Executive Summary
Objective
This review was conducted to assess the effectiveness of repetitive transcranial magnetic stimulation (rTMS) in the treatment of major depressive disorder (MDD).
The Technology
rTMS is a noninvasive way to stimulate nerve cells in areas of the brain. During rTMS, an electrical current passes through a wire coil placed over the scalp. The current induces a magnetic field that produces an electrical field in the brain that then causes nerve cells to depolarize, resulting in the stimulation or disruption of brain activity.
Researchers have investigated rTMS as an option to treat MDD, as an add-on to drug therapy, and, in particular, as an alternative to electroconvulsive therapy (ECT) for patients with treatment-resistant depression.
The advantages of rTMS over ECT for patients with severe refractory depression are that general anesthesia is not needed, it is an outpatient procedure, it requires less energy, the simulation is specific and targeted, and convulsion is not required. The advantages of rTMS as an add-on treatment to drug therapy may include hastening of the clinical response when used with antidepressant drugs.
Review Strategy
The Medical Advisory Secretariat used its standard search strategy to locate international health technology assessments and English-language journal articles published from January 1996 to March 2004.
Summary of Findings
Some early meta-analyses suggested rTMS might be effective for the treatment of MDD (for treatment-resistant MDD and as an add-on treatment to drug therapy for patients not specifically defined as treatment resistant). There were, however, several crucial methodological limitations in the included studies that were not critically assessed. These are discussed below.
Recent meta-analyses (including 2 international health technology assessments) have done evidence-based critical analyses of studies that have assessed rTMS for MDD. The 2 most recent health technology assessments (from the Oxford Cochrane Collaboration and the Norwegian Centre for Health Technology Assessment) concluded that there is no evidence that rTMS is effective for the treatment of MDD, either as compared with a placebo for patients with treatment-resistant or nontreatment-resistant MDD, or as an alternative to ECT for patients with treatment-resistant MDD. This mainly due to the poor quality of the studies.
The major methodological limitations were identified in older meta-analyses, recent health technology assessments, and the most recently published trials (Level 2–4 evidence) on the effectiveness of rTMS for MDD are discussed below.
Small sample size was a limitation acknowledged by many of the authors. There was also a lack of a priori sample size calculation or justification.
Biased randomization may have been a problem. Generally, the published reports lacked detailed information on the method of allocation concealment used. This is important because it is impossible to determine if there was a possible influence (direct or indirect) in the allocation of the patients to different treatment groups.
The trials were single blind, evaluated by external blinded assessors, rather than double blind. Double blinding is more robust, because neither the participants nor the investigators know which participants are receiving the active treatment and which are getting a placebo. Those administering rTMS, however, cannot be blinded to whether they are administering the active treatment or a placebo.
There was patient variability among the studies. In some studies, the authors said that patients were “medication resistant,” but the definitions of resistant, if provided, were inconsistent or unclear. For example, some described “medication resistant” as failing at least one trial of drugs during the current depressive episode. Furthermore, it was unclear if the term “medication resistant” referred to antidepressants only or to combinations of antidepressants and other drug augmentation strategies (such as neuroleptics, benzodiazepine, carbamazepine, and lithium). Also variable was the type of depression (i.e., unipolar and/or bipolar), if patients were inpatients or outpatients, if they had psychotic symptoms or no psychotic symptoms, and the chronicity of depression.
Dropouts or withdrawals were a concern. Some studies reported that patients dropped out, but provided no further details. Intent-to-treat analysis was not done in any of the trials. This is important, because ignoring patients who drop out of a trial can bias the results, usually in favour of the treatment. This is because patients who withdraw from trials are less likely to have had the treatment, more likely to have missed their interim checkups, and more likely to have experienced adverse effects when taking the treatment, compared with patients who do not withdraw. (1)
Measurement of treatment outcomes using scales or inventories makes interpreting results and drawing conclusions difficult. The most common scale, the Hamilton Depression Rating Scale (HDRS) is based on a semistructured interview. Some authors (2) reported that rating scales based on semistructured interviews are more susceptible to observation bias than are self-administered questionnaires such as the Beck Depression Inventory (BDI). Martin et al. (3) argued that the lack of consistency in effect as determined by the 2 scales (a positive result after 2 weeks of treatment as measured by the HDRS and a negative result for the BDI) makes definitive conclusions about the nature of the change in mood of patients impossible. It was suggested that because of difficulties interpreting results from psychometric scales, (4) and the subjective or unstable character of MDD, other, more objective, outcome measures such as readmission to hospital, time to hospital discharge, time to adjunctive treatment, and time off work should be used to assess rTMS for the treatment of depression.
A placebo effect could have influenced the results. Many studies reported response rates for patients who received placebo treatment. For example, Klein et al. (5) reported a control group response rate as high as 25%. Patients receiving placebo rTMS may receive a small dose of magnetic energy that may alter their depression.
Short-term studies were the most common. Patients received rTMS treatment for 1 to 2 weeks. Most studies followed-up patients for 2 to 4 weeks post-treatment. Dannon et al. (6) followed-up patients who responded to a course of ECT or rTMS for up to 6 months; however, the assessment procedure was not blinded, the medication regimen during follow-up was not controlled, and initial baseline data for the patient groups were not reported. The long-term effectiveness of rTMS for the treatment of depression is unknown, as is the long-term use, if any, of maintenance therapy. The cost-effectiveness of rTMS for the treatment of depression is also unknown. A lack of long-term studies makes cost-effectiveness analysis difficult.
The complexity of possible combinations for administering rTMS makes comparing like with like difficult. Wasserman and Lisanby (7) have said that the method for precisely targeting the stimulation in this area is unreliable. It is unknown if the left dorsolateral prefrontal cortex is the optimal location for treatment. Further, differences in rTMS administration include number of trains per session, duration of each train, and motor threshold.
Clinical versus statistical significance. Several meta-analyses and studies have found that the degree of therapeutic change associated with rTMS across studies is relatively modest; that is, results may be statistically, but not necessarily clinically, significant. (8-11). Conventionally, a 50% reduction in the HDRS scores is commonly accepted as a clinically important reduction in depression. Although some studies have observed a statistically significant reduction in the depression rating, many have not shows the clinically significant reduction of 50% on the HDRS. (11-13) Therefore, few patients in these studies would meet the standard criteria for response. (9)
Clinical/methodological diversity and statistical heterogeneity. In the Norwegian health technology assessment, Aarre et al. (14) said that a formal meta-analysis was not feasible because the designs of the studies varied too much, particularly in how rTMS was administered and in the characteristics of the patients. They noted that the quality of the study designs was poor. The 12 studies that comprised the assessment had small samples, and highly variable inclusion criteria and study designs. The patients’ previous histories, diagnoses, treatment histories, and treatment settings were often insufficiently characterized. Furthermore, many studies reported that patients had treatment-resistant MDD, yet did not listclear criteria for the designation. Without this information, Aarre and colleagues suggested that the interpretation of the results is difficult and the generalizability of results is questionable. They concluded that rTMS cannot be recommended as a standard treatment for depression: “More, larger and more carefully designed studies are needed to demonstrate convincingly a clinically relevant effect of rTMS.”
In the Cochrane Collaboration systematic review, Martin et al. (3;15) said that the complexity of possible combinations for administering rTMS makes comparison of like versus like difficult. A statistical test for heterogeneity (chi-square test) examines if the observed treatment effects are more different from each other than one would expect due to random error (or chance) alone. (16) However, this statistical test must be interpreted with caution because it has low power in the (common) situation of a meta-analysis when the trials have small sample sizes or are few. This means that while a statistically significant result may indicate a problem with heterogeneity, a nonsignificant result must not be taken as evidence of no heterogeneity.
Despite not finding statistically significant heterogeneity, Martin et al. reported that the overall mean baseline depression values for the severity of depression were higher in the treatment group than in the placebo group. (3;15) Although these differences were not significant at the level of each study, they may have introduced potential bias into the meta-analysis of pooled data by accentuating the tendency for regression to the mean of the more extreme values. Individual patient data from all the studies were not available; therefore, an appropriate adjustment according to baseline severity was not possible. Martin et al. concluded that the findings from the systematic review and meta-analysis provided insufficient evidence to suggest that rTMS is effective in the treatment of depression. Moreover, there were several confounding factors (e.g., definition of treatment resistance) in the studies, thus the authors concluded, “The rTMS technique needs more high quality trials to show its effectiveness for therapeutic use.”
Conclusion
Due to several serious methodological limitations in the studies that have examined the effectiveness of rTMS in patients with MDD, it is not possible to conclude that rTMS either is or is not effective as a treatment for MDD (in treatment-resistant depression or in nontreatment-resistant depression).
Objective
The primary objective was to perform an evidence-based analysis of the effectiveness and cost-effectiveness of rTMS for the treatment of major depressive disorder.
Background
Clinical Need – Target Population and Condition
Mood disorders are characterized by a disturbance in the regulation of mood, behaviour, and affect. Mood disorders are subdivided into depressive disorders, bipolar disorders, and depression associated with medical illness or alcohol and substance abuse. (17) Depressive disorders are differentiated from bipolar disorders by the absence of a manic or hypomanic episode.
Major depressive disorder (MDD) is defined as depressed mood on a daily basis for a minimum of 2 weeks. An episode may be characterized by sadness, indifference, apathy, or irritability. It is usually associated with changes in sleep patterns, appetite, and weight; motor agitation or retardation; fatigue; impairment in concentration and decision making; feelings of shame or guilt; and thoughts of death or dying. (17)
MDD frequently follows a recurrent or chronic course and impairs quality of life considerably. By 2020, the World Health Organization predicts that major depression will be second only to ischemic heart disease as a cause of disability, ahead of common health problems such as infectious diseases, cancer, and accidents. (18)
The average age of onset of MDD ranges from the early 20s to the early 30s. Major depression is increasingly recognized as primarily a chronic and recurrent disease with frequent episode relapses and recurrences. (18) Recovery (i.e., symptom remission) occurs in about 50% of men and women after 1 year. However, recurrence of depressive episodes in people who recover from an index episode of major depression is extremely common. As many as 85% of people who have recovered from MDD have another episode within 15 years, as do 58% of people who remain well for at least 5 years after recovery. (18;19)
Predictors of recurrence include female sex, a longer depressive episode before treatment, more prior episodes, and never marrying. (19) Risk of recurrence tends to decrease as the length of time a person has remained well increases. People hospitalized for a first episode of depression have a 50% lifetime risk of readmission, and those who have had previous hospitalizations have a 50% risk of readmission within the next 3 years after discharge. (18-20)
In Ontario, an expert consultant estimated that about 6% to 7% of the population has experienced an episode of major depression the last year. About 50% of these people will have recurrent depression (the rest will have 1 or 2 lifetime episodes only). Of the 50% with recurrent depression, about 25% will not respond to at least 2 medications; therefore, they will be considered resistant. This means about 0.8% of the population (96,000 people per year if the population is 12 million) has had an episode of treatment-resistant depression in the past year.
Of these, 80% would not have been considered for electroconvulsive therapy (ECT), the gold standard for treatment-resistant depression, for the following reasons:
Anesthetic contraindications
Unavailability of ECT
Unavailability of inpatient facilities
Requested an alternative antidepressant treatment
About 10% of these people would be eligible for and accept a course of ECT in 1 year (about 1,800 patients). It is estimated that close to 10% of these patients may have also received ECT in the past, leaving about 1,500 new patients who may have received ECT in 1 year.
Existing Treatment Options Other Than Technology Being Reviewed
Treatment requires coordinating short-term symptom remission with longer-term maintenance strategies designed to prevent recurrence. According to the American Psychiatric Association guidelines, (21) treatment for MDD may include the following:
Drug therapy (e.g., selective serotonin reuptake inhibitors, tricyclic antidepressants, monoamine oxidase inhibitors, venlafaxine, bupropion, mirtazapine, buspirone, pindolol, noradrenergic tricyclic agents, alpha 2 antagonists, atypical antipsychotics, thyroid augmentation, and lithium). During treatment, positive response to antidepressant medication should be evident to some degree by 4 weeks, with full response by 8 to 12 weeks. (22) Patients with depression that has not responded positively by 4 weeks should be re-evaluated. The diagnosis should be reconsidered, the patient’s adherence to medication should be assessed, or adjunctive psychotherapy should be considered. Usually, some sort of further intervention is required. Strategies for patients whose depression does not respond to a given drug after 4 weeks include switching to a different antidepressant, adding a second antidepressant, or augmenting therapy with a drug such as lithium. (22)
Psychotherapy
Drug therapy plus psychotherapy
ECT
ECT is an established treatment for severe, incapacitating forms of treatment-refractory depression. (17) During ECT, an electric current is passed through the brain by electrodes that have been placed on the scalp. This induces generalized seizure activity. During the procedure, the patient is placed under general anesthetic and given muscle relaxants to prevent body spasms. ECT electrodes are placed on one or both sides of the head. Unilateral placement is usually on the nondominant side of the brain to reduce adverse cognitive effects. The number of sessions in a course of ECT ranges from 6 to 12. ECT is usually administered twice a week. It is rarely given as continuation or maintenance therapy to prevent the relapse of symptoms, and it can be either an inpatient or an outpatient procedure.
A systematic review and meta-analysis of the efficacy and safety of ECT to treat depressive disorders was conducted by the United Kingdom ECT Review Group. (23) Although many of the trials were old, and most were small, the evidence revealed that in the short-term (i.e., at the end of a course of treatment), ECT is an effective treatment for adult patients who have depressive disorders and no substantial cormorbidity.
Rose et al. (24) did a descriptive systematic review of physician- and patient-led research to examine patients’ views on the benefits of, and possible memory loss from, ECT. Although the various studies did not use consistent definitions or standardized ratings for memory loss, Rose et al. concluded that one-third of patients reported experiencing memory loss after ECT.
The management of depression in patients with bipolar depression differs in some important aspects from those with unipolar depression. For example, antidepressants are used more cautiously, in case they trigger a manic relapse. Also, a manic relapse may lead to an apparent “improvement” in depression scores and assessment. Finally, most patients with established bipolar disorder are also taking a mood stabilizer, such as lithium. (25)
Treatment-Resistant or Refractory Depression
The term refractory depression does not distinguish among treatment resistance, chronicity, relapse, or recurrence and it does not account for psychosocial factors that may prevent recovery. (25) Moreover, the term does not take into account if the patient has had an adequate course of (or any) psychotherapy.
In 2002, Stimpson et al. (26) systematically reviewed randomized controlled trials (RCTs) that assessed the efficacy of a pharmacological or psychological intervention for treatment-refractory depression. Inclusion criteria were RCTs that included adults aged 18 to 75 years, with a diagnosis of unipolar depression, and who had not responded to a 4-week course of a recommended dose of an antidepressant.
They identified 16 RCTs. The strategy that had received the most investigation was augmentation of existing antidepressant medication. There were no studies of psychological treatment. It was possible to do a meta-analysis using the results from 2 trials that investigated lithium and 3 that studied pindolol. All the trials were too small to detect an important clinical response. The authors concluded that treatment-refractory depression is a common clinical problem. Furthermore, they noted that the lack of evidence on effectiveness is reflected by an absence of consensus among clinicians and in the vagueness of current guidelines.
A limitation to this study was that treatment-resistant depression was defined according to the 1974 World Psychiatric Association definition, which used a 4-week criterion. Importantly, most other definitions of treatment resistance require patients to have failed to respond to more than a single course of antidepressants. (27)
Up to now, there has been little guidance on the management of treatment-refractory depression. (28) Current guidelines suggest increasing the dose of antidepressants, switching to a different class of antidepressants, adding psychotherapy, or augmenting antidepressant therapy with lithium or ECT. Stimpson et al. (26) argued that the lack of guidance is reflected by variation in the management of treatment-refractory depression. They supported their argument by stating the following:
One-third of psychiatrists in the Northeastern United States preferred lithium augmentation. (29)
Canadian psychiatrists had an equal preference for a second tricyclic antidepressant, augmentation with a monoamine oxidase inhibitor, and augmentation with lithium. (28)
The most popular choice in the United Kingdom was to increase the dose or to change the class of antidepressant. However, 39% of respondents in that study said they were not confident when treating this condition. (30)
Thase and Rush (27) estimated that 60% to 70% of patients able to tolerate an antidepressant will respond to their drug of first choice, and 5% to 10% will remain depressed despite multiple interventions. This poorly responsive group has been variously described as resistant, refractory, or intractable in the literature. Thase and Rush proposed that the term “treatment resistance” be clarified by a stage as shown in Table 1:
Table 1: Stage of Treatment Response in Clinical Depression*.
| Stage | Treatment Response |
|---|---|
| 0 | Has not had a single adequate trial of medication |
| 1 | Nonresponse to an adequate trial of one medication (monotherapy) |
| 2 | Failure to respond to two different adequate monotherapy trials of medications with different pharmacological profiles (e.g., TCA and SSRI)† |
| 3 | Stage 2 plus failure to respond to one augmentation strategy (e.g., lithium or thyroid augmentation of one of the monotherapies) |
| 4 | Stage 3 plus a failure on a second augmentation strategy |
| 5 | Stage 4 plus failure to respond to ECT† |
Thase and Rush (27)
TCA represents tricyclic antidepressants; SSRI, selective serotonin reuptake inhibitors; ECT, electroconvulsive therapy
New Technology Being Reviewed: Repetitive Magnetic Transcranial Stimulation
Transcranial magnetic stimulation (TMS) is a noninvasive way to stimulate nerve cells in areas of the brain. During TMS, an electrical current passes through a wire coil placed over the scalp (Figures 1 and 2 in Appendix 1). The current induces a magnetic field that produces an electrical field in the brain, which then causes nerve cells to depolarize, resulting in the stimulation or disruption of brain activity. (31)
TMS can be applied once or repeated many times per second with variation in intensity, site, and orientation of the magnetic field. In most studies, figure-8 coils are used (Figure 2 in Appendix 1). (31) Figure-8 coils are 2 round coils that, when placed side by side, produce a focal stimulation. Coils with a smaller diameter have a more focused field of stimulation but require greater stimulation intensity to produce similar depth-of-field penetration. Fitzgerald et al. (31) have said that highly focused stimulation is essential for many research applications, although it is unclear if this property will prove of use in clinical situations where less focused stimulation may better compensate for variations in disease localization and differences in anatomy among people.
The frequency of cortical stimulation varies. Rapid-rate or repetitive TMS (rTMS) usually refers to the application of TMS for a train of minutes at frequencies above 1 Hertz (Hz) and is commonly used in treatment studies. TMS at less than or equal to 1Hz is referred to as slow or low-frequency TMS. Hasey (32) has suggested that the ability to stimulate the brain either at either high or low frequency is important, because high-frequency rTMS (e.g., 20 Hz) may increase cerebral blood flow and neuronal excitability in the region of the cortex under the coil, but low-frequency rTMS (less than or equal to1Hz) may have the opposite effect.
The magnetic pulse is further described by its intensity in proportion to the motor threshold of the individual. The motor threshold is the lowest intensity of stimulation that, when applied to the motor cortex, causes a standard contraction of a muscle in at least 5 of 10 consecutive trials. (9) When rTMS is administered, the number of pulse trains per daily session is usually described, as are the intertrain interval, the number of daily sessions, the site of stimulation, the type of coil used, and the orientation of the coil relative to the site on the scalp. (9)
Fitzgerald et al. (31) noted that the design and subsequent interpretation of therapeutic trials using TMS may be complicated by confounding variables such as the following:
Prior response to medication therapies
Current medication therapy
Illness duration
Severity
Treatment location (i.e., on an inpatient or outpatient basis)
Diagnosis
Intensity, frequency, and location of stimulation
Number of stimuli applied in each train of stimulation
Number of trains per session
Fitzgerald and colleagues also noted that many studies have been done based on 5-days-per-week stimulation, although this choice appears to have arisen out of convenience rather than a proven advantage. Patients receiving rTMS are usually fully awake and sitting. Sessions can last from 20 minutes to 1 hour. (9)
The control subjects in TMS studies may also present a problem. In crossover research designs, a scalp muscle effect with placebo stimulation may compromise the blind condition. (31) Ideally, a placebo for TMS provides scalp and nose sensation but has minimal cortical stimulation. However, the higher the degree of sensation achieved, the higher the likelihood of cortical stimulation.
In 1994, it was suggested that the prefrontal cortex may be an effective target area of the brain for TMS (Figures 3 and 4 in Appendix 2). This brain region was selected because of evidence of an association between the response to ECT and changes in prefrontal cortex function, and because authors of imaging studies had reported abnormalities in the prefrontal cortex in patients with depression. (31)
rTMS has been most studied in the left prefrontal cortex (Figure 4 in Appendix 2). More recently, there has been interest in the therapeutic effects of slow TMS administered to the right prefrontal cortex. Some researchers have speculated that rTMS boosts underlying activity levels (e.g., increasing activity on the left in depression and on the right in mania), whereas slow TMS decreases underlying activity levels (thereby reducing a relative hyperactivity on the right side in depression). (31)
The method to determine the location of the left dorsolateral prefrontal cortex (LDLPFC) was introduced by George et al. (33) It involves determining the optimal site of stimulation over the motor cortex to elicit motor evoked potentials in the abductor pollicus brevis the thumb muscle). The coil is then moved 5 cm forward on the parasagittal plane. It is then presumed to be over the dorsolateral prefrontal cortex (DLPFC). The magnetic stimulus intensity for the treatment is typically set as a percentage of the motor threshold. As noted, Burt et al. (9) have argued that this method is inexact, because it does not account for individual differences in brain size and anatomy. In basic science research, magnetic resonance imaging-guided 3-dimensional stereotactic methods have been used to provide more precise coil positioning relative to specific anatomic locations, but this technique has not been used in many therapeutic trials. (9)
To date, there is a presumption of a strong association between the motor threshold and the intensity needed to produce the physiological response in the DLPFC using rTMS. (9) Because the cortex-to-coil distance is the major determinant of local induced current density and has shown a relationship to the motor threshold, factors such as cortical atrophy may introduce variability in the distance between the coil and the motor cortex and the coil and the DLPFC. Originally, the use of the motor threshold to determine the intensity of stimulation over the DLPFC was introduced as a safety precaution, (34) because highly intense rTMS elicited seizures in a few healthy volunteers.
Potential clinical uses of rTMS may be as an add-on to drug therapy and as a replacement for ECT for patients who have treatment-resistant MDD. Possible advantages of rTMS as an add-on to drug treatment may be a hastened clinical response in conjunction with antidepressants. The advantages of rTMS over ECT for patients with treatment-resistant depression may include the following:
No general anesthesia is given.
It is an outpatient procedure.
The energy requirement is lower. Hair, skin, and skull are not good electrical conductors; therefore, to reach brain tissue, large currents must pass through skin-surface electrodes during ECT. However, the skull and other tissues are more “transparent” to the magnetic field created by a TMS coil, and lower energy is needed to alter neuronal activity. (32) Also, as some researchers have noted, “the magnetic field can be highly focused, thereby reducing the side effects caused by the large currents that probably flow diffusely through neuronal structures during ECT.” (32)
There is no social stigma of having to receive ECT. (35)
The stimulation is specific and targeted.
Convulsion is not part of the procedure.
There are minimal adverse effects, such as mild headache and discomfort at the site of stimulation.
There are no known adverse effects on cognition.
Regulatory Status
The following TMS devices are licensed by Health Canada:
Multipulse Cortical Stimulator (Digitmer Limited, Welwyn Garden City, United Kingdom). This is a Class 2 device(Licence 6887) indicated by Health Canada for “transcranial stimulation with trains of electrical shock permit rapid assessment of the functional continuity of the motor pathways.”
Digitimer’s Web site states that that the Multipulse cortical stimulator is considered “transcranial electrical stimulation” and a “safe and efficient transcranial cortical (electrical) motor evoked potential (TceMEP) generation for effective spinal cord monitoring.” In addition, it states, “TceMEPs are now used worldwide during intraoperative spinal cord monitoring to help prevent postoperative paraplegia and our unique D185 Multipulse stimulator is the only stimulator cleared by the FDA for this application.”
Another company in the United Kingdom, The Magstim Company Limited (Whitland, Carmarthenshire, Wales), makes 3 devices that are licensed by Health Canada for use as: “A nerve stimulator. Intended for the assessment of neuromuscular function.” They are as follows:
Magstim Rapid (Class 2; licence 62504).
Magstim Model 2002 (Class 2; licence 62505).
Magstim Model 200 (Class 2; licence 62506).
Also overseas, Medtronic A/S (Charlottenlund, Denmark) makes the following devices that are licensed by Health Canada:
Magpro Magnetic Stimulator (Class 3; licence 7355). This is indicated by Health Canada for use as “magnetic stimulation of the central nervous system.”
Magpro X100 Series (Class 3; licence 60608). This is indicated by Health Canada for use as: “A non-invasive way of stimulating nerves in the central and peripheral nervous system. Used short-term to examine the physiology of the motor pathways, functional aspects of motor nerves stimulation, human cortical physiology, to change muscle function in a therapeutic manner and to change brain activity in a therapeutic manner.”
Maglite Magnetic Stimulator (Class 3; licence 12164). This is indicated by Health Canada for use as “magnetic stimulation of the central nervous system.”
According to the Medtronic product monograph for Magpro, the indication for its use states, “It is also intended for investigating the effects of rTMS.”
As of this review, no device using cortical magnetic stimulation to treat refractory MDD has been approved for use in the United States by the United States Food and Drug Administration.
Literature Review on Effectiveness
Objective
To assess the effectiveness and cost-effectiveness of rTMS for MDD.
Questions Asked
Is rTMS more effective than a placebo for treatment-resistant MDD?
Is rTMS more effective or as effective as ECT for treatment-resistant MDD?
What adverse effects are associated with rTMS treatment?
Is rTMS (with or without drugs or psychotherapy) more effective than only drugs or psychotherapy for the treatment of MDD?
Methods
Inclusion criteria
English-language articles (January 1996–March 2004)
Journal articles that report primary data on the effectiveness or cost-effectiveness of rTMS obtained in a clinical setting, or analysis of primary data maintained in registries or databases
Study design and methods that are clearly described
Systematic reviews, RCTs, non-RCTS or cohort studies that have ≥20 patients, and cost-effectiveness studies
Exclusion criteria
Duplicate publications (superseded by another publication by the same investigator group, with the same objective and data)
Non-English-language articles
Non-systematic reviews, letters, and editorials
Animal and in-vitro studies
Case reports
Studies that did not examine the outcomes of interest
Intervention
rTMS
Controls do not undergo rTMS but receive optimal conventional medical management
Literature Search
Cochrane database of systematic reviews
ACP Journal Club
DARE
INAHTA
EMBASE
MEDLINE
Reference section from reviews and extracted articles
Outcomes of Interest
Adverse effects
Length of time depression-free or relapse-free
Decrease in depressive symptoms
Change in antidepressant usage
Time to adjunctive treatment
Time to when patients go back to work
Time to hospital admissions/time to discharge
Economic analysis data
Results of Literature Review on Effectiveness
Summary of Existing Health Technology Assessments
The Cochrane and INAHTA databases yielded 7 health technology assessments or systematic reviews on rTMS. An overview is shown in Table 2. Each study is described in detail below.
Table 2: Overview of Existing Health Technology Assessments and Systematic Reviews on Repetitive Transcranial Magnetic Stimulation.
| Publication Year | Author | Type | Date Literature Search Ended |
|---|---|---|---|
| 2004 | Martin et al. (3;15) | Cochrane systematic review and meta-analysis | June 2001; updated March 2002 |
| 2003 | Aarre et al. (14;36) | Norwegian health technology assessment | February 2001 |
| 2002 | Kozel and George (8) | Systematic review and meta-analysis | April 2002 (rTMS* versus placebo only) |
| 2002 | Burt et al. (9) | Systematic review and meta-analysis | Not stated (Article accepted by journal for publication December 5, 2001) |
| 2001 | McNamara et al. (37) | Systematic review and meta-analysis | January 2000 |
| 2001 | Holtzheimer et al. (10) | Systematic review and meta-analysis | No dates of literature search reported. No dates of acceptance by journal for publication. Article published in 2001 autumn issue. |
| 1999 | Alberta Heritage Foundation for Medical Research (38) | Technology scan | No dates of literature search reported. Date of report was May 1999. |
rTMS represents repetitive transcranial magnetic stimulation
Cochrane Systematic Review
Martin et al. (15) conducted a literature search for publications up to June 2001. They also searched unpublished data and grey literature. They selected RCTs that assessed the therapeutic efficacy and safety of TMS for depression. They also published an update to their meta-analysis that expanded the literature search up to March 2002. (3) However, this update only included a meta-analysis of LDLPFC rTMS compared with placebo treatment. It did not include analyses of rTMS variations (e.g., right prefrontal cortex) or an alternative treatment (e.g., ECT) that the initial Cochrane review provided.
Sixteen trials were included in the initial review; 14 contained data in a suitable form for quantitative analysis. Two studies could not be analyzed due to methodological problems. Forty-five studies were excluded from the quantitative analysis for the following reasons: they had no or inadequate randomization, they were narrative reviews, or they were descriptive studies or studies with healthy volunteers. Two studies had not matured sufficiently for analysis.
Randomization and allocation concealment: None of the studies included in the statistical analysis of the initial review provided sufficient detail about accrual.
Blinding of interventions in patients and researchers: Twelve of the 16 studies included in the initial review were clearly described as double blind or double masked. Martin et al. noted that in these interventions it is impossible to blind the professional who applies the technique; therefore, they cannot be double blinded.
Follow-up: All studies included in the quantitative evaluation described the withdrawals during the study. (39) Only 2 studies (40;41) used intent-to-treat analysis (the method where the last observation is carried forward). The rest of the studies, which had withdrawals, did not include these patients in the analyses. Most of the trials had small sample sizes, and some of these trials noted that there were no withdrawals.
Three studies (42-44) had a period of post-treatment follow-up of 2 weeks, and 1 study (41) had a period of post-treatment follow-up of 1 week between the first and second phase of the crossover design.
Evaluation of depressive symptoms: The score on the Hamilton Depression Rating Scale (HDRS) was used as the primary outcome variable in all of the studies. The Beck Depression Inventory (BDI) was the most used as a secondary self-administered evaluation.
Methods of evaluating: Most of the studies in the first review reported higher baseline values on the HDRS in patients in the treatment group compared with those in the placebo group, due either to a small sample size or to inappropriate randomization. An overall analysis (rTMS compared with placebo) of all of the studies was done to determine if there were baseline differences. All 14 studies analyzed showed statistical homogeneity (χ213 = 13.37, P=.42). Treated patients had statistically significantly higher (worse) HDRS scores at baseline than did those in the placebo group. The standardized mean difference (SMD) obtained with a fixed-effect model was 0.30 (95% confidence interval [CI], 0.06–0.54; P=.02). Martin et al. did not comment further on reconciling the difference between a finding of statistical homogeneity for the studies and a finding of significant baseline differences in depression scores between groups.
Due to this significant difference, and to prevent more regression to the mean (45) in the treatment group compared with the placebo group evaluating the change in scores from baseline to the final assessment for the 2 groups was not recommended. Instead, it was decided to use only the final values of both groups. Therefore, the clinical interpretation of this comparison was the difference in the level of depression in the patients at the end of the studies between groups. However, this choice of analysis by the authors raises the question as to the initial appropriateness of even comparing 2 groups when the randomization in the RCTs may have been flawed.
Regression to the mean is the principle that unusual events are likely to recur. It occurs when a nonrandom sample is selected from a population and 2 imperfectly correlated variables are measured, such as 2 consecutive blood pressure measurements. (45) The less correlated the 2 variables, the larger the effect of regression to the mean. Also, the more extreme the value from the population mean, the more room there is to regress to the mean. Regression to the mean often occurs whenever a group is selected with extreme values for one variable, and another variable is then measured.
