Abstract
Aims: Peroxiredoxins (PRXs) are a newly characterized family of peroxide scavenging enzymes that not only help maintain cellular redox homeostasis but also may directly engage in a variety of intracellular signaling pathways. PRX2 is a neuronal-specific PRX believed to participate in cerebral antioxidant responses in several neurodegenerative diseases. This study investigates the potential neuroprotective effect and the underlying mechanism of PRX2 in models of ischemic neuronal injury. Results: Transgenic mice overexpressing PRX2 showed reduced brain injury and improved neurological recovery up to 3 weeks after transient focal cerebral ischemia compared to wild-type littermates. In primary cultures of cortical neurons, transfection of PRX2 but not the loss-of-catalytic-site PRX2 mutant conferred neuroprotection against cell death induced by oxygen glucose deprivation. PRX2 exhibited potent pro-survival effects in ischemic neurons by maintaining thioredoxin (Trx) in its reduced state, thereby preventing oxidative stress-mediated activation of apoptosis signal–regulating kinase 1 (ASK1) and the downstream MKK/JNK pro-death signaling pathway. PRX2 failed to provide additional neuroprotection against ischemic injury in Trx- or ASK1-knockdown neuron cultures and in mice treated with a JNK inhibitor. Innovation: This study provides evidence that neuronal overexpression of PRX2 confers prolonged neuroprotection against ischemic/reperfusion brain injury. Moreover, the results suggest a signaling pathway by which PRX2 suppresses ischemia-induced neuronal apoptosis. Conclusions: Enhanced neuronal expression and activity of PRX2 protect against ischemic neuronal injury by directly modulating the redox-sensitive Trx-ASK1 signaling complex. Antioxid. Redox Signal. 17, 719–732.
Introduction
Neurons are highly aerobic cells with a limited antioxidant capacity, which makes them vulnerable targets of oxidative damage during aging or under pathological conditions (30). Oxidative stress-mediated neuronal death are likely obligatory to the pathogenesis of various neurological disorders, including ischemic/reperfusion brain injury (4, 28). After an ischemic episode, overproduced reactive oxygen species (ROS) (40) can attack macromolecules such as lipids, DNA, and proteins, and trigger redox state-sensitive pro-death signaling pathways, ultimately leading to mitochondrial dysfunction and neuronal demise (5, 22, 43). Thus, studies aiming to understand the key mechanisms that maintain intracellular redox homeostasis in neurons are an essential step toward developing novel antioxidant-based therapeutics for stroke.
Peroxiredoxins (PRXs) are a newly characterized family of six antioxidant enzymes (PRX1–6) ubiquitously expressed in mammalian cells. In the brain, the expression of PRX isozymes shows distinct distribution patterns in different regions, cell types, and subcellular compartments, which may be related to their cell-specific functional roles (1, 11, 13, 34). All PRXs have the capacity of decomposing peroxides such as H2O2 and alkyl peroxides using thioredoxin (Trx) or other electron donors (30). However, in addition to their common peroxide scavenging activity, a unique feature of PRXs is that certain PRX isoforms may directly engage in divergent intracellular signaling pathways that govern specific biological processes, including proliferation, differentiation, gene expression, and apoptosis (14).
PRX2 is a relatively abundant neuron-specific PRX in brain (1, 13, 34). Increased expression of PRX2 is found in aging brain and in frontal cortex Alzheimer's disease, Parkinson's disease, and Down syndrome postmortem brains, suggesting that PRX2 is involved in the elevated neuronal antioxidant response under oxidative stress (30). Homozygous mice deficient in PRX2 show remarkably increased accessibility to oxidative stress-induced tissue damage, indicating a crucial role for PRX2 in endogenous antioxidant defense (19). Moreover, a direct neuroprotective effect by PRX2 against cell death has been demonstrated in cultures of PRX2-overexpressing neurons challenged by growth factor depletion or ischemic/oxidative injury (2). PRX2 catalyzes the reduction of peroxides through the oxidation of its two active cysteine (Cys) residues into the sulfenic acid form, which is then recycled back to a thiol using one of several cell-specific disulfide oxido-reductases, such as the highly efficient thioredoxin/thioredoxin reductase (Trx/TrxR) system (30). Because PRX2 utilizes Trx for recycling from its oxidized forms in the PRX2-catalyzed peroxide scavenging reaction, we have recently speculated that over-consumption of PRX2 under oxidative stress could deplete the reduced Trx in neurons, resulting in the activation of pro-death signaling molecules such as apoptosis signal–regulating kinase 1 (ASK1) that are normally kept at an inactive state by reduced Trx (33).
Innovation.
Despite the importance of oxidative stress in the pathogenesis of ischemic/reperfusion brain injury (7, 21), numerous antioxidative drugs targeted at scavenging overproduced free radicals have failed in clinical trials. An improved understanding of the mechanisms that maintain intracellular redox homeostasis in neurons is therefore imperative to identify novel therapeutic targets for stroke management. The current study documents an active involvement of peroxiredoxin 2 (PRX2), an antioxidant enzyme, in the defense against neuronal death after ischemia/reperfusion. We demonstrate for the first time that neuronal overexpression of PRX2 potently reduced brain infarct and improved neurological outcomes evaluated up to 3 weeks after ischemia/reperfusion. Further study indicates that in addition to its commonly recognized role as an antioxidant enzyme, PRX2 is able to exert neuroprotection through a novel mechanism whereby this redoxsensitive molecule interferes with thioredoxin-apoptosis signal–regulating kinase 1 (ASK1) interactions and the activation of the ASK1-JNK pro-death signaling pathway. Taken together, our research results shed light on the pro-death signaling regulation by PRX2 in neurons under ischemia/reperfusion conditions. PRX2 may represent a potential target for stroke intervention.
In this study, using a newly created PRX2 transgenic (Tg-PRX2) model, we have investigated the neuroprotective effect and the underlying mechanism of PRX2 against ischemic/reperfusion brain injury. Novel evidence is presented to demonstrate that PRX2 overexpression confers marked and prolonged neuroprotection by suppressing the ASK1/JNK-mediated mitochondrial apoptosis signaling pathway.
Results
Characterization of Tg-PRX2 mice
Several independent lines of Tg-PRX2 mice were confirmed by polymerase chain reaction genotyping (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertonline.com/ars). Western blot analysis of forebrain cortical tissues revealed that the low-expressing Tg-PRX2 line (Tg-PRX2L) yielded about 2.5-fold increases in PRX2 protein expression, whereas the high-expressing Tg-PRX2 line (Tg-PRX2H) yielded about 5.5-fold increases in the cortex and striatum (Supplementary Fig. S1B). Double-label immunofluorescence of brain sections showed that transgenic expression of PRX2 was predominantly in neurons (Supplementary Fig. S1C).
The offspring of breeding between heterozygous Tg-PRX2 mice showed genotypes in a ratio consistent with mendelian transmission without disproportional pre- or perinatal lethality. Determined at 8 and 12 weeks of age, respectively, there was no significant difference in either body weight or brain weight between Tg-PRX2H mice and wild-type (WT) littermates (Supplementary Fig. S2A). Overexpression of PRX2 did not significantly affect the expression levels of PRX1, PRX3, or PRX4 in the forebrain cortex of Tg-PRX2H mice (Supplementary Fig. S2B) and did not significantly change the numbers of microglia (Iba-1 positive) or astrocytes (GFAP positive) in proportion to neurons (Supplementary Fig. S2C–E).
