Transplantation Proceedings. 2002;34:866–7. doi: 10.1016/s0041-1345(02)02645-3. [DOI] [PubMed] [Google Scholar]
- 28.Pinna AD, Weppler D, Nery JR, Khan F, Ruiz P, Kato T, et al. Induction therapy for clinical intestinal transplantation: Comparison of four different regimens. Transplantation Proceedings. 2000;32:1193–4. doi: 10.1016/s0041-1345(00)01179-9. [DOI] [PubMed] [Google Scholar]
- 29.Florman S, Gondolesi G, Schiano T, Tschernia A, Leleiko N, Kaufman SS, et al. Improved results in small bowel transplantation using sirolimus. Transplantation Proceedings. 2002;34(936) doi: 10.1016/s0041-1345(02)02678-7. [DOI] [PubMed] [Google Scholar]
- 30.Grant D. Intestinal transplantation: 1997 Report of the international registry. Transplantation. 1999;67:1061–4. doi: 10.1097/00007890-199904150-00021. [DOI] [PubMed] [Google Scholar]
- 31.The 9th Keio University International Symposium for Life Sciences and Medicine: Current issues in liver/small bowel transplantation, corpauthor. Keio J. Med. 2001 2001 Mar 23-24;50(Suppl 1):12–34. Tokyo, Japan. Abstracts. [PubMed] [Google Scholar]
- 32.Reyes J, Bueno J, Kocoshis S, Green M, Abu-Elmagd K, Furukawa H, et al. Current status of intestinal transplantation in children. J. Pediatr. Surg. 1998;33:243–54. doi: 10.1016/s0022-3468(98)90440-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iyer K, Horslen S, Iverson A, Sudan D, Fox I, Shaw B, et al. Nutritional outcome and growth of children after intestinal transplantation. Journal of Pediatric Surgery. 2002;37:464–6. doi: 10.1053/jpsu.2002.30864. [DOI] [PubMed] [Google Scholar]
- 34.Farmer DG, McDiarmid SV, Yersiz H, Cortina G, Amersi F, Vargas J, et al. Outcome after intestinal transplantation: Results from one center’s 9-year experience. Archives of Surgery. 2001;136:1027–32. doi: 10.1001/archsurg.136.9.1027. [DOI] [PubMed] [Google Scholar]
- 35.Sudan DL, Iverson A, Weseman RA, Kaufman S, Horslen S, Fox IJ, et al. Assessment of function, growth and development, and long-term quality of life after small bowel transplantation. Transplantation Proceedings. 2000;32:1211–2. doi: 10.1016/s0041-1345(00)01190-8. [DOI] [PubMed] [Google Scholar]
- 36.Nucci AM, Barksdale Jr EM, Beserock N, Yaworski JA, Iurlano K, Kosmach-Park B, et al. Long-term nutritional outcome after pediatric intestinal transplantation. Journal of Pediatric Surgery. 2002;37:460–3. doi: 10.1053/jpsu.2002.30863. [DOI] [PubMed] [Google Scholar]
- 37.Cicalese L, Sileri P, Green M, Abu-Elmagd K, Fung JJ, Starzl TE, et al. Bacterial translocation in clinical intestinal transplantation. Transplantation Proceedings. 2000;32(1210) doi: 10.1016/s0041-1345(00)01189-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farmer DG, McDiarmid SV, Winston D, Yersiz H, Cortina G, Dry S, et al. Effectiveness of aggressive prophylatic and preemptive therapies targeted against cytomegaloviral and Epstein-Barr viral disease after human intestinal transplantation. Transplantation Proceedings. 2002;34:948–9. doi: 10.1016/s0041-1345(02)02710-0. [DOI] [PubMed] [Google Scholar]
- 39.Abu-Elmagd KM, Reyes J, Fung JJ, Mazariegos G, Bueno J, Martin D, et al. Clinical intestinal transplantation in 1998: Pittsburgh experience. Acta Gastro-Enterologica Belgica. 1999;62:244–7. [PMC free article] [PubMed] [Google Scholar]
- 40.Sigurdsson L, Reyes J, Putnam PE, del Rosario JF, Di Lorenzo C, Orenstein SR, et al. Endoscopies in pediatric small intestinal transplant recipients: five years experience. American Journal of Gastroenterology. 1998;93:207–11. doi: 10.1111/j.1572-0241.1998.00207.x. [DOI] [PubMed] [Google Scholar]
- 41.Abu-Elmagd K, Reyes J, Todo S, Rao A, Lee R, Irish W, et al. Clinical intestinal transplantation: new perspectives and immunologic considerations. J. Am. Coll. Surg. 1998;186:512–25. doi: 10.1016/s1072-7515(98)00083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nalesnik M, Jaffe R, Reyes J, Mazariegos G, Fung JJ, Starzl TE, et al. Posttransplant lymphoproliferative disorders in small bowel allograft recipients. Transplantation Proceedings. 2000;32(1213) doi: 10.1016/s0041-1345(00)01191-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sudan D, Iyer K, Horslen S, Shaw JB, Langnas A. Assessment of quality of life after pediatric intestinal transplantation by parents and pediatric recipients using the child health questionnaire. Transplantation Proceedings. 2002;34:963–4. doi: 10.1016/s0041-1345(02)02718-5. [DOI] [PubMed] [Google Scholar]
- 44.Rovera GM, DiMartini A, Schoen RE, Rakela J, Abu-Elmagd K, Graham TO. Quality of life of patients after intestinal transplantation. Transplantation. 1998;66:1141–5. doi: 10.1097/00007890-199811150-00005. [DOI] [PubMed] [Google Scholar]
- 45.Cicalese L, Baum C, Brown M, Sileri P, Smith D, Abcarian H, et al. Segmental small bowel transplant from adult living-related donors. Transplantation Proceedings. 2001;33(1553) doi: 10.1016/s0041-1345(00)02590-2. [DOI] [PubMed] [Google Scholar]
- 46.Cameron EA, Binnie JA, Jamieson NV, Pollard S, Middleton SJ. Quality of life in adults following small bowel transplantation. Transplantation Proceedings. 2002;34:965–6. doi: 10.1016/s0041-1345(02)02719-7. [DOI] [PubMed] [Google Scholar]
- 47.Buchman AL. Complications of long-term home total parenteral nutrition: their identification, prevention and treatment. Dig. Dis. Sci. 2001;46:1–18. doi: 10.1023/a:1005628121546. [DOI] [PubMed] [Google Scholar]
- 48.Howard L. A global perspective of home parenteral and enteral nutrition. Nutrition. 2000;16:625–8. doi: 10.1016/s0899-9007(00)00310-5. [DOI] [PubMed] [Google Scholar]
- 49.Scolapio JS, Fleming CR, Kelly DG, Wick DM, Zinsmeister AR. Survival of home parenteral nutrition-treated patients: 20 years of experience at the Mayo Clinic. Mayo Clin. Proc. 1999;74:217–22. doi: 10.4065/74.3.217. [DOI] [PubMed] [Google Scholar]
- 50.Abu-Elmagd KM, Reyes J, Fung JJ, Mazariegos G, Bueno J, Janov C, et al. Evolution of clinical intestinal transplantation: improved outcome and cost effectiveness. Transplantation Proceedings. 1999;31:582–4. doi: 10.1016/s0041-1345(98)01565-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghanekar A, Grant D. Small bowel transplantation. Curr. Opin. Crit Care. 2001;7:133–7. doi: 10.1097/00075198-200104000-00013. [DOI] [PubMed] [Google Scholar]