The updated meta-analysis by Martin et al. (3) further discussed the rationale for this approach and will also be discussed in detail in the summary of the paper by Martin et al.
Description of the Scales
Hamilton Depression Rating Scale (HDRS)
It is generally used in pharmacotherapy studies of people with depression. Various versions exist, each with a different number of items.
The objective of the scale is to quantify the results of a semistructured interview.
It gives more importance to somatic and behavioural symptoms than to psychological manifestations of depression.
Low values indicate less depression.
Beck Depression Inventory (BDI)
It is a self-administered questionnaire. Various versions exist.
It gives more importance to cognitive components than to somatic and behavioural components of depression.
Low values indicate less depression.
Global Assessment Scale (GAS)
Scores range from 0 to 100.
It gives a global measure of functioning and symptomatology.
High values indicate better functioning.
Clinical Global Impression Scale (CGI)
It evaluates the severity of the illness.
It has 3 subscales.
Low values indicate a decrease in the severity of an illness and/or significant recuperation.
Analysis of Efficacy
The first analysis of efficacy compared high-frequency LDLPFC rTMS with placebo TMS. (15)
Outcome: Depressive Symptoms Measured by the Hamilton Depression Rating Scale
11 studies (N=197: 109 in the treatment group and 88 in the placebo group).
No statistically significant differences between groups after 1 week of treatment, 1 week of treatment plus 1 week of post-treatment follow-up, or 2 weeks of treatment plus 2 weeks of post-treatment follow-up.
There was a statistically significant difference between groups after 2 weeks of treatment.
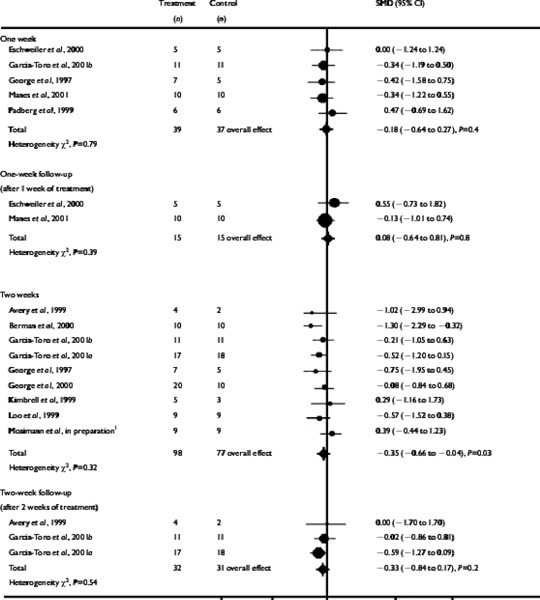
The time variable reported in studies most often was 2 weeks of treatment. After 1 week of treatment (measured in 3 studies), the SMD for rTMS compared with the placebo was 0.02 (95% CI, -0.66–0.70; P=0.9). After 1 week of treatment plus 1 week of post-treatment follow-up (measured in 1 study), the weighted mean difference (WMD) between rTMS and the placebo was 3.80 (95% CI, -4.01–11.61; P=.3). After 2 weeks of treatment (9 studies), the SMD was -0.35 (95% CI, -0.66–0.04; P=.03), in favour of rTMS. After 2 weeks of post-treatment follow-up (3 studies), there was no longer a statistically significant difference (SMD, -0.33 [95% CI, -0.84–0.17], P=.2).
Outcome: Depressive Symptoms Measured by the Beck Depression Inventory
7 studies (N= 145: 81 in the treatment group and 64 in the placebo group).
The same subcategories of analysis were performed for the same periods as in the previous analysis.
No statistically significant differences between rTMS and placebo TMS were found for any of the periods.
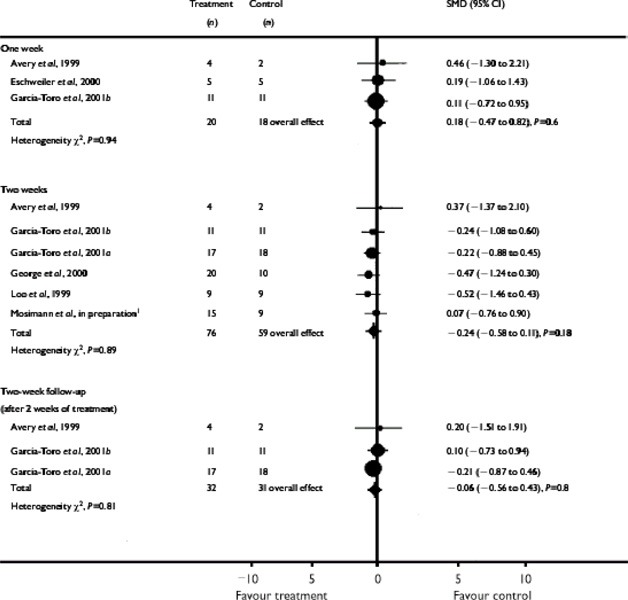
After 1 week of treatment (measured in 3 studies), the SMD for rTMS compared with placebo TMS was 0.18 (95% CI, -0.47–0.82; P=.6). After 1 week of post-treatment follow-up, the WMD (measured in only 1 study) was 6.60 (95% CI, -7.54–20.74; P=.4). The SMD after 2 weeks of treatment (3 studies) was -.24 (95% CI, -0.56–0.43; P=.8).
Outcome: Clinical Global Improvement
1 study (N= 30: 20 in the treatment group and 10 in the placebo group).
No statistically significant differences were found.
The WMD for rTMS compared with placebo TMS after 2 weeks of treatment was -0.70 (95% CI, -1.77–0.37; P=.2).
The second analysis of efficacy compared low-frequency LDLPFC rTMS with placebo TMS. (15)
Outcome: Depressive Symptoms Measured by the Hamilton Depression Rating Scale
2 studies (N= 20: 11 in the treatment group and 9 in the placebo group).
There were no statistically significant differences after 1 or 2 weeks of treatment.
After 1 week of treatment (measured in 1 study), the WMD for rTMS compared with placebo was -2.00 (95% CI, -13.49–9.49; P=.7). After 2 weeks of treatment (measured in 1 study), the WMD was 2.53 (95% CI, -13.53–18.59; P=.8).
The third analysis of efficacy compared low-frequency RDLPFC rTMS with placebo TMS. (15)
Outcome: Depressive Symptoms Measured by the Hamilton Depression Rating Scale
1 study (N= 67: 35 in the treatment group and 32 in the placebo group).
There was a statistically significant difference in favour of rTMS after 2 weeks of treatment but not after 1 week.
After 1 week of treatment, the WMD for rTMS compared with placebo TMS was -4.20 (95% CI -8.44–0.04; P=.05). After 2 weeks of treatment, the WMDL was -6.00 (95% CI -10.69—1.31; P=.01).
Outcome: Clinical global improvement
There was no statistically significant difference between groups.
After 1 week of treatment, the WMD for rTMS compared with placebo TMS was -0.50 (95% CI, -1.11–0.11; P=.11). After 2 weeks of treatment, the WMD was -0.70 (95% CI, -0.40–0.00; P=.05).
The fourth analysis of efficacy compared high-frequency LDLPFC rTMS with low-frequency LDLPFC rTMS. (15)
Outcome: Depressive Symptoms Measured by the Hamilton Depression Rating Scale
2 studies (N= 22: 11 in high-frequency group and 11 in low-frequency group).
After 1 or 2 weeks of treatment, no statistically significant difference between the 2 forms of rTMS was found.
After 1 week of treatment (measured in 1 study), the WMD for the rTMS high vs. low frequency was -.60 (95% CI, -10.76–5.56; P=.5). After 2 weeks of treatment (measured in 1 study), the WMD was -0.40 (95% CI, -13.84-13.04; P=0.9).
The fifth analysis of efficacy compared vertex motor localization +0.3 tesla rTMS with vertex motor localization -0.3 tesla rTMS (15)
Outcome: Depressive Symptoms Measured by the Hamilton Depression Rating Scale
1 study (N= 10: 5 in +0.3 group and 5 in -0.3 group).
There were no statistically significant differences between groups.
The WMD for the rTMS +0.3 tesla group compared with the rTMS -0.3 tesla group after 1 week of treatment was 6.40 (95% CI, -1.71–14.51; P=.12).
Outcome: Clinical Global Improvement
There were no statistically significant differences between groups.
The WMD for the rTMS +0.3 tesla group compared with the rTMS -0.3 tesla group after 1 week of treatment was 0.40 (95% CI, -1.33–2.13; P=.6).
The sixth analysis of efficacy compared high-frequency LDLPFC rTMS with ECT. (15)
Outcome: Depressive Symptoms Measured by the Hamilton Depression Rating Scale
1 study (N= 40: 20 in rTMS and 20 in ECT).
The study did 3 comparisons: whole sample, only patients with psychotic symptoms, and only patients without psychotic symptoms.
Looking at the whole sample, there were no statistically significant differences. After 2 weeks of treatment, the WMD for rTMS compared with ECT was 1.7 (95% CI, -3.27–6.67; P=.5). There was no statistically significant difference between the treatment groups after 4 weeks either (WMD, 4.20 [95% CI, -0.74–9.14], P=.10).
No statistically significant differences were found looking at only patients without psychotic symptoms either. After 2 weeks of treatment, the WMD for rTMS compared with ECT was -3.90 (95% CI, -10.90–3.10; P=.3). After 4 weeks of treatment, the WMD was -2.90 (95% CI, -10.26–4.46; P=.4).
Analyzing only patients with psychotic symptoms resulted in a statistically significant difference in favour of ECT. After 2 weeks of treatment, the WMD for rTMS compared with ECT was 7.90 (95% CI, 1.98–13.82; P=.009), favouring ECT. After 4 weeks, there was still a significant difference between the treatment groups favouring ECT (WMD, 12.40 [95% CI, 7.77–17.03], P<.00001).
Outcome: Clinical Global Improvement
Looking at the whole sample, no statistically significant differences were found.
After 2 weeks of treatment, the WMD for rTMS compared with ECT was -2.30 (95% CI, -12.22–7.62; P=.6). After 4 weeks, the WMD was -10.50 (95% CI, -22.85–1.85; P=.10).
No statistically significant differences were found for only patients without psychotic symptoms either. After 2 weeks, the WMD was 8.40 (95% CI, -5.10–21.90; P=.2). After 4 weeks, the WMD was 3.00 (95% CI, -14.61–20.61; P=.7).
Analyzing only patients with psychotic symptoms resulted in a statistically significant difference in favour of ECT. After 2 weeks, the WMD was -14.50 (95% CI, -27.04–1.96; P=.02). After 4 weeks, the WMD was -26.10 (95%CI, -41.12–11.08; P=.0007).
The final analysis of efficacy compared any method of application of rTMS with placebo TMS. (15)
Outcome: Withdrawals During the Study (for any reason)
5 studies (N= 184: 99 in the treatment group and 85 in the placebo group).
There were no statistically significant differences between groups.
The relative risk using a fixed-effect model for rTMS (any method of administration) compared with placebo TMS was 0.81 (95% CI, 0.36–1.83; P=.6).
The authors stated that the limitations in the studies included the following:
The studies had small sample sizes, and no sample size calculations or justifications were provided. Apart from one study that had 70 patients, the rest had sample sizes of 6 to 40 patients (median, 19).
The process of randomization was biased. None of the studies discussed the method of concealment used. Instead, they said only that a study was randomized or described only the generation of the allocation sequence (e.g., with a computer). Therefore, it is impossible to determine if an influence (direct or indirect) existed in the allocation of the patients to different treatment groups. For example, this can happen if the patients who are most likely to respond are put in only the active treatment group. This can influence the results by overestimating a possible treatment effect.
The allocation to groups was not strictly double blind. In Berman, (40) patients guessed their groups in 10 of 15 cases. The evaluators deduced the patients’ treatment groups in 12 of 15 cases.
The HDRS is the most commonly used scale in clinical studies of depression. However, scales based on semistructured interviews, like the HDRS, are more susceptible to an observation bias than are self-administered questionnaires. All of the studies in the review used the HDRS as a primary outcome variable. In the analysis of high-frequency LDLPFC rTMS versus placebo, there was a positive effect of treatment in the HDRS score after 2 weeks of treatment. However, the positive effect disappeared using the BDI (although the BDI comparison used fewer studies, because some trials lacked numerical data). The only study that found an effect in favour of rTMS using the HDRS (40) showed no difference between groups in the BDI scores.
Diversity of pathology (definition of treatment resistance, chronicity).
The techniques to treat patients were diverse (e.g., high-frequency, low-frequency, location of coil, motor threshold).
The observed placebo effect is not well understood. Patients who received placebo rTMS may have received a small dose of magnetic energy that may have altered their depression.
Reviewers’ Conclusions: Martin et al. concluded, “There is no strong evidence for a possible efficacy of TMS for the treatment of depression, although these results do not exclude the possibility of benefit.” (15)
Currently in the United Kingdom, the NHS Research and Development Health Technology Assessment Programme is sponsoring a multicentre randomized clinical trial to assess the effectiveness and cost-effectiveness of rTMS compared with ECT for severe depression. (46) The project started in August 2001, and its results are expected to be published mid 2005.
The aims of this study are to carry out a multicentre RCT with 6 months follow-up for rTMS compared with ECT in patients with severe depression. Ninety patients will be entered into each arm of the trial, which is sufficient to obtain a 95% CI to demonstrate equivalence or a subtle difference between rTMS and ECT. The objectives of the RCT are as follows:
To determine if rTMS is as effective as ECT
To determine if rTMS is associated with fewer adverse effects than ECT
To identify patient characteristics indicative of a beneficial response to rTMS
To ascertain if patients prefer rTMS or ECT
The objectives of the cost-effectiveness analysis of rTMS compared with ECT are as follows:
To calculate the short- and longer-term costs of treatment of rTMS and ECT
To establish if there are economic as well as therapeutic advantages of rTMS compared with ECT in the immediate and long term
Martin et al. Updated Literature Search
In another publication, Martin et al. (3) reported an updated literature search extending up to March 2002. Included were RCTs that compared rTMS at any frequency and at any localization with a placebo intervention. Patients were of any age and sex and had a diagnosis of depression (depressive disorders or bipolar disorders in depressed phase), with or without psychotic symptoms, according either to the Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV; American Psychiatric Association, 1994) or to the ICD-10 (World Health Organization, 1993).
Eighty-five references were identified, of which 48 were excluded for the following reasons:
17 trials had no control group.
7 trial reports whose outcomes efficacy outcomes had been published elsewhere.
9 studies used healthy volunteers.
1 had no report of any randomization process.
1 was a descriptive study.
2 studied outcomes other than depression.
2 gave rTMS after a previous intervention of sleep deprivation.
16 studies were still in progress: either the data were incomplete or the authors were awaiting data.
Five studies were excluded from the identified trials because there was either no placebo comparison group or because although a placebo group was included with 2 randomly allocated active-treatment groups, the control group itself had not been generated by a randomization process. The detailed analysis of the 5 studies was included as part of the larger Cochrane review previously published by Martin et al.
Among the 16 RCTs there was clinical heterogeneity with respect to 4 variables:
Localization of rTMS application (LDLPFC, RPFC, vertex, or multiple sites)
Frequency of rTMS (high or low)
Duration of treatment (10 consecutive working days [2 weeks] or 5 consecutive working days [1 week])
Number of interventions per day (1 or more)
The process of inclusion of studies is presented in Figure 1. Two studies (47;48) were awaiting evaluation of design data and methodological quality to be included; additional quantitative information was needed for these studies to be analyzed. (3)
Figure 1: The Process of Inclusion of Studies for Review and Analysis*,†.
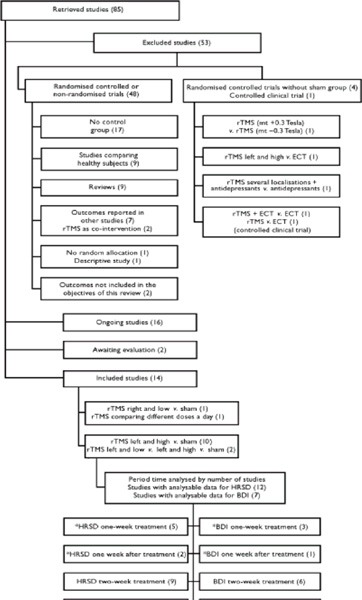
Some studies of 2 weeks duration measured outcomes at both 1 and 2 weeks.
HDRS represents Hamilton Depression Rating Scale; BDI, Beck Depression Inventory; ECT, electroconvulsive therapy, rTMS, repetitive transcranial magnetic stimulation.
Used with permission from the Royal College of Psychiatrists; Martin JL, Barbanoj MJ, Schlaepfer TE, Thompson E, Perez V. Repetitive transcranial magnetic stimulation for the treatment of depression: systematic review and meta-analysis. Br J Psychiatry 2003; 182:480-491 (3)
Fourteen studies met the inclusion criteria. (3) The majority of the studies (13/14) compared LDLPFC with a group receiving a placebo, but 1 study compared it with low-frequency RPFC. The duration of the treatment was 2 weeks in 9 of the LDLPFC studies and 1 week in the remaining 3 studies. A summary of the studies is presented in Table 3.
Table 3: Summary of Studies Comparing the Effectiveness of rTMS With a Placebo*.
| Study | Participants | Intervention | |||||
|---|---|---|---|---|---|---|---|
| Author | Design | n (withdrawals) | Pathology | Mean age (s.d.) |
Male/female | Type1 | Duration of treatment period (week)2 |
| Eschweiler et al. 2000 | Cross-over | 12 (3) | Major depression (DSM–IV) | 57 (8) | 4/8 | Left-side 10Hz, 90% motor threshold, 20tps of 10s | 1 (first phase) |
| Padberg et al. 1999 | Parallel | 18 (0) | Major depression (DSM–IV) | 51.2(16.1) | 7/11 | Left-side10 or 0.3Hz 90% motor threshold, 5 or 10tps of 5s | 1 |
| George et al. 1997 | Cross-over | 12 (0) | Major depression (DSM–IV) | 41.8 (12.4) | 1/11 | Left-side 20Hz, 80% motor thershold, 20tps of 2s | 2 (first phase) |
| Avery et al. 1999 | Parallel | 6(0) | Major depression or bipolar disorder (de pressed phase) (DSM–IV) | 44.5 (8.48) | 1/5 | Left-side 10Hz, 80% motor threshold, 20tps of 5s | 2 (+2 follow-up) |
| Berman et al. 2000 | Parallel | 20 (3) | Major depressive episode (DSM–IV) | 42.3 (10.1) | 14/6 | Left-side, 20Hz, 80% motor threshold, 20tps of 2s | 2 |
| García-Toro et al, 2001b | Parallel | 28 (3 TMS, 3 sham) | Major depression (DMS–IV) | TMS 43.2 (13.1). Sham 45.0(18.3) | 10/12 | Left-side, 20Hz, 90% motor threshold, 30tps of 2s | 2 (+2 follow-up) |
| García-Toro et al, 2001a | Parallel | 40 (3 TMS, 2 sham) | Major depression (DMS–IV) | TMS 51.5 (15.9). sham 50.0 (11.0) | 20/15 | Left-side, 20Hz, 90% motor threshold, old, 30tps of 2s | 2 (+2 follow up) |
| George et al. 2000 | Parallel | 32 (2) | Major depression or bipolor disorder (de pressed phase) (DSM–IV) | 44.5 (8.4) | 11/19 | Lift-side, 5 or 20Hz, 100% motor threshold, 40tps of 80or 2s | 2 |
| Kimbrell et al. 1999 | Cross-over | 8 (0) | Major depression (DSM–IV) | 42.46 (15) | 6/7 | Left-side, 20Hz, 80% motor threshold, 20tps of 2s | 2 (fisrt phase) |
| Loo et al. 1999 | Parallel | 18 (0) | Major depressive episode(DSM–IV) | TMS 45.7 (14.7). sham 50.9 (14.7) | 9/9 | Left-side, 10Hz, 110% motor threshold, 30tps of 5s | 2 |
| Mosimamn et al in preparation3 | Parallel | 24 (0) | Major depression (DSM–IV) | 60.87 (13.25) | 8/16 | Lift-side, 20Hz, 100% motor threshold, 40tps of 2s | 2 |
| Klein et al. 1999 | Parallel | 70 (3) | Major depression (DSM–IV) | 58.2 (17.2) | 17/53 | Right-side, 1Hz, 110% motor threshold, 2tps of 60s | 2 |
| Manes et al. 2001 | Parallel | 20 (0) | Major or minor depression (DSM–IV) | 60.7 (9.8) | 10/10 | Left-side, 20Hz, 80% motor threshold, 20tps of 2s | 1 (+1 follow-up) |
| Szuba et al. 2001 | Parallel | 16 (2) | Major depression (DSM–IV) | TMS 39.7 (12.1) sham 33.4 (9.3) | 6/8 | Left-side, 10Hz, 100% motor threshold, 20tps of 5s | 2 |
Left-side, left dorsolateral perfrontal cortex; right-side, right dor solateral prefrontal cortex; tips trains per session.
Working days: 1 week=5 days; 2 weeks=10 days.
Used with permission from the Royal College of Psychiatrists; Martin JL, Barbanoj MJ, Schlaepfer TE, Thompson E, Perez V. Repetitive transcranial magnetic stimulation for the treatment of depression: systematic review and meta-analysis. Br J Psychiatry 2003; 182:480-491 (3)
Only 3 studies reported that patients were free of antipsychotic medication for 1 week before the study and during the study itself. In 7 of the 14 studies, the patients were described as “medication resistant” (i.e., having failed at least 1 trial of pharmacotherapy during the current depressive episode). In some studies, pharmacotherapy was continued, whereas in others it was not.
Most studies excluded patients at high risk of suicide. Some studies recruited only outpatients. Others recruited only inpatients. Some recruited both; others did not specify the pattern of recruitment (Table 4).
Table 4: Patient Medication Regimens During the Studies.
| Eschweller et al, 20001 | All patients received constant doses of at least one antidepressant, five patients also received neuroleptics, four received benzodiazepines and two received lithium |
| Padberg et al, 1999 | Three patients remained unmedicated. Least antidepressant was kept at a stable dose for 15 patients |
| George et al. 19971 | Antidepressant medication tapered for 9 patients. Three patients had exper lenced a partial response to a 10 week, stable-dose antidepressant trial; these regimens were continued |
| Avery et al, 1999 | Patients taking stable, lneffective doses of medication for at least 6 weeks continued on the same doses during the study. Patients on no medication for at least 6 weeks continued on no medication during the study |
| Berman et al, 2000 | All patients free of antidepressants, neurolepices and benzodiazepines for the week prlor to initialising rTMS treatment, with tapers beginning earlier as necessary. Chloral hydrate given when required for lnsomnia to in-patients |
| Garcia-Toro et al, 2001b1 | All undrewent 1 week wash out, off all medication. All patients started on sertraline (50 mg for 2 weeks, later increased, if necessery, depending on clinical response) at same times as rTMS treatment. All except two taking benzodiazepines at study entry |
| Garcia-Toro et al, 2001a | Patients had taken the same antidepressant medication for 6 weeks before study and continued this throughout the study |
| George et al. 20001 | Patients free of antidepressant medications at least 2 weeks before study entry, three patients with bipolar disorder required ongoing mood stabilisers or benzodiazepins for anxiety |
| Kimbrell et al, 19991 | Nine medication-free patients with unipolar depression; one patient with bipolar ll disorder, on lizhium; three with bipolar l disorder, one on lithium and carbamazepine one on lithium and la motrigine and one matication-free. Patients with bipolar disorder who had had previous relapes during a major depressive episode when on mood stabilisers remained on these medication |
| Loo et al, 1999 | Five patients had antidepressants withdrawn 5 days before rTMS. Patients on steady doses of antidepressant falling to show an effect were maintained for 2 weeks before and throughout the study; nine patients received venlafaxine; four recieved nefazadone |
| Mosimann et al, In preparation2 | Antidepressant medication not an execlution criterion. Dose stable for at least 2 weeks and no new psychoactive drug started for at least 6 weeks before the first rTMS |
| Klein et al, 19991 | Patients:none were treatment-resistant. Patients were maintained with their previous medication regimen throughout the course of the study |
| Manes et al, 2001 | All patients were withdrawn over 5 days form all antidepressant medications. They were durg-free 4 days before treatment |
| Szuba et al, 20011 | Patients were free of any psychotropic medication for 7 days before study treatment(30 days fluoxetine, monoamine oxidase inhibitor antidepressants or neuroleptics)or electroconvulsive treatment in the pervious 30 days |
Used with permission from the Royal College of Psychiatrists; Martin JL, Barbanoj MJ, Schlaepfer TE, Thompson E, Perez V. Repetitive transcranial magnetic stimulation for the treatment of depression: systematic review and meta-analysis. Br J Psychiatry 2003; 182:480-491
The updated results from the systematic review by Martin et al. (3) are similar to the initial results that were reported in the Cochrane review. (49)
Hamilton Depression Rating Scale (LDLPFC high frequency vs. placebo)
Subgroup analyses were done for duration of treatment (1 or 2 weeks) and for length of follow-up (1 or 2 weeks). (3)
After 2 weeks of treatment (measured in 9 studies), the SMD for rTMS compared with placebo treatment was -0.35 (95% CI, -0.66–0.04; P=.03), showing a difference in favour of rTMS. For studies that reported data after 1 week of treatment or only gave treatment for 1 week (5 studies), the SMD for rTMS compared with placebo treatment was not statistically significant (SMD, -0.18 [95% CI, -0.64–0.27]; P=.4).
After 1 week of post-treatment follow-up (measured in 2 studies), the SMD was 0.08 (95% CI, -0.64–0.81); P=.8). After 2 weeks of post-treatment follow-up (3 studies), the SMD was not statistically significant (SMD, -0.33 [95% CI, -0.84–0.17]; P=.2).
Results for the HDRS scores are presented in Table 5.
Table 5: Effect Size (Remission of Symptoms) for the Fixed-effect Model of rTMS Compared With Placebo rTMS for Depression on the HDRS Scale*.
 |
Subgroup analyses pooled by time; rTMS represents repetitive transcranial magnetic stimulation; HDRS, Hamilton Depression Rating Scale.
Used with permission from the Royal College of Psychiatrists; Martin JL, Barbanoj MJ, Schlaepfer TE, Thompson E, Perez V. Repetitive transcranial magnetic stimulation for the treatment of depression: systematic review and meta-analysis. Br J Psychiatry 2003; 182:480-491 (3)
Beck Depression Inventory (LDLPFC high frequency vs. placebo)
There was no difference between rTMS and placebo for any of the times using the BDI. After 1 week of treatment (measured in 3 studies), the SMD for the rTMS compared with placebo treatment was 0.18 (95% CI, -0.47–0.82; P=.6). After 2 weeks of treatment (6 studies), the SMD was -0.24 (95% CI, -0.58–0.11; P=.18). The SMD after 2 weeks of post-treatment follow-up (3 studies) was -0.06 (95% CI, -0.56–0.43; P=.8). Results for the BDI are presented in Table 6.
Table 6: Effect Size (Remission of Symptoms) in the Fixed-effect Model of rTMS Compared With Placebo rTMS for Depression on the Beck Depression Inventory*.
 |
Subgroup analyses pooled by time; rTMS represents repetitive transcranial magnetic stimulation.
Used with permission from the Royal College of Psychiatrists; Martin JL, Barbanoj MJ, Schlaepfer TE, Thompson E, Perez V. Repetitive transcranial magnetic stimulation for the treatment of depression: systematic review and meta-analysis. Br J Psychiatry 2003; 182:480-491 (3)
Acceptability of treatment
Four studies reported withdrawals of patients during intervention, with a total sample size of 114 patients (63 in the treatment group and 51 in the placebo group). The relative risk using a fixed-effect model for rTMS compared with placebo rTMS for all patients was not significant (SMD, 0.88 [95% CI, 0.37–2.13]; P=.8).
Methodological Considerations
All of the studies had serious major methodological weaknesses including the following:
Small sample size (median, 19)
Uncontrolled variables that may influence outcomes may not be sufficiently evenly distributed between treatment and control groups.
In all except 3 of the studies, all or a proportion of the patients (in the treatment and control arms) were taking some form of psychotropic medication. This may have affected the results.
In some cases, the authors stated that patients were “medication resistant,” but the definition of resistance was unclear; therefore, the potential for concurrent medication to interfere with the performance of the rTMS procedure cannot be ruled out.
None of the included studies provided information on the method of allocation concealment used. (e.g., the patients who are most likely to respond could have been included only in the active treatment arm).
Rather than using the term “double blind,” it is more accurate to consider the trials as having been single blind with an evaluation by external blinded assessors.
There is the potential for the patients to guess their group allocation through nonverbal communication with the administrator of the intervention.
Wasserman and Lisanby (7) reported that, depending on the way a placebo is delivered, the physical sensation experienced can differ for placebo and active treatment, thereby effectively alerting the patient to the treatment being given.
Measurement of treatment outcomes using scales or inventories.
The most common scale, the HDRS, is based on a semistructured interview. Some authors (2) have reported that rating scales based on semistructured interviews are more susceptible to observation bias than are self-administered questionnaires such as the BDI. Martin et al. (3) argued that the lack of consistency in effect as determined by the 2 scales (a positive score after 2 weeks of treatment as measured by the HDRS and a negative scored on the BDI) makes definitive conclusions about the nature of the change in mood of the patients impossible.
Martin and colleagues suggested that, because of difficulties interpreting results from psychometric scales, (4) and the subjective or unstable character of MDD, the use of other, more objective, outcome measures should be taken into account in the assessment of rTMS for depression. These include readmission to hospital, time to hospital discharge, time to adjunctive treatment, and time off work.
Complexity of possible combinations for administering rTMS makes comparison of like versus like difficult.
Martin et al. (3) categorized the 3 main variations in administration methods: localization on the skull, frequency of treatment, and duration of treatment. In most of the included studies, rTMS was applied to the LDLPFC, but Wasserman and Lisanby (7) have stated that the method for precisely targeting the stimulation in this area is unreliable.
Moreover, it is unknown if the LDLPFC is the optimal localization for treatment.
Differences that Martin and colleagues did not categorize include the shape of the coil, the number of trains per session, and the duration of each train.
Data Analysis Considerations
Martin et al. (3) noted that in 8 of the 12 studies in the updated meta-analysis for the HDRS, and in 6 of the 7 studies in the updated meta-analysis for the BDI, the baseline mean values for the severity of depression were higher in the treatment group than in the placebo group. They said, “Although these differences were not statistically significant at the level of each individual study, they would have introduced a potential bias within the meta-analysis of pooled data by accentuating the tendency for regression to the mean of the more extreme values.” If the patients who are most likely to respond (i.e., the sickest patients) are only in the active treatment arm, it can influence the final results of the studies by overestimating a possible effect of treatment.
Martin et al. acknowledged their study was limited because individual patient data were not available from all the studies, and an appropriate adjustment according to baseline severity was not possible. However, to reduce potential bias caused by these differences in baseline values, Martin et al. only compared final values on depression severity between the active and control groups. The authors defended their analysis by stating that they used the means and standard deviations because, “We considered that owing to probable baseline imbalance between the studies, these estimates reflect a more precise effect size than a dichotomous measure such as the rate of improvement...derived from the continuous data of the rating scales.” However, this choice of analysis by the authors raises the question as to the initial appropriateness of even comparing 2 groups when the randomization process may have been flawed.