Tg-PRX2 mice are highly resistant to focal cerebral ischemia
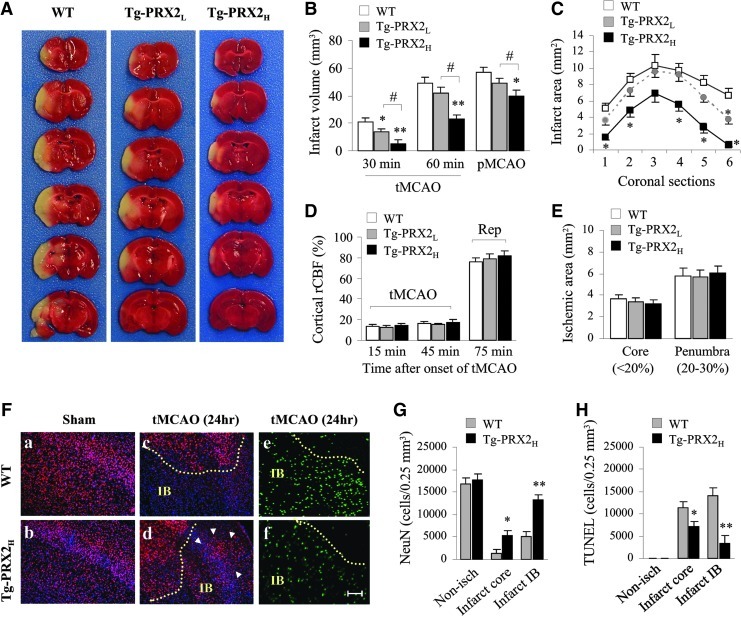
To determine the effect of PRX2 overexpression on ischemic brain injury, we performed either transient middle cerebral artery occlusion (30- or 60-min tMCAO) or permanent middle cerebral artery occlusion (pMCAO) in Tg-PRX2 mice and WT littermates. In all experimental paradigms, PRX2 overexpression decreased infarct volume in a dose-dependent manner at 48 h after MCAO (Fig. 1A–C). In particular, Tg-PRX2H mice showed greater neuroprotection against each ischemic condition compared to Tg-PRX2L mice (Fig. 1B). Regional cerebral blood flow as determined using laser Doppler flowmetry (Fig. 1D) or a laser speckle imager (Fig. 1E) was not significantly different between the transgenic and WT groups. Moreover, neither brain surface blood vessels in the MCA territory nor posterior communicating artery plasticity was significantly affected by PRX2 overexpression (Supplementary Table 1). Physiological parameters (blood pressure, core temperature, blood gases, etc.) did not differ significantly between the transgenic and WT groups (data not shown).
FIG. 1.
Peroxiredoxin 2 (PRX2) overexpression protects against ischemia induced by middle cerebral artery occlusion (MCAO). (A) Representative 2,3,5-triphenyltetrazolium chloride (TTC) stains showing smaller infarct in a PRX2 transgenic (Tg-PRX2) mouse brain than in wild-type (WT) mouse brain at 48 h after 60 min of tMCAO. (B) Infarct volume determined in WT, low-expressing Tg-PRX2 (Tg-PRX2L) and high-expressing Tg-PRX2 (Tg-PRX2H) mice at 48 h after 30 or 60 min transient MCAO (tMCAO), or permanent MCAO (pMCAO). n=8/group. (C) Infarct areas of consecutive coronal sections every 1 mm throughout the MCA territory at 48 h after 60-min tMCAO. (D, E) The neuroprotective effect of PRX2 overexpression is independent of regional cerebral blood flow (rCBF) changes after focal ischemia. (D) Changes in rCBF were determined using laser Doppler flowmetry at 15 and 45 min after tMCAO, and 15 min after reperfusion (Rep, or 75 min after the onset of MCAO). Data are expressed as percentages of preischemic baseline levels. n=8/group. (E) WT, Tg-PRX2L, and Tg-PRX2H mice demonstrated similar sizes of ischemic zones, including ischemic core (<20% of baseline levels) and penumbra (20%–30% of baseline levels), as determined using laser speckle imaging. n=6/group. (F) Brain sections from WT or Tg-PRX2H mice showing NeuN immunoreactivity (red, a–d) and apoptotic DNA fragmentation as determined by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay (green, e, f) at 24 h after tMCAO. Arrows indicate the increased number of surviving neurons (NeuN-positive) within the inner boundary (IB) of infarction in Tg-PRX2 brain compared to WT brain after ischemia. Scale bars, 100 μm. (G, H) The number of NeuN immunoreactive cells (G) and TUNEL-positive cells (H) were quantified using a nonbiased stereological method at 24 h of reperfusion. n=6/group. All data are presented as mean±standard error of the mean (SEM), *p<0.05, **p<0.01 versus WT mice; #p<0.05 between the indicated groups. Comparisons among multiple groups were performed with one-way analysis of variance (ANOVA), followed by Bonferroni's/Dunn's post hoc analysis. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
The protective effect of PRX2 against ischemic cell death was further confirmed by stereology-assisted quantification of NeuN-immunoreactive or DNA-fragmented cells on brain sections at 24 h after 60 min of tMCAO. tMCAO resulted in robust loss of NeuN-positive cells and induction of DNA fragmentation in both infarct core and infarct inner boundary regions in WT mice (Fig. 1F–H), whereas these changes were significantly attenuated in Tg-PRX2H mice. The high-expressing Tg-PRX2H line was used in all subsequent studies.
PRX2 overexpression improves long-term neurological recovery after tMCAO
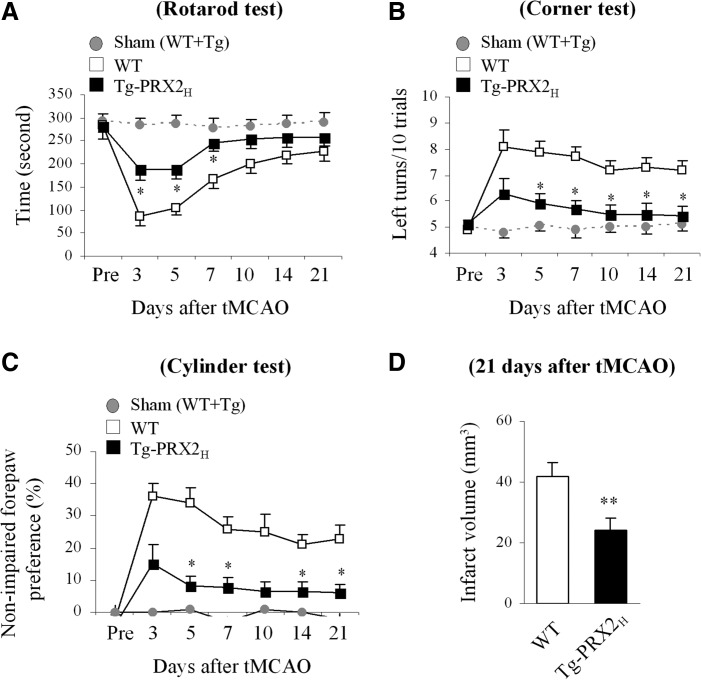
To further determine the impact of PRX2 overexpression on neurofunctional outcomes after stroke, neurobehavioral assays including the rotarod test, corner test, and cylinder test were administered during the 21-day recovery period after 60-min tMCAO. Sham-operated mice displayed no significant difference in sensorimotor performance regardless of their transgenic phenotypes. Postischemic sensorimotor dysfunction was significantly improved in Tg-PRX2H mice compared with WT littermates up to 21 days after ischemia (Fig. 2A–C). At the end of the 21-day recovery period, infarct volume was measured to determine the long-term effect of PRX2 against ischemia. Tg-PRX2H mice showed significantly smaller infarct volume than WT mice (Fig. 2D), indicating that PRX2 overexpression conferred long-lasting neuroprotection against tMCAO instead of simply delaying ischemic injury.
FIG. 2.
Transgenic PRX2 overexpression improves neurological functional recovery after focal ischemia and confers long-lasting neuroprotection. (A–C) Assessment of sensorimotor function in sham control mice and ischemic mice (n=8 per group). Tg-PRX2H or WT mice were subjected to rotarod test (A), corner test (B), and cylinder test (C) before and at 3, 5, 7, 10, 14, and 21 days after 60-min tMCAO. Transgenic PRX2 oxerexpression improved ischemia-induced acute sensorimotor dysfunction and long-lasting sensorimotor asymmetry. The performance on the rotarod test was expressed as the mean duration on rotarod out of five trials per day. Corner test performance was expressed by the number of left turns out of 10 turn trials per day. The cylinder test data are expressed as nonimpaired forepaw preference, which is calculated using the formula described in Materials and Methods. (D) Infarct volume determined in WT or Tg-PRX2H mice at 21 days after ischemia in cresyl violet-stained brain sections (n=8 per group). All data are presented as mean±SEM, *p<0.05, **p<0.01 versus WT mice. Multiple comparisons for (A–C) were performed with repeated ANOVA, followed by Bonferroni's/Dunn's post hoc analysis; comparison between the two groups in (D) was done using Student's unpaired t-test.
PRX2 prevents ischemia-induced ASK1 activation by maintaining Trx at a reduced state
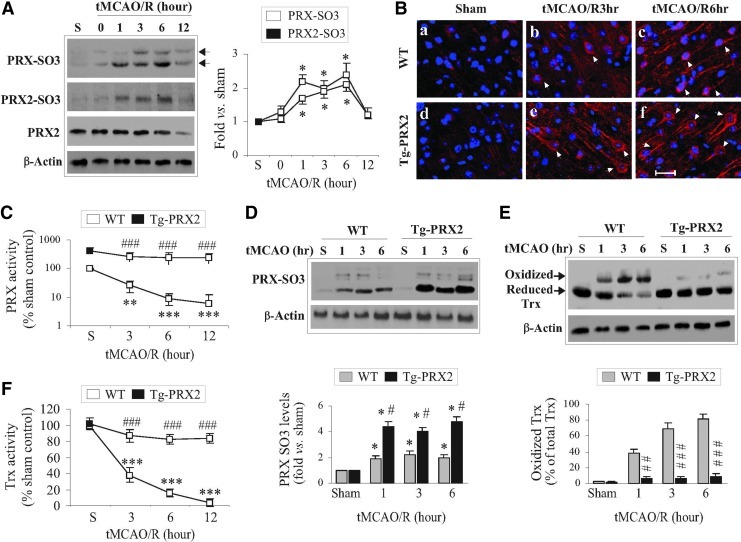
When catalyzing the reduction of peroxides, PRXs are oxidized at active cysteine residues to generate sulfenic acid (30) that is slowly recycled back to a reduced state by the Trx/TrxR system (24). However, under oxidative stress, the sulfenic acid may be overoxidized to generate cysteine sulfinic acid (Cys-SO2) and cysteine sulfonic acid (Cys-SO3) (24), which are thought to be irreversible (7). Oxidative modification of PRXs results in a reduction in antioxidative activity, and thus dramatically influences their cytoprotective capacity (20). Using an antibody recognizing overoxidized PRX (PRX-SO3), we showed that PRX-SO3 immunoreactivity was barely detectable in nonischemic brains, but was significantly augmented after tMCAO (Fig. 3A, B). To further determine the PRX2-specific oxidative modification, tissue lysates from ischemic brains were subjected to immunoprecipitation (IP) (31) using the PRX2 antibody followed by immunoblotting against PRX-SO3. Similar to total PRX-SO3, the PRX2-derived PRX-SO3 (PRX2-SO3) was elevated up to 6 h after tMCAO (Fig. 3A). The increases in PRX2-SO3 appeared to contribute to a large portion of total PRX-SO3 following tMCAO.
FIG. 3.
Transgenic PRX2 overexpression prevents the depletion of PRX activity and thioredoxin (Trx) activity after ischemia. (A) Ischemia induces PRX overoxidation in brains. WT mice were subject to 60 min of tMCAO and reperfusion, and forebrain cortical lysates were processed for immunoblotting against overoxidized PRX (PRX-SO3) or PRX2 immunoprecipitation (IP) followed by immunoblotting against PRX-SO3 at the indicated time points. Note that ischemia induced two PRX-SO3 bands (arrows), which were likely due to the overoxidation of PRX1-4, whereas the lower band is corresponding to PRX2-SO3 [also see (D)]. The graph in the right panel summarizes the temporal profiles of changes in total PRX-SO3 and PRX2 IP-derived PRX-SO3 (PRX2-SO3). Both PRX-SO3 and PRX2-SO3 were significantly increased as early as 1 h and peaked at 6 h after 60 min tMCAO. Data are expressed as the fold changes over sham controls. n=4 per condition. (B) Representative fluorescence images demonstrate the increased immunofluorescence for PRX-SO3 (red) in brain sections from WT or Tg-PRX2 mice at 3 h (b and e) and 6 h (c and f) after 60 min of tMCAO. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; blue). Arrows indicate PRX-SO3-positive cells. The expression levels of PRX-SO3 increased in Tg-PRX2 brain (a–c) compared to in the WT brain (d–f) after ischemia. Scale bar, 30 μm. (C, D) PRX2 overexpression reinstates PRX activity and increases PRX oxidation after ischemia. Cortical tissue extracts obtained from Tg-PRX2 and WT mice at 3, 6, and 12 h after tMCAO were processed for PRX activity (C) and immunoblotting against PRX-SO3 (D). n=5–6 per group. (E, F) PRX2 overexpression reduces Trx oxidation and reinstates Trx activity after ischemia. Brain tissue extracts obtained from Tg-PRX2 and WT mice at 3, 6, and 12 h after tMCAO were processed for Trx activity (F) and immunoblotting using a Trx antibody that recognizes both oxidized (higher molecular weight) and reduced (lower molecular weight) forms (E). n=5–6 per group. *p<0.05, **p<0.01, ***p<0.001 versus sham controls; #p<0.05, ##p<0.01, ###p<0.001 versus WT mice. Statistical analysis was performed using one-way ANOVA, followed by Bonferroni's/Dunn's post hoc analysis. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
The results of PRX activity analysis revealed dramatic decreases in cellular PRX activity (an index for PRX antioxidant reserves) following tMCAO (Fig. 3C) in parallel with the increases in PRX2-SO3 (Fig. 3A), indicating a severe exhaustion of brain PRXs after ischemia. In contrast to WT mice, Tg-PRX2 mice showed approximately fivefold increases in PRX activity in nonischemic brain, and maintained PRX activity at significantly elevated levels after tMCAO (Fig. 3C). Interestingly, significantly higher levels of PRX-SO3 were observed after tMCAO in Tg-PRX2H mice compared to WT mice (Fig. 3C, D). These results suggest that exogenously overexpressed PRX2 actively participated in peroxide scavenging in ischemic brain, ultimately preventing the exhaustion of endogenous PRXs reserves after tMCAO.
Since Trx serves as a reducing enzyme both in PRX2-catalyzed peroxide-scavenging reaction and in recycling the oxidized PRX2 under oxidative stress (24), we hypothesized that exhaustion of PRX reserves after tMCAO could deplete the endogenous reduced Trx. We further hypothesized that depletion of reduced Trx might lead to ASK1 activation in ischemic neurons as Trx oxidation and subsequent dissociation from ASK1 are prerequisites for ASK1 activation under oxidative stress (33). Indeed, tMCAO resulted in marked accumulation of oxidized Trx but a decrease in reduced Trx in brain (Fig. 3E), which was accompanied by significant decreases in cellular Trx activity (Fig. 3F), an index of Trx reserves. In contrast to WT mice, tMCAO-induced oxidation of Trx and decreases in Trx activity were significantly attenuated in Tg-PRX2H mice (Fig. 3E, F), suggesting that PRX2 overexpression preserved reduced Trx from overconsumption after tMCAO.