Regression to the mean occurs whenever a nonrandom sample is selected from a population and 2 imperfectly correlated variables are measured, such as 2 consecutive blood pressure measurements. (45) The less correlated the variables, the larger the effect of regression to the mean. Also, the more extreme the value from the population mean, the more room there is to regress to the mean. Regression to the mean occurs whenever a group is selected with extreme values for one variable and another variable is then measured. Given the limitations of the data to date, it would be useful to conduct an RCT and do sample size calculations with patient groups that are appropriately randomized and comparable at baseline on the severity of their depression.
Discussion of Results
Martin et al.(3) stated that the results to date are not very encouraging. However, they also suggested that their results should not be a reason to abandon rTMS as a treatment for depression altogether.
The number of patients included in studies of the efficacy of rTMS falls short of the number of patients registered in trials for new drug treatments. In addition, longer-term results are required for patients receiving rTMS.
Technical details such as where to stimulate, at what frequency, the total number of stimuli, and the duration of the treatment have yet to be resolved.
There is a need for “thorough randomized controlled multicentre studies involving large numbers of patients.” (3)
Clinical Implications
The findings from the systematic review and meta-analysis provide insufficient evidence to suggest that rTMS is effective in the treatment of depression.
There were several confounding factors in the included studies that should be kept in mind before considering rTMS for clinical use.
The rTMS technique needs more high-quality trials to show its effectiveness for therapeutic use.
Limitations
There was a lack of pragmatic variables in the studies, such as time to further treatment, time off work, readmission to hospital, or hospital discharge.
Individual patient data from all the studies were not available; therefore, an appropriate adjustment according to baseline severity was not possible.
There was poor follow-up evaluation in the studies.
Norway – Aarre et al.
Aarre et al. (14;36) systematically reviewed all published evidence on the treatment of depression with rTMS. Inclusion criteria were RCTs that compared rTMS with placebo rTMS or with ECT. Case reports and uncontrolled studies were excluded. This report by Aarre et al. (14) is an abbreviated version of a lengthy report published in Norwegian by the Norwegian Centre for Health Technology Assessment. (36)
Results: Of 225 reports identified in a literature search up to the end of February 2001, 12 studies were considered to be of sufficient scientific quality to include in the review. Aarre and colleagues said that a formal meta-analysis was not feasible, because the studies varied too much in design, in the way that rTMS was administered, and in patient characteristics.
Efficacy in Depression: Of the 12 studies included, the results were inconsistent. Most studies showed modest efficacy, with patients somewhat improved after receiving rTMS, although still in need of further treatment. Some studies found no clinically significant effects of rTMS, but 2 studies found robust efficacy in patients with severe depression. Aarre et al. stated that the inconsistent findings may be due to differences in study design, the kind of rTMS administered, and patient characteristics.
They also stated that no study has yet shown that an effect for TMS persists beyond 2 weeks following treatment cessation, and that few studies have long-term follow-up data.
Furthermore, they noted that the quality of the study designs was poor. The 12 included studies had small samples, and highly variable inclusion criteria and study designs. The patients were often insufficiently characterized as to previous history, diagnosis, treatment history, and treatment setting. In addition, many studies stated that the patients had treatment-resistant depression without outlining any clear criteria for this designation. Without this information, Aarre et al. suggested that the interpretation of the results is difficult and that the generalizability is questionable.
Stimulus site, Frequency, and Intensity: Most trials studied the effect of stimulating the LDLPFC. Stimulus frequency varied among studies: 20, 10, 1, and 0.1 Hz were used. Stimulus intensity also varied, from 110% to 80% of the motor threshold. Aarre et al. could draw no conclusions about the optimal stimulus site, frequency, or intensity.
Placebo Condition and Blinding: In most of the reviewed studies, placebo rTMS was administered by placing the coil tangential or at an angle of 45 degrees to the scalp. This positioning does not elicit the twitching in scalp muscles that is often seen with real rTMS. This difference in effect may have allowed some of the patients to guess which treatment they had received. This problem was addressed in Berman et al. (40) In that study, 67% of patients correctly identified the treatment they had received. As well, just generally, some placebo conditions may have active properties. (9)
Masking the treatment is a concern in studies using a crossover design. Bohlan et al. (50) have shown that patients are able to discriminate between active and placebo rTMS.
Blinding is very difficult, if not impossible, to achieve in a rTMS study when ECT is the comparator. (14) This is unavoidable, because ethical issues would arise in trying to approve a study that anesthetizes patients unnecessarily just to achieve appropriate blinding for scientific purposes.
Use of Active Comparators: Aarre et al. (14) noted that it might be premature to compare rTMS with an active comparator. They argued that when there is a lack of consistency as to the efficacy of the treatment, RCTs with active comparators should not be done unless sample sizes are large enough to make type II errors unlikely. In the studies with the small sample sizes that were reviewed, it is highly probable that type II errors were made. If so, this would have led to the conclusion that there is no difference between treatments, where indeed such differences may exist. Therefore, trials with active comparators would expose more patients to possibly inferior treatments than would be the case with placebo-controlled trials.
Aarre and colleagues concluded that rTMS cannot be recommended as a standard treatment for depression: “More, larger and more carefully designed studies are needed to demonstrate convincingly a clinically relevant effect of rTMS.”
Meta-Analysis of LDLPFC rTMS versus placebo
Kozel and George (8) did a meta-analysis to assess the use of rTMS over the LDLPFC to treat depression. The authors analyzed rTMS over the LDLPFC because most of the studies in the literature have treated the LDLPFC part of the brain. The literature was searched for RCTs up to April 2002.
To reduce a possible confounding order effect of prior knowledge of TMS or placebo rTMS affecting the effectiveness of the masking, only data for patients who said they had never received rTMS or placebo TMS were used. For studies that used crossover designs, only data from the initial randomized group were used. The outcome variable for all studies was the change in scores on the HDRS. A positive effect was defined as an improvement in depressive symptoms during the trial (i.e., baseline HDRS score minus the post-rTMS/placebo HDRS score = positive value). Effect sizes were calculated (using Hedges’ d).
Twelve studies met the inclusion criteria. (See Table 7.) The details of these trials follow.
Table 7: Double-Blind Placebo-Controlled Treatment Trials of LDLPFC rTMS*.
| Study | Effect Size Hedges’d |
Non-Parametric Variance |
Total N |
Frequency in Hz |
Days of Treatment |
Total Daily Stimulation |
Percent Motor Threshold |
|---|---|---|---|---|---|---|---|
| George et al. 199715 | 1.3691 | 0.3429 | 12 | 20 | 10 | 800 | 80% |
| Avery et al. 199616 | 0.7644 | 0.7500 | 6 | 10 | 10 | 1000 | 80% |
| Kimbrell et al. 199717 | 0.3188 | 0.2000 | 13 | 1/20 | 10 | 800/800 | 80% |
| Loo et al. 19996 | –0.1797 | 0.2222 | 18 | 10 | 10 | 1500 | 110% |
| Padberg et al. 199918 | 0.4440 | 0.2500 | 18 | 0.3/10 | 5 | 250/250 | 90% |
| Berman et al. 200019 | 1.0504 | 0.2000 | 20 | 20 | 10 | 800 | 80% |
| Eschweiler et al. 200020 | 0.2.68 | 0.3429 | 12 | 10 | 10 | 2000 | 90% |
| Garcia-Toro et al. 200121 | 1.0783 | 0.1144 | 35 | 20 | 10 | 2000 | 90% |
| Garcia-Toro et al. 200122 | 0.1364 | 0.1818 | 22 | 20 | 10 | 1200 | 90% |
| George et al. 200023 | 0.6539 | 0.1500 | 30 | 5/20 | 10 | 1600/1600 | 100% |
| Lisanby et al. 20017 | 0.2841 | 0.1683 | 24 | 10 | 10 | 1600 | 110% |
| Manes et al. 200024 | 0.3003 | 0.2000 | 20 | 20 | 5 | 800 | 80% |
LDLPFC represents left dorsolateral prefrontal cortex; rTMS, repetitive transcranial stimulation. From: Kozel and George (8)
Kozel and George (8) calculated that the average change in mean HDRS scores from before rTMS to after rTMS was 7.20 (SE, 0.96) for the active group and 3.58 (SE,_0.89) for the control group. Because the mean change in HDRS scores includes responders and nonresponders, the group change for those who received active rTMS was relatively small but significantly greater (t 3.89, P=.0025) than for those who received a placebo. The test for heterogeneity was not significant (Qtotal 10.65, χ211 0.47). Therefore, Kozel and George used a fixed-effects model to calculate the summary statistic.
The summary analysis of all 12 studies (n=230) revealed a cumulative effect size of 0.53 (95% CI, 0.24–0.82). The summary analysis is presented in Figure 2.
Figure 2: Forrest plot of Hedges’ d effect size and 95% confidence interval for LDLPRC rTMS treatment of depression. The horizontal axis indicates Hedges’ d effect size.*.
LDLPFC represents left dorsolateral prefrontal cortex; rTMS, repetitive transcranial stimulation. From: Kozel and George (8)
A funnel plot evaluation was used to analyze the potential impact of unpublished studies. In this type of evaluation, if the plot does not have a symmetric funnel shape, it indicates there are likely to be unpublished studies that may provide nonsignificant results. According to the authors, the funnel plot shown in Figure 3 indicated that a publication bias might have been possible.
Figure 3: A Funnel Plot Evaluation To Examine the Effect of Unpublished Studies*.
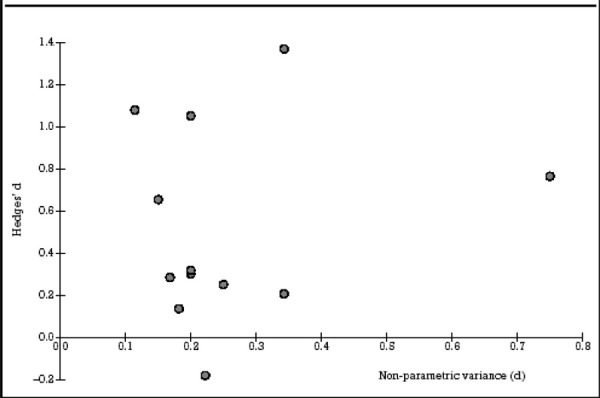
The plot indicates that there are likely unpublished studies that found nonsignificant results since the plot does not have a symmetric funnel shape tapering down as the variance (horizontal axis) gets smaller. Smaller variances indicate larger sample sizes. The studies with larger sample sizes would have less variability in results versus the studies with smaller sample sizes. One would expect the graphed points to have a “funnel” shape if all studies using rTMS including those showing no effect had been published. From Kozel and George. (8)
Orwin’s formula was used to calculate the number of studies with findings of nonsignificance that would be required to shift the result of the meta-analysis from significant to. Kozel and George estimated that about 20 unpublished studies with no significant findings would be required to render the overall summary statistic nonsignificant.
Kozel and George concluded that the following are still unknown about rTMS treatment for depression:
The duration of improvement after acute treatment
Whether maintenance rTMS could be used to prolong benefits
Relationship between pharmacotherapy use and rTMS
The limitations of Kozel and George’s meta-analysis include the following:
All of the limitations applicable to the meta-analysis by Martin et al. (3)
Varying TMS parameters were used. These include waveform, coil type, size and orientation, stimulus intensity, pulse frequency, train duration, inter-train interval, number of trains, and number of treatment sessions. According to Kammer et al. (51), there was variability in rTMS delivery according to device type (Dantec Magpro, Magstim 200, and Magstim Rapid).
2 studies were not included in the analysis due to inability to determine required data from the publication or response from the first author.
The results may be statistically significant, but they are not necessarily clinically significant.(8) The mean change in HDRS scores for patients who received active rTMS was small but significantly different from that for patients who received a placebo. Conventionally, many studies consider a successful clinical response to be a 50% decrease in the HDRS score.
Meta-analysis of rTMS Compared With Various Other Interventions
Burt et al. (9) did a meta-analysis to assess the treatment effect of rTMS for 3 categories of studies of patients with depression: open and uncontrolled trials; placebo or otherwise controlled trials; and comparisons of rTMS and ECT. For each study, the percentage change in HDRS scores were reported. One study, by Pridmore et al., (52) reported scores from the Montgomery-Asberg Depression Rating Scale (MADRS).
Burt et al. did not report any details of the literature search they did for the meta-analysis. Weighted mean effect sizes combining studies were reported for both Cohen’s d and Hedges’ adjusted g. Weighting was based on a function of a study’s sample size and the precision of the effect size estimate. The Hedges’ g statistic provides a more conservative estimate of the combined effect size.
Open and Uncontrolled Trials
The first two tables in Appendix 3 summarize the open and uncontrolled studies of rTMS in the treatment of major depression.
Across the 9 studies that reported changes in depression scores, the combined weighted effect size (Cohen’s d) was statistically significant (d=1.37). There was no evidence of heterogeneity in the effect size for either Cohen’s d (Q8=6.69, P=.57) or Hedges’ g (Q8=5.41, P=.71). (9) (See Figure 4)
Figure 4: Effect Size (d) and 95% Confidence Interval for Open and Uncontrolled Studies of TMS and rTMS in the Treatment of Depression*.
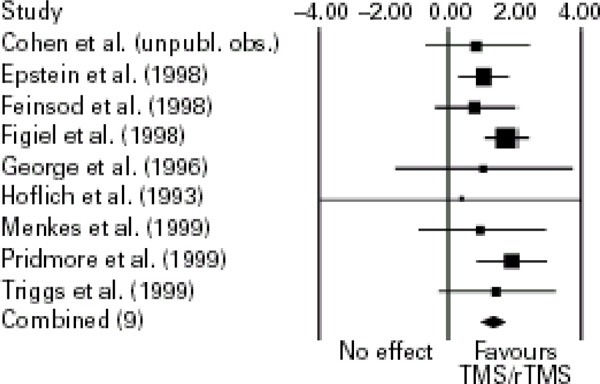
rTMS represents repetitive transcranial stimulation. The size of the boxes is proportional to the sample size. The overall combined effect size is indicated by a diamond.
Burt T, Lisanby SH, Sackeim HA. Neuropsychiatric applications of transcranial magnetic stimulation: a meta analysis. International Journal of Neuropsychopharmacology 2002; 5(1):73-103; Subject to the copyright notice provided by Cambridge University Press, Reprinted with the permission of Cambridge University Press and the author (9)
The patient sample reported by Epstein et al. (1998) overlapped with the study by Figiel et al. (1998). When the authors excluded the study by Epstein et al., the effect size (Cohen’s d) for the remaining 8 studies increased to 1.45.(9)
Despite the size of the effect, the degree of therapeutic change across these studies was relatively modest. The unweighted mean reduction in the HDRS or MADRS scores was 37.03% (standard deviation [SD], 29.23). Conventionally, as noted earlier in this report, the outcome of interest in trials is a 50% reduction of the HDRS score. Therefore, relatively few patients in these studies would meet the standard criteria for response or remission. The findings from the open and uncontrolled studies suggested that slow or fast rTMS may have antidepressant properties, but the clinical significance of this effect is uncertain.
Placebo and Other Controlled Trials
The third and fourth tables in Appendix 3 summarize the placebo- or otherwise-controlled studies of TMS and rTMS to treat depression. Several studies included a single placebo condition and 2 active TMS/rTMS conditions. The authors included each of these comparisons as individual observations. Therefore, because patients who received placebos were compared to each active condition, the total number of patients is artificially large and duplicates patients. Similarly, in studies that used a crossover design, each phase of the study is presented as a separate comparison. Two studies did not involve a placebo comparison. Conca et al. (55) compared a group assigned to TMS plus medication with a group treated with medication only. Kimbrell et al. (57) compared high-frequency LDLPFC rTMS (20 Hz) with low-frequency LDLPFC rTMS (1Hz). Because this latter study had 2 active treatments, its inclusion in the meta-analysis may be questionable. However, Burt et al. included it because the predominant hypothesis in the field was that high-frequency LDLPFC rTMS would be more effective.
The combined effect size (Cohen’s d) for the 23 comparisons was 0.67, which indicates a moderate to large effect. As shown in Figure 5 below, Cohen’s d also indicated that there was significant heterogeneity in the effect size (Q22=47.08, P=.001); however, the detection of heterogeneity was marginal with the more conservative Hedges’ g (Q22=33.21, P=.06).
Figure 5: Effect Size (d) and 95% Confidence Interval for Randomized Controlled Trials of TMS and rTMS in the Treatment of Depression*.
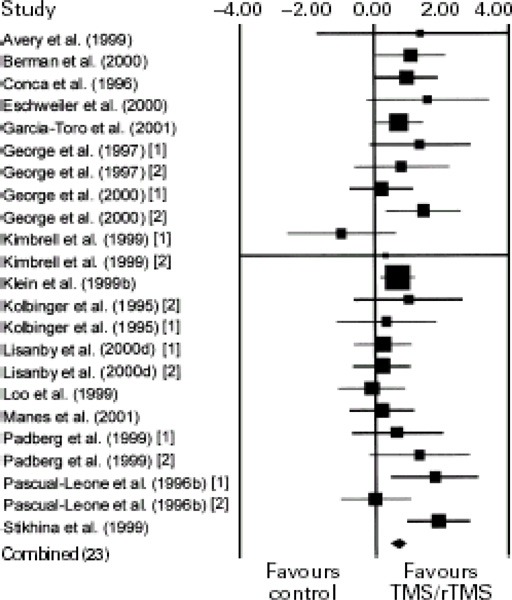
rTMS represents repetitive transcranial stimulation. The size of the boxes is proportional to the sample size. The overall combined effect size is indicated by a diamond. Figures within brackets following the study’s authors refer to specific comparisons within a study.
Burt T, Lisanby SH, Sackeim HA. Neuropsychiatric applications of transcranial magnetic stimulation: a meta analysis. International Journal of Neuropsychopharmacology 2002; 5(1):73-103; Subject to the copyright notice provided by Cambridge University Press, Reprinted with the permission of Cambridge University Press and the author (9)
Stikhina et al. (1999) reported an especially large effect size. In this study, rTMS was administered at an intensity that is unlikely to have biological effects (0.015 tesla) to patients who were characterized as having “neurotic depression.” Removing this study did not influence the overall results.
Kimbrell et al. (57) found that low-frequency rTMS (1 Hz) over the LDLPFC had a superior outcome to high-frequency rTMS (20 Hz) over the same location. If this study was removed, the heterogeneity in combined effect size would still be significant for Cohen’s d (Q21=37.96, P=.01) but not for Hedges’ g (Q21=27.45, P=.16).
Burt et al. also examined if the effect sizes differed in studies using slow rTMS (<1 Hz) or fast rTMS (>1Hz). Five comparisons involved slow rTMS, and 18 comparisons involved fast rTMS. The point estimate for Hedges’ g was higher for slow rTMS (g=0.68, SE=0.17, z=3.93, P=.0001) than for fast rTMS (g=0.58, SE=0.13, z=4.37, P<.0001). These analyses suggest that higher-frequency stimulation dos not necessarily enhance the antidepressant properties of TMS.
Moreover, the magnitude of the effect was of doubtful clinical significance. The authors re-examined the therapeutic effect after excluding 3 questionable comparisons that may have biased these results: the Pascual-Leone et al. (1996b) use of rTMS over the RDLPFC, the Kimbrell et al. (1999) (57) comparisons of 20 Hz and 1 Hz rTMS/TMS over the LDLPFC, and the Stikhina et al. (1999) use of 0.015 tesla stimulation over the LDLPFC. The mean unweighted percentage improvement in the HDRS scores in the active conditions of the remaining studies was 28.94% (SD, 23.19). The mean percentage improvement with placebo was 6.63% (SD, 25.56). Again, the therapeutic change was modest with rTMS; furthermore, relatively few patients met the standard criteria for successful response (50% reduction in HDRS score) or remission (final HDRS ≤8). (9)
Burt et al. concluded that the modest therapeutic effects of rTMS in major depression may suggest that its primary role might be as an add-on or augmentation strategy. Most of the studies in Tables 3 and 4 of Appendix 3) limited rTMS administration to either 5 or 10 sessions, corresponding to approximately 1 or 2 weeks of treatment. Antidepressant medications typically have a delayed onset of action. Burt et al. suggested that a role for either slow or fast rTMS might be to provide some symptom relief while patients await the full impact of antidepressant medications. They classified the 23 comparisons according to whether or not the sample was medication free. If most of the patients were not receiving antidepressant medications, the comparison was classified as medication free. For 8 comparisons of medication-free patients, Hedges’ g point estimate was 0.71 (SE= 0.12, z=5.92, P<.0001). For 15 comparisons of patients receiving medication, Hedges’ g point estimate was 0.60 (SE=0.20, z=3.03, P=.003). Based on these results, it did not appear that concomitant pharmacotherapy either enhanced or detracted from the therapeutic effects of rTMS.
Burt et al. stated that the issue of medication resistance deserves greater attention. The studies to date have recruited patients reported to have medication-resistant depression, or they have comprised mostly patients with medication-resistant depression. Medication resistance is a negative predictor of response to ECT. Burt et al.suggested that whether slow or fast rTMS has greater clinical potential when administered to patients earlier in the course of antidepressant treatment still needs to be determined. Furthermore, the relationship between degree and specific forms of medication resistance and rTMS response need to be determined. The authors supported their claim by stating that there is evidence that failure to respond to adequate treatment with a selective serotonin reuptake inhibitor has little predictive value for ECT response. Conversely, failure to respond to adequate treatment with a tricyclic antidepressant predicts a lower response probability.
Comparisons With Electroconvulsive Therapy
The fifth and sixth tables of Appendix 3 summarize the 3 studies that randomized patients to treatment with either rTMS or ECT. According to Burt et al. (9), a meta-analysis of the 3 studies (n=112) yielded a combined Cohen’s d of 0.21 favouring ECT. No plot of the combined effect size or 95% CI was reported by the authors. There was no significant heterogeneity in effect size (i.e., no data were reported).
The mean improvement in HDRS scores in rTMS conditions was 47.13%, about double the degree of therapeutic improvement observed in the 23 comparisons of the controlled studies in Tables 3 and 4. However, the reasons for this greater therapeutic effect (which was also of greater clinical significance) are unknown. Burt and colleagues considered 2 possibilities:
The studies provided longer courses of rTMS (20 sessions in the studies by Grunhaus et al. (unpublished data) and the number of treatments based on degree of clinical progression in the study by Pridmore et al. (52) Therefore, it is possible that extended treatment with rTMS may have greater antidepressant effects.
The samples in these studies were selected for receiving ECT. Typically, patients selected for ECT are unique in the severity of their depressive symptoms and the presentation of endogenous or melancholic features. They also have a high rate of psychotic depression. A more favourable rTMS response may have existed in these patients with severe depression.
Additionally, the mean improvement in HDRS scores with ECT was only 54.47%, which is relatively low for this form of treatment. Previous studies on high-dosage ECT reported a 69% to 73% improvement in HDRS scores immediately after treatment. When patients with psychotic depression were excluded, these values were 75.66% (SD, 29.68) for high-dose bilateral ECT and 72.92% (SD, 25.37) for high-dose right unilateral ECT. Therefore, Burt et al. suggested that the degree of improvement observed with ECT in the rTMS/ECT comparisons was suboptimal. The reason for this is unknown, but Burt and colleagues suggested an underestimation of the therapeutic effects of ECT relative to prolonged courses of rTMS.
TMS in the treatment of depression: age
Kozel et al.(53) suggested that prefrontal atrophy advances at a greater rate with aging, which may result in the underdosing of magnetic stimulation to older patients.
Limitations to the meta-analysis of Burt et al. (9) included the following:
No report of the details of the literature search they did for the meta-analysis
All of the limitations previously mentioned in Martin et al. (3)
Small size of trials; no sample size calculation or justification
No discussion of the baseline depression values among the study groups
No discussion of randomization bias
Meta-analysis of rTMS compared to placebo, McNamara et al.
McNamara et al. (37) conducted a systematic review and literature search up to January 2000. The aim of the study was to evaluate the evidence for the effectiveness of rTMS in mood disorders. Placebo-controlled RCTs were included. Explicit exclusion criteria were not reported.
Results
Sixteen published clinical trials of rTMS for depression were identified. Eight studies were excluded because they did not have a randomized control group. One study was excluded because people in the control group were treated with ECT. (54) Another study was excluded because there was no placebo TMS treatment included as part of the trial protocol. (55) Yet another trial, which had 18 patients and failed to show any significant benefit of rTMS, was excluded because the “individual responses” of patients in the TMS and control groups were not available. (56)
Therefore, 5 studies were included in the meta-analysis. The standardized placebo treatment was application of the TMS coil at an ineffective angle of 45 or 90 degrees on the scalp. All of the trials included only patients who met DSM criteria for a major depressive episode. In 4 of the 5 studies, the patients remained on antidepressant medication. In the remaining study, medication had been withdrawn for at least 1 week prior to commencing the trial.
The authors reported that “the overall chi squared test of association was 13.3 (df=1), implying a statistically significant benefit of rTMS (P<.001).” There was no significant heterogeneity in the studies (Q4 = 5.3, P>.1). The difference between the undefined “rate of improvement” in the treated group and that in the control group was 43% (95% CI, 25%–61%). The estimated number-needed-to-treat was 2.3 (95% CI, 1.6–4.0). McNamara et al. did not further discuss the number needed to treat. The duration of the therapeutic response was not defined in any of these studies.
Adverse Effects
There were no withdrawals from treatment due to adverse effects. No seizures were reported. In all the studies, patients reported getting transient headaches after the TMS sessions. In all cases, it was reported that the headache resolved spontaneously or responded to treatment with analgesics. The only other adverse effect recorded was local scalp discomfort at the site of the treatment during the session.
McNamara et al. stated that further large trials will be needed if rTMS is to be considered as an alternative to ECT in treating selected patients with depression. Specifically, they noted that the following questions will need to be answered in future trials:
Is rTMS as effective as ECT in depression?
What is the most effective treatment regimen for rTMS?
Are there any subgroups of patients who do particularly well with rTMS?
Limitations to the meta-analysis by McNamara et al. included the following:
Some patients may not have been blinded by the placebo condition (orientation of coil to scalp).
Duration of antidepressant effect was not defined in any of the studies.
Small size of trials; lack of sample size calculation or justification.
No discussion of the differences between the studies.
No discussion of the baseline depression values among the groups.
No discussion of randomization bias.
Meta-analysis of rTMS compared with placebo, Holtzheimer et al.
Holtzheimer et al. (10) conducted a meta-analysis of rTMS in the treatment of major depression. The scope of the study was limited to placebo-controlled studies of rTMS applied to the prefrontal cortex in the treatment of major depression. A literature search was conducted, but no cut-off dates were reported. Inclusion criteria consisted of the following:
Placebo-controlled study design (crossover or parallel groups); studies that used another treatment for depression as a control (such as medication alone or ECT) were excluded
Patients with depression (MDD or bipolar disorder, depressed)
Reported means and standard deviations for HDRS scores before and after treatment; the 17-, 21-, or 25-item HDRS was accepted
Prefrontal cortical stimulation (left or right) as the treatment condition
No overlap in patient population with another included study
Thirteen placebo-controlled studies were initially identified. Of these, one did not provide the information necessary to determine its adequacy for inclusion or to calculate an effect size. Therefore, 12 studies were included in the meta-analysis. This allowed for the calculation of 16 individual SMD effect sizes. Initial results for the overall weighted mean effect size failed homogeneity testing, so a random-effects model was applied.
The overall weighted mean effect size, based on the raw decrease in HDRS scores in the 12 studies (16 effect sizes) was 0.81 (95% CI, 0.42–1.20, P<.001). The weighted mean effect size for those studies using LDLPFC stimulation (11 studies, 14 effect sizes) was 0.89 (95% CI, 0.44–1.35, P<.001). The weighted mean effect size for those studies using LDLPFC stimulation in a parallel-groups design (7 studies, 9 effect sizes) was 0.88 (95% CI, 0.22–1.54, P<.01). In the last analysis, it was noted that 1 study was a distinct outlier. (40) The weighted mean effect size excluding this study (6 studies, 8 effect sizes) was 0.63 (95% CI, 0.15–1.11, P<.01).
Eleven of the 12 studies reported the number of responders, which was defined as a more than 50% decrease in HDRS scores. Overall, 25 (13.7%) of the 183 patients treated with rTMS were classified as responders compared with 10 (7.9%) of 127 patients given the placebo stimulation. These data appeared to have been heavily influenced by one study in which a relatively large proportion of patients in both of the conditions responded. (5) When this study was excluded, 7 (4.7%) of 148 patients treated with rTMS and 2 (2.1%) of 95 patients given placebo stimulation were classified as responders.
Four of the 12 studies included in this analysis commented on the duration of antidepressant effects of rTMS when present. Pascual-Leone et al. (47) reported that the antidepressant effects seen with LDLPFC stimulation “tapered off” after 14 days, and HDRS scores essentially returned to baseline. Avery et al. (42) followed-up on a patient who showed a robust response to rTMS (at 3 weeks post-treatment, the patient had an HDRS score of 0, and at 1 year post-treatment she continued to rate herself as very much improved). Kimbrell et al. (57) noted that most patients with an initially robust response to rTMS with one stimulation frequency tended to have worse depression when they were crossed over to treatment with the other frequency. It was also noted that 1 patient who showed a partial response to rTMS sustained this response for 6 weeks; however, it is unclear if the patient received rTMS during this follow-up period. Finally, Padberg et al. (58) reported that 1 patient with a very robust response to rTMS (a decrease in the 21-item HDRS score from 47 to 7) maintained this response at 2 months follow-up.
Holtzheimer et al. (10) acknowledged that the studies included in the analysis differed from one another on multiple design parameters. In fact, when studies were segregated into groups with homogenous design characteristics so that a correlation with effect size could be calculated, the groups were too small to allow meaningful analysis. The authors stated that certain design characteristics (stimulation frequency, intensity, and number of sessions) were analyzed individually and showed no significant correlation with effect size.
According to Holtzheimer and colleagues the mean effect size statistic, which expresses the difference between treatment and control in terms of a pooled SD, does not clearly address the clinical significance of rTMS in the treatment of depression. Many studies of rTMS have revealed statistically significant antidepressant effects, but these effects were usually modest. Of note, none of the studies in the meta-analysis have demonstrated a mean decrease of more than 50% in HDRS scores in the rTMS group. Overall, only 22% of the patients treated with rTMS across all of the included studies showed a more than 50% decrease in HDRS scores, compared with 7.9% of patients treated with placebo.
Table 8: Number of Responders (>50% Decrease in HDRS Score) by Study*†.
| Study | rTMS† (n/N) |
Placebo (n/N) |
|---|---|---|
| Pascual-Leone et al. | 4/17 | 0/17 |
| Pascual Leone et al. | 0/17 | 0/17 |
| George et al. | 1/12 | 0/12 |
| Avery et al. | 1/4 | 0/2 |
| Klein et al. | 17/35 | 8/32 |
| Loo et al. | 0/9 | 0/9 |
| Kimbrell et al. | 0/13 | 0/3 |
| Kimbrell et al. | 1/10 | 0/3* |
| Padberg et al. | 0/6 | 0/6 |
| Padberg et al. | 0/6 | 0/6 |
| Berman et al. | 1/10 | 0/10 |
| Eschweiler et al. | NA | NA |
| George et al. | 3/10 | 0/10 |
| George et al. | 6/10 | 0/10 |
| Garcia-Toro et al. | 5/17 | 1/18 |
| Avery et al. | 2/7 | 1/8 |
| Overall | 41/183 (22%) | 10/127 (7.9%) |
HDRS represents Hamilton Depression Rating Scale; rTMS, repetitive transcranial magnetic stimulation
Used with permission from the authors; Holtzheimer PE, III, Russo J, Avery DH. A meta-analysis of repetitive transcranial magnetic stimulation in the treatment of depression. Psychopharmacology Bulletin 2001; 35(4):149-169. Incorporates erratum reported in Psychopharmacology Bulletin 2003, Spring; 37(2): 5. (10)
In the study with the largest individual effect size, (40) patients in the rTMS group had a mean post-treatment 25-item HDRS score of 23.2, despite a mean drop of 14 points. Two studies reported on a patient with remission (defined as a post-treatment HDRS score <8) following rTMS that persisted for at least 2 months in 1 study (58) and for 1 year in the other. (42) The studies did not report the necessary follow-up data to determine the overall duration of any antidepressant effects of rTMS.