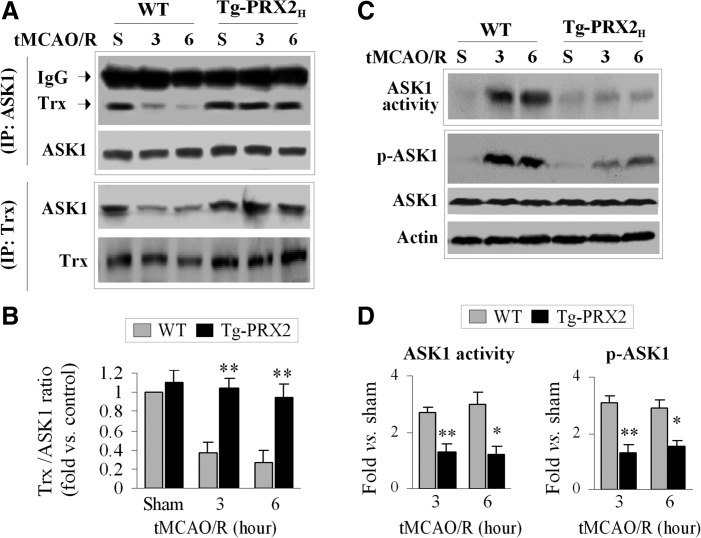
To examine the interactions between Trx and ASK1 after tMCAO, co-IP was performed using brain extracts from Tg-PRX2H mice and WT littermates. An association between Trx and ASK1 was readily detectable in sham control mice regardless of genotype (Fig. 4A, B). However, a marked dissociation of Trx from ASK1 occurred at 3 and 6 h after tMCAO in WT mice but not in Tg-PRX2H mice (Fig. 4A, B). Consistent with the co-IP results, tMCAO-induced activation of ASK1 (increased phosphorylation at Thr845 and increased ASK1 activity) was significantly attenuated in Tg-PRX2H mice compared to WT mice (Fig. 4C, D). Taken together, these results suggest that PRX2 suppresses ischemia-induced ASK1 activation by maintaining Trx at a reduced state.
FIG. 4.
Transgenic PRX2 overexpression inhibits ischemia-induced apoptosis signal–regulating kinase 1 (ASK1) activation. (A, B) PRX2 prevents the dissociation between Trx and ASK1 after ischemia. (A) Cortical protein extracts were prepared from Tg-PRX2 or WT mice at 3 and 6 h after 60-min tMCAO. Co-IP was performed using antibodies against ASK1 and Trx, respectively. (B) Quantitative analysis of (A); data are expressed as the relative amounts of Trx that bound to ASK1. Note that transient ischemia resulted in the dissociation of Trx from ASK1, as revealed by decreased Trx density in the ASK1 precipitates. Transgenic PRX2 overexpression prevents dissociation of Trx and ASK1. n=4–5 per group. (C, D) PRX2 inhibits ischemia-induced ASK1 activation. (C) Under the experimental conditions described in (A), cortical protein extracts were processed for immunoblotting against p-ASK1 or IP for ASK1 followed by ASK1 kinase activity assay. (D) Quantitative analysis of (C); data are expressed as the fold changes in p-ASK1 and ASK1 activity over sham control. n=4–5 per group. *p<0.05, **p<0.01 versus WT mice. Multiple comparisons were made using one-way ANOVA, followed by Bonferroni's/Dunn's post hoc analysis.
The 2-C catalytic sites are essential for PRX2-afforded neuroprotection in cultured neurons
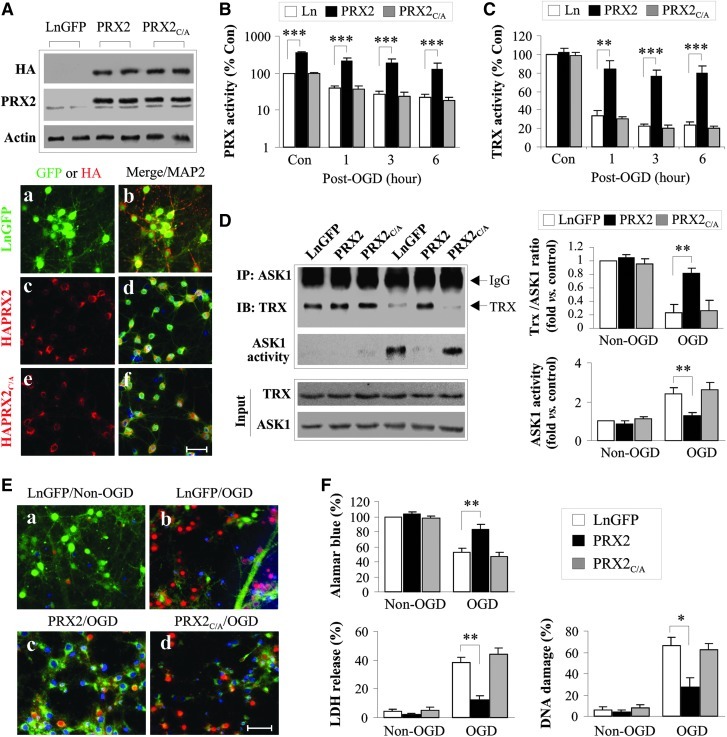
To test the hypothesis that the peroxide-scavenging activity possessed by PRX2 is essential for its neuroprotective effect via interaction with the Trx-ASK1 complex, we constructed lentiviral (Ln) vectors expressing either WT human PRX2 or its catalytically inactive mutants in which the two cysteine residues at amino acids 51 and 172 were mutated to alanine (PRX2C/A, Supplementary Fig. S3). Incubation of vectors in primary cultures of rat cortical neurons yielded highly efficient gene transfection of PRX2 and PRX2C/A, respectively (Fig. 5A). As predicted, overexpression of PRX2, but not PRX2C/A, markedly elevated the peroxide-scavenging activity in neurons before and after oxygen and glucose deprivation (OGD) (Fig. 5B). As a result, the otherwise diminished Trx activity following OGD was restored to near preischemic levels in PRX2-transfected but not in PRX2C/A-transfected neurons (Fig. 5C).
FIG. 5.
Lentiviral over-expression of PRX2 enhances Trx-ASK1 association and protects against oxygen and glucose deprivation (OGD)-induced neuronal injury in cortical neuronal cultures. Primary cortical neurons at 9 days in vitro were infected for 3 days with empty lentivirus or lentiviral (Ln) vector carrying GFP (LnGFP), or with hemagglutinin (HA)-tagged human PRX2 (PRX2) or mutant PRX2 in which Cys51 and Cys172 were replaced by alanine (PRX2C/A). (A) Lentivirus-mediated overexpression of PRX2 in neurons was confirmed by Western blotting against the HA tag and PRX2, respectively, and by triple-label immunofluorescence [GFP (a,b) or HA (c, d), MAP2 (b,d,f), and DAPI (b,d,f)]. Scale bar, 50 μm. (B, C) Transfection of PRX2 enhances PRX and Trx activity after OGD. Infected neurons were subjected to OGD for 60 min and, at the indicated time points after OGD, cytosolic proteins were processed for PRX activity (B) and Trx activity (C) assays. Note that transfection of PRX2 but not of PRX2C/A reinstated both PRX and Trx activities to nearly preischemia levels at 1, 3, and 6 h after OGD. Data are expressed as percentage changes over control (Con) cell cultures without OGD challenge. (D) Transfection of PRX2 but not PRX2C/A enhances Trx-ASK1 association and inhibits ASK1 activation after OGD. Protein extracts were prepared from infected cells at 3 h after OGD and processed for ASK1 IP followed by immunoblotting against Trx or the ASK1 activity assay. Semi-quantitative data are expressed as the fractions of Trx that bound to ASK1 (right upper panel) or the fold changes of ASK1 activity over control cultures (right lower panel). Data are based on three to four independent experiments. (E, F) Transfection of PRX2 but not of PRX2C/A protects against neuronal cell death at 24 h after 60 min of OGD. Representative triple-label fluorescence images for DNA damage are presented in (E), where neurons were triple-labeled for GFP (green, a, b) or HA (green, c, d) with TUNEL (red) and DAPI (blue). Scale bar, 50 μm. (F) Cell viability was quantified using the Alamar blue assay (left panel); cell death was assessed by measuring lactate dehydrogenase (LDH) release (upper right panel) and by counting TUNEL-positive cells (lower right panel). n=12 from 3 independent experiments. All quantitative data are presented as mean±SEM, *p<0.05, **p<0.01, ***p<0.001 versus Ln- or LnGFP-infected cell cultures under the indicated experimental conditions. Multiple comparisons were made using one-way ANOVA, followed by Bonferroni's/Dunn's post hoc analysis. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
To determine the effect of PRX2 on the interaction between Trx and ASK1 after OGD, co-IP was performed. Consistent with the role of PRX2 in protecting Trx against the loss of reducing activity under oxidative stress, PRX2, but not PRX2C/A, overexpression inhibited OGD-induced dissociation of Trx from ASK1 and, consequently, ASK1 activation (Fig. 5D).
Using three independent assays for cell death/survival, we evaluated the neuroprotective effect of PRX2 transfection against OGD in primary rat neurons. Transfection of PRX2 but not PRX2C/A significantly increased neuronal survival in OGD-challenged cultures (Fig. 5E, F). In separate experiments, Ln transfection of PRX2 in primary cortical neurons derived from mouse embryos also significantly increased neuronal viability after OGD (Supplementary Fig. S4), indicating that PRX2 protects against ischemic injury in both rat and mouse neurons.
Critical role of the Trx-ASK1 signaling complex in mediating the neuroprotective effect of PRX2
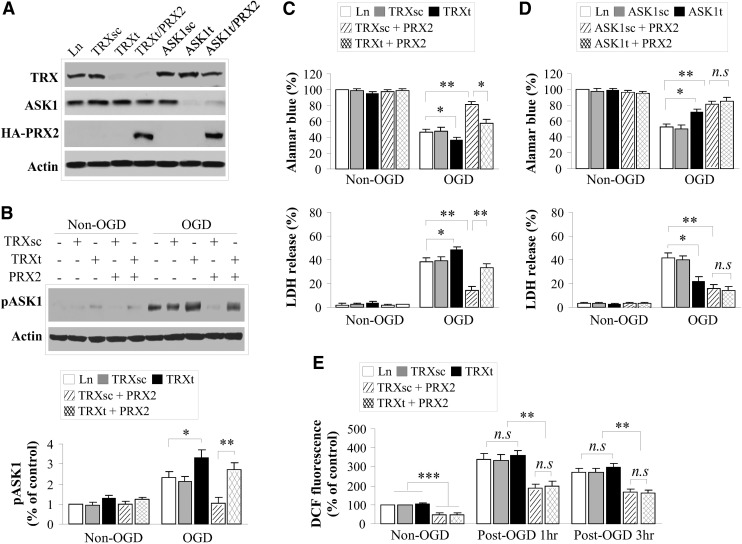
To determine if the Trx-ASK1 signaling complex and PRX2 interact with different pro-death signaling pathways after ischemia, we performed lentivirus-short hairpin RNA (shRNA)-mediated knockdown of Trx and ASK1, respectively, in primary rat neuron cultures (Fig. 6A and Supplementary Fig. S5). In all transfection experiments, the scramble sequences or empty vector served as controls. As shown (Fig. 6B, C), Trx knockdown significantly enhanced OGD-induced ASK1 activation and neuronal death in cultures. Furthermore, Trx knockdown markedly impaired the ability of PRX2 to suppress OGD-induced ASK1 activation and neuronal death (Fig. 6B, C). Similarly, ASK1 knockdown was protective against OGD-induced neuronal death, whereas PRX2 overexpression did not confer further neuroprotection in ASK1-deficient neurons (Fig. 6D). These results suggest that the Trx-ASK1 signaling complex is a major target for PRX2-afforded neuroprotection against ischemic injury.
FIG. 6.
Critical role of the Trx/ASK1 signaling complex in mediating the neuroprotective effect of PRX2 in cortical neuronal cultures. (A) Knockdown of Trx or ASK1 in neurons. Primary cortical neurons were infected for 3 days with Ln vectors containing short hairpin RNA (shRNA) targeting the Trx sequence (TRXt) or ASK1 sequence (ASK1t), their scrambled sequence (TRXsc and ASK1sc), or the empty lentivirus, or were co-infected with TRXt or ASK1t and lentivirus containing PRX2 cDNA. Immunoblotting results confirmed the knockdown of Trx and ASK1 expression, respectively, and showed that co-transfection of PRX2 did not affect the knockdown effect of TRXt or ASK1t. (B) Knockdown of Trx expression enhances OGD-induced ASK1 activation. Infected neurons were challenged with 60 min of OGD and, at 3 h after OGD, cell extracts were subjected to immunoblotting for p-ASK1. Note that Trx knockdown enhanced OGD-induced ASK1 activation and impaired the inhibitory effect of PRX2 overexpression on ASK1 activation. Data are based on three to four independent experiments. (C) Knockdown of Trx expression exacerbates neuronal death 24 h after OGD. Cell viability was quantified using the Alamar blue assay (upper panel); cell death was assessed by measuring LDH release (lower panel). Note that Trx knockdown enhanced OGD-induced neuronal death, but impaired the neuroprotective effect of PRX2. n=12 from 3 independent experiments. (D) ASK1 knockdown in neurons protects against OGD-induced cell death, but fails to offer additional neuroprotective effect in PRX2-transfected neurons. n=12 from 3 independent experiments. (E) PRX2 overexpression inhibits OGD-induced reactive oxygen species production independent of Trx expression. At 1 or 3 h after 60 min of OGD, neurons were incubated with CM-H2DCFDA (10 μM), a relatively specific sensor for H2O2, at 37°C in the dark for 30 min. dichlorofluorescein (DCF) fluorescence was measured on a fluorescence plate reader using 485-nm excitation and 530-nm emission. The data are presented as the percentage changes of DCF fluorescence over the non-OGD control (empty vector infection). n=4/group. Note that OGD increased DCF fluorescence, whereas PRX2 overexpression significantly decreased DCF fluorescence in both pre- and post-OGD neurons. There was no significant difference in DCF fluorescence intensity in PRX2-overexpressed neurons with or without Trx knockdown. All quantitative data are presented as mean±SEM, no significant (n.s.) p>0.05, *p<0.05, **p<0.01, ***p<0.001 between the indicated groups; based on multiple comparisons using one-way ANOVA, followed by Bonferroni's/Dunn's post hoc analysis.
Assessment of intracellular ROS using the H2O2-sensitive dichlorofluorescein fluorescence assay showed that while PRX2 overexpression significantly reduced ROS before or after OGD, Trx knockdown had an insignificant effect on the levels of H2O2 in neurons with or without PRX2 transfection (Fig. 6E). Thus, the impaired neuroprotective effect by PRX2 in Trx-deficient neurons could not be attributed to enhanced oxidative stress due to Trx knockdown. Alternatively, these results emphasize the importance of Trx as a specific and redox-sensitive endogenous inhibitor for the ASK1-dependent signaling pathway.