Holtzheimer et al. (10) suggested that the patients included in these studies had such severe and/or treatment-resistant depression that little improvement could have been expected. They also suggested that rTMS in less severely depressed or less treatment-resistant patients might result in more substantial antidepressant effects. Klein et al. (5) assessed patients who were not treatment resistant and who received rTMS. They found one of the largest overall drops in HDRS scores and reported the largest response rate (49%) compared with other studies. However, the placebo group also showed significant improvement and had a 25% response rate.
Holtzheimer et al. said they were unable to explain why their results showed that rTMS is an effective treatment for depression although many of the included studies did not show this. They suggested that by using a random-effects model, the heterogeneity among studies was explained statistically, but not clarified. They concluded, “rTMS has real antidepressant effects that can be large at times but are generally modest.” However, they did not clarify the clinical versus statistical significance.
Limitations of their study are similar to those in Martin et al., (3) McNamara et al., (37) and Kozel and George. (8)
Alberta Heritage Foundation for Medical Research
In May 1999, the commentary from a technology scan of rTMS by the Alberta Heritage Foundation for Medical Research (38) stated:
“Use of rTMS to treat depression is based on potential to replace some ECT treatments currently used for severely depressed patients. The technology is in the early states of assessment. Efficacy is not established. Longer term data, including comparison with other treatments, are needed.”
Summary of Medical Advisory Secretariat Review
The Medical Advisory Secretariat searched MEDLINE and EMBASE after the literature search cutoff date for the most recent systematic review, from June 2001 to April 2004, for articles comparing rTMS with alternative treatments (including ECT). Additionally, the literature was searched from April 2002 to April 2004_for articles that compared rTMS with a placebo. This search yielded 383 studies of which 13 met the inclusion criteria. The quality of the included articles is presented in Table 9.
Table 9: Quality of Evidence.
| Study Design | Level of Evidence |
Number of Eligible Studies |
|---|---|---|
| Large RCT,* systematic reviews of RCT | 1 | |
| Large RCT unpublished but reported to an international scientific meeting | 1(g)* | |
| Small RCT | 2 | 11 |
| Small RCT unpublished but reported to an international scientific meeting | 2(g) | |
| Non-RCT with contemporaneous controls | 3a | 1 |
| Non-RCT with historical controls | 3b | |
| Nonrandomized study presented at international conference | 3(g) | |
| Surveillance (database or register) | 4a | |
| Case series (multisite) | 4b | |
| Case series (single site) | 4c | 1 |
| Retrospective review, modeling | 4d | |
| Case series presented at international conference | 4(g) |
RCT refers to randomized controlled trial; g, grey literature
Update of rTMS versus ECT
O’Connor et al.(11) compared the effects of rTMS (n=14) and ECT (n=14) on mood and cognition in patients with MDD (Level 3a evidence). All patients met DSM-IV criteria for MDD. Only patients with pretreatment HDRS scores of 18 or higher were included. Patients enrolled in the rTMS protocol underwent at least a 2-week washout of all psychotropic drugs and were considered “medication free” at the time rTMS was initiated. However, patients who received ECT continued to take medication; therefore, they received ECT as an add-on treatment. Exclusion criteria were as follows:
Psychosis
Acute suicidality
Other current Axis I diagnoses in DSM-IV
Known central nervous system pathology
Pacemakers
Electronic or metallic implants
Severe cardiac pathology
Personal or first-degree family history of a seizure disorder
Inability to give informed consent
There were no statistically significant between-group differences for age, education, baseline memory performance, and estimates of verbal IQ. However, the groups differed in their levels of baseline depression. Before treatment, patients in the rTMS group had a mean HDRS score of 29 compared with patients in the ECT group who had a mean HDRS score of 39 (P=.001).
ECT (right unilateral) was conducted 3 times per week. The number of sessions varied according to clinical response (range, 6–12). The dosage intensity was 2.5 times the patient’s seizure threshold. Testing of ECT patients was conducted a minimum of 2 hours after treatment to minimize the effects of anesthesia and post-ECT confusion (range 2–4 hours).
rTMS was administered in daily sessions of 1600 stimuli in 20 trains of 8-second duration with 24-second intertrain intervals. Stimulation parameters were 10 Hz at an intensity of 90% of the motor threshold. A focal 70 mm double cone coil was centered over the LDLPFC based on distance from the motor cortex. rTMS sessions, 5 per week, occurred for 2 weeks.
Patients were tested on 3 occasions. Baseline testing was done on the first day of treatment with ECT or rTMS. The second test was at the end of the treatment course. For patients receiving ECT, the end of treatment varied according to clinical outcome. It ranged from 2 to 4 weeks (6–12 sessions) after the first treatment session. The end of rTMS treatment was fixed by the research protocol. Patients were tested after the 10-day trial of rTMS (2 weeks) without regard to their clinical response. The third test was 2 weeks after the final ECT or rTMS treatment. For rTMS, it occurred 4 weeks after the first rTMS treatment. For ECT, it happened 4 to 6 weeks after the initial ECT. A research assistant blinded to the hypotheses, but not to treatment allocation, did the testing.
Statistical Analysis
Baseline group differences on mood ratings and cognitive tasks were compared using unpaired t tests. Treatment-related differences were analyzed according to a 2x3 repeated measure analysis of variance (ANOVA). Between-subject factors were the 2 treatments (ECT or rTMS). The test sessions (baseline, end of treatment, and follow-up) were the within-subject factors. (11)
Mood Ratings
Both groups demonstrated reduced depression over the course of treatment, as measured by scores on the HDRS. Patients receiving ECT showed a decline in severity of depression from baseline (mean 39, SD 7.25) to the end of treatment (mean 15.3, SD 11.7; no P value provided). At the 2-week follow-up, these patients continued to show a reduction in depression (mean 20.4, SD 9.5). Patients receiving rTMS had a modest reduction in severity of depression from baseline (mean 29.33, SD 4.90) to end of treatment (mean 25.6, SD7.7, no P value provided). This was also seen at the 2-week follow-up session (mean 24.8, SD 9.5, no P value provided). None of the participants in the rTMS group demonstrated a 50% reduction in depression severity on the HDRS.
Figure 6: Severity of Depression: Mean Score on the Hamilton Depression Rating Scale. From O’Connor et al.(11).
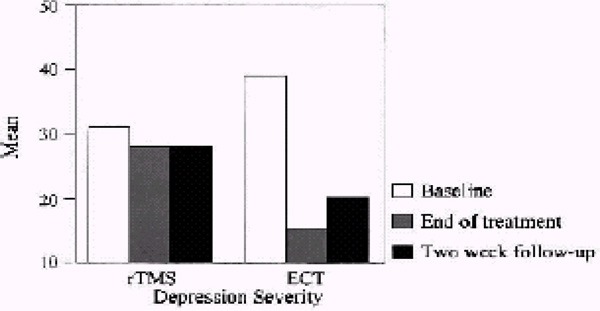
The repeated-measure ANOVA indicated that there was a significant effect of treatment session on changes in depression levels (P<.01), but there were no significant differences between groups (P>.05). There was, however, a significant interaction between the treatment session and treatment group with respect to changes in levels of depression (P<.01).
Working Memory – Letter Numbering (LN) Sequencing
Comparisons were based on 13 ECT and 14 rTMS patients (1 missing due to a scheduling conflict). There was no difference between the groups at baseline (ECT mean, 10.92, SD 2.49; rTMS mean, 10.42, SD, 3.00). At the end of ECT treatment, there was a small decline (mean, 9.23, SD, 1.83; no P value provided) and improvement at the 2 weeks follow-up (mean, 11.15, SD 1.46; no P value provided). Those who received ECT did not show a statistically significant difference in LN results when baseline performance was compared directly with the 2-week follow-up results (P>.05). The patients who received rTMS showed mild improvement on the LN test over all sessions (end of treatment mean, 10.71, SD, 3.83; 2-week follow-up mean, 11.14, SD, 3.08; no P values provided).
The repeated-measure ANOVA revealed a main effect of treatment session on LN (P<.05) but there was not a main effect of treatment group (P>.05). There was no significant interaction between treatment session and groups on LN (P>.05).
New Learning – Acquisition
It is assumed that 14 ECT patients and 14 rTMS patients were examined for this outcome. At baseline, both groups performed similarly (ECT mean, 43.78, SD 11.07; rTMS mean, 43.71, SD, 12.09). Patients in the ECT group showed reduced acquisition at the end of treatment (mean, 29.14, SD 7.93) and improved acquisition at the 2-week follow-up session (mean, 46.92, SD, 10.80). The difference between baseline acquisition and performance on the acquisition task at the 2-week follow-up session was not significant (P>.05). Patients in the rTMS group showed similar acquisition across the test sessions (end-of-treatment mean, 43.00, SD 10.09; 2-week follow-up mean, 44.07, SD 10.43).
Repeated measure ANOVA yielded a significant main effect of treatment session (P<.01), but there was not a significant main effect of treatment group (P>.05). The treatment session by treatment group interaction was significant (P<.01).
Figure 7: New Learning: Mean Number of Words Recalled on the Rey Auditory Verbal Learning Test Over 5 trials*.
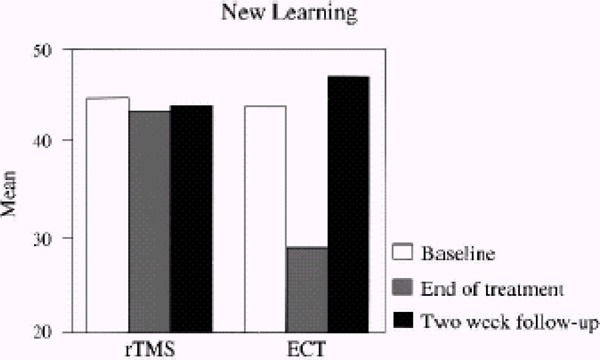
From O’Connor et al. (11)
New Learning – Retention
It is assumed that 14 ECT and 14 rTMS patients were available for this testing. The patient groups did not differ in baseline retention (ECT mean, 8.07, SD, 4.49; rTMS mean 9.76, SD, 3.08). Those in the ECT group showed reduced retention at the end of treatment (mean, 2.15, SD, 1.99) and subsequent improvement at the 2-week follow-up (mean, 8.92, SD, 4.14). There was no statistically significant difference for these patients when baseline performance was compared directly with the 2-week follow-up results (P>.05). Patients in the rTMS group showed stable retention across test sessions (end-of-treatment mean, 8.23, SD, 2.80; 2-week follow-up mean, 8.31, SD, 4.07).
Repeated-measure ANOVA indicated a significant effect of treatment session (P<.01) and a significant effect of treatment group (P<.05) on retention. Repeated measures ANOVA also indicated a significant interaction between treatment group and session (P<.01).
New Learning – Retrograde Memory
Due to scheduling conflicts, data were available for only 10 patients in the ECT group and 11 in the rTMS group. The groups did not differ according to baseline retrograde memory performance (ECT mean, 64.30, SD, 19.4; rTMS mean, 55.63, SD, 18.12; no P value was provided). At the end of treatment, patients in the ECT group showed reduced recall (mean, 39.10, SD, 13.21) and subsequent improvement at the end of the 2-week follow-up (mean, 59.20, SD, 20.67). Patients in the rTMS group demonstrated improvement in retrograde memory at the end of treatment (mean, 57.81, SD, 18.33) and the 2-week follow-up (mean 61.54, SD, 19.12).
Repeated-measures ANOVA yielded a significant main effect of treatment session (P<.01), but there was no significant main effect of group (P>.05). A significant interaction between treatment group and treatment session was reported (P<.01).
Figure 8: Remote Memory: Mean Number of Items Recalled From the Transient News Events Test*.
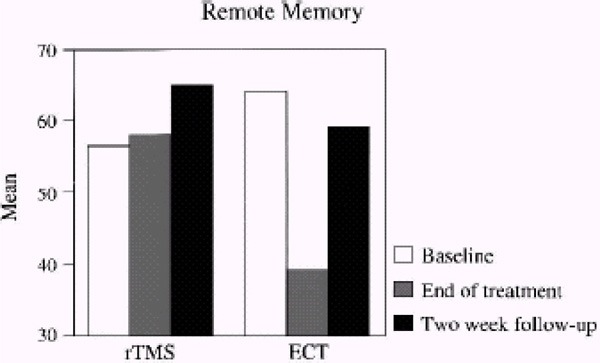
From O’Connor et al. (11)
Authors’ Conclusions
ECT resulted in a significant reduction in depressive symptoms, and this effect was sustained 2 weeks after the end of treatment. ECT was associated with transient negative effects on some components of memory and persistent effects on one task of retrograde amnesia.
For rTMS, the magnitude of therapeutic response was small, and none of the patients in this group demonstrated a 50% reduction in severity of depression. However, the authors concluded that rTMS does not adversely affect attention and memory.
Limitations
There was no discussion of sample size requirements, calculations, or what the study was statistically designed and/or powered to answer and/or not answer specifically.
The reporting of P values was inconsistent and sporadic.
There was no discussion of how patients were allocated to treatment. The authors said that patients referred to the ECT service or to the Laboratory of Magnetic Stimulation at Beth Israel Deaconess Medical Center were offered participation in the study. No further information was provided.
There were differences in depression severity between the patient groups at baseline. Patients in the ECT group were more depressed at baseline; however, they also showed more improvement at the end of treatment and at the 2-week follow-up than did patients in the rTMS group.
The test for retrograde memory used in the study is sensitive to practice effects. Therefore, improvement on repeat administrations may have been caused by being previously exposed to items. The authors said, “Because only a limited number of...items were available, the same test was administered on all three sessions.” (11) In addition, the patient may have become more familiar with the examiner and more relaxed in the testing situation.
It was a short study (2 weeks follow-up) with a very small sample size.
For ECT, treatment continued until relief from depression was observed. However, for rTMS, treatment went according to a previously established research protocol, so that treatment was terminated after 10 days regardless of therapeutic response. Results might have been different if rTMS was carried out according to clinical outcome rather than to the “protocol requirements.”
Efficacy data were obtained at the end of treatment for both groups, rather than in accordance with a fixed schedule.
The treatment arms were unbalanced. In the ECT group, patients received ECT as an add-on to drug therapy. In the rTMS group, patients received rTMS without the adjunct of drugs. O’Connor et al. (43) argued it is unlikely that the concurrent medications would have been significantly more effective, because a previous trial of rTMS with concurrent medication showed only discrete antidepressant efficacy.
Randomization was not discussed.
Intent-to-treat analysis was not discussed.
In 2003, Grunhaus et al.(59) reported on an RCT of ECT (n=20) and rTMS (n=20) of patients with severe and resistant nonpsychotic depression (Level 2 evidence). All of the patients were referred for ECT by their treating physician after they failed at least one course of antidepressants (at adequate levels and for at least 4 weeks of treatment). Inpatients and outpatients were included. Inclusion criteria were as follows:
Diagnosis of MDD (unipolar) according to DSM-IV criteria. Diagnosis was reached following detailed and semistructured clinical interviews by senior clinicians.
A score of 18 or more in the 17 item HDRS.
Not meeting any of the exclusion criteria stipulated in the safety guidelines for TMS and rTMS as stated by Wasserman.
Being over 18 years of age.
MDD was not secondary to a general medical condition or substance abuse.
Exclusion criteria were any additional Axis I diagnoses, including MDD with psychosis.
Patients were evaluated at baseline, 2 weeks into treatment, and at the end of treatment with clinical ratings that included the following the following:
17-item HDRS
Brief Psychiatric Rating Scale (BPRS)
Global Assessment of Function Scale (GAF)
Global Depression Scale (GDR)
Pittsburgh Sleep Quality Index (PSQI)
Mini-Mental State Examination (MMSE).
Patients were assigned to rTMS or ECT based on a previously defined random list. Ratings were performed by trained research assistants blind to treatment modality. (This was done by hiring assistants that did not regularly work with the program.) Patients in both groups were progressively withdrawn from psychotropic medications. The tapering process was finished within 3 days of starting the study. During the study, the only medication allowed was lorazepam (up to 3 mg per day). Patients in both groups were required to delay ingestion of lorazepam until after their treatment for that day.
A trained psychiatrist administered rTMS 5 times per week for 4 weeks (total, 20) over the LDLPFC. Stimulation was done at 10Hz and 90% motor threshold with 1200 pulses each day (20 6-second trains with a 30-second interval between the trains).
Treatments with ECT were continued until the treating physician considered that a therapeutic response had been obtained or that no further therapeutic benefit could be expected. Patients were required to have at least 6 ECT treatments, unless the course was suspended owing to an early therapeutic response. Patients received a mean of 10.25 (SD, 3.1) ECT treatments.
Patients were randomized to either rTMS or ECT groups based on a computer-generated list. The primary outcome variables were treatment response and the change in the HDRS scores. Additional outcomes variables were changes in the scores of the BPRS, GAF, GDR, PSQI, and MMSE. A sample size calculation or justification was not reported.
Baseline ratings for most demographic and clinical variables were similar in both groups. Patients in the ECT group had significantly worse scores on the BPRS (P=.036) and the GAF (P=.007).
Positive response to treatment was defined as a decrease of 50% or more in HDRS scores, or a final rating of 10 or less on the HDRS, and a final GAF rating of 60 or more. Remission was defined as a final HDRS score of 8 or less.
The endpoints assessed were as follows:
Response rate
Change of scores with treatment (ANOVA). In rating scales with baseline differences between the groups (BPRS and GAF), ANOVA with repeated measures of change scores was used.
Extent of clinical response in those patients who improved with treatment was tested by comparing the final ratings of both groups (t test).
The overall response rate for both groups was 58% (23/40). The primary outcome variable of response to treatment was not statistically significant between ECT (12 responded, 8 did not) and rTMS (11 responded, 9 did not), P>.05.
Looking at the HDRS, GDR, PSQI, and MMSE, the ANOVA with repeated measures of absolute scores showed a significant effect of treatment but not of group or interaction. This suggests that scores changed similarly with either ECT or rTMS. Identical findings were found for the ANOVA with repeated measures of change scores for the BPRS and GAF.
Table 10: ANOVAs for Clinical Ratings in Patients With Major Depression Treated With ECT or rTMS, From Grunhaus et al. (59).
| Group Effect | Time Effect | Interaction | |||||
|---|---|---|---|---|---|---|---|
| Rating Scale | F (df) | P | F (df) | P | F (df) | P | |
| ANOVA with Repeated Measures of Absolute Scores | |||||||
| HRSD | .1(1, 38) | ns | 60.9(2, 76) | .01 | .1(2, 76) | ns | |
| GDR | .1(1, 38) | ns | 69.3(2, 76) | .01 | .3(2, 76) | ns | |
| PSQI | .02(1, 38) | ns | 5.3(2, 76) | .01 | 2.5(2, 76) | ns | |
| MMSE | 3.1(1, 34 | ns | .9(2, 68) | ns | .5(2, 68) | ns | |
| ANOVA with Repeated Measures of Change Scoresa | |||||||
| BPRS | 1.9(1, 38) | ns | 3.7(1, 38) | .05 | .05(1, 38) | ns | |
| GAF | 2.7(1, 38) | ns | 5.0(1, 38) | .05 | .3(1, 38) | ns | |
ANOVA, analysis of variance; ECT electroconvulsive therapy; rTMS, repetitive transcranial magnetic stimulation; HRSD, Hamilton Depression Rating Scale; GDR, Global Depression Scale; PSQL, Pittsburgh Sleep Quality Index; MMSE, Mini-Mental State Examination; BPRS, Brief Psychiatric Rating Scale, GAF, Global Assessment of Function.
Performed on scales with significant baseline differences.
Reprinted from Biological Psychiatry, 53(4), Grunhaus L, Schreiber S, Dolberg OT, Polak D, Dannon PN. A randomized controlled comparison of electroconvulsive therapy and repetitive transcranial magnetic stimulation in severe and resistant nonpsychotic major depression, p. 324-331, Copyright 2003, with permission from the Society of Biological Psychiatry
The final scores were similar between groups, with the exception of the GAF score in which the patients who received rTMS had higher scores. Although final GAF scores were higher in these patients, change scores (the difference between the baseline and final score) were similar in both groups (P>.05). The rate of remission was 30%, equal in both groups.
Table 11: Final Ratings for Responders Onlya From Grunhaus et al.(59).
| ECT Group (n =12) |
TMS Group (n =11) |
t | CI | P | |
|---|---|---|---|---|---|
| HRSD | 9.0 ± 3.8 | 6.8 ± 2.7 | 1.6 | (–.6, 5.1) | ns |
| BPRS | 24.3 ± 3.2 | 22.4 ± 2.2 | 1.5 | (–.5, 4.3) | ns |
| GAF | 69.3 ± 7.4 | 77.2 ± 4.1 | –3.0 | (–.13.2,–2.5) | .006 |
| GDR | .25 ± .45 | 0 ± .3 | .9 | (–.1,.4) | ns |
| PSQI | 5.5 ± 3.2 | 8.5 ± 4.6 | –1.7 | (–6.4,.4) | ns |
| MMSEb | 27.4 ± 2.1 | 28.8 ± .7 | –2 | (–2.8,.1) | ns |
Data are mean ± SD. ECT, electroconvulsive therapy; rTMS, repetitive transcranial magnetic stimulation; HRSD, Hamilton Depression Rating Scale; BPRS, Brief Psychiatric Rating Scale; GAF, Global Assessment of Function; GDR, Global Depression Scale; PSQL, Pittsburgh Sleep Quality Index; MMSE, Mini-mental State Examination.
HRSD decreased ≥50% or more from baseline or HRSD ≤ 10, and the final GAS ≥ 60.
n = 11.
Reprinted from Biological Psychiatry, 53(4), Grunhaus L, Schreiber S, Dolberg OT, Polak D, Dannon PN. A randomized controlled comparison of electroconvulsive therapy and repetitive transcranial magnetic stimulation in severe and resistant nonpsychotic major depression, p. 324-331, Copyright 2003, with permission from the Society of Biological Psychiatry
The adverse effects reported by the rTMS-treated patients were mild: 3 patients had a mild headache, and 2 required a benzodiazepine for sleep disturbances. No patient in the ECT-treated group had his or her course interrupted due to adverse effects.
The authors drew the following conclusions:
The clinical responses to rTMS and ECT were indistinguishable, which suggests that both treatments were effective for severe and resistant nonpsychotic MDD. This suggestion was supported by the findings on the primary and secondary outcomes.
The extent of the clinical response as assessed by the final ratings of the responders to treatment only was similar in both groups. The GAF was higher for the rTMS-treated patients; however, when delta scores were calculated, this difference disappeared.
The rate of remission (final HDRS <8) was 30%, equal in both groups.
Of note, Grunhaus et al. performed rTMS for up to 20 days. In many studies, rTMS has been done for 1 or 2 weeks only, and some studies have applied only 80% of the motor threshold. Grunhaus and colleagues suggested that shorter periods of stimulation or less intense paradigms of stimulation may be associated with weaker response rates.(60)
Limitations to the study by Grunhaus et al. were as follows:
The results apply to the short term only.
There was no masked or placebo comparison group. It is possible that the effects of rTMS could be secondary to placebo effects or to interactions between the treating psychiatrist and patient. However, a “true” placebo rTMS has yet to be finalized because placebo positioning of rTMS coils (placed at a 45 or 90 degree angle) can create excitability changes in the cortex.
The clinical method for determining the exact location of the LDLPFC is not ideal. Anatomical differences between patients may create positioning effects that may negatively affect the outcome. Using external navigational landmarks or neuronavigational methods (e.g., MRI) may be helpful.
The BPRS and GAF scores favoured patients in the rTMS group. The authors suggested that future studies might need to incorporate matching strategies using scores of depression, agitation/psychosis, and functional state.
The power of stimulation was based on the determination of the motor threshold in each case. This method may be inaccurate, especially in elderly patients, because it has been found that the scalp-to-cortex distance increases with age especially in the frontal cortex. Therefore, studies that provide stimulation based only on the motor threshold may be underpowered.
The sample size was small, and there was no sample size calculation. The authors said that the power of the statistics may have been insufficient.
Studies with more thorough cognitive batteries are needed. The MMSE is a crude measure of the cognitive effects of treatment. The MMSE results were similar between the rTMS and ECT groups at baseline and after treatment.
Dannon et al.(6) reported 3- and 6-month outcomes of a group of patients randomly assigned to ECT or rTMS (Level 2 evidence). Inclusion criteria were as follows:
Over 18 years of age
DSM-IV diagnosis of MDD with or without psychotic features (17-item HDRS ≥18)
No personal or first-degree family history of seizure
No major medical, neurological, or neurosurgical disorders
Dannon et al. noted that patients diagnosed with MDD with or without psychotic features and referred to ECT were randomly assigned to receive either ECT or rTMS. However, the authors did not report how many patients were initially diagnosed with MDD and referred to ECT. Patients who responded to a course of ECT (n=20) or rTMS (n=23) agreed to remain for follow-up at the outpatient program. Furthermore, the authors did not say how many patients did not respond to ECT or rTMS, and if all the patients who did respond agreed to be followed up. Response was defined as having HDRS scores of 10 or less, or showing a 60% drop in HDRS scores and a final Global Assessment Scale (GAS) score of 60 or more. Two patients in the rTMS group dropped out of the follow-up program; reasons were not provided.
Treatments were delivered at 90% motor threshold over the LDLPFC and administered daily for 20 days. Patients received 1200 pulses each day at 10 Hz.
To assess improvement during the treatment period, patients were evaluated with the HDRS and the GAS. The follow-up after ECT or rTMS was performed “naturalistically” with patients visiting the outpatient clinic once a month.
All patients received antidepressants, mainly selective serotonin reuptake inhibitors, at the end of treatment.
The main outcome of interest was the presence or absence of relapse at 3 and 6 months. Relapse was defined as a return of depressive symptoms meting DSM-IV criteria for MDD with a HDRS score of 16 or more points.
Results showed both groups were similar on all the parameters at the end of treatment. Over the 6 months, 8 patients (4 in ECT and 4 in rTMS) relapsed. The authors acknowledged that the small sample size made it difficult to judge whether the relapse rate (20%) is lower than that reported in other studies of ECT-treated patients. There was no statistically significant difference in the relapse rates between the ECT and rTMS groups. Two patients from the ECT group, and 1 patient from the rTMS group, relapsed during the first 3 months. By the sixth month, 2 patients from the ECT group and 3 from rTMS group had relapsed.
The clinical ratings of the HDRS and GAS at 3 and 6 months were comparable between the groups (Table 12).
Table 12: Clinical Variables at 3 and 6 Months Follow-Up.
| ECT group | rTMS group | t | df | C-I | p | |
|---|---|---|---|---|---|---|
| X ± SD | X ± SD | |||||
| (n=20) | (n=21) | |||||
| HRSD post 3 month | 7.71 ± 5.03 | 6.40 ± 4.91 | .846 | 39 | –1.83, 4.46 | NS |
| GAS post 3 month | 75.52 ± 13.81 | 79.75 ± 12.92 | –1.01 | 39 | –12.69, 4.23 | NS |
| HRSD post 6 month | 8.40 ± 5.60 | 7.90 ± 7.14 | .246 | 39 | –3.61, 4.61 | NS |
| GAS post 6 month | 72.80 ± 11.94 | 77.75 ± 17.13 | –1.06 | 39 | –14.40, 4.50 | NS |
| MATS 3 month | 1.92 ± 1.04 | 2.28 ± 1.07 | –.92 | 29 | –1.14,.43 | NS |
| MATS 6 month | 1.82 ± 0.98 | 2.44 ± 1.03 | –1.563 | 25 | –1.44,.20 | NS |
ECT, electroconvulsive therapy; GAS, Global Assessment Scale; HRSD, Hamilton Rating Scale for Depression; MATS, Michigan Adequacy of Treatment; rTMS, repetitive transcranial magnetic stimulation.
Reprinted from Biological Psychiatry, 51(8), Dannon PN, Dolberg OT, Schreiber S, Grunhaus L. Three and six-month outcome following courses of either ECT or rTMS in a population of severely depressed individuals—preliminary report. Biological Psychiatry, 687-690, Copright 2002, with permission from the Society of Biological Psychiatry (6)
The limitations to the study by Dannon et al. were as follows:
Few patients were studied.
The authors did not report how many patients were initially diagnosed with MDD and referred to ECT. Patients who responded to a course of ECT (n=20) or rTMS (n=23) agreed to remain for follow-up at the outpatient program. The authors did not report how many patients did not respond to ECT or rTMS and if all the patients who did respond agreed to be followed-up.
Initial baseline data for the patient groups (prior to any treatment) was not reported.
An intent-to-treat analysis was not discussed.
The follow-up period was brief.
Uncontrolled nature of the medication regimen during follow-up.
The assessment procedure was not blinded.
One-year relapse rates of patients with resistant MDD or psychotic MDD can be as high as 50%. Dannon et al. reported a relapse rate of approximately 20%. They said that further study on this issue is warranted.
In another study, Janicak et al. (61) compared the efficacy of rTMS with ECT for patients who had major depression and for whom ECT would be considered appropriate in a general clinical setting (Level 2 evidence). Inclusion criteria were as follows:
Aged 18 to 75 years
DSM-IV criteria for major depression (unipolar or bipolar)
Deemed clinically appropriate for a course of ECT by treating psychiatrist
Exclusion criteria were as follows:
Serious medical conditions that would preclude ECT or rTMS
History of clinically significant substance or alcohol abuse/dependency within the previous 3 months
Women who were pregnant or of childbearing potential and not taking contraception
Intracranial metallic or magnetic implants, or a pacemaker
All of the patients in the study had a chronic illness, scored more than 20 on the HDRS, and had multiple medication trials. Twenty-five patients were randomized either to rTMS or to ECT. One patient in the ECT group withdrew from the study after receiving 3 courses of treatment and before any clinical effect or assessment. One patient crossed over from ECT to rTMS (this patient “inadvertently received low-energy right unilateral ECT”). One patient withdrew from rTMS treatment after 4 sessions and before any clinical effect or assessment. Therefore, all analyses related to treatment are based on 22 patients. No further details of these patients were reported.
Following the initial randomized treatment study, patients who did not meet response criteria could crossover to the alternative arm. There were no differences between the rTMS and ECT treatment groups on age, number of previous hospitalizations, age at first episode, length of episode, length of medication washout, and baseline symptoms. For ECT, patients received up to 12 courses of treatments using bitemporal stimulus electrode placement. For rTMS, patients underwent 10 to 20 treatment sessions.
Before treatment, 20 of the 22 patients completed a brief medication washout (mean, 4 days; SD, 3 days). There were no differences between groups in medications received before washout. No patients were receiving depot neuroleptics. Those previously treated with fluoxetine had not received that medication for at least 1 month before starting the trial. Assessments comprised the 24-item HDRS, the BPRS, the Young Mania Rating Scale (YMRS), and the CGI. Response was defined a priori as a 50% decrease in HDRS score from baseline and a total HDRS score 8 or less. Every attempt was made to minimize the use of concomitant rescue medications.
A paired t test was used to compare baseline to end-of-treatment ratings for each group. A continuous measure of improvement was obtained by calculating percent change on the rating scale total scores ([pretreatment – post-treatment]/pretreatment). Categorization of responders and nonresponders was according to the criteria noted above.
Both the ECT (n=13, P<.000) and rTMS (n=9, P<.001) groups showed significant improvement. However, there were no significant differences between ECT and rTMS based on percent change scores from baseline to end of treatment. (Table 13.)
Table 13: Mean HDRS Scores and Percent Change.
| Baseline | End of treatment |
Percent change |
|
|---|---|---|---|
| rTMS (SD) (n = 13) |
32.2 (6.8) | 13.9 (11.1) | 55% (36) |
| ECT (SD) (n = 9) |
31.4 (8.5) | 10.9 (9.5) | 64% (30) |
| TOTAL (SD) (n = 22) |
31.9 (7.4) | 12.8 (10.4) | 59% (33) |
HDRS, Hamilton Depression Rating Scale; rTMS, repetitive transcranial magnetic stimulation; ECT, electroconvulsive therapy.
Reprinted from Biololgical Psychiatry, 51(8), Janicak PG, Dowd SM, Martis B, Alam D, Beedle D, Krasuski J et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: preliminary results of a randomized trial, p. 659-667, Copyright 2002, with permission from the Society of Biological Psychiatry (61)
For the a priori outcome, there was no significant difference in response rates between the groups. (Table 14)
Table 14: Number of Responders by Treatment Group.
| ≥50; ≤8 | <50%; >8 | Response rate | |
|---|---|---|---|
| rTMS (n = 13) | 6 | 7 | 46% |
| ECT (n = 9) | 5 | 4 | 56% |
Fisher’s Exact test; p = ns.
HDRS, Hamilton Depression Rating Scale; rTMS, repetitive transcranial magnetic stimulation; ECT, electro convulsive therapy.
Reprinted from Biololgical Psychiatry, 51(8), Janicak PG, Dowd SM, Martis B, Alam D, Beedle D, Krasuski J et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: preliminary results of a randomized trial, p. 659-667, Copyright 2002, with permission from the Society of Biological Psychiatry (61)
No differences were observed between the study groups on the secondary measures.
There were no significant adverse events (e.g., seizure). Generally, only mild adverse effects were reported in the rTMS group. Subjectively, patients reported various effects localized to the stimulation site, including mild pain or discomfort (n=6), feelings of warmth (n=3), a tapping sensation (n=2), and headache (n=1). There were no serious or unexpected adverse events associated with ECT, either. Adverse effects with bitemporal ECT included short-term memory impairment, drowsiness shortly after treatment, and anesthesia-induced confusion. (Specific patient numbers were not reported.)
The limitations to the study by Janicak et al. were as follows:
It had a small sample size and was a pilot study. No sample size calculation or justification was reported. The authors said that a larger study is warranted.
An intent-to-treat analysis was not discussed.
In a published comment to the article by Janicak et al., Kellner et al. stated that the sample size in the study was statistically inadequate to determine equivalence of treatments. In addition, Kellner et al. questioned the remission rate in the study, as it appeared to be lower than that commonly established to date.
Update of rTMS: Comparisons to Placebo or Different Frequencies or Different Locations
Studies Explicitly Stating Patients Were Treatment Resistant
Fitzgerald et al.(62) conducted a double-blind randomized placebo-controlled trial (n=60) to evaluate the efficacy of high-frequency rTMS on the LDLPFC and low-frequency rTMS on the RPFC in treatment-resistant depression (Level 2 evidence). Sixty patients with treatment-resistant depression who had failed to respond to therapy with multiple antidepressant medications were divided into 3 groups of 20 that did not differ in age, sex, or any clinical variables. All patients completed the double-blind phase.
rTMS was administered daily for 2 weeks using 20 5-second high-frequency LDLPFC trains at 10 Hz and 5 60-second low-frequency RPRC trains at 1 Hz. Placebo stimulation was applied with the coil angled at 45 degrees from scalp resting on the side of one wing of the coil. The main outcome of interest was the score on the MADRS.
Patients and raters were blind to treatment, but the clinician administering rTMS was not.
After the 10th session, a blinded assessment was made, and the patients, but not the raters, were told their treatment group. Patients who had a reduction in MADRS score of more than 20% in the active treatment arms could continue with the same rTMS condition for another 10 sessions. Patients who did not achieve this improvement were offered the option of crossing over to the other active treatment. Patients initially randomized to placebo were subsequently randomized to 1 of the 2 active treatments after initial review.
During the second phase of study, raters remained blind to treatment type. Patients were not deliberately withdrawn from medication before the trial, but their doses could not have changed in the 4 weeks before beginning the study, or during the study.
There was a significant difference between the high-frequency LDLPFC (reduction in mean score 13.5%, SD, 16.7%) and placebo (0.76%, SD, 16.2%) groups and between the low-frequency RPFC (15.0%, SD, 14.1%) and placebo groups (P<.005 for all). There was no difference between the 2 treatment groups (P=.91).
Concurrent use of medication did not have a statistically significant effect on primary outcome (P=.23).
After the randomized phase of the study, 7 (11%) of the 60 patients reported site discomfort or pain during rTMS, and 6 (10%) reported a headache after rTMS. There was no difference in the incidence of these adverse effects (P=.08).
Twenty-nine (48%) patients correctly guessed their type of treatment before blinding was removed, 17 (42%) of 40 in the active treatment group (P=.34), and 12 (60%) of 20 in the placebo group (P=.37). The degree of response was the main reason given for the guess.
The limitations to the study by Fitzgerald et al. were as follows:
No a priori sample size calculation or justification was reported.
It was a short-term study. The a priori primary outcome of interest was measured after 2 weeks of treatment.
An intent-to-treat analysis was not discussed.
Instead of “double blind,” the trial was single blind with an evaluation by external blinded assessors.
Any analyses after 2 weeks have limited validity, because patients were aware of their treatment.
In another study, Boutros et al.(63) did an RCT to examine the efficacy and safety of rTMS as an augmentation strategy in 22 treatment-refractory patients with depression without any modifications to their current drug therapy (Level 2 evidence).
Twenty-two patients diagnosed with MDD were recruited. Patients had to have failed 2 prior medication attempts and have a score of at least 20 on the HDRS. Patients were assessed at days 3, 5, 6, 8, and 10. A research assistant blinded to the treatment modality administered the depression score testing. An unblinded psychiatrist administered the rTMS. The blind was broken after the 10th session. Following completion of the placebo course, patients were offered open-label active rTMS. Treatment consisted of 10 left-sided stimulation sessions administered over 10 consecutive weekdays. Each session consisted of 20 2-second stimulation trains, with a 20 Hz frequency applied over 20 minutes and 58-second intervals between trains. Stimulations were delivered at 80% motor threshold. During placebo, the coil was angled 90 degrees to the head.
One patient dropped out of placebo rTMS group after the first session; data from that patient was excluded from the analysis. Twelve patients received active rTMS, and 9 received a placebo. The HDRS scores at baseline were not significantly different between the 2 groups.
Analyses included a comparison between the HDRS scores following the last treatment with baseline as a covariate (ANCOVA). No statistically significant difference between the 2 groups was found. A repeated-measures ANOVA found the slope for depression over time did not differ significantly between the 2 groups, but there was a significant time effect (P=.001).
The authors concluded, “The data provided above strongly suggest that the rTMS parameters used in this study for the treatment of depression (chosen as these were the prevailing parameters at the time of designing the study) in severely ill and treatment-resistant patients are not effective.”
Patients in both groups reported adverse effects (8 in active and 5 in placebo). The most frequent complaint was headache during or after the session. Three patients in the active and 1 in the placebo group reported transient scalp tenderness.
The limitations to the study by Boutros et al. were as follows:
No a priori sample size calculation or justification was reported.
The sample size used in the study was small.
It was a short-term study of 2 weeks.
An intent-to-treat analysis was not conducted.
Padberg et al.(13) conducted a parallel-design controlled study of 31 patients with MDD that was pharmacotherapy resistant (Level 2 evidence). The authors wanted to test the hypothesis that the antidepressant efficacy of rTMS is related to the stimulation intensity applied. Patients were randomized into a 2-week trial of LDLPFC rTMS either at 100% motor-threshold intensity (n=10), at 90% motor threshold (n=10), or at a low-intensity placebo (n=10). Placebo was angled 90 degrees to the head.
The dose of the currently unsuccessful antidepressant treatment was kept constant for at least 3 weeks before rTMS. Patients were examined by a psychiatrist uninvolved in rTMS treatment and blinded to the rTMS condition. A 7-point CGI severity scale was used as overall outcome measure.
No significant differences were found between the groups for baseline HDRS, MADRS, or CGI scores. One patient dropped out due to withdrawal of consent after the second rTMS session. Across the treatment groups, depression scores significantly declined during treatment (P<.001). However, the main effect of treatment group was not significant. Interaction of treatment group with time was significant for MADRS scores (P<.05) but not for HDRS scores.
The linear effect on the MADRS difference scores was significant (P<.05). Expressed as percent decrease of MADRS scores, receiving the placebo yielded a 4.1% (SE 5.2) reduction, 90% motor threshold resulted in 15.1% (SE 6.6) decrease, and 100% motor threshold reduced MADRS scores by 33.2% (SE 8.9).
The linear effect on HDRS scores was not statistically significant. Percent reductions of HDRS scores were 7.1% (SE 5.8) after placebo rTMS, 14.9% (SE 8.9) after 90% motor threshold, and 29.6% (SE 8.7) after 100% motor threshold.
The duration of hospital stay after rTMS and the number of antidepressant trials until discharge from the hospital were retrospectively assessed based on the patients’ charts. (Table 15)
Table 15: Antidepressant Trials and Duration of Hospital Stay by Treatment Group.
| After rTMS* | 100% MT* rTMS | 90% MT rTMS | Placebo rTMS |
|---|---|---|---|
| Antidepressant trials (No.) | 1.4±0.2 | 1.6±0.2 | 3.6±0.6 |
| Duration of hospital stay (days) | 42.6±10.2 | 60.6±12.7 | 135.0±38.0 |
rTMS represents repetitive transcranial magnetic stimulation; MT, motor threshold
Reprinted by permission from Neuropsychopharmcology, 27(4), Padberg F, Zwanzger P, Keck ME, Kathmann N, Mikhaiel P, Ella R et al. Repetitive transcranial magnetic stimulation (rTMS) in major depression: relation between efficacy and stimulation intensity., 638-645, Copyright 2002 Macmillan Publishers Ltd. Available at:. http://www.nature.com/npp/index.html
One-way ANOVA showed significant differences between groups for the duration of hospital stay (P<.05) and the number of required antidepressant trials after rTMS (P<.001).
No severe adverse effects of rTMS were observed.
The authors concluded, “The clinical improvement after rTMS significantly increased across the 3 groups, with best results at MT intensity, little improvement after subthreshold stimulation and no improvement after low intensity stimulation.”
The limitations to the study by Padberg et al. were as follows:
No a priori sample size calculation or justification was reported.
“Small sample size.”(13)
It was a short-term study (2 weeks).
No intent-to-treat analysis was discussed.
The authors examined the use of rTMS in patients with MDD who were pharmacotherapy resistant. However, one of the secondary endpoints of the study was to assess the number of antidepressant trials patients underwent until discharge from hospital. It is unclear how they defined drug resistance.
The authors said they “regard findings as preliminary.” Furthermore, they said, “As the hypothesized relationship between intensity and efficacy has major implications for the therapeutic use of rTMS, further studies are needed to prove this hypothesis and investigate rTMS over a larger range of MT related intensities.”
Conca et al. (64) conducted an RCT to investigate the augmentation properties of rTMS. They combined low and high frequencies of rTMS in 36 medicated inpatients who had severe depression (Level 2 evidence). Medication conditions were defined using the classification of Thase and Rush. (27) All patients had to be classified at stage 4 of treatment resistance, indicating a failure to respond to 2 different adequate monotherapy trials of medications with different pharmacological profiles and a failure to respond to a second augmentation strategy. For inclusion into the study, a minimum HDRS score of 24 had to be achieved.
Psychiatrists blinded to treatment methods administered the 21-item HDRS 1 day before the first treatment, 1 day after treatment and 1 day after completion of the entire rTMS course. The CGI scale was administered 1 day after rTMS.
Patients were randomly assigned to one of 3 rTMS treatment modalities while on stable drugs for 5 consecutive days at 1 session per day. The group protocols were as follows:
Group 1 (n=12)
110% motor threshold, 10 Hz, 10 trains, train duration 10 seconds, each with a train interval of 60 seconds over the LDLPFC. Over the RDLPFC: 110% motor threshold at 1 Hz, 1 train at 300 seconds.
Group 2 (n=12)
Only the LDLPFC was stimulated at 110% motor threshold, 10Hz, 10-second train duration alternating with 110% motor threshold, 1 Hz, 30-second train, with an interval of 6 seconds for 10 times per session.
Group 3 (n=12)
Standard stimulation over the LDLPFC using 110% motor threshold, 10 Hz, 13 trains, and 10-second train duration.
Therapeutic outcome was based on CGI scores. Patient response was defined as achieving at least moderate improvement (CGI improvement score >4) and being no more than mildly depressed (CGI severity score <4).
There were no statistically significant differences in clinical outcome between the groups. None of the demographic, illness, diagnosis, or co-diagnosis-related data revealed any influence on the response rate except for handedness: right-handed patients showed a weak statistical tendency to greater therapeutic response (nonresponders versus responders, P<.08).
No seizures were observed in any of the patients. Seven (19.1%) of the 36 patients experienced a mild headache during the first session. The headaches remitted spontaneously.
The limitations to the study by Conca et al. were as follows:
No a priori sample size calculation or justification was reported.
The sample size was small.
“Preliminary findings”. (64)
There was no placebo group.
Conca et al. was the only study identified in the updated Medical Advisory Secretariat literature search that fully defined “medication resistance” according to a detailed classification system developed by Thase and Rush. (27) (All patients had to be classified at “stage 4” of treatment resistance, indicating a failure to respond to 2 different adequate monotherapy trials of medications with different pharmacological profiles, and a failure to respond to a second augmentation strategy.)
Update of rTMS: Comparisons to Placebo or Different Frequencies or Different Locations
Studies Not Explicitly Stating Patients Were Treatment Resistant, and/or Using rTMS as Add-On Treatment
Hausmann et al. (66) conducted a single-centre, prospective, double-blind placebo-controlled add-on trial (n=41) to assess if bilateral rTMS is superior to unilateral rTMS and if there is a “speeding up” effect in the add-on group compared with the group receiving antidepressants alone (Level 2 evidence).
Forty-one medication-free patients with MDD without psychotic features admitted to a psychiatric unit were consecutively randomized into 3 groups. Group A1 (n=12) received unilateral active stimulation (20 Hz, 100% motor threshold, 10 trains of 10-second duration with a 90 second intertrain interval) of high-frequency rTMS over the LDLPFC and subsequent placebo low-frequency rTMS over the RDLPFC. Group A2 (n=13) received simultaneous bilateral active stimulation of high-frequency rTMS over the LDLPFC and low-frequency RDLPFC rTMS (1 Hz for 10 min, 120% motor threshold). Group C (n=13) received bilateral placebo stimulation. rTMS was administered on 10 consecutive workdays. The optimal LDLPFC and RDLPFC positions were determined by three-dimensional MR imaging.
All patients received rTMS on 10 consecutive workdays. Antidepressants were started on the first day of stimulation and were maintained throughout the stimulation period. Dosage remained constant throughout the trial. At entry, patients underwent a 5 x half life washout period. Patients were evaluated by blinded trained psychiatrists using the 21-item HDRS and the BDI on days 0, 1, 3, 5, 7, 10, 14, and 28.
Of the 41 patients recruited, 38 completed the 2-week protocol. At baseline, no significant differences in HDRS and BDI scores were observed among the 3 groups or between the pooled treatment groups (A1 and A2) and the placebo group.
There were no statistically significant differences between the 2 active-treatment groups for HDRS and BDI scores over the course of treatment (days 7, 14, and 28). Groups A1 and A2 were pooled for comparison with Group C. At days 14 and 28, there were no statistically significant differences between rTMS (A1 and A2) and controls (Group C) in mean percentage decrease of the HDRS total score and the BDI.
When testing the time course of the outcome variables HDRS and BDI (days 0, 7, 14, 28) by repeated-measures ANOVA, there was a significant effect of time on HDRS (P<.001) and BDI (P<.001), but there were no significant group differences for a group by time interaction.
The limitations to the study by Hausmann et al. were as follows:
No a priori sample size calculation or justification was reported.
Antidepressant regimens were variable.
The authors said, “Three patients, each per group, terminated the study prematurely.” No further details were given about the withdrawals, nor was using intent-to-treat analysis discussed.
It was a short-term study. Duration of treatment was 10 days, and outcome was measured up to 28 days from baseline.
Instead of being “double blind,” the trial was single blind with an evaluation by external blinded assessors
Hoppner et al. (67) compared the clinical effects of 2 different stimulation procedures with placebo stimulation as add-on treatments in patients with depressive disorders (Level 2 evidence). Thirty patients with depression were recruited. Each received an antidepressant in a constant dose over 2 weeks before and during the stimulation period. Patients were randomly allocated to receive high-frequency LDLPFC (20, 20 Hz trains of 2-second duration with an intertrain interval of 60 seconds), low-frequency RDLPFC (2, 1 Hz trains of 60-second duration with an intertrain interval of 3 minutes), or placebo stimulation on 10 out of 12 days (2 weeks with 5 sessions each week). Ten patients were in each group. Placebo stimulation consisted of the same conditions as the 20 Hz rTMS, except that the coil was placed at a 90-degree angle to the head.
Severity of depression was assessed by the 21-item HDRS and the BDI. The rater was a psychiatrist blind to the stimulation procedure. Clinical response was defined as at least_50% improvement from baseline scores (HDRS, BDI) from pre to post-treatment assessment.
Twenty-nine of the 30 patients initially included in the study completed the treatment phase. One patient from the high-frequency treatment group refused to continue after 6 days because of insufficient effectiveness and headache. The other 29 patients did not report adverse effects.
Only 1 of the patients was classified as a responder according to both BDI and HDRS score criteria.
Treatment response according to HDRS
Five patients in the 20 Hz group were HDRS responders, compared with 3 patients in the 1 Hz group and 5 patients who were placebo stimulated. The average reduction of the HDRS baseline score was 61.8%.
HDRS scores were significantly reduced among the 20 Hz group (day 5, P=.03; end of treatment, P=.015) and the placebo stimulation group (day 5, P=.017; end of treatment, P≤.001), but they were not significantly reduced in the 1 Hz group.
Treatment response according to BDI
Two of the 9 patients treated by 20 Hz rTMS were “responders” based on the BDI-score criterion. One patient given treatment with 1 Hz rTMS “responded”. Two placebo-stimulated patients “responded.” In the 2 stimulation groups, reduction of BDI scores was observed after 5 days (20 Hz, day 5, P=.008, end of treatment, P=.011; 1 Hz, day 5, P=.039, end of treatment P=.029). For the placebo group, improvement was statistically significant only at the end of treatment (P=.005).
The limitations to the study by Hoppner et al. were as follows:
No a priori sample size calculation or justification was reported.
There was no discussion of using intent-to-treat analysis. One patient from the high-frequency group refused to continue after 6 days because of insufficient effectiveness and headache.
-
The Authors noted: (67)
“Preliminary and explorative comparison study.”
“Small sample size”
The enhanced placebo effect of rTMS was due to its “impressive name, its ability to cause involuntary movements as if by magic, its discomfort, and its bulky and sophisticated looking equipment.”
“Results of preliminary data points to the necessity of further research to be able to answer the still open questions regarding stimulation procedures of rTMS, location of stimulation and of the number of treatment sessions.”
Nahas et al. (12) examined the safety, feasibility, and potential efficacy of using TMS to treat the depressive symptoms of bipolar affective disorder (BPAD) (Level 2 evidence). Twenty-three patients with BPAD (12 BPI depressed state and 9 BPII depressed state, 2 BPI mixed state) were enrolled. Patients were assigned using “an urn randomization based on age and gender” to receive daily LDLPFC rTMS (5 Hz, 110% motor threshold, 8 seconds on, 22 seconds off over 20 min) or placebo (coil angled 45 degrees off head) each weekday morning for 2 weeks. Blinded assessments on the HDRS and Young Mania Rating Scale (YMRS) were obtained weekly. Patients with persistent depression could take carbamazepine or valproate, but the dose had to be stable for 2 weeks prior to beginning treatment. All other psychotropic medications (especially antidepressants) were tapered over a 2-week washout period (longer for fluoxetine). Patients taking lithium or lamotrigine were excluded.
The primary outcome variable was percentage change in HDRS at 2 weeks compared with day 1 of treatment (clinical response defined as >50% decline in HDRS or a score of <10). Following the last day of the 2-week period, the blind was broken for each patient. Patients initially randomized to placebo were offered the option of 2 weeks of active treatment using the same parameters. Patients who responded to either the active or later open TMS phase were offered the option of weekly maintenance rTMS treatments over the next year.
Eleven patients received rTMS. All patients guessed their status based on their clinical response (all responders guessed they were receiving active TMS and all clear nonresponders guessed placebo). No adverse cognitive effects of the TMS occurred as measured by subjective complaints. There were no dropouts. No patients stopped the study due to hypomania or mania, and active rTMS treatment did not cause a statistically significant within-group increase on the YMRS (P=.49).
In the active rTMS group, 4of the 11 patients were considered responders. In the placebo group, 4 of the 12 patients were responders. Each group had one patient who was a “remitter.” There was no significant difference between the 2 groups in HDRS change from baseline over the 2 weeks (P=.83). The mean percent change in HDRS was 25% (SD, 32%) for the active rTMS group and 25% (SD, 31%) for the placebo rTMS group.
The limitations to the study by Nahas et al. were as follows:
No a priori sample size calculation or justification was reported.
The sample size was small, and it was a pilot study. The authors said, “Due to the small numbers, limited conclusions can be made from this result...”(12)
It was a short-term study. The treatment administration (and total study duration) was 2 weeks.
There was a high response to placebo.
Schule et al. (68) conducted an open trial over 2 weeks to determine if antidepressant pharmacotherapy can stabilize clinical improvement after rTMS (administered without any concurrent drugs) in 26 patients (Level 4 evidence). rTMS treatment consisted of 10 to 13 sessions, 10 Hz, 15 trains (each 10 seconds with 30-second intertrain intervals), LDLPFC at 100% motor threshold. Patients were followed-up during standardized antidepressant pharmacotherapy with mirtazapine for a further 4 weeks. Severity of depression was assessed using the 21-item HDRS. HDRS ratings were obtained before rTMS treatment (day 0), after 1 week (day 7), and after 2 weeks (day 14). Raters were not involved in administering rTMS. Response to rTMS was defined as a reduction of at least 50% of the HDRS score after 2 weeks of rTMS compared to baseline. An overall response (rTMS followed by mirtazapine treatment, week 0–6) was defined as a decrease of at least 50% in HDRS score after 6 weeks compared to baseline. Remission was defined as a HDRS score of 9 or less after 2 or 6 weeks.
Ten (39%) out of the 26 patients responded to rTMS, as indicated by at least a 50% reduction in their HDRS scores. Six (23%) of the patients were remitters (HDRS score <9). During subsequent antidepressant treatment, response and remission rates were further increased: after week 6, 20 (77%) of the patients achieved clinical remission.
The differences in the HDRS scores between rTMS responders and nonresponders became significant (P<.05) after 1 week of rTMS monotherapy and were observed up to the second week of mirtazapine treatment. After 3 weeks of drug therapy, there were no more significant differences between rTMS responders and nonresponders. Of the nonresponders to rTMS, 69% (11/16) converted into 6-week responders.
rTMS responders and nonresponders did not differ in the severity of their depressive symptoms at baseline, number of depressive episodes, number of failed antidepressant trials during the current episode, and duration of the current episode. Week 6 responders and nonresponders were comparable in baseline HDRS scores and number of depressive episodes. However, week 6 nonresponders were characterized by a significantly higher number of failed antidepressant trials before entering the study (P=.025) and a significantly longer duration of the current episode (P=.037), compared with week 6 responders.
A significant deterioration of depressive symptoms occurred between the last rTMS treatment and the first administration of mirtazapine in rTMS responders (P=.002), but not in rTMS nonresponders (P=.375). There was an overall low but significant correlation (P=.025) between worsening on the HDRS score and duration of treatment interruption. The deterioration in rTMS responders was reversible in most cases: 9/10 rTMS responders were stabilized during subsequent mirtazapine treatment (weeks 3–6). One rTMS responder became worse during pharmacotherapy.
The limitations to the study by Schule et al. (68) were as follows:
No a priori sample size calculation or justification was reported.
There was no control group.
It was an open-study design.
Half of the patients also received lithium, carbamazepine, or neuroleptics.
Herwig et al. (69) investigated the efficacy of neuronavigated rTMS that was guided according to the prefrontal metabolic state determined by positron emission tomography (PET). A double-blind randomized placebo-controlled study design (n=25) was used. Thirteen patients received rTMS, and 12 patients received placebo rTMS (Level 2 evidence).
Prior to rTMS, PET scans were obtained. In order to state that a DLPFC was hypometabolic, a PET scan had to show at least 5% lower activity in the mean value compared to the contralateral hemisphere. In addition, the mean values of the region of interest in the slices of interest on both sides had to be significantly different in a paired t test. When these criteria were fulfilled, the site with the lower metabolism was stimulated in the active treatment group. If no difference was detected, patients were assigned alternating to either a left- or a right-sided stimulation.
A statistical parametric mapping analysis of the PET scan was performed for all patients at the end of the study (since according to the authors, this technique was methodologically established at the end of the study). However, for the last 3 patients of the study, parametric mapping analysis was performed prior to the active stimulation for determination of the stimulation site replacing the region of interest analysis. In 4 patients assigned to placebo stimulation, PET scans were unavailable.
Stimulation parameters were 15 Hz at 110% motor threshold. A neuronavigational system (where the coil is navigated according to individual anatomy visualized by MR imaging) was used to place the magnetic coil above each individual’s selected cortical region (active treatment: DLPFC; placebo treatment: midline parieto-occipital; intensity 90% of motor threshold). Double blinding was defined as neither the raters nor the patients being informed about the stimulation condition.
rTMS was administered as an add-on to drugs. Patients had to have been on stable antidepressant medication for at least 3 weeks prior to stimulation onset. However, in 6 patients, the stimulation was commenced on the day of onset of a new antidepressant medication. Furthermore, patients were permitted to be prescribed other drugs in a “naturalistic manner,” such as low dose hypnotics, low dose olanzapine as antidepressant augmentation or lithium. Depression-related symptoms were rated with the BDI, HDRS, and MADRS. Responders were defined by a 50% reduction in the mean HDRS and MADRS ratings. Ratings were performed 5 times:
Before stimulation
After 4 stimulations
After 7 stimulations
At the end of the stimulation sessions
In responders, 2 weeks after the stimulation sessions
The initial rating scores did not differ between the placebo and active-stimulation groups. There were significant differences between the placebo and active stimulation groups in terms of the relative changes in scores (at the end of stimulations compared with the initial scores normalized to 100%), in percent in HDRS (P=.002) and MADRS (P<.001), but not for the self-rating BDI (P=.1).
Four of the 13 patients in the active treatment group responded, compared with none of the 12 patients in the placebo group. The ratings done 2 weeks after the stimulation sessions in the 4 patients that responded showed a persisting effect with a mean HDRS of 48% and mean MADRS of 44% of the initial rating scores.
Five of the 13 patients in the active treatment group showed a relatively lower DLPFC metabolism in the initial PET region of interest analysis and were stimulated accordingly. Only 1 of the patients was a responder. PET-guided stimulation revealed no difference in antidepressant efficacy compared with the non-PET-guided stimulation (BDI, P=.9; HDRS, P=.8; MADRS, P=.7).
The post-hoc PET parametric mapping analysis revealed prefrontal hypometabolism that was not detected in the initial region of interest analysis in 3 more patients in the active treatment group and did not confirm the prior analysis in 1 patient. Additional testing of PET- and non-PET guided showed no statistically significant differences (BDI, P=.9, HDRS, P=.3; MADRS, P=.7).
Patients did not report any severe adverse effects of rTMS treatment.
The authors concluded, “The principle finding of the presented study is a moderate antidepressant efficacy according to HDRS and MADRS of the neuronavigated DLPFC rTMS, which does not seem to depend on the prefrontal metabolic state.” (69)
The limitations to the study by Herwig et al. were as follows:
No a priori sample size calculation or justification was reported.
Patients were taking a wide variety of drugs.
PET technique used by the authors was not consistent throughout the study.
It was not directly tested if the navigational approach led to a better outcome than at the traditional “5 cm rule.” In fact, the effect was not better than previously reported in earlier studies that used the “5 cm rule.”
“Small sample size.” (69)
Some authors (2) reported that rating scales based on semi structured interviews (e.g., the HDRS and MADRS) are more susceptible to observation bias than are self-administered questionnaires like the BDI. In Herwig et al., BDI scores were not statistically different between the placebo and active treatment groups; however, the ratings for the HDRS and MADRS were significantly different between groups.
Summary of Findings
Some early meta-analyses suggested that rTMS may be effective for the treatment of MDD (for treatment-resistant MDD or as an add-on to pharmacotherapy for patients who are not specifically defined as treatment resistant). There were, however, several crucial methodological considerations and limitations in the included studies that were not critically assessed. These are discussed below.
More recent meta-analyses, including 2 international health technology assessments, conducted an evidence-based critical analysis of studies that assessed rTMS for MDD. The 2 most recent health technology assessments (from the Oxford Cochrane Collaboration and the Norwegian Centre for Health Technology Assessment) concluded that there is no evidence that rTMS is effective for the treatment of MDD, either compared with a placebo in patients with either treatment-resistant or nontreatment-resistant depression, or as an alternative to ECT in patients with treatment-resistant depression. This is mainly due to the poor quality of the studies to date.
Major methodological limitations identified in the older meta-analyses, the recent health technology assessments, and the more recently published trials (Level 2–4 evidence) that examined the effectiveness of rTMS in patients with MDD to date include the following:
Limitations
Small sample sizes
Lack of a priori sample size calculation or justification.
Many of the authors from recent studies acknowledged that the small sample sizes were limitations of their studies. This is because uncontrolled variables that may influence outcomes may not be evenly distributed between treatment and control groups.
For studies that compared rTMS with ECT, there was no explicit methodological discussion or consideration of the study design for the testing of equivalence between rTMS and ECT.
The number of patients included in studies of the efficacy of rTMS in the treatment of depression falls short of the number of patients registered in trials for new drug treatments. (3)
Biased randomization
There is a lack of detailed information in the published reports on the method of allocation concealment used.
Many studies indicated only that they were randomized, or they only described the generation allocation sequence (e.g., with a computer). This is important, because it is impossible to determine if a possible influence (direct or indirect) existed in the allocation of the patients to different treatment groups. For example, this may happen if the patients who are most likely to respond are included only the in the active treatment arm. This can influence the results of the studies by overestimating a possible effect of treatment.
Regression to the mean occurs whenever a nonrandom sample is selected from a population and 2 imperfectly correlated variables are measured, such as 2 consecutive blood pressure measurements. (45) In the systematic review by the Cochrane Collaboration, the mean baseline depression values in most of the studies were higher in the treatment group than in the control group. The authors said, “Although these differences were not statistically significant at the level of each individual study, they would have introduced a potential bias within the meta-analysis of pooled data by accentuating the tendency for regression to the mean of the more extreme values.” (3) If the patients who are most likely to respond (i.e., the sickest patients) are included only in the active treatment arm, then it can influence the final results of the studies by overestimating a possible effect of treatment.
Instead of being “double blind,” the trials were single blind with an evaluation by external blinded assessors
Double blinding means the participants do not know if they are receiving the treatment of interest or a placebo, and the investigator assessing the patients does not know if the patients are receiving the treatment of interest or a placebo.