Attenuation of the MKK4/JNK signaling cascade in Tg-PRX2H mice
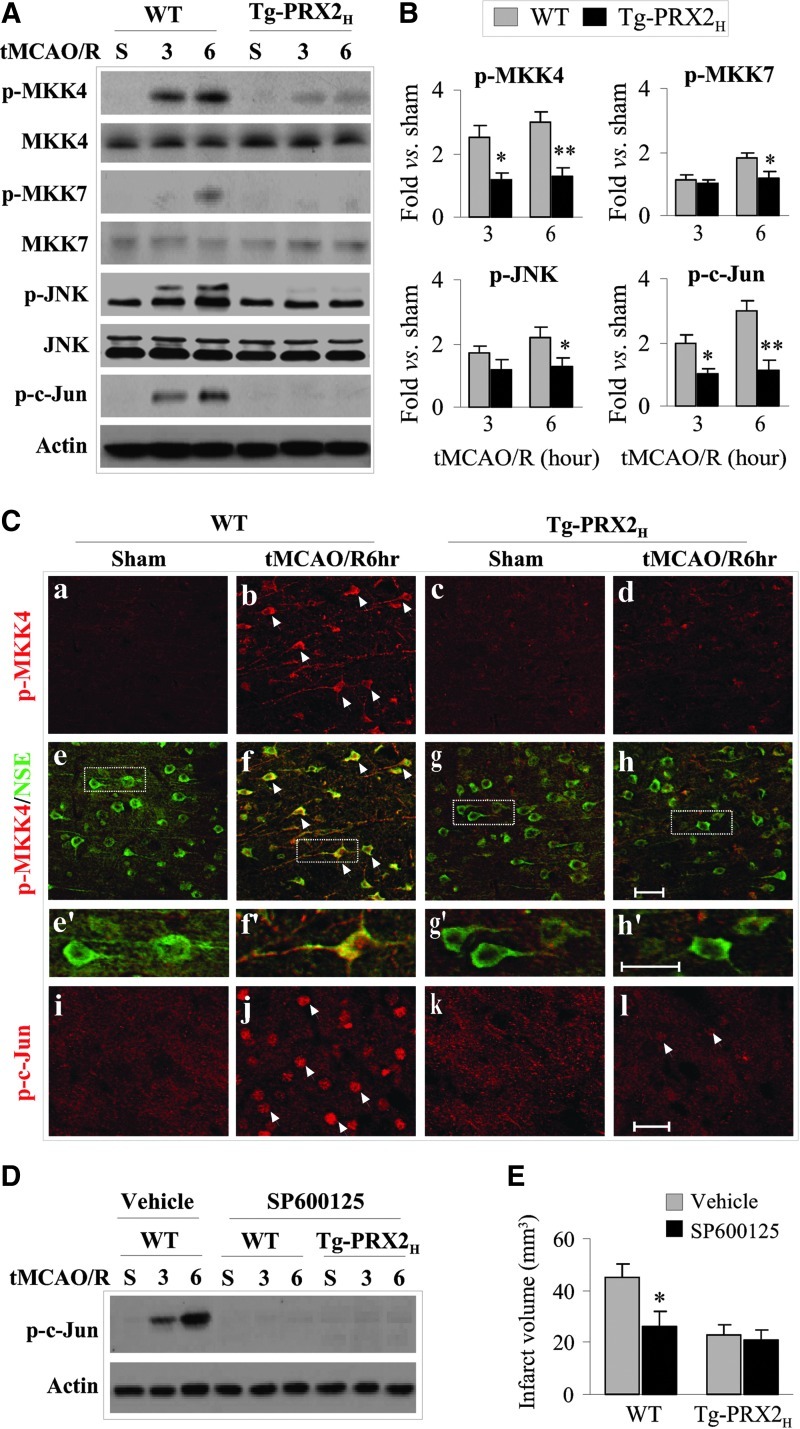
We further determined the effect of PRX2 overexpression on the MKK/JNK pathway, the main signaling pathway downstream of ASK1 activation that contributes to ischemic neuronal death (8, 15, 18, 29, 37). Determined using Western blots, tMCAO robustly activated MKK4 (increased phospho-Ser257) and, to a lesser extent, MKK7 (phospho-Ser271/Thr275) in brain of WT mice; tMCAO also activated JNK (phospho-Thr183/Tyr185) and its specific target c-Jun (phospho-Ser63). In contrast to WT mice, Tg-PRX2H mice showed nearly complete attenuation in cerebral activation of the MKK4/JNK/c-Jun signaling cascade after tMCAO (Fig. 7A, B). Immunofluorescent staining for phospho-MKK4 and phospho-c-Jun on brain sections, which showed predominant neuronal localizations, confirmed the inhibitory effect of PRX2 overexpression on MKK4/JNK activation after tMCAO (Fig. 7C).
FIG. 7.
Transgenic PRX2 overexpression inhibits the JNK signaling pathway after tMCAO. (A) Tg-PRX2 mice and their WT littermates were subjected to 60 min of tMCAO or sham operation. At 3 and 6 h after reperfusion, cortical tissue extracts were subjected to immunoblotting against p-MKK4, p-MKK7, p-JNK, and p-c-Jun or the total of each protein. Semi-quantitative data are illustrated in (B). Data are presented as mean±SEM, n=4–5 per group, *p<0.05, **p<0.01 versus WT mice. (C) Double-label immunofluoroscent staining for p-MKK4 (red, a–h and e′–h′) and NSE (green, e–h and e′–h′) in brain sections from Tg-PRX2 (c, d, g, h, low magnification; g′, h′, high magnification) or WT mice (a, b, e, f, low magnification; e′, f’, high magnification) with sham surgery or subjected to tMCAO and 6 h of reperfusion. Note that transgenic PRX2 expression attenuated ischemia-induced p-MKK4 immunofluorescence in ischemic neurons. Immunofluoroscent staining for p-c-Jun (red, i–l, low magnification) showed reduced p-c-Jun staining in Tg-PRX2 mice 6 h after tMCAO. Scale bars: low magnification, 50 μm; high magnification, 25 μm. (D-E) Attenuation of JNK activation mediates PRX2-afforded neuroprotection. JNK inhibitor SP600125 was administered before tMCAO or sham operation. At the dose of 10 mg/kg, SP600125 treatment abolished JNK activation at 3 and 6 h after reperfusion as demonstrated by immunoblotting against p-c-Jun. Determined at 48 h after tMCAO. SP600125 treatment significantly decreased infarct volume in WT mice, but failed to further reduce infarct volume in the Tg-PRX2H mice. Data are presented as mean±SEM, n=6 per group, *p<0.05 versus vehicle-treated WT mice; based on one-way ANOVA, followed by Bonferroni's/Dunn's post hoc analysis. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
To determine if attenuation of MKK4/JNK has a major role in mediating the neuroprotective effect of PRX2, WT, or Tg-PRX2H mice were administered the JNK inhibitor SP600125 (10 mg/kg) before tMCAO. SP600125 treatment attenuated JNK activation (Fig. 7D) and significantly decreased infarct volume after tMCAO in WT mice (Fig. 7E), but failed to reduce infarct volume in the Tg-PRX2H mice (Fig. 7E). These results suggest that inhibition of MKK4/JNK signaling is an integral component of the mechanism underlying PRX2-afforded neuroprotection against ischemic brain injury.
Discussion
Currently, the only established therapy for ischemic stroke, intravenous administration of recombinant tissue plasminogen activator, benefits just a small fraction of patients because of its limited time window of efficacy. Moreover, thrombolytic reperfusion may augment oxidative stress and the risk of intracranial hemorrhage in the ischemic brain (16). Thus, it is imperative to develop alternative therapeutic strategies for ischemic/reperfusion injury, such as aiming to limit ROS-mediated neuronal damage (5). In this study, we demonstrate for the first time that neuronal overexpression of PRX2 significantly reduced brain infarct and improved neurological outcomes up to 3 weeks after ischemia/reperfusion. We further show that PRX2 down-regulates a redox-dependent mitochondrial pro-death pathway via enhancing Trx-ASK1 interaction and thus prevents the activation of the ASK1-JNK signaling cascade in ischemic neurons.
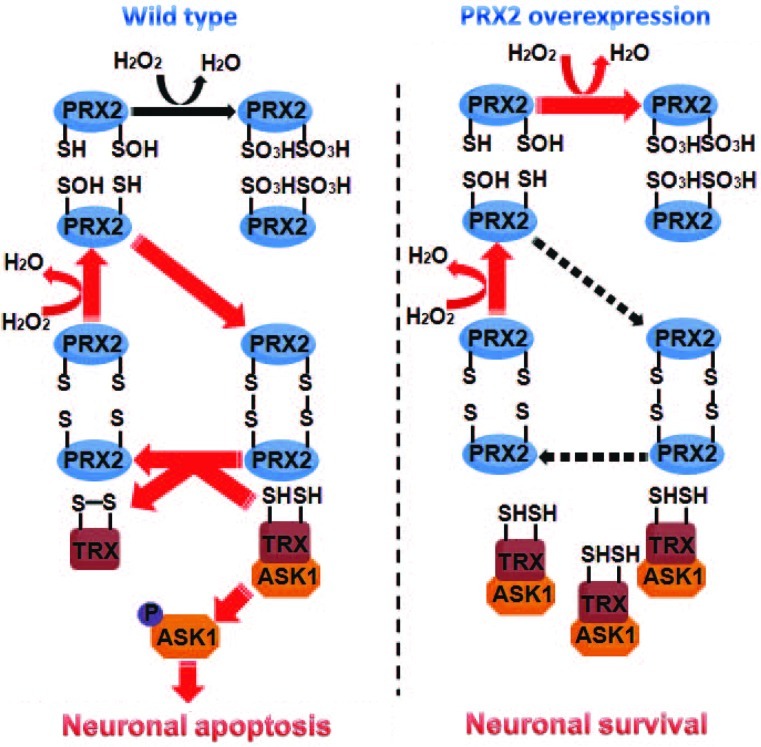
The cytoprotective effect of PRX2 has recently been observed in neuronal cultures under ischemia-like conditions (2), and this PRX2-afforded protection is thought to be attributed to its antioxidant property. Indeed, PRX2 actively participates in the peroxide-reducing reactions after ischemia/reperfusion, resulting in remarkably increased PRX2 over-oxidation but decreased PRX activity reserve in ischemic neurons. This observation is consistent with a previous study showing increased production of PRX-SO3 after brain ischemia (10). However, the neuroprotective effect of PRX2 against ischemic/reperfusion could not be fully explained by its antioxidant actions per se, as PRX2 overexpression only partially reduced the levels of ROS in ischemic neurons. Moreover, knockdown of Trx expression, which alone did not augment oxidative stress in neurons, almost completely abolished PRX2-afforded neuroprotection against ischemia, supporting the notion that PRX2 achieves a neuroprotective effect through a Trx-dependent mechanism. Our results thus suggest an additional action of PRX2 via direct engagement in Trx/ASK1-dependent apoptosis-regulatory signaling. The following model is proposed: PRX2 overexpression, by maintaining Trx in a reduced state, enhances the interaction between Trx and ASK1 that keeps ASK1 at its inactive form, thus preventing the activation of ASK1 and of the ASK1-dependent pro-death signaling pathway after ischemia (Fig. 8).
FIG. 8.
Schematic diagram illustrating the deduced pathway through which PRX2 interacts with the Trx-ASK1 signaling complex. Left panel: after ischemic insult, PRX2 is oxidized when scavenging free radicals, which results in a reduction in its antioxidative activity. Oxidized PRX can regain its activity at the expense of reduced Trx, yielding oxidized Trx. The reduced Trx normally binds to and inhibits ASK1; however, oxidation of Trx sets free ASK1, leading to the activation of subsequent apoptotic cascades. Right panel: overexpression of PRX2 results in increases of reduced PRX2 to battle ischemia-induced oxidative stress. Increased amount of PRX2 at its reduced form inhibits the cysteine thiol–disulfide exchange between PRX2 and Trx, thus maintaining Trx at its reduced state that inhibits the activation of ASK1 and cell death. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
The peroxide-reducing activity of the active site Cys51 and Cys172 on PRX2 is essential for the functional interaction between PRX2 and the Trx/ASK1 complex. Cys51 on PRX2 is most accessible to oxidation under peroxide-imposed oxidative stress (41). Accordingly, under oxidative stress Cys51 is first oxidized to Cys51-SOH, which then is overoxidized to the irreversible form Cys51-SO2H or Cys51-SO3H; alternatively, Cys51-SOH forms a disulfide with Cys172 on another PRX2 molecule, and the disulfide can be reduced back to the active thiol form by the Trx/TrxR system (41, 42). Consistent with this concept, we found that mutation of Cys51 to alanine avoided any oxidation of PRX2 by hydrogen peroxide and that PRX2 with mutations at both Cys51 and Cys172 completely lost its ability to interact with the Trx/ASK1 complex or to inhibit ASK1 in ischemic neurons. Our results also show that overconsumption of PRX2 after ischemia/reperfusion led to depletion of reduced Trx in neurons, whereas PRX2 overexpression preserved Trx at its reduced state.
It has been well established that ischemia/reperfusion-induced overproduction of ROS causes neuronal demise by either directly targeting cellular macromolecules and/or triggering various pro-death signaling pathways (32, 36). Thus, in principle, antioxidant therapy should hold great promise in translation into an effective clinical treatment for stroke. However, such a therapeutic strategy, exemplified by the disufenton sodium (NXY-059) trials, so far has failed to produce beneficial effects in stroke patients (21, 35). Although many factors could contribute to the unsuccessful clinical trials, one issue worthy of consideration is that an untitrated suppression of ROS may deprive the normal physiological functions of free radicals and potentially result in unwanted effects. In contrast to classic antioxidant agents, PRXs possess a moderate ROS-scavenging property and yet are capable of potently inhibiting redox-sensitive pro-death signaling pathways that critically determine the fate of ischemic neurons. Several PRXs, including PRX1 and PRX2, are potentially inducible proteins in the brain (12, 17, 27). Conceivably, brain levels of PRX1 and/or PRX2 can be enhanced by PRX inducers or carrier-based protein delivery. Taken together, the results of this study demonstrating long-lasting neuroprotective effect by PRX2 warrant further investigation into PRX2's potential therapeutic value in experimental stroke, which would also include a poststroke treatment regimen.
While the current study focuses on PRX2, it does not exclude the possibility that PRX2 acts in conjunction with other PRX isoforms in neuroprotection. All mammalian PRX isoforms have been detected in the CNS and each isoform could have a unique neuroprotective role depending on its specific cellular distribution. For example, it is plausible that the glia-enriched PRX1 could influence stroke outcome by modulating astroglial and/or microglial responses (26). It would be interesting to determine if enhanced levels of both PRX2 and PRX1 offer additive neuroprotective effects against stroke. Moreover, future studies would also need to compare the neuroprotective effect of PRX2 in male versus female animals, as emerging evidence supports the important concept that there may be sex differences in response to neuroprotective agents in stroke models (23, 39).
In summary, transgenic overexpression of PRX2 confers long-term neuroprotection against ischemic/reperfusion brain injury in mice. This study also characterizes a potential mechanism underlying PRX2-afforded neuroprotection whereby PRX2 inhibits the ASK1-mediated pro-death signaling cascade in a Trx-dependent manner. Our results suggest that PRX2 may represent a potential target for stroke intervention.
Materials and Methods
Details beyond the descriptions here are provided in Supplementary Materials and Methods.
Models of cerebral ischemia
All animal experiments were approved by the Institutional Animal Care and Use Committee of Capital Medical University and University of Pittsburgh, respectively, and performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Male 8- to 12-week-old mice were randomly assigned to various experimental groups with the surgeon blinded to the genotypes of the mice. Focal cerebral ischemia was produced by intraluminal occlusion of the left MCA as described previously (3). To calculate infarct volume, brains were removed at either 2 or 21 days after MCAO, and subjected to 2,3,5-triphenyltetrazolium chloride (TTC) staining and MAP-2 immunohistochemical straining, respectively. Infarct volume was determined using MCID™ imaging with correction for edema.
To model ischemia-like conditions in vitro, primary cortical neuronal cultures were exposed to OGD for 60 min followed by reperfusion (37). Neuronal viability/death was quantified using the Alamar blue assay, lactate dehydrogenase release assay, and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) (37).
Neurobehavioral tests
Neurobehavioral tests were performed before surgery and 3–21 days after MCAO by a researcher who was blinded to genotypes of the mice. The assessment for sensorimotor deficits consisted of three different tests: the rotarod test, the corner test, and the cylinder test. Rotarod and corner tests were performed as described previously (37). The cylinder test was adapted for use in mouse to assess forepaw use and rotation asymmetry. The mouse was placed in a cylinder 9 cm in diameter and 15 cm in height, and videotaped for 5 min. Videotapes were analyzed, and forepaw (left/right/both) use of the first contact against the cylinder wall after rearing and during lateral exploration was recorded. Nonimpaired forepaw (right) preference is expressed as the relative proportion of right forepaw contacts, which was calculated as: (right−left)/(right+left+both)×100 (6).