The people who administer rTMS cannot be blinded as to whether they are administering the active treatment or a placebo intervention.
There is also the potential for patients to guess which treatment they receive. In Berman et al., (40) patients deduced their treatment group in 10 of 15 cases, whereas the evaluators deduced the patient treatment group in 12 of 15 cases.
Patient variability between studies
In some studies, the authors said that patients were “medication resistant,” but the definition of resistant, if provided, was inconsistent or unclear. Some studies described medication resistant as failing at least one trial of drugs during the current depressive episode. It is unclear if medication resistance included antidepressants only or combinations of antidepressants plus any other drug augmentation strategy or strategies (such as neuroleptics, benzodiazepine, carbamazepine, or lithium).
In the updated Cochrane systematic review, Martin et al. (3) reported that in 7 of the 14 studies, the patients were described as medication resistant (having failed at least one trial of dugs during the current depressive episode), but in some of these studies drugs were continued, and in other studies, drugs were not continued.
Conca et al. (65) was the only study identified in the updated Medical Advisory Secretariat literature search that fully defined medication resistance according to a detailed classification system developed by Thase and Rush. (27) All patients had to be classified at “stage 4” of treatment resistance, indicating the failure to respond to 2 different adequate monotherapy trials of medications with different pharmacological profiles, and the failure to respond to a second augmentation strategy.
Padberg et al. (13) examined the use of rTMS in patients with MDD who were pharmacotherapy resistant. However, one of the secondary endpoints of the study was to assess the number of antidepressant trials patients underwent until discharge from hospital. It is unclear how they defined drug resistance specifically.
The potential for concurrent medications (in both treatment and control arms) alone or in combination (e.g., antidepressants and/or lithium and/or neuroleptics and/or benzodiazepine and/or carbamazepine) to interfere with the performance of the rTMS procedure cannot be ruled out.
There was patient variability in the studies. Some had unipolar depression, and some had bipolar depression. Some were inpatients; others were outpatients. Some had psychotic symptoms, whereas others had none. They also differed as to their levels of chronicity.
Dropouts or withdrawals
Some studies reported that patients dropped out of the study, but provided no further details.
An intent-to-treat analysis was not discussed in any of the trials.
This is important because ignoring patients who drop out of a trial can bias the results, usually in favour of the intervention. It has been reported that patients who withdraw from trials are less likely to have taken the treatment, and more likely to have missed their interim checkups and to have had adverse effects when taking the treatment, compared with those patients who do not withdraw. (1)
The standard practice is to analyze the results of RCTs on an intent-to-treat basis. This means that all data on patients originally allocated to the intervention arm of the study (including patients who withdrew before the trial finished and those who did not take the treatment) should be analyzed along with data on the patients who followed the trial protocol. This means patients who withdrew from the placebo arm of the trial should be analyzed with the patients who continued with placebo treatment until the end of the trial.
Measurement of treatment outcomes using scales or inventories
The most common scale, the HDRS, is based on a semistructured interview. Some authors (2) reported that rating scales based on semistructured interviews are more susceptible to observation bias than are self-administered questionnaires like the BDI. Martin et al. (3) argued that the lack of consistency in effect as determined by the 2 scales (a positive result after 2 weeks of treatment as measured by the HDRS and a negative result for the BDI) makes definitive conclusions about the nature of the change in the mood of patients impossible.
Martin et al. (3) also suggested that, because of difficulties interpreting results from psychometric scales, (4) and the subjective or unstable character of MDD, other, more objective, outcome measures such as readmission to hospital, time to hospital discharge, time to adjunctive treatment, and time off work should be used to assess the effectiveness of rTMS for depression. Padberg et al. (13) examined duration of hospital stay and number of antidepressant trials until discharge from hospital in 31 drug-resistant patients who received rTMS or placebo. However, these were secondary endpoints retrospectively assessed from the charts of the patients with drug-resistant depression.
Placebo effect
Many studies reported response rates in patients who received placebo treatment. For example, Klein et al. (5) reported a placebo group response rate as high as 25%.
It is possible that patients receiving placebo rTMS receive a small dose of magnetic energy that may alter their depression.
Short-term studies
In the studies, patients received rTMS treatment for 1 to 2 weeks.
Most studies followed-up patients for 2 to 4 weeks post-treatment. Dannon et al. (6) followed-up patients who responded to a course of ECT or rTMS up to 6 months; however, the assessment procedure was not blinded, the medication regimen during follow-up was not controlled, and initial baseline data for the patient groups were not reported.
The long-term effectiveness of rTMS for the treatment of depression is unknown.
The long-term use, if any, of maintenance therapy is unknown.
The cost-effectiveness of rTMS for the treatment of depression is unknown. The lack of long-term studies makes doing a cost-effectiveness analysis difficult.
Complexity of possible combinations for administering rTMS makes comparison of like versus like difficult
Martin et al. (3) categorized the 3 major variations in administration methods (localization on the skull, frequency, and duration of treatment period). In the majority of included studies, rTMS was applied to the LDLPFC, but Wasserman and Lisanby (7) stated that the method for precisely targeting the stimulation in this area is unreliable.
It is unknown if the LDLPFC is the optimal localization for treatment.
Further differences in rTMS administration include number of trains per session, duration of each train, and motor threshold.
Clinical versus statistical significance
Several meta-analyses and individual studies indicated that the degree of therapeutic change associated with rTMS across studies was relatively modest. (8-11).
Conventionally, an accepted reduction in depression (i.e., response) is considered a 50% reduction in the Hamilton Depression Rating Scale. Some studies may have observed a significantly reduced depression rating; however, many did not demonstrate a 50% reduction. (11-13) Therefore, few patients in these studies would meet the standard criteria for response. (9)
Clinical/methodological diversity and statistical heterogeneity
Clinical diversity among studies in a systematic review is defined as variability in the types of participants, interventions, and outcomes studied. (16) Methodological diversity is defined as variability in trial design and quality. (16) Statistical heterogeneity is defined as variability in the treatment effects being evaluated in the different trials and is a consequence of clinical and/or methodological diversity. (16)
In the Norwegian health technology assessment, Aarre et al. (14) stated that a formal meta-analysis was not feasible because the studies varied too much in design, in the way that rTMS was administered, and in patient characteristics. They noted that the quality of the study designs was poor. The 12 included studies had small samples, and highly variable inclusion criteria and study designs. The patients were often insufficiently characterized as to previous history, diagnosis, treatment history, and treatment setting. In addition, many studies stated that the patients were treatment resistant without any clear criteria for such a designation. Without this information, Aarre et al. suggested that the interpretation of the results is difficult and the generalizability is questionable. They concluded that rTMS cannot be recommended as a standard treatment for depression. “More, larger and more carefully designed studies are needed to demonstrate convincingly a clinically relevant effect of rTMS.” (14)
In the Cochrane Collaboration systematic review, Martin et al. (3;15) stated that the complexity of possible combinations for administering rTMS makes comparison of like versus like difficult. The chi-square test is a statistical test for heterogeneity; the observed treatment effects being more different from each other than one would expect due to random error (or chance) alone. (16) However, the chi-square test must be interpreted with caution because it has low power in the (common) situation of a meta-analysis when the trials have small sample sizes or are few in number. This means that while a statistically significant result may indicate a problem with heterogeneity, a nonsignificant result must not be taken as evidence of no heterogeneity.
Despite not finding statistically significant heterogeneity, Martin et al. reported that the overall mean baseline depression values for the severity of depression were higher in the treatment group than in the placebo group. (3;15) Although these differences were not significant at the level of each individual study, they may have introduced potential bias within the meta-analysis of pooled data by accentuating the tendency for regression to the mean of the more extreme values. Individual patient data from all the studies were not available; therefore, an appropriate adjustment according to baseline severity was not possible. Martin et al. concluded that the findings from the systematic review and meta-analysis provide insufficient evidence to suggest that rTMS is effective in the treatment of depression. Moreover, there were several confounding factors (e.g., definition of treatment resistance) in the included studies signifying, “The rTMS technique needs more high quality trials to show its effectiveness for therapeutic use.” (3;15)
Economic Analysis
Literature Review
No economic analyses of rTMS for the treatment of depression were identified in the literature search.
Ontario-Based Economic Analysis
For Ontario, an expert consultant estimated about 6% to 7% of the population experienced an episode of major depression the last year, and about 0.8% of the population has had a treatment resistant depression in the past year. Of these, 80% would not be considered for electroconvulsive therapy (ECT), the gold standard, due to the following considerations:
Anesthetic contraindications
Unavailability of ECT
Unavailability of inpatient facilities
Request alternative antidepressant treatment
About 10% would be eligible and would accept a course of treatment in a year. Therefore about 1,800 patients may receive ECT in a year. It is estimated that about 10% of these patients may have also received ECT in the past, leaving around 1,500 new patients who may have received ECT in a year.
According to Provider Services Branch of the Ministry of Health and Long-Term Care, the number of ECT procedures for the fiscal year 2002/2003 was as follows:
Inpatient: 8,973 services
Outpatient: 5,058 services
The data was extracted using the following criteria:
Fee-for-service claims only.
Ontario registered physicians only.
The data is for the physician who performed the therapy. Anesthetist claims were excluded.
According to the manufacturer, pricing for an rTMS device is as follows (in Canadian currency):
Devices: $34,000 to $74,000 including an integral computer and software. For the treatment of depression, pricing is at the high end of the range.
Coils: $6,000 to $10,000 each, and users usually have 2 or more coils per device. They are replaced at intervals, usually every 12 to 24 months, depending on usage. Note that their cost is not included in the device price.
Other accessories: $7,500 for cables, carts, power transformers, flexible arms for holding coils – with the purchase of a new device.
Installation and training: $2,000; additional training days are $1,000. Offsite training costs (e.g., travel to another site for training) are the responsibility of the purchaser
A private clinic that treated 80 patients over 1.5 years estimated that 1 machine could be used to treat about 13 patients per day. Accordingly, the coils required replacement 5 to 10 times per year. (As more patients received treatment, more coils required replacement.)
A technician can administer rTMS to a patient; however, a physician is required to be in close proximity (“on the floor”) when a patient receives treatment.
Existing Guidelines for Use of Technology
The American Psychiatric Association Practice Guideline For The Treatment of Patients With Major Depressive Disorder, 2000 (70) does not mention of rTMS (or TMS) for the treatment of depression.
The Canadian Psychiatric Association and the Canadian Network for Mood and Anxiety Treatments partnered to produce clinical guidelines for psychiatrists for the treatment of depressive disorders in 2001. (71) They said:
“Potential clinical uses include the treatment of medication-refractory patients (perhaps in place of ECT) or because of its rapid onset of effect, to hasten clinical response in conjunction with antidepressants. There is still however, no clinical consensus about the optimal parameters of treatment including frequency and anatomic location of stimulation. Most of the positive results have not yet been replicated, the follow-up periods have been short and there are no long term or maintenance studies.
“Transcranial magnetic stimulation...are promising new biological treatments but there is too little evidence to warrant recommendations for general clinical use (Level 2 and 3 evidence).”
The Royal Australian and New Zealand College of Psychiatrists in October 2003 (72) produced a position statement. A compilation of quotations taken from that report is listed below.
“rTMS presents the RANZCP with a unique situation. The Therapeutic Goods Administration (TGA) in Australia and Medsafe in New Zealand assess new medications with respect to both safety and efficacy before they are accepted for use in the community. However, TMS machines are classified as ‘listable’ electromedical devices and, under current legislation, these bodies would not assess their efficacy.... “It is therefore important that the RANZCP accepts the responsibility for making recommendations concerning the use of rTMS in clinical psychiatry and for monitoring such use.”
“On the basis of currently available data it is reasonable to conclude that rTMS has antidepressant efficacy which may be clinically significant in at least a subgroup of depressed patients. Further studies are required to explore the question of the robustness of the clinical response and to identify those patients who are likely to respond and to determine the most appropriate treatment parameters and site of application. Given its favourable safety profile it is reasonable therefore to recommend that rTMS should be available to certain patients in the clinical setting, outside of research, subject to the conditions outlined below.”
“Patients for whom rTMS might be offered outside a research protocol include:
a) those suffering from Major Depression (DSM-IV) who have failed or who are intolerant of other suitable treatments.
b) previous responders to rTMS who have relapsed and are not eligible for an existing research protocol.
c) patients whose clinical presentation suggests that ECT is indicated, but who prefer to try rTMS before ECT, or for whom ECT might present an unusually high risk.”
“Patients with a history of epilepsy or with surgically implanted metal in the head or neck should not have rTMS.”
“In view of the absence of efficacy and safety data in pregnancy, pregnant women should not have rTMS outside of a properly conducted and ethically approved clinical trial.”
“In view of the paucity of information relating to the use of TMS in patients under the age of 18, TMS should only be used outside of an ethically approved clinical trial after careful evaluation of the individual situation by an appropriate child and adolescent service.”
“Until further data are available, for psychiatric illnesses other than depression rTMS should only be used within an approved research protocol.”
“Patients having rTMS in the clinical setting should sign an appropriate consent form for the treatment. The consent form should detail the possible side effects and adverse events, including the possibility of seizures and the induction of mania. The consent form should inform the patient that the potential benefits of rTMS in depression may be limited, that it may not work for a particular individual, and that the most efficacious manner of administering rTMS has not yet been established.”
“It is recommended that rTMS should not be an office based procedure, but should only be performed in a hospital setting such as an academic unit or tertiary referral unit or in other hospital based units, such as in a private psychiatric hospital, provided that a protocol for the use of rTMS outside research has been developed by an interested and properly trained psychiatrist or group of psychiatrists who undertake to supervise and regulate the administration of rTMS in that hospital or clinic. Such a protocol should be approved by an appropriate local body such as the Medical Advisory Committee or Ethics Committee or similar. Medical staff and resuscitation equipment such as oxygen and a simple ventilation apparatus should be at hand in the event of a seizure.”
“rTMS is considered to be a medical procedure and should only be administered by a medical practitioner or by trained nursing staff who are under the supervision of a medical practitioner. Personnel administering rTMS should be adequately trained in first aid and resuscitation to deal with an unexpected seizure.”
“Psychiatrists who are intending to administer rTMS, either in the clinical or research setting, should be properly trained in the theory and technique of the treatment and the safe operation of TMS devices.”
“Although it is reasonable for certain patients to have access to rTMS outside of research for the treatment of depression, it is recognised that as a treatment in psychiatry rTMS is in the early stage of development and that much research is required to establish its place in the management of psychiatric illness. For this reason it is envisaged that for the most part, rTMS in Australia and New Zealand should continue to be done within a research protocol. In any event, it is important that clinical information about the usefulness and safety of rTMS is collected. Centres who wish to use rTMS should register their interest with the RANZCP, through the Committee for Psychotropic Drugs and Other Physical Treatments. Basic efficacy and safety data should be collected by each centre, regardless of whether patients are being treated clinically or in a research trial. These data should then be forwarded to the Committee, at least annually, to enable a form of centralized monitoring of rTMS practice and outcomes in Australia and New Zealand. It is particularly important that any adverse events, such as seizure or the induction of mania, is reported to the Committee without delay.”
The guidelines of the National Institute for Clinical Excellence (NICE), in the United Kingdom, 2003, (73) does not mention rTMS (or TMS) for the treatment of depression.
The Beyondblue Guidelines for Treating Depression in Primary Care in Australia, 2002, (74) does not mention rTMS (or TMS) for the treatment of depression.
On the National Guideline Clearinghouse Web site (www.guideline.gov) (May 11, 2004), there were no guidelines identified specific to the treatment of depression with TMS.
The International Society for Transcranial Stimulation (ISTS) Consensus Statement on Managing the Risks of rTMS. (Adopted by ISTS Consensus Committee July, 2002) (75) said:
“Those who administer rTMS should be trained as “first responders” in order to render appropriate care in the event of seizure.
rTMS should be performed in a medical setting with appropriate emergency facilities to manage seizures and their consequences. Patients and research subjects should be continuously monitored during the administration of rTMS for signs of epileptic activity or other adverse effects by a trained individual, according to criteria established in the clinical or experimental protocol. This monitoring may include electrophysiological recording and/or visual inspection.
During the informed consent process, patients and study participants should be informed of the risk of seizure and its possible medical and social consequences.
The dosage of rTMS should generally be limited by published safety guidelines (e.g., Wassermann, Clin Neurophysiol, 1998;108:1 or any subsequent updates). If there is a compelling scientific or clinical reason to exceed such guidelines, the rationale for doing so should be considered carefully, documented and the patients or study participants should be informed that they may be at higher risk for seizure.
The long-term risks of rTMS are not known. However, the limited data available at this time (2002) from repeated application of high intensity, time-varying magnetic fields to humans, as in magnetic resonance imaging, do no suggest that they are significant.
The use of rTMS should comply with regulations put forward by local regulatory bodies, medical professional organizations, and medical licensing boards.”
Ongoing Trials
In addition to the ongoing trial of rTMS compared with ECT in the United Kingdom, 2 trials comparing rTMS with a placebo for the treatment of major depression are underway. Neuronetics, a manufacturer of TMS technology, is sponsoring a multicentre RCT (76) of 286 patients with treatment-resistant major depression who receive either active rTMS or placebo rTMS. Patients will undergo 30 outpatient treatment sessions of about 45 minutes each over a 6-week period.
The National Institute of Mental Health is sponsoring a trial of rTMS for major depression (Grant Number 5R01MH062154-03). (77) Eighty-six drug-free patients with MDD who have failed at least 2 trials of antidepressant medication will be studied. Patients will be randomized to either active LDLPFC or placebo LDLPFC rTMS. Each patient will receive 10 sessions with 32 trains per session over 2 weeks. Blind assessment will take place before the first rTMS session, after the fifth and 10th sessions. and 1 and 2 weeks post-treatment. In the second phase of the study, responders will be placed on maintenance antidepressant medication 2 weeks after the last rTMS session and evaluated at 1 to 6 months after the last rTMS session. The project was reported to have started August 15, 2000 and ended July 31, 2004. (77)
Appraisal
Policy Considerations
Patient outcomes
Potential advantages of rTMS compared with ECT for treatment-resistant MDD are as follows:
No general anesthesia
Outpatient procedure
Lower energy is required. Hair, skin, and skull are not good electrical conductors; therefore, to reach brain tissue, large currents must pass through skin-surface electrodes during ECT. However, the skull and other tissues are more “transparent” to the magnetic field created by a TMS coil; therefore, lower energy is needed to alter neuronal activity. (32) In addition, “The magnetic field can be highly focused, thereby reducing the side effects caused by the large currents that probably flow diffusely through neuronal structures during ECT.” (32)
No social stigma of having to receive ECT (35)
Specific targeted stimulation
No convulsion requirement as part of procedure
Minimal adverse effects such as mild headache and discomfort at the site of stimulation
No known adverse effects on cognition
A potential advantages of rTMS as an add-on treatment is hastening of the clinical response in conjunction with antidepressants.
The long-term effectiveness of rTMS for severe depression is unknown.
The use, if any, of continued maintenance therapy is unknown.
Long-term pragmatic patient outcomes, such as time to further treatment, time to adjunctive therapy, time off work, readmission to hospital, or hospital discharge, are unknown.
Demographics
For Ontario, an expert consultant estimated that about 6% to 7% of the population has experienced an episode of major depression the last year. About 0.8% of the population has had treatment-resistant depression in the past year. Of these, 80% would not be considered for ECT, the gold standard, due to the following:
Anesthetic contraindications
Unavailability of ECT
Unavailability of inpatient facilities
Request alternative antidepressant treatment
About 10% would be eligible for, and accept a course of, treatment in a year. Therefore, about 1,800 patients may receive ECT in a year. It is estimated that about 10% of these patients may have also received ECT in the past, leaving around 1,500 new patients who may have received ECT in a year.
Diffusion
In the United States, rTMS for treatment of depression is not approved for clinical use by the United States Food and Drug Administration.
In the United States, insurers such as Aetna, Blue Cross Blue Shield Georgia, and the Regence Group consider TMS investigational for the treatment of major depression. (78-80)
In the United Kingdom, a large RCT examining rTMS versus ECT for treatment-resistant depression is expected to be published in mid 2005.
In 2003, Agence d’evaluation des technologies et des mode d’intervention en sante (AETMIS) in Quebec stated that TMS appears to be promising as an ECT substitute technology; however, according to the current state of knowledge, TMS is considered experimental. (81)
A private rTMS clinic based in Western Canada recently opened a new clinic in Toronto to treat patients with major depression. The clinic charges about $4,000 (Cdn) for a course of treatment (about 5, 1-hour sessions per week for 4 weeks).
Cost
According to the manufacturer, pricing for an rTMS device is as follows (in Canadian currency):
Devices: $34,000 to $74,000 including an integral computer and software. For the treatment of depression, pricing is at the high end of the range.
Coils: $6,000 to $10,000 each, and users usually have 2 or more coils per device. They are replaced at intervals, usually every 12 to 24 months, depending on usage. Note that their cost is not included in the device price.
Other accessories: $7,500 for cables, carts, power transformers, flexible arms for holding coils – with the purchase of a new device.
Installation and training: $2,000; additional training days are $1,000. Offsite training costs (e.g., travel to another site for training) are the responsibility of the purchaser
A private clinic that treated 80 patients over 1.5 years estimated that 1 machine could be used to treat about 13 patients per day. Accordingly, the coils required replacement 5 to 10 times per year. (As more patients received treatment, more coils required replacement.)
A technician can administer rTMS to a patient; however, a physician is required to be in close proximity (“on the floor”) when a patient receives treatment.
Stakeholder Analysis
If rTMS is not approved for funding, psychiatrists with an interest in administering rTMS for depression may discontinue research.
System Pressures
If rTMS is approved for funding, technicians or psychiatrists administering rTMS will require training, which is provided by the device manufacturer.
If rTMS is not approved for funding, hospitals may discontinue rTMS research programs in depression.
If rTMS is approved for funding, patient volumes should be maintained at selected centres to define, improve, and refine the administration technique.
Appendices
Appendix 1
Figure 1: TMS equipment.
Used with permission from Magstim Company, available at http://www.magstim-us.com
Figure 2: TMS being administered to a patient.

Used with permission from From Dr. Thomas E. Schlaepfer, University Hospital Bonn; Available at: http://pni.unibe.ch/artwork/Brainweek.gif
Appendix 2
Figure 3: Location of prefrontal cortex.
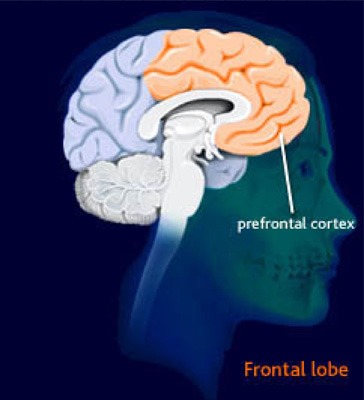
Used with permission from the Lundbeck Institute; Available at Brain Explorer: http://www.brainexplorer.org/glossary/prefrontal_cortex.shtml
Figure 4: Dorsolateral prefrontal cortex (outside view).
Image provided courtesy of Amen Clinics Inc., www.amenclinics.com; Available at http://www.brainplace.com/bp/brainsystem/prefrontal.asp
Appendix 3
Table 1. Open TMS studies in major depression: therpaeutic effects and effect size.
| Study | Treatment | n | Depression type | Precent change in HRSD |
s.d. | Effect Size(d) |
Lower | Upper | p value |
|---|---|---|---|---|---|---|---|---|---|
| Hoflich et al. (1993) | Verlex TMS | 2 | MDD | 10.3 | 14.6 | 0.71 | –6.22 | 7.03 | 0.52 |
| George et al. (1995) | LDLPFC rTMS | 6 | 1 MDD/5 BPD | 26.5 | 19.6 | 1.35 | –1.63 | 3.79 | 0.02 |
| Grisaru et al. (1995) | Motor TMS | 10 | 5 MDD/3 BPD/2 schizoaffective depressed | na (see comments; Table 2) | na | na | na | na | na |
| Geller et al. (1997) | LPFC and RPFC TMS | 10 | 6 MDD/3 BPD/1 schizoaffective depressed | na (see comment; Table 2 | na | na | na | na | na |
| Epstein et al. (1998) | LDLPFC rTMS | 32 | 25 MDD/3 BPD | 52.0 | 46.4 | 1.12 | 0.31 | 1.87 | 0.0001 |
| Figiel et al. (1998) | LDLPFC rTMS | 56 | 53 MDD/3 BPD | 44.4 | 25.0a | 1.78 | 1.12 | 2.39 | 0.0001 |
| Feinsod et al. (1998) | RDLPFC TMS | 14 | MDD | 30.8 | 35.8 | 0.86 | –0.42 | 2.03 | 0.01 |
| Menkes et al. (1999) | RF TMS | 8 | MDD/dysthymia | 42.4 | 37.4 | 1.13 | –0.94 | 2.91 | 0.02 |
| Pridmore (1999) | LDLPFC rTMS | 12 | MDD | na(see comments; Table 2) | na | na | na | na | na |
| Pridmore et al. (1999) | LDLPFC rTMS | 22 patients in 24 episodes | MDD with melancholia | 58.1b | 29.5 | 1.97 | 0.85 | 2.95 | 0.0000 |
| Triggs et al. (1999) | LDLPFC rTMS | 10 | MDD | 40.5 | 25.0a | 1.62 | –0.29 | 3.21 | 0.0009 |
| Eschweiler et al. (2000) | LDLPFC rTMS (n = 14) and RDLPFC TMS (n = 2) |
16 (all later received ECT) | MDD and schizeaffective depressed | na(see comments Table 2) | na | na | na | na | na |
| Cohen et al. (unpubl. obs.) | Bilateral TMS: LDLPFC rTMS followed by RDLPFC TMS | 10 | MDD | 28.3 | 29.8 | 0.95 | –0.71 | 2.42 | 0.02 |
LDLPFC, left dorsolateral prefrontal cortex; RDLPFC, right dorsolateral prefrontal cortex; RPFC, right prefrontal cortex.
Indicates that the s.d was estimated.
In Pridmore et al. (1999), the outcome measure was change in Montgomery–Asberg (MADRS) scores.
Burt T, Lisanby SH, Sackeim HA. Neuropsychiatric applications of transcranial magnetic stimulation: a meta analysis. International Journal of Neuropsychopharmacology 2002; 5(1):73-103; Subject to the copyright notice provided by Cambridge University Press, Reprinted with the permission of Cambridge University Press and the author (9)
Table 2. Open TMS studies in major depression: patient characteristics, treatment parameters and comments.
| Medication | Stimulus intensity |
Pulse freq.(Hz) |
Train duration(s) |
Number of trains |
Pulses per session |
Total session |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Treatment | Age | Resist | Free | Comment | ||||||
| Hoflich et al. (1993) |
Vertex TMS |
42.0 | Yes | No | 105–130% MT | 0.3 | na | na | 250 | 10 | One patient had slight improvement. |
| George et al. (1995) |
LDLPFC rTMS |
46.5 | Yes | 4/6 | 80% MT | 20 | 2 | 20 | 800 | 5+ | Two robust responders. |
| Grisaru et al. (1995) |
Motor TMS |
39.4 | na | No | 2 T | 0.017 | 3600 | 1 | 60 | 1 | Outcome assessed after single session; 4 mild improvement, 1 worse, 5 no change. |
| Geller et al. (1997) | LPFC and RPFC TMS | 39.4 | na | No | 2.5 T | 0.033 | 900 | 1 | 30 | 1 | Outcome assessed after single session; 3 immediate, lifeting of mood; 2 possible improvement; 1 worsening 4 no change. |
| Epstein et al. (1998) | LDLPFC rTMS | 40.0 | Yes | Yes | 110% MT | 10 | 5 | 10 | 250 | 5 | Age < 65, 4 dropouts, rTMS resulted in HRSD < 10 in 50% of sample. 8/10 with previous favourable response to ECT responded to rTMS (HRSD < 10). Non-responders older than rTMS responders. |
| Figiel et al. (1998) | LDLPFC rTMS | 59.9 | 53/56 | 50/56 | 110% MT | 10 | 5 | 10 | 500 | 5 | Sample overlaps with Epistein study, but includes new sample≥65. Results calculated on 50 patients who completed study. Only 2.3% of ≥65 responded; 56% of those < 65 responded (< 60%HRSD reduction with maximal post score of 16). Only 2 of 8 patients (25%) with psychotic depression responded. |
| Feinsod et al. (1998) | RDLPFC TMS | 58.0 | na | 4/14 | 1 T, 0.1 ms | 1 | 60 | 2 | 120 | 10 | By CGI 6 of 14 (42.9%) MDD patients showed marked improvement. |
| Menkes et al. (1999) | RF TMS | 33.3 | No | No | 100% MT | 0.5 | 40 | 5 | 800 | 8 | Included 6 healthy controls who had no change in HRSD score(mean 0.7). |
| Pridmore (1999) | LDLPFC rTMS | 57 | Yes | No | 90–100% MT | 10 | 5 | 20 | 1000 | 10–14 | All 12 patients were dexamethasone test (DST) non-suppressors at baseline. 6 Of 12 normalized the DST after rTMS. These 6 had strong clinical improvement (MADRS decreased from 31 to 9; 70.0%)and maintained their response for at least 4 wk. The remaining 6 patients showed at best moderate improvement that was not sustained. |
| Pridmore et al. (1999) | LDLPFC rTMS | 52.5 | Yes | 5/24 | 90-100% MT | 10 | 5 | 25 | 1250 | 12–14 | Patients were characterized as melancholic by CORE criteria. Only 3 went on to receive ECT. In 19 of 24 episodes (79.2%) MADRS scores decreased by < 50%. The mean time from treatment to relapes was 20 wk. |
| Triggs et al. (1999) | LDLPFC rTMS | 52.0 | 9/10 | Yes | 80% MT | 20 | 2 | 40 | 2000 | 10 | 5/10 had at least 50% redution in HRSD. Motor-evoked potnetial threshold decreased during treatment in 9/10. |
| Eschweiler et al.(2000) | LDLPFC RTMS (n = 2) RDLPFC TMS (n = 2) |
50.0 | Unknown | Unknown | LDLPFC: 90–100% MT; RDLPFC: 130% MT |
LDLPFC 10%; RDLPFC: 1 |
LDLPFC: 5–6.5; RDLPFC: 50 |
LDLPFC: 20; RLDPFC: 20 |
LDLPFC: 1000–1300; RLDPFC: 1000 |
5–15 | 38% of patients were responders with CGI score indicating much or very much improved. Non-responters and patients who relapsed received RUL ECT after an average of 14.3 ± 153 d;12 of 16 responded to ECT. The inclued all 6 TMS responders. The 4 ECT non-responders did not respond to earlier TMS (p < 0.05). |
| Cohen et al. (unpubl. obs.) | Bilateral TMS: LDLPFC rTMS and RDLPFC TMS | 45 | Yes | No | LDLPFC: 100% MT; RLDPFC: 100% MT |
LDLPFC: 20; RDLPFC 1 |
LDLPFC: 1.5; RLDPFC: 60 |
LDLPFC: 20; RLDPFC: 2 |
LDPFC: 600; RDLPFC: 120 |
5–10 | 4/10 (40%)patients showed a 50% reduction in HRSD scores, but change in CGI and self-ratings wwre slight. There was a trent for younger patients to have stronger therapeutic response. |
Burt T, Lisanby SH, Sackeim HA. Neuropsychiatric applications of transcranial magnetic stimulation: a meta analysis. International Journal of Neuropsychopharmacology 2002; 5(1):73-103; Subject to the copyright notice provided by Cambridge University Press, Reprinted with the permission of Cambridge University Press and the author (9)
Table 3. Randomized, controlled TMS trials in major depression: therapeutic outcome and effect size.
| Study | Design | Group 1 | N1 | % HRSD change |
s.d | Group 2 | N2 | % HRSD change |
s.d | Effect (d) | Lower | Upper | Total (n) | p value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kolbinger et al.(1995)[1] | Parallel | Above thershold rTMS (vertex) | 5 | 16.0 | 19.9 | Sham | 5 | 5.7 | 33.4 | 0.34 | –1.14 | 1.82 | 10 | 0.567 |
| Kolbinger et al.(1995)[2] | Parallel | Below threshold rTMS(vertex) | 5 | 35.5 | 17.8 | Sham | 5 | 5.7 | 33.4 | 1.01 | –0.60 | 2.61 | 10 | 0.116 |
| Conca et al.(1996) | Parallel | TMS(8 sites: frontal, temporal and parietal)+ medication | 12 | 57.5 | 25.0a | Medication only | 12 | 32.4 | 25.0a | 0.97 | 0.07 | 1.87 | 24 | 0.003 |
| Pascual-Leone et al.(1996b)[1] | Cross-over | LDLPFC rTMS | 17 | 48.0 | 30.0 | LDLPFC sham | 17 | 20 | 17.0 | 1.76 | 0.49 | 3.03 | 17 | 0.002 |
| Pascual-Leone et al.(1996b)[2] | Cross-over | RDLPFC rTMS | 17 | 2.0 | 20.0 | RDLPFC sham | 17 | 2.0 | 20.0 | 0.00 | –1.04 | 1.04 | 17 | 1.000 |
| George et al.(1997)[1] | Parallel | LDLPFC rTSM | 7 | 23.9 | 23.1 | Sham | 5 | –15.2 | 30.9 | 1.36 | –0.15 | 2.87 | 12 | 0.031 |
| George et al.(1997)[2] | Cross-over | LDLPFC rTMS | 5 | 5.6 | 26.0 | Sham | 7 | –15.8 | 22.5 | 0.83 | –0.56 | 2.21 | 12 | 0.158 |
| Avery et al.(1999) | Parallel | LDLPFC rTMS | 4 | 42.5 | 20.0a | Sham | 2 | 10.0 | 15.0a | 1.38 | –1.68 | 4.43 | 6 | 0.118 |
| Kimbrell et al.(1999)[1] | Cross-over | LDLPFC rTMS (20Hz) | 10 | –26.2 | 63.9 | LDLPFC TMS (1 Hz) | 10 | 18.8 | 21.6 | –0.99 | –2.59 | 0.61 | 10 | 0.120 |
| Kimbrell et al(1999)[2] | Cross-over | LDLPFC rTMS (20Hz) | 3 | 24.7 | 10.0 | Sham | 3 | 0.9 | 17.5 | 0.32 | –5.54 | 6.18 | 3 | 0.632 |
| Klein et al.(1999b) | Parallel | RDLPFC TMS | 35 | 46.9 | 33.1 | Sham | 32 | –7.9 | 33.1 | 0.69 | 0.19 | 0.19 | 67 | 0.007 |
| Loo et al.(1999) | Parallel | LDLPFC rTMS | 9 | 20.0 | 25.0a | Sham | 9 | 22.7 | 25.0a | –0.11 | –1.11 | 0.89 | 18 | 0.822 |
| Padberg et al.(1999)[1] | Parallel | LDLPFC rTMS | 6 | 5.6 | 9.5 | Sham | 6 | –5.9 | 21.2 | 0.70 | –0.63 | 2.03 | 12 | 0.254 |
| Padberg et al.(1999)[2] | Parallel | LDLPFC TMS | 6 | 19.5 | 14.0 | 6 | –5.9 | 21.2 | 1.41 | –0.03 | 2.85 | 12 | 0.035 | |
| Stikhina et al.(1999) | Parallel | LDLPFC TMS+ | 15 | 62.4 | 25.0a | Sham+ psychotherapy | 14 | 14.5 | 25.0a | 1.65 | 0.75 | 2.55 | 29 | 0.000 |
| Berman et al.(2000) | Parallel | LDLPFC rTMS | 10 | 31.5 | 23.4 | Sham | 10 | –0.2 | 31.7 | 1.14 | 0.12 | 2.15 | 20 | 0.020 |
| Eschweiler et al.(2000) | Cross-over | LDLPFC rTMS | 10 | 24.2 | 43.1 | Sham | 10 | –9.2 | 43.1 | 1.77 | 0.05 | 3.50 | 10 | 0.023 |
| George et al.(2000)[1] | Parallel | LDLPFC rMTS (20Hz) | 10 | 26.4 | 28.7 | Sham | 10 | 21.2 | 16.0 | 0.21 | –0.73 | 1.16 | 20 | 0.623 |
| George et al.(2000)[2] | Parallel | LDLPFC rTMS(5Hz) | 10 | 48.1 | 19.2 | sham | 10 | 21.2 | 16.0 | 1.46 | 0.37 | 2.54 | 20 | 0.003 |
| Garcia-Toro et al.(2001) | Parallel | LDLPFC rTMS | 17 | 26.0 | 20.0a | Sham | 18 | 12.6 | 15.0a | 0.76 | 0.05 | 1.47 | 35 | 0.031 |
| Lisanby et al.(2001b)[1] | Parallel | LDLPFC rTMS+ sertraline | 12 | 20.7 | 24.9 | Sham + sertraline | 12 | 13.3 | 34.6 | 0.24 | –0.61 | 1.09 | 24 | 0.554 |
| Lisanby et al.(2001b)[2] | Parallel | RDLPFC TMS+ sertaline | 12 | 19.5 | 26.1 | sham +sertaline | 12 | 13.3 | 34.6 | 0.20 | –0.65 | 1.04 | 24 | 0.625 |
| Manes et al.(2001) | Parallel | LDLPFC rTMS | 10 | 36.6 | 25.0a | Sham | 10 | 31.7 | 25.0a | 0.19 | –0.75 | 1.14 | 20 | 0.670 |
LDLPFC, left dorsolateral prefrontal cortex; RDLPFC, right dorsolateral prefrontal cortex; Effect (d), effect size of difference between Group 1 and Group 2; Lower and Upper are estimates of lower and upper 95% confidence intervals for the effect size.
Figures within brackets following the study’s authors refer to specific comparison within a study.
Indicates that the s.d was estimated.
Burt T, Lisanby SH, Sackeim HA. Neuropsychiatric applications of transcranial magnetic stimulation: a meta analysis. International Journal of Neuropsychopharmacology 2002; 5(1):73-103; Subject to the copyright notice provided by Cambridge University Press, Reprinted with the permission of Cambridge University Press and the author (9)
Table 4. Randomized, controlled TMS studies: patient characteristics and treatment parameters.
| Study | Design | Age | Medication resistant |
Medication free |
TMS intensity |
Pulse frequency (Hz) |
Train duration(s) |
No.of trains |
Total pulses per session |
No.of sessions |
|---|---|---|---|---|---|---|---|---|---|---|
| Kolbinger et al. (1995)[1] | Parallel | 49.0 | Unknown | No | 110%MT | 0.25–0.50 | na | 1 | 250 | 5 |
| Kolbinger et al. (1995)[2] | Parallel | 49.0 | Unknown | No | 90%MT | 0.25–0.50 | na | 1 | 250 | 5 |
| Conca et al. (1996) | Parallel | 42.7 | Unknown | No | 1.9T | 0.17 | 30 | 8 | 40 | 10–14 |
| Pascual-Leone et al. (1996b)[1] | Cross-over | 48.6 | All | No | 90%MT | 10 | 10 | 20 | 2000 | 5 |
| Pascual-Leone et al. (1996b)[2] | Cross-over | 48.6 | All | No | 90%MT | 10 | 10 | 20 | 2000 | 5 |
| George et al. (1997)[1] | Parallel | 42.0 | All | 9/12 | 80%MT | 20 | 2 | 20 | 800 | 10 |
| George et al. (1997)[2] | Cross-over | 42.0 | All | 9/12 | 80%MT | 20 | 2 | 20 | 800 | 10 |
| Avery et al. (1999) | Parallel | 44.5 | All | 2/6 | 80%MT | 10 | 5 | 20 | 1000 | 10 |
| Kimbrell et al. (1999)[1] | Cross-over | 42.1 | All | 7/10 | 80%MT | 20 | 2 | 20 | 800 | 10 |
| Kimbrell et al. (1999)[2] | Cross-over | 45.1 | All | 2/3 | 80%MT | 20 | 2 | 20 | 800 | 10 |
| Kelin et al. (1999) | Parallel | 59.0 | Most | 24/70 | 110%MT | 1 | 60 | 2 | 120 | 10 |
| Loo et al. (1999) | Parallel | 48.3 | Most | 5/18 | 110%MT | 10 | 5 | 30 | 1500 | 10 |
| Padberg et al. (1999)[1] | Parallel | 51.2 | All | 2/12 | 90%MT | 10 | 5 | 5 | 250 | 5 |
| Padberg et al. (1999)[2] | Parallel | 51.2 | All | 2/12 | 90%MT | 0.3 | 8.3 | 10 | 250 | 5 |
| Stikhina et al. (1999) | Parallel | 37.5 | Some | Yes | 0.015 T | 40 | 600 | 2 | 4800 | 10 |
| Berman et al. (2000) | Parallel | Unknown | Most | Yes | 80%MT | 20 | 2 | 20 | 800 | 10 |
| Eschweiler et al. (2000) | Cross-over | 57.0 | Unknown | No | 90%MT | 10 | 10 | 20 | 2000 | 10 |
| George et al. (2000)[1] | Parallel | 44.5 | Most | Yes | 100%MT | 20 | 2 | 40 | 1600 | 10 |
| George et al. (2000)[2] | Parallel | 45.4 | Most | Yes | 100%MT | 5 | 8 | 40 | 1600 | 10 |
| Garcia-Toro et al. (2001) | Parallel | 50.8 | All | No | 90%MT | 20 | 2 | 30 | 1200 | 10 |
| Lisanby et al. (2001d)[1] | Parallel | 48.2 | Most | All recd 50 mg sertraline |
110%MT | 10 | 8 | 20 | 1600 | 10 |
| Lisanby et al. (2001d)[2] | Parallel | 45.9 | Most | All recd 50 mg sertraline |
110%MT | 1 | 1600 | 1 | 1600 | 10 |
| Manes et al. (2001) | Parallel | 60.7 | All | Yes | 80%MT | 20 | 2 | 20 | 800 | 5 |
MT, Motor threshold.
Figures within brackets following the study’s authors refer to specific comparisons within a study.
Burt T, Lisanby SH, Sackeim HA. Neuropsychiatric applications of transcranial magnetic stimulation: a meta analysis. International Journal of Neuropsychopharmacology 2002; 5(1):73-103; Subject to the copyright notice provided by Cambridge University Press, Reprinted with the permission of Cambridge University Press and the author (9)
Table 5. Randomized trials contrasting rTMS and ECT in major depression: therapeutic effects and effects size.
| Study | Treatment groups |
Design | n | Depression type |
Percent change in HRSD |
s.d | Effect (d) |
lower | Upper | Group different in p value |
|---|---|---|---|---|---|---|---|---|---|---|
| Grunhaus et al (2000) | LDLPFC rTMS | Open and randomized | 20 | MDD(11 psychotic) | 40.3 | na | 0.54 | –0.11 | 1.19 | 0.09 |
| 12 RUL ECT only; 8 RUL and BL ECT | 20 | MDD(10 psychotic) | 60.6 | na | ||||||
| Pridmore et al. (2000) | LDLPFC rTMS | Single-masked eaters and randomized | 16 | 11 MDD 5 BPD | 55.6 | 30.2 | 0.33 | –0.40 | 1.06 | 0.40 |
| RUL ECT | 16 | 15 MDD 1BPD | 66.4 | 33.6 | ||||||
| Grunhaus et al. (unpubl.obs) | LDLPFC rTMS | Single-masked raters and randomized | 20 | MDD(non-psychotic) | 45.5 | na | 0.04 | –0.06 | 0.68 | 0.10 |
| 13 RUL ECT only;7 RUL and BL ECT | 20 | MDD(non-psychotic) | 48.2 | na |
LDLPFC, left dorsolateral prefrontal cortex; RUL, right unilateral ECT; BL, bilateral ECT; MMD, major depressive disorder; BPD, bipolar depressed; Effect (d), effect size of difference between ECT and rTMS groups. Lower and Upper are estimates of lower and upper 95% confidence intervals for the effect size.
Burt T, Lisanby SH, Sackeim HA. Neuropsychiatric applications of transcranial magnetic stimulation: a meta analysis. International Journal of Neuropsychopharmacology 2002; 5(1):73-103; Subject to the copyright notice provided by Cambridge University Press, Reprinted with the permission of Cambridge University Press and the author (9)
Table 6. Randomized trials contrasting rTMS and ECT in major depression: patient characteristics, treatment parameter, and comments.
| Study | Age | Medication resistant |
Medication free |
Stimulus intensity |
Pulse freq.(Hz) |
Train duration (s) |
No.of trains |
Pulses per session |
Total session |
Comment |
|---|---|---|---|---|---|---|---|---|---|---|
| Grunhaus et al. (2000) | 58.4 63.6 |
5/15 10/10 |
Clonazepam 1–2 mg/d No |
90%MT 2.5 × seizure threshold and increased progressively |
10 | 2 (8 patients) 6(12 patients) |
20 | 400–1200 | 20 9.6 (range 7–14) |
Pyschotic MDD patients had a superior response to ECT than rTMS (73.3 vs.27.5% reduction in HRSD, p = 0.005). Non-psychotic patients showed comparable reduction with ECT and rTMS (44.8 vs 53.2%). The degree of symptomatic improvment in non-psychotic patients was unusual for an ECT trial |
| Pridmore et al.(2000) | 44.0 41.5 |
All All |
No No |
100%MT 504 mC |
20 | 2 | 30–35 | 1200–1400 30 |
10–14 12.2±3.4 |
ECT was superior to rTMS in multivariate analyses across depression measures (p = 0.04), with the diffrent most market for the Beck Depression Inventory (BDI)(69.1 vs 45.5% improvment, p = 0.03). HOwever, no difference noted on change in HRSD. An equivalent number of patients in each group (11 of 16) achieved remission criteria (final HRSD < 8). |
| Grunhaus et al. (unpubl. obs.) | 57.6 61.4 |
na na |
Lorazepam (up to 3 mg/d) Lorazepam (up to 3 mg/d) |
90% MT 2.5 × seizure threshold and increased progressively |
10 | 6 | 10 | 1200 | 20 10.3 ± 3.1 (range 4–13) |
ECT and rTMS were equivalent in efficacy in all deoression measures. 12 of 20 ECT patients met response critiria (52% descrease in HRSD or a final rating < 10 and final GAF < 60; 11 of 20 rTMS patients met response criteria. As in the previous study by Grunhaus et al.(2000) the degree of impronement was unusually ioe for an ECT sample. |
Burt T, Lisanby SH, Sackeim HA. Neuropsychiatric applications of transcranial magnetic stimulation: a meta analysis. International Journal of Neuropsychopharmacology 2002; 5(1):73-103; Subject to the copyright notice provided by Cambridge University Press, Reprinted with the permission of Cambridge University Press and the author (9)
Table 7. rTMS studies (comparator is placebo, different intensities, or different cranial locations) published from 2002 to April 2004.
| Study (year, author, country) | Objective | Method | Results | Limitations |
|---|---|---|---|---|
| 2003 Fitzgerald et al.(62) Australia | To prospectively evaluate the efficacy of high-frequency LDLPFC and low-frequency RPFC in treatment resistant depression and compared with a placebo-treated control group. | Double-blind randomized placebo-controlled trial. Primary outcome measured after 2 weeks treatment. Participants: 60 patients with treatment resistant depression who had failed to respond to therapy with multiple antidepressant medications were divided into 3 groups of 20 that did not differ in age, sex or any clinical variables. All patients completed the double-blind phase of the study. Intervention: 20, 5-second high-frequency LDLPFC trains at 10 Hz and 5 60 second low-frequency RPRC trains at 1 Hz applied daily for 2 weeks. Placebo stimulation applied with coil angled at 45 degrees from scalp resting on the side of one wing of the coil. Main outcome measure: score on Montgomery-Asberg Depression Rating Scale. Patients and raters were blind to treatment, but the clinician administering rTMS was aware of the treatment group. After the 10th session, a blinded assessment was made and the patients but not the raters were informed of their treatment group. Patients who had a reduction in MADRS score of >20% in the active treatment arms could continue with the same TMS condition for another 10 sessions. Patients not achieving this improvement offered option of crossing over to the other active treatment. Patients initially randomized to placebo were subsequently randomized to 1 of the 2 active treatments after initial review. During 2nd phase of study, raters remained blind to treatment type. Patients were not deliberately withdrawn from medication before the trial, but their doses were not allowed to have changed in the 4 weeks before commencement of or during the trial. Figure-8 coil. |
Significant difference in response among the 3 groups with a significant difference between the high-frequency LDLPFC [reduction in mean score 13.5%±SD16.7%] and placebo [0.76%±16.2%] groups and between the low-frequency RPFC [15.0%±14.1%] and placebo groups (P<.005 for all) but not between the 2 treatment groups (P=.91). Concurrent use of medication did not have a statistically significant effect on primary outcome (P=.23). After phase 1, 7 (11%) of the 60 patients reported site discomfort or pain during rTMS and 6 (10%) reported a headache after rTMS. No difference n the incidence of these adverse effects (P=.08). 29 (48%) of patients correctly guessed their type of treatment before disclosure, 17 (42%) of 40 in the active treatment group (P=.34) and 12 (60%) of 20 in the placebo group (P=.37). The degree of response was the predominant reason given for the guess. |
No a priori sample size calculation or justification reported. The a priori primary outcome of interest was measured after 2 weeks of treatment. After 2 weeks, the study is no longer considered double blind. Any analyses after the double-blind phase of the study is limited since patients were aware of their treatment. |
| 2004 Hausmann et al.(66) Austria | To assess whether bilateral rTMS is superior to unilateral rTMS and that there is a speeding up effect in the add on group in comparison to the usual care group medicated with antidepressants alone. | Single centre, prospective double-blind placebo-controlled “add on” trial. 41 medication free patients with MDD without psychotic features admitted to a psychiatric unit were consecutively randomized into 3 groups. Group A1 (n=12) received unilateral active stimulation (20 Hz, 100% MT, 10 trains of 10 sec duration with a 90 sec intertrain interval) consisting of high-frequency rTMS over the LDLPFC and subsequent placebo low-frequency rTMS over the RDLPFC. Group A2 (n=13) received simultaneous bilateral active stimulation consisting of high-frequency rTMS over the LDLPFC and low-frequency RDLPFC rTMS (1 Hz for 10 min, 120% MT). Group C (n=13) received bilateral placebo stimulation. Stimulation was performed on 10 consecutive workdays. Antidepressants were started on the first day of stimulation and maintained throughout the stimulation period. Dosage remained constant throughout the trial. At entry patients underwent a 5 x half life washout period. The optimal LDLPFC and RDLPFC positions were determined by 3D MRI. Figure-8 coil. |
Of 41 patients recruited, 38 completed the 2 week protocol. No significant differences between the 3 groups as well as between the pooled treatment groups (A1 and A2) and the placebo group regarding HDRS and BDI scores at baseline. No significant differences between the 2 active treatment groups in terms of HDRS and BDI scores over the course of treatment (day 7, 14, and 28). Groups A1 and A2 were pooled for comparison to Group C. At day 14 and 28 there was no statistically significant difference between rTMS (A1 and A2) and controls (Group C) in mean percentage decrease of the HDRS total score and the BDI. When testing the time course of the outcome variables, HDRS and BDI (days 0, 7, 14, 28) by repeated measures ANOVA, there was a significant effect of time on outcome variables in both groups, whereas there were no significant group differences in terms of a group by time interaction. The size of the interaction term for the HDRS amounted to 2.8 (95% CI -2.8 to 8.5) for day 14 versus baseline, and 3.0 (95% CI - 3.8 to 9.8) for day 28 versus baseline, where a positive value indicates a favour for group A1+A2. The corresponding values for BDI were 5.0 (95% CI - 3.2 to 13.2) and 5.7 (95% CI - 3.8 to 15.0) respectively. |
No a priori sample size calculation or justification reported. Antidepressant regimens were variable. Duration of treatment was 10 days, outcome measured up to 28 days from baseline. |
| 2003 Hoppner et al.(67) Germany | To compare clinical effects of 2 different stimulation procedures with placebo stimulation as add on treatments in patients with depressive disorders. | 30 depressed patients. Every patient received an antidepressant in a constant dose over 2 weeks before and during the stimulation period. Patients were randomly allocated to receive high-frequency LDLPFC (twenty 20 Hz trains of 2 sec duration with intertrain interval of 60 sec), low-frequency FDLPFC (two 1 Hz trains of 60 sec duration with an intertrain interval of 3 minutes) or placebo stimulation on 10 out of 12 days (2 weeks with 5 sessions each week). Ten patients were included in each group. Placebo stimulation consisted of same conditions as the 20 Hz rTMS except that the coil was placed at a 90 degree angle to the head. Severity of depression was assessed by HDRS (21 items) and BDI. The rater was a psychiatrist who was blind to the stimulation procedure. Clinical response was defined as >50% improvement of baseline scores (HDRS, BDI) between pre and post treatment assessments. Figure-8 coil. |
29/30 patients initially included in the study completed the treatment phase. One patient from the high-frequency treatment group refused to continue after 6 days because of insufficient effectiveness and headache. The other 29 patients did not report adverse effects. 5 patients in the 20Hz group were responders compared to 3 patients in the 1 Hz group and 5 patients who were placebo stimulated. Average reduction of baseline score was 61.8%. Only one of the patients was classified as responder according to both BDI and HDRS score criteria. HDRS scores significantly reduced among the 20 Hz group (day 5 P=.03; end of treatment P=.015) and placebo stimulation group (day 5 P=.017; end of treatment P≤.001) but was not statistically significant in the 1 Hz group. 2/9 patients treated by 20 Hz were “responders” based on BDI. One patient from 1 Hz “responded”. Two placebo stimulated patients “responded”. Within the 2 stimulation groups, reduction was observed after 5 days (20 Hz day 5, P=.008, end of treatment, P=.011; 1 Hz day 5, P=.039, end of treatment P=.029). For the placebo treatment group, improvement was statistically significant only at the end of treatment (P=.005). |
No a priori sample size calculation or justification reported. Authors stated: 1) “Preliminary and explorative comparison study”. 2) “Small sample size” 3) Enhanced placebo effect of rTMS due to its “impressive name, its ability to cause involuntary movements as if by magic, its discomfort, and its bulky and sophisticated looking equipment.” 4) “Results of preliminary data points to the necessity of further research to be able to answer the still open questions regarding stimulation procedures of rTMS, location of stimulation and of the number of treatment sessions.” |
| 2003 Nahas et al.(12) United States | To determine the safety feasibility and potential efficacy of using TMS to treat the depressive symptoms of bipolar affective disorder (BPAD). | 23 depressed BPAD patients (12 BPI depressed state and 9 BPII depressed stated, 2 BPI mixed state) were enrolled. Patients assigned using “an urn randomization based on age and gender) to receive daily LDLPFC rTMS (5 Hz, 110% MT, 8 sec on, 22 sec off over 20 min) or placebo (coil angled 45 degrees off head) each weekday morning for 2 weeks. Blinded HDRS and Young Mania Rating Scales (YMRS) obtained weekly. Patients could take carbamazepine or valproate but eth dose had to be stable for 2 weeks prior to beginning treatment with persisting depression. All other psychotropic medications (especially antidepressants) were tapered over a 2 week washout period, longer for fluoxetine). Patients on lithium and lamotrigine were also excluded. Primary outcome variable: percentage change in HDRS at 2 weeks compared with day 1 of treatment (clinical response defined as >50% decline in HDRS or <10). Following last day of the 2 week period, the blind was broken for each patient. Patients initially randomized to placebo were offered the option of 2 weeks of active treatment at the same parameters. Treatment responders to either the active or later open TMS phase were offered the option of weekly maintenance TMS treatments over the next year. Figure-8 coil. |
11 patients received rTMS. All patients guessed their status based on their clinical response (all responders guessed they were receiving active TMS and all clear nonresponders guessed placebo). No adverse cognitive effects of the TMS as measured by subjective complaints. No drop outs from the study. No patients stopped the study as a result of hypomania or mania and active rTMS did not cause a statistically significant within group increase in the YMRS (P=.49). 4/11 active TMS patients were responders. 4/12 placebo TMS patients were responders. Each group had one “remitter”. There was no significant difference between the 2 groups in HDRS change from baseline over the 2 weeks (P=.83). Mean % change in HDRS was 25% (SD, 32%) for the active TMS and 25% (SD, 31%) for the placebo TMS. |
No a priori sample size calculation or justification reported. Small sample size. Two weeks duration. Only 2 week drug washout period. High placebo response. |
| 2002 Boutros et al.(63) United States | “To provide efficacy and safety data for use of subthreshold rTMS as an augmentation strategy in treatment refractory depressed patients without any modifications on their current pharmacological therapy in a double-blind randomized fashion.” | 22 patients diagnosed with MDD. Patients had to have failed 2 prior medication attempts. A score of at least 20 on the HDRS was required. Patients randomized into either placebo or active rTMS. Patients assessed at days 3, 5, 6, 8, and 10. A research assistant blinded to the treatment administered the depression score testing. Unblinded psychiatrist administered the rTMS. Blind broken following treatment #10. Following completion of the placebo course, patients were offered open label active rTMS. Treatment consisted of 10 left sided stimulation sessions administered over 10 consecutive weekdays. Each session consisted of 20, 2sec stimulation trains, with a 20 Hz frequency applied over 20 min with 58 second intervals between trains. Stimulations were delivered at 80% MT. Figure-8 coil. During placebo, the coil was angled 90 degrees to the head. |
One patient dropped out of placebo rTMS after the first session – data from that patient was excluded from the analysis. 12 patients were randomized to receive active rTMS and 9 to placebo. There was no statistically significant difference between HDRS scores at baseline between the 2 groups. Analyses performed included a comparison between the HDRS scores following the last treatment with baseline as a covariate (ANCOVA). No difference between the 2 groups was found. A repeated measures ANOVA found that the slope associated with depression over time did not differ significantly between the 2 groups but a significant time effect (P=.001) occurred. Patients in both groups reported adverse effects (8 in active and 5 in placebo). Most frequent complaint was headache during o rafter the session. 3 patients in the active and 1 in the placebo reported transient scalp tenderness. |
No a priori sample size calculation or justification reported. “Sample size used in the study was small”. Two week randomized duration. |
| 2002 Padberg et al.(13) Germany | To test the hypothesis that antidepressant efficacy of rTMS is related to the stimulation intensity applied. | Parallel design controlled study of 31 patients with MDD who were pharmacotherapy resistant prior to rTMS. Patients randomized into a 2 week trial of LDLPFC rTMS either at 100% MT intensity (n=10) at 90% MT (n=10) or at a low intensity placebo condition (n=10). One patient dropped out due to withdrawal of consent after the second rTMS session. The dosage of the current unsuccessful antidepressant treatment was kept constant for at least 3 weeks prior to rTMS. Patients were examined by a psychiatrist uninvolved in rTMS treatment and blinded to the rTMS condition. Placebo was angled 90 degrees to the head. A 7 point clinical global impression (CGI) of severity scale was used as overall outcome measure. Figure-8 coil. |
No significant differences were found between the groups for baseline HDRS, MADRS or CGI scores. Across treatment groups, depression scores significantly declined during rTMS (P<.001). The main effect of treatment group was not significant. Interaction of treatment group with time was significant for MADRS scores (P<.05) but not for HDRS scores. The linear effect on MADRS difference scores was significant (P<.05). Expressed in percent decrease of MADRS scores, placebo yielded a 4.1% (SE 5.2) reduction, 90% MT resulted in 15.1% (SE 6.6) decrease and 100% MT reduced MADRS scores by 33.2% (SE 8.9) The linear effect on HDRS scores showed was not statistically significant. Percent reductions of HDRS scores were 7.1% (SE 5.8) after placebo rTMS, 14.9% (SE 8.9) after 90% MT and 29.6% (SE 8.7) after 100% MT. One way ANOVA showed significant differences between groups for both duration of the hospital stay (P<.05) and the number or required antidepressant trials after rTMS (P<.001). No severe adverse effects of rTMS were observed. |
No a priori sample size calculation or justification reported. “Small sample size”. “Regard findings as preliminary”. |
| 2002 Conca et al.(65) Austria | To investigate the augmentation properties of rTMS combining low and high frequencies. | Recruited 36 severely depressed medicated in patients. Medication conditions defined using the classification of Thase and Rush (1995) – all patients had to be classified at stage 4 of treatment resistance indicating the failure to respond to 2 different adequate monotherapy trials of medications with different pharmacological profiles and the failure to respond to a 2nd augmentation strategy. Psychiatrists blinded to treatment methods completed the 21 item HDRS 1 day before the first treatment and 1 day after completion and 1 day after completion of the rTMS course. For inclusion, a minimum score of ≥24 had to be achieved. Clinical Global Improvement (CGI) completed 1 day after rTMS. Patients remained on the last prescribed pharmacotherapy. Patients randomly assigned to 3 different rTMS treatment modalities while on stable drugs for 5 consecutive days. rTMS administered on 5 consecutive days at 1 session/day. Group 1 (n=12) = 110% MT, 10Hz, 10 trains, train duration 10 seconds, each with a train interval of 60 seconds over the LDLPFC. Over the RDLPFC: 110% MT at 1 Hz, 1 train at 300 seconds. Group 2 (n=12) = only the LDLPFC was stimulated at 110% MT, 10Hz, 10 second train duration alternating with 110% MT, 1 Hz, 30 second train, with an interval of 6 seconds for 10 times/session. Group 3 (n=12) = standard stimulation over LDLPFC using 110% MT, 10 Hz, 13 train and 10 second train duration. Therapeutic outcome measurement was based on CGI scores. Patient response was defined as achieving at least moderate improvement (CGI improvement score >4) and being no more than mildly ill (CGI severity score <4). Figure-8 coil. |
No statistical differences in clinical outcome between the groups. None of the demographic, illness, diagnosis, or co-diagnosis related data revealed any influence on the response rate except for handedness; right handed patients showed a weak statistical tendency to greater therapeutic response (non responders vs responders, P<.08). No seizures were observed. 7/36 patients (19.1%) experienced a mild headache during the first session which remitted spontaneously. |
No a priori sample size calculation or justification reported. Small sample size “Preliminary findings”. No control group |
| 2003 Schule et al.(68) Germany | To examine whether antidepressant pharmacotherapy can stabilize clinical improvement after rTMS monotherapy | 26 drug-free patients. Open trial over 2 weeks 10-13 sessions, 10 Hz, 15 trains (each 10 seconds) with 30 second intertrain intervals) LDLPFC at 100% MT. Patients followed-up during standardized antidepressant pharmacotherapy with mirtazapine for a further 4 weeks. Severity of depression assessed using 21 item HDRS. Ratings performed before rTMS treatment (day 0) and after one week (day 7) and after 2 weeks of rTMS (day 14). Raters were not involved in rTMS treatment. Response to rTMS was defined by a reduction of at least 50% of the HDRS score after 2 weeks of rTMS compared to baseline. An overall response (rTMS followed by mirtazapine treatment) week 0-6) was defined by a decrease of at least 50% in the HDRS score after 6 weeks compared with baseline (week 6 response). Remission was defined as a HDRS score of 9 or less after 2 weeks or 6 weeks respectively. |
10/26 (39%) of patients responded to rTMS by at least 50% reduction in the HDRS. 23% (6/26) were remitters (HDRS score ≤9). During subsequent antidepressant treatment, response and remission rates were further increased: after week 6, 77% of the patients (20/26) achieved clinical remission. The differences in the HDRS scores between rTMS responders and nonresponders became significant (P<.05) after 1 week of rTMS monotherapy and were observed up to the 2nd week of mirtazapine treatment. After 3 weeks of drug therapy, there were no more significant differenced between rTMS responders and nonresponders. 69% of the rTMS nonresponders (11/16) converted into 6-week responders. rTMS responders and nonresponders did not differ in severity of depressive symptoms at baseline, number of depressive episodes, and number of failed antidepressant trials during the current episode and duration of the current episode. Week-6 responders and nonresponders were comparable n baseline HDRS scores and number of depressive episodes. However, week-6 nonresponders were characterized by a significantly higher number of failed antidepressant trials before entering the study (P=.025) and a significantly longer duration of the current episode (P=.037) compared with week-6 responders. A significant deterioration of depressive symptoms occurred between the last rTMS treatment and the first administration of mirtazapine in rTMS responders (P=.002), but not in rTMS nonresponders (P=.375). An overall low but significant correlation (P=.025) between worsening on the HDRS score and duration of treatment interruption. The deterioration in rTMS responders was reversible in most cases: 9/10 rTMS responders showed an at least stabilized course during subsequent mirtazapine treatment (week 3-6). One rTMS responder became worse during pharmacotherapy. |
No a priori sample size calculation or justification reported. No control group. Open study design. Half of the patients also received lithium, carbamazepine, or neuroleptics. |
| 2003 Herwig et al.(69) Germany | To investigate the efficacy of neuronavigated rTMS guided according to the prefrontal metabolic state determine by PET. | Double-blind randomized placebo controlled pilot study 25 patients with MDD. 13 patients rTMS 12 patients placebo. Prior to rTMS, PET scans obtained. For stimulation, the DLPFC with lower metabolic activity compared to the contralateral hemisphere was selected. Stimulation parameters 15 Hz, 110% MT. A neuronavigational system (navigate the coil according to individual anatomy visualized by MRI) was used to place the magnetic coil above each individuals selected cortical region (active treatment: DLPFC; placebo treatment: midline parieto-occipital, intensity 90% of MT). rTMS was administered as an add-on to drugs. Depression related symptoms rated with BDI, HDRS, and MADRS scales. Responders were defined by a 50% reduction of the mean of the HDRS and MADRS ratings. Double blind=neither the raters nor the patients were informed about the stimulation condition. Ratings performed 5 times: Before stimulation After 4 stimulations After 7 stimulations At the end of the stimulation sessions and In responders 2 weeks after the stimulations sessions. Figure-8 coil. |
Severe adverse effects not observed. Initial rating scores of the placebo and real stimulated patients did not differ between the groups. There were significant differences between the placebo and active stimulation groups in terms of the relative changes in scores (at the end of stimulations compared with the initial scores normalized to 100%) in percent in HDRS (P=.002) and MADRS (P<.001) but not for the self rating BDI (P=0.1). Responder rate=4/13 in active treatment group and 0/12 in the placebo group. The ratings performed 2 weeks after the stimulation sessions in the 4 responding real stimulated patients showed a persisting effect with a mean HDRS of 48% and mean MADRS of 44% of the initial rating scores. Authors concluded “preliminary results indicate that rTMS of prefrontal hypometabolism may not be advantageous to stimulation irrespective of the metabolic state.” |
No a priori sample size calculation or justification reported. Not directly tested if the navigational approach led to better outcome than at the “5 cm rule”. “small sample size” |
Suggested Citation
This report should be cited as follows:
Medical Advisory Secretariat. Repetitive transcranial magnetic stimulation for the treatment of major depressive disorder: an evidence-based analysis. Ontario Health Technology Assessment Series 2004;4(7).
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to MASinfo.moh@ontario.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: www.health.gov.on.ca/ohtas.
Print copies can be obtained by contacting MASinfo.moh@ontario.ca.
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All Medical Advisory Secretariat analyses are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Contact Information
The Medical Advisory Secretariat
Ministry of Health and Long-Term Care
20 Dundas Street West, 10th floor
Toronto, Ontario
CANADA
M5G 2N6
Email: MASinfo.moh@ontario.ca
Telephone: 416-314-1092
ISSN 1915-7398 (Online)
ISBN 978-1-4249-7279-1 (PDF)
About the Medical Advisory Secretariat
The Medical Advisory Secretariat is part of the Ontario Ministry of Health and Long-Term Care. The mandate of the Medical Advisory Secretariat is to provide evidence-based policy advice on the coordinated uptake of health services and new health technologies in Ontario to the Ministry of Health and Long-Term Care and to the healthcare system. The aim is to ensure that residents of Ontario have access to the best available new health technologies that will improve patient outcomes.
The Medical Advisory Secretariat also provides a secretariat function and evidence-based health technology policy analysis for review by the Ontario Health Technology Advisory Committee (OHTAC).
The Medical Advisory Secretariat conducts systematic reviews of scientific evidence and consultations with experts in the health care services community to produce the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, the Medical Advisory Secretariat systematically reviews available scientific literature, collaborates with partners across relevant government branches, and consults with clinical and other external experts and manufacturers, and solicits any necessary advice to gather information. The Medical Advisory Secretariat makes every effort to ensure that all relevant research, nationally and internationally, is included in the systematic literature reviews conducted.
The information gathered is the foundation of the evidence to determine if a technology is effective and safe for use in a particular clinical population or setting. Information is collected to understand how a new technology fits within current practice and treatment alternatives. Details of the technology’s diffusion into current practice and input from practicing medical experts and industry add important information to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist policy makers to make timely and relevant decisions to optimize patient outcomes.
If you are aware of any current additional evidence to inform an existing evidence-based analysis, please contact the Medical Advisory Secretariat: MASinfo.moh@ontario.ca. The public consultation process is also available to individuals wishing to comment on an analysis prior to publication. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Disclaimer
This evidence-based analysis was prepared by the Medical Advisory Secretariat, Ontario Ministry of Health and Long-Term Care, for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation, and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data, and information provided by experts and applicants to the Medical Advisory Secretariat to inform the analysis. While every effort has been made to reflect all scientific research available, this document may not fully do so. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of publication. This analysis may be superseded by an updated publication on the same topic. Please check the Medical Advisory Secretariat Website for a list of all evidence-based analyses: http://www.health.gov.on.ca/ohtas.
Abbreviations
- ANCOVA
Analysis of covariance
- ANOVA
Analysis of variance
- BDI
Beck Depression Inventory
- BPRS
Brief Psychiatric Rating Scale
- CGI
Clinical Global Impression Scale
- CI
Confidence interval
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders IV
- ECT
Electroconvulsive therapy
- DLPFC
Dorsolateral prefrontal cortex
- GAF
Global Assessment of Function
- GAS
Global Assessment Scale
- GDR
Global Depression Scale
- HDRS
Hamilton Depression Rating Scale
- Hz
Hertz
- LDLPFC
Left dorsolateral prefrontal cortex
- MADRS
Montgomery-Asberg Depression Rating Scale
- MDD
Major depressive disorder
- MMSE
Mini-Mental State Examination
- PET
Positron emission tomography
- PSQI
Pittsburg Sleep Quality Index
- RPFC
Right prefrontal cortex
- rTMS
Repetitive transcranial magnetic stimulation
- SD
Standard deviation
- SMD
Standardized mean difference
- TMS
Transcranial magnetic stimulation
- WMD
Weighted mean difference
- YMRS
Young Mania Rating Scale
Glossary
- Dorsal:
Denoting a position more toward the back surface than some other object of reference.
- Electroconvulsive therapy (ECT):
Psychiatric treatment that briefly passes an electrical current through the brain in order to produce a convulsion. Treatment can be delivered bilaterally (electrodes placed on both sides of the skull) or unilaterally (on the right side of the skull).
- Hertz (Hz):
Unit of frequency.
- Lateral:
Denoting a position farther from the median plane or midline of the body or of a structure.
- Prefrontal cortex:
The anterior part of the frontal cortex of the brain.
- Recovery:
Remission for at least 6 consecutive months.
- Regression to the mean:
Occurs whenever a nonrandom sample is selected from a population and 2 imperfectly correlated variables are measured such as 2 consecutive blood pressure measurements. It occurs whenever a group is selected with extreme values for one variable and another variable is then measured.
- Remission:
Attainment of a virtually asymptomatic status (e.g., HRSD score < 7) for at least 2 consecutive weeks.
- Repetitive transcranial magnetic stimulation:
The application of pulsed magnetic fields to the cortical surface of the brain with the aim of stimulating or disrupting ongoing brain activity.
References
- 1.Sackett DL, Haynes RB, Guyatt GH, Tugwell P. Clinical epidemiology – a basic science for clinical medicine. London: Little Brown. 1991 [Google Scholar]
- 2.Hotopf M, Chruchill R, Lewis G. Pragmatic randomized controlled trials in psychiatry. Br J Psychiatry. 1999;175:217–223. doi: 10.1192/bjp.175.3.217. [DOI] [PubMed] [Google Scholar]
- 3.Martin JL, Barbanoj MJ, Schlaepfer TE, Thompson E, Perez V. Repetitive transcranial magnetic stimulation for the treatment of depression: systematic review and meta-analysis. Br J Psychiatry. 2003;182:480–491. doi: 10.1192/bjp.182.6.480. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg R. Outcome measures of antidepressive therapy. Acta Psychiatr Scand. 2000;101:41–44. doi: 10.1034/j.1600-0447.2000.02607.x. [DOI] [PubMed] [Google Scholar]
- 5.Klein E, Kreinin I, Chistyakov A, Koren D, Mecz L, Marmur S, et al. Therapeutic efficacy of right prefrontal slow repetitive transcranial magnetic stimulation in major depression: a double-blind controlled study.[see comment] Archives of General Psychiatry. 1999;56(4):315–320. doi: 10.1001/archpsyc.56.4.315. [DOI] [PubMed] [Google Scholar]
- 6.Dannon PN, Dolberg OT, Schreiber S, Grunhaus L. Three and six-month outcome following courses of either ECT or rTMS in a population of severely depressed individuals--preliminary report. Biological Psychiatry. 2002;51(8):687–690. doi: 10.1016/s0006-3223(01)01274-4. [DOI] [PubMed] [Google Scholar]
- 7.Wassermann EM, Lisanby SH. Therapeutic application of repetitive transcranial magentic stimulation: a review. Clin Neurophysiol. 2001;112:1367–1377. doi: 10.1016/s1388-2457(01)00585-5. [DOI] [PubMed] [Google Scholar]
- 8.Kozel FA, George MS. Meta-analysis of left prefrontal repetitive transcranial magnetic stimulation (rTMS) to treat depression. Journal of Psychiatric Practice. 2002;8(5):270–275. doi: 10.1097/00131746-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Burt T, Lisanby SH, Sackeim HA. Neuropsychiatric applications of transcranial magnetic stimulation: a meta analysis. International Journal of Neuropsychopharmacology. 2002;5(1):73–103. doi: 10.1017/S1461145702002791. [DOI] [PubMed] [Google Scholar]
- 10.Holtzheimer PE, III, Russo J, Avery DH. A meta-analysis of repetitive transcranial magnetic stimulation in the treatment of depression. Psychopharmacology Bulletin. 2001;35(4):149–169. [PubMed] [Google Scholar]
- 11.O’Connor M, Brenninkmeyer C, Morgan A, Bloomingdale K, Thall M, Vasile R, et al. Relative effects of repetitive transcranial magnetic stimulation and electroconvulsive therapy on mood and memory: a neurocognitive risk-benefit analysis. Cognitive & Behavioral Neurology. 2003;16(2):118–127. doi: 10.1097/00146965-200306000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Nahas Z, Kozel FA, Li X, Anderson B, George MS. Left prefrontal transcranial magnetic stimulation (TMS) treatment of depression in bipolar affective disorder: a pilot study of acute safety and efficacy. Bipolar Disorders. 2003;5(1):40–47. doi: 10.1034/j.1399-5618.2003.00011.x. [DOI] [PubMed] [Google Scholar]
- 13.Padberg F, Zwanzger P, Keck ME, Kathmann N, Mikhaiel P, Ella R, et al. Repetitive transcranial magnetic stimulation (rTMS) in major depression: relation between efficacy and stimulation intensity. Neuropsychopharmacology. 2002;27(4):638–645. doi: 10.1016/S0893-133X(02)00338-X. [DOI] [PubMed] [Google Scholar]
- 14.Aarre TF, Dahl AA, Hohansen JB, Kjonniksen I, Neckelmann D. Efficacy of repetitive transcranial magnetic stimulation in depression: a review of the evidence. Nordic Journal of Psychiatry. 2003;57:227–232. doi: 10.1080/08039480310001409. [DOI] [PubMed] [Google Scholar]
- 15.Martin JLR, Barbanoj MJ, Schlaepfer TE, Clos S, Perez V, Kulisevsky J, et al. Transcranial magnetic stimulation for treating depression. Cochrane Database of Systematic Reviews. 2004;(1) doi: 10.1002/14651858.CD003493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cochrane Statistical Methods Group, corpauthor. Analysing and Presenting Results. In: Deeks J, Higgins J, Altman D, editors. Cochrane Reviewers’ Handbook 4.2.2. Oxford: Cochrane Collaboration; 2004. pp. 68–139. [Google Scholar]
- 17.Reus VI. Mental Disorders. Harrison’s Principles of Internal Medicine. In: Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, editors. New York: McGraw-Hill; 2001. pp. 2542–2556. [Google Scholar]
- 18.Parikh SV, Lam RW the CANMAT Depression Work Group, corpauthor. Canadian Journal of Psychiatry. 2001;46:13S–19S. Clinical guidelines for the treatment of depressive disorders. I. Definitions, prevalence and health burden. [PubMed] [Google Scholar]
- 19.Mueller TI, Leon AC, Keller MB, Solomon DA, Endicott J, Coryell W, et al. Recurrence after recovery from major depressive disorder during 15 years of observational followup. Am J Psychiatry. 1999;156:1000–1006. doi: 10.1176/ajp.156.7.1000. [DOI] [PubMed] [Google Scholar]
- 20.Lee AS, Murray RM. The long term outcome of Maudsley depressives. Br J Psychiatry. 1988;153:741–751. doi: 10.1192/bjp.153.6.741. [DOI] [PubMed] [Google Scholar]
- 21.Anonymous, corpauthor. American Psychiatric Association practice guideline for the treatment of patients with major depressive disorder. Am J Psychiatry. 2000;157(Suppl 4):1–45. [PubMed] [Google Scholar]
- 22.Kennedy SH, Lam RW, Morris B. for the CANMAT Depression Work Group. Can Fam Physician. 2003;49:489–491. Clinical guidelines for depressive disorders. summary of recommendations relevant to family physicians. [PMC free article] [PubMed] [Google Scholar]
- 23.UK ECT Review Group, corpauthor. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361:799–808. doi: 10.1016/S0140-6736(03)12705-5. [DOI] [PubMed] [Google Scholar]
- 24.Rose D, Wykes T, Leese M, Bindman J, Fleischmann P. Patients’ perspectives on electroconvulsive therapy: systematic review. Br Med J. 2003;326:1363–1367. doi: 10.1136/bmj.326.7403.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Institute of Clinical Evidence, corpauthor. Depression: the management of depression in primary and secondary care. NICE guideline. 2003 2003 Dec;:1–58. Draft for Second Consultation. [Google Scholar]
- 26.Stimpson N, Agrawal N, Lewis G. Randomised controlled trials investigating pharmacological and psychological interventions for treatment refractory depression. Br J Psychiatry. 2002;181:284–294. doi: 10.1192/bjp.181.4.284. [DOI] [PubMed] [Google Scholar]
- 27.Thase ME, Rush AJ. Treatment resistant depression. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. New York: Raven Press Ltd.; 1995. pp. 1081–1097. [Google Scholar]
- 28.Chaimowitz GA, Links PS, Padgett RW, et al. Treatment resistant depression: a survey of practice habits of Canadian psychiatrists. Canadian Journal of Psychiatry. 1991;36:353–356. doi: 10.1177/070674379103600507. [DOI] [PubMed] [Google Scholar]
- 29.Nierenberg AA, White K. What next? A review of pharmacologic strategies for treatment resistant depression. Psychopharmacology Bulletin. 1990;26:429–460. [PubMed] [Google Scholar]
- 30.Shergill SS, Katona CLE. Pharmacological choices after one antidepressant fails: a survey of UK psychiatrists. J Affect Disord. 1997;43:19–25. doi: 10.1016/s0165-0327(96)00100-0. [DOI] [PubMed] [Google Scholar]
- 31.Fitzgerald PB, Brown TL, Daskalakis ZJ. The application of transcranial magnetic stimulation in psychiatry and neurosciences research. Acta Psychiatr Scand. 2002;105:324–340. doi: 10.1034/j.1600-0447.2002.1r179.x. [DOI] [PubMed] [Google Scholar]
- 32.Hasey G. Transcranial magnetic stimulation in the treatment of mood disorder: a review and comparison with electroconvulsive therapy. Canadian Journal of Psychiatry. 2001;46:720–727. doi: 10.1177/070674370104600804. [DOI] [PubMed] [Google Scholar]
- 33.George MS, Wassermann EM, Williams WA, Callahan A, Ketter TA, Basser P, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6:1853–1856. doi: 10.1097/00001756-199510020-00008. [DOI] [PubMed] [Google Scholar]
- 34.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: Report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation. Electroencephalography & Clinical Neurophysiology: Evoked Potentials. 1996 1998 Jun 5-7;108(1):1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- 35.Kirkcaldie M, Pridmore S, Reid P. Bridging the skull: Electroconvulsive therapy (ECT) and repetitive transcranial magnetic stimulation (rTMS) in psychiatry. Convuls Ther. 1997;13(2):83–91. [PubMed] [Google Scholar]
- 36.Dahl AA, Aarre TF, Johansen JB, Neckelmann D, Kjonniksen I. Oslo: The Norwegian Centre for Health Technology Assessment (SMM); 2001. Transcranial magnetic stimulation in the treatment of depressions. [Google Scholar]
- 37.McNamara B, Ray JL, Arthurs OJ, Boniface S. Transcranial magnetic stimulation for depression and other psychiatric disorders. Psychol Med. 2001;31(7):1141–1146. doi: 10.1017/s0033291701004378. [DOI] [PubMed] [Google Scholar]
- 38.Alberta Heritage Foundation for Medical Research, corpauthor. Edmonton: Alberta Heritage Foundation for Medical Research (AHFMR); 1999. Repetitive transcranial magnetic stimulation. [Google Scholar]
- 39.Martin JLR, Barbanoj MJ, Schlaepfer TE, Clos S, Perez V, Kulisevsky J, et al. Cochrane Database of Systematic Reviews. 2004;(1) doi: 10.1002/14651858.CD003493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berman RM, Narasimhan M, Sanacora G, Miano AP, Hoffman RE, Hu XS, et al. A randomized clinical trial of repetitive transcranial magnetic stimulation in the treatment of major depression. Biological Psychiatry. 2000;47(4):332–337. doi: 10.1016/s0006-3223(99)00243-7. [DOI] [PubMed] [Google Scholar]
- 41.Eschweiler GW, Wegerer C, Schlotter W, Spandl C, Stevens A, Bartels M, et al. Left prefrontal activation predicts therapeutic effects of repetitive transcranial magnetic stimulation (rTMS) in major depression. Psychiatry Research. 2000;99(3):161–172. doi: 10.1016/s0925-4927(00)00062-7. [DOI] [PubMed] [Google Scholar]
- 42.Avery DH, Claypoole K, Robinson L, Neumaier JF, Dunner DL, Scheele L, et al. Repetitive transcranial magnetic stimulation in the treatment of medication-resistant depression: preliminary data. Journal of Nervous & Mental Disease. 1999;187(2):114–117. doi: 10.1097/00005053-199902000-00009. [DOI] [PubMed] [Google Scholar]
- 43.GarciaToro M, PascualLeone A, Romera M, Gonzalez A, Mico J, Ibarra O, et al. Prefrontal repetitive transcranial magnetic stimulation as add on treatment in depression. Journal of Neurology, Neurosurgery & Psychiatry. 2001;71(4):546–548. doi: 10.1136/jnnp.71.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.GarciaToro M, Mayol A, Arnillas H, Capllonch I, Ibarra O, Crespi M, et al. Modest adjunctive benefit with transcranial magnetic stimulation in medication-resistant depression. Journal of Affective Disorders. 2001;64(2-3):271–275. doi: 10.1016/s0165-0327(00)00223-8. [DOI] [PubMed] [Google Scholar]
- 45.Morton V, Torgerson DJ. Effect of regression to the mean on decision making in health care. Br Med J. 2003;326:1083–1084. doi: 10.1136/bmj.326.7398.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.National Health Service Research and Development Health Technology Assessment Programme, corpauthor. Clinical effectiveness and cost of repetitive transcranial magnetic stimulation versus ECT in severe depression: a multicentre randomized controlled trial and economic analysis. 2004 doi: 10.3310/hta11240. http://www.hta.nhsweb.nhs.uk/projectdata/1_project_record_notpublished.asp?PjtId=1203&Search . [DOI] [PubMed] [Google Scholar]
- 47.PascualLeone A, Rubio B, Pallardo F, Catala MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression.[see comment] Lancet. 1996;348(9022):233–237. doi: 10.1016/s0140-6736(96)01219-6. [DOI] [PubMed] [Google Scholar]
- 48.Speer AM, Repella JD, Figueras S, Demian NK, Kimbrell TA, Wasserman EM. Lack of adverse cognitive effects of 1 Hz and 20 Hz repetitive transcranial magnetic stimulation at 100% of motor threshold over left prefrontal cortex in depression. Journal of ECT. 2001;17(4):259–263. doi: 10.1097/00124509-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Martin JLR, Barbanoj MJ, Schlaepfer TE, Clos S, Perez V, Kulisevsky J, et al. Cochrane Database of Systematic Reviews. 2004;(1) doi: 10.1002/14651858.CD003493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boylan LS, Pullman SL, Lisanby SH, Spicknall KE, Sackeim HA. Repetitive transcranial magnetic stimulation to SMA worsens complex movements in Parkinson’s disease. Clin Neurophysiol. 2001;112:259–264. doi: 10.1016/s1388-2457(00)00519-8. [DOI] [PubMed] [Google Scholar]
- 51.Kammer T, Beck S, Thielscher A, Laubis-Herrmann U, Topka H. Motor thresholds in humans: a transcranial magnetic stimulation study comparing different pulse waveforms, current directions and stimulator types. Clin Neurophysiol. 2001;112:250–258. doi: 10.1016/s1388-2457(00)00513-7. [DOI] [PubMed] [Google Scholar]
- 52.Pridmore S. Rapid transcranial magnetic stimulation and normalization of the dexamethasone suppression test. Psychiatry & Clinical Neurosciences. 1999;53(1):33–37. doi: 10.1046/j.1440-1819.1999.00467.x. [DOI] [PubMed] [Google Scholar]
- 53.Kozel FA, Nahas Z, DeBrux C, Molloy M, Lorberbaum JP, Bohning D, et al. How coil-cortex distance relates to age, motor threshold, and antidepressant response to repetitive transcranial magnetic stimulation. Journal of Neuropsychiatry & Clinical Neurosciences. 2000;12(3):376–384. doi: 10.1176/jnp.12.3.376. [DOI] [PubMed] [Google Scholar]
- 54.Grunhaus L, Dannon PN, Schreiber S, Dolberg OH, Amiaz R, Ziv R, et al. Repetitive transcranial magnetic stimulation is as effective as electroconvulsive therapy in the treatment of nondelusional major depressive disorder: an open study. Biological Psychiatry. 2000;47(4):314–324. doi: 10.1016/s0006-3223(99)00254-1. [DOI] [PubMed] [Google Scholar]
- 55.Conca A, Koppi S, Konig P, Swoboda E, Krecke N. Transcranial magnetic stimulation: a novel antidepressive strategy? Neuropsychobiology. 1996;34(4):204–207. doi: 10.1159/000119312. [DOI] [PubMed] [Google Scholar]
- 56.Loo C, Mitchell P, Sachdev P, McDarmont B, Parker G, Gandevia S. Double-blind controlled investigation of transcranial magnetic stimulation for the treatment of resistant major depression. American Journal of Psychiatry. 1999;156(6):946–948. doi: 10.1176/ajp.156.6.946. [DOI] [PubMed] [Google Scholar]
- 57.Kimbrell TA, Little JT, Dunn RT, Frye MA, Greenberg BD, Wassermann EM, et al. Frequency dependence of antidepressant response to left prefrontal repetitive transcranial magnetic stimulation (rTMS) as a function of baseline cerebral glucose metabolism. Biological Psychiatry. 1999;46(12):1603–1613. doi: 10.1016/s0006-3223(99)00195-x. [DOI] [PubMed] [Google Scholar]
- 58.Padberg F, Zwanzger P, Thoma H, Kathmann N, Haag C, Greenberg BD, et al. Repetitive transcranial magnetic stimulation (rTMS) in pharmacotherapy-refractory major depression: comparative study of fast, slow and placebo rTMS. Psychiatry Research. 1999;88(3):163–171. doi: 10.1016/s0165-1781(99)00092-x. [DOI] [PubMed] [Google Scholar]
- 59.Grunhaus L, Schreiber S, Dolberg OT, Polak D, Dannon PN. A randomized controlled comparison of electroconvulsive therapy and repetitive transcranial magnetic stimulation in severe and resistant nonpsychotic major depression. Biological Psychiatry. 2003;53(4):324–331. doi: 10.1016/s0006-3223(02)01499-3. [DOI] [PubMed] [Google Scholar]
- 60.Gershon AA, Dannon PN, Grunhaus L. Transcranial magnetic stimulation in the treatment of depression. Am J Psychiatry. 2003;160:835–845. doi: 10.1176/appi.ajp.160.5.835. [DOI] [PubMed] [Google Scholar]
- 61.Janicak PG, Dowd SM, Martis B, Alam D, Beedle D, Krasuski J, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: preliminary results of a randomized trial.[see comment] Biological Psychiatry. 2002;51(8):659–667. doi: 10.1016/s0006-3223(01)01354-3. [DOI] [PubMed] [Google Scholar]
- 62.Fitzgerald PB, Brown TL, Marston NA, Daskalakis ZJ, De Castella A, Kulkarni J. Transcranial magnetic stimulation in the treatment of depression: a double-blind, placebo-controlled trial. Archives of General Psychiatry. 2003;60(10):1002–1008. doi: 10.1001/archpsyc.60.9.1002. [DOI] [PubMed] [Google Scholar]
- 63.Boutros NN, Gueorguieva R, Hoffman RE, Oren DA, Feingold A, Berman RM. Lack of a therapeutic effect of a 2-week sub-threshold transcranial magnetic stimulation course for treatment-resistant depression. Psychiatry Research. 2002;113(3):245–254. doi: 10.1016/s0165-1781(02)00267-6. [DOI] [PubMed] [Google Scholar]
- 64.Conca A, Di Pauli J, Beraus W, Hausmann A, Peschina W, Schneider H, et al. Combining high and low frequencies in rTMS antidepressive treatment: preliminary results. Human Psychopharmacology. 2002;17(7):353–356. doi: 10.1002/hup.422. [DOI] [PubMed] [Google Scholar]
- 65.Conca A, Peschina W, Hausmann A, Koenig P. Combining 1-Hz and 10-Hz rTMS in the treatment of depressives. European Psychiatry: the Journal of the Association of European Psychiatrists. 2002;17(Suppl 1):218. [Google Scholar]
- 66.Hausmann A, Kemmler G, Walpoth M, Mechtcheriakov S, KramerReinstadler K, Lechner T, et al. No benefit derived from repetitive transcranial magnetic stimulation in depression: a prospective, single centre, randomised, double blind, sham controlled “add on” trial. Journal of Neurology, Neurosurgery & Psychiatry. 2004;75(2):320–322. [PMC free article] [PubMed] [Google Scholar]
- 67.Hoppner J, Schulz M, Irmisch G, Mau R, Schlafke D, Richter J. Antidepressant efficacy of two different rTMS procedures. High frequency over left versus low frequency over right prefrontal cortex compared with sham stimulation. European Archives of Psychiatry & Clinical Neuroscience. 2003;253(2):103–109. doi: 10.1007/s00406-003-0416-7. [DOI] [PubMed] [Google Scholar]
- 68.Schule C, Zwanzger P, Baghai T, Mikhaiel P, Thoma H, Moller HJ, et al. Effects of antidepressant pharmacotherapy after repetitive transcranial magnetic stimulation in major depression: an open follow-up study. Journal of Psychiatric Research. 2003;37(2):145–153. doi: 10.1016/s0022-3956(02)00101-2. [DOI] [PubMed] [Google Scholar]
- 69.Herwig U, Lampe Y, Juengling FD, Wunderlich A, Walter H, Spitzer M, et al. Add-on rTMS for treatment of depression: a pilot study using stereotaxic coil-navigation according to PET data. Journal of Psychiatric Research. 2003;37(4):267–275. doi: 10.1016/s0022-3956(03)00042-6. [DOI] [PubMed] [Google Scholar]
- 70.Anonymous, corpauthor. American Psychiatric Association practice guideline for the treatment of patients with major depressive disorder (revision) Am J Psychiatry. 2000;157(Suppl 4):1–45. [PubMed] [Google Scholar]
- 71.Kennedy SH, Lam RW, Cohen NL, Ravindran AV the CANMAT Depression Work Group, corpauthor. Clinical Guidelines for the treatment of depressive disorders. IV. 2001;46:38S–58S. Medications and other biological treatments. Canadian Journal of Psychiatry. [PubMed] [Google Scholar]
- 72.Royal Australian and New Zealand College of Psychiatrists, corpauthor. Transcranial Magnetic Stimulation (TMS) Position Statement # 40. 2003 www.ranzcp.org/pdffiles/posstate/ps40.pdf . [Google Scholar]
- 73.National Institute of Clinical Evidence, corpauthor. Depression: the management of depression in primary and secondary care. NICE guideline. 2003 2003 Dec;:1–58. Draft for Second Consulation. [Google Scholar]
- 74.Ellis PM, Smith DAR. Treating depression: the beyondblue guidelines for treating depression in primary care. Med J Aust. 2002;176:S77–S83. [PubMed] [Google Scholar]
- 75.Belmaker B, Fitzgerald P, George MS, Lisanby SH, Pascual-Leone A, Schlaepfer TE, et al. Managing the risks of repetitive transcranial stimulation. Cns Spectrums. 2003;8:489. doi: 10.1017/s1092852900018952. [DOI] [PubMed] [Google Scholar]
- 76.ECRI, corpauthor. Magnetic therapy under study to treat major depression. Health Technology Forecast. 2004 [Google Scholar]
- 77.Avery DH. Project Title: TMS treatment of major depression. Grant Number 5R01MH062154-03. 2004 http://crisp.cit.nih.gov . [Google Scholar]
- 78.Aetna Clinical Policy Bulletin. Transcranial Magnetic Stimulation and Cranial Electrical Stimulation. 1469. 2004 [Google Scholar]
- 79.The Regence Group, corpauthor. Transcranial Magnetic Stimulation. 2004 Feb 3; www.regence.com/printFriendly.jsp . [Google Scholar]
- 80.Blue Cross Blue Shield Georgia, corpauthor. Transcranial magnetic stimulation as a treatment of depression and other neuropsychiatric disorders. 2003 Jun 25; www.provider.bcbsga.com/provider/medpolicy/policies/behavioral_health/magnetic_stim_depress.html . [Google Scholar]
- 81.Agence d’evaluation des technologies et des mode d’intervention en sante (AETMIS), corpauthor The use of electroconvulsive therapy in Quebec. 2003 [Google Scholar]