Construction of viral vectors
Ln vectors carrying either the human full-length (LnPRX2) or catalytically inactive mutant (LnPRX2C/A) PRX2 cDNA (also encoding a Ha tag) were constructed as described previously (9). To construct Ln vectors expressing shRNA against rat Trx or ASK1, the gene-specific targeting sequence (TRXt or ASK1t) or its counterpart scramble sequence (TRXsc or ASK1sc) was inserted into the transfer vector FSW under the control of the U6 promoter. Large-scale production, purification, and titration of the recombinant lentivirus was performed using a protocol described previously (38).
PRX and Trx activity assay
Peroxidase activity was determined as previously described (9). The PRX activity assay kit (Redoxica, Little Rock, AR) was used for the measurement according to the manufacturer's instructions. Trx activity was determined by the insulin disulfide reduxing assay as previously described (25). The data were expressed as percentage changes in PRX or Trx activity over control noninjured cell cultures or animals.
ASK1 kinase assay
Tissue or cell lysates were prepared under nondenaturing conditions as described. To assay for ASK1 activity, the lysates (150 μg total protein) were first subjected to ASK1 capture using the specific anti-ASK1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), and then incubated with recombinant myelin basic protein (MBP) in the presence of [γ-32P]ATP, and MBP phosphorylation was detected using autoradiogram. The data were expressed as fold changes in ASK1 activity over control noninjured cell cultures or animals.
Statistical analysis
Results are reported as the mean±standard error of the mean (SEM). The significance of difference between means was assessed by Student's t-test (single comparisons) or by analysis of variance with post hoc Bonferroni's/Dunn's tests (for multiple comparisons). A value of p<0.05 was considered statistically significant.
Supplementary Material
Abbreviations Used
- ANOVA
analysis of variance
- ASK1
apoptosis signal–regulating kinase 1
- Cys
cysteine
- DCF
dichlorofluorescein
- HA
hemagglutinin
- IB
inner boundary
- IP
immunoprecipitation
- LDH
lactate dehydrogenase
- Ln
lentiviral
- MBP
myelin basic protein
- MCAO
middle cerebral artery occlusion
- n.s.
no significant
- OGD
oxygen and glucose deprivation
- pMCAO
permanent MCAO
- PRX
peroxiredoxin
- PRX-SO3
overoxidized PRX
- rCBF
regional cerebral blood flow
- ROS
reactive oxygen species
- shRNA
short hairpin RNA
- Tg-PRX2
PRX2 transgenic
- tMCAO
transient MCAO
- Trx
thioredoxin
- TrxR
thioredoxin reductase
- TTC
2,3,5-triphenyltetrazolium chloride
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- WT
wild-type
Acknowledgments
This work was supported by Chinese Natural Science Foundation Grant #30870854 (to X.J.), Chinese Ministry of Education Grant NCET-08-0625 (to XJ), and NIH Grants NS036736, NS043802, and NS045048 (to JC). Xiaoming Hu is supported by AHA grant 10POST4150028. We thank Carol Culver and Susan Giegel for their excellent editorial assistance.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Aon-Bertolino ML. Romero JI. Galeano P. Holubiec M. Badorrey MS. Saraceno GE. Hanschmann EM. Lillig CH. Capani F. Thioredoxin and glutaredoxin system proteins-immunolocalization in the rat central nervous system. Biochim Biophys Acta. 2011;1810:93–110. doi: 10.1016/j.bbagen.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Boulos S. Meloni BP. Arthur PG. Bojarski C. Knuckey NW. Peroxiredoxin 2 overexpression protects cortical neuronal cultures from ischemic and oxidative injury but not glutamate excitotoxicity, whereas Cu/Zn superoxide dismutase 1 overexpression protects only against oxidative injury. J Neurosci Res. 2007;85:3089–3097. doi: 10.1002/jnr.21429. [DOI] [PubMed] [Google Scholar]
- 3.Cao G. Luo Y. Nagayama T. Pei W. Stetler RA. Graham SH. Chen J. Cloning and characterization of rat caspase-9: implications for a role in mediating caspase-3 activation and hippocampal cell death after transient cerebral ischemia. J Cereb Blood Flow Metab. 2002;22:534–546. doi: 10.1097/00004647-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Chan PH. Mitochondrial dysfunction and oxidative stress as determinants of cell death/survival in stroke. Ann N Y Acad Sci. 2005;1042:203–209. doi: 10.1196/annals.1338.022. [DOI] [PubMed] [Google Scholar]
- 5.Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Chang YS. Mu D. Wendland M. Sheldon RA. Vexler ZS. McQuillen PS. Ferriero DM. Erythropoietin improves functional and histological outcome in neonatal stroke. Pediatr Res. 2005;58:106–111. doi: 10.1203/01.PDR.0000163616.89767.69. [DOI] [PubMed] [Google Scholar]
- 7.Claiborne A. Yeh JI. Mallett TC. Luba J. Crane EJ., 3rd Charrier V. Parsonage D. Protein-sulfenic acids: diverse roles for an unlikely player in enzyme catalysis and redox regulation. Biochemistry. 1999;38:15407–15416. doi: 10.1021/bi992025k. [DOI] [PubMed] [Google Scholar]
- 8.Gao Y. Signore AP. Yin W. Cao G. Yin XM. Sun F. Luo Y. Graham SH. Chen J. Neuroprotection against focal ischemic brain injury by inhibition of c-Jun N-terminal kinase and attenuation of the mitochondrial apoptosis-signaling pathway. J Cereb Blood Flow Metab. 2005;25:694–712. doi: 10.1038/sj.jcbfm.9600062. [DOI] [PubMed] [Google Scholar]
- 9.Hu X. Weng Z. Chu CT. Zhang L. Cao G. Gao Y. Signore A. Zhu J. Hastings T. Greenamyre JT. Chen J. Peroxiredoxin-2 protects against 6-hydroxydopamine-induced dopaminergic neurodegeneration via attenuation of the apoptosis signal-regulating kinase (ASK1) signaling cascade. J Neurosci. 2011;31:247–261. doi: 10.1523/JNEUROSCI.4589-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang IK. Yoo KY. Kim DW. Choi JH. Lee IS. Won MH. Hyperoxidized peroxiredoxins and glyceraldehyde-3-phosphate dehydrogenase immunoreactivity and protein levels are changed in the gerbil hippocampal CA1 region after transient forebrain ischemia. Neurochem Res. 2007;32:1530–1538. doi: 10.1007/s11064-007-9345-6. [DOI] [PubMed] [Google Scholar]
- 11.Ichimiya S. Davis JG. O'Rourke DM. Katsumata M. Greene MI. Murine thioredoxin peroxidase delays neuronal apoptosis and is expressed in areas of the brain most susceptible to hypoxic and ischemic injury. DNA Cell Biol. 1997;16:311–321. doi: 10.1089/dna.1997.16.311. [DOI] [PubMed] [Google Scholar]
- 12.Ishii T. Itoh K. Takahashi S. Sato H. Yanagawa T. Katoh Y. Bannai S. Yamamoto M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem. 2000;275:16023–16029. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- 13.Jin MH. Lee YH. Kim JM. Sun HN. Moon EY. Shong MH. Kim SU. Lee SH. Lee TH. Yu DY. Lee DS. Characterization of neural cell types expressing peroxiredoxins in mouse brain. Neurosci Lett. 2005;381:252–257. doi: 10.1016/j.neulet.2005.02.048. [DOI] [PubMed] [Google Scholar]
- 14.Kalinina EV. Chernov NN. Saprin AN. Involvement of thio-, peroxi-, and glutaredoxins in cellular redox-dependent processes. Biochemistry (Mosc) 2008;73:1493–1510. doi: 10.1134/s0006297908130099. [DOI] [PubMed] [Google Scholar]
- 15.Kamada H. Nito C. Endo H. Chan PH. Bad as a converging signaling molecule between survival PI3-K/Akt and death JNK in neurons after transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2007;27:521–533. doi: 10.1038/sj.jcbfm.9600367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly PJ. Morrow JD. Ning M. Koroshetz W. Lo EH. Terry E. Milne GL. Hubbard J. Lee H. Stevenson E. Lederer M. Furie KL. Oxidative stress and matrix metalloproteinase-9 in acute ischemic stroke: the Biomarker Evaluation for Antioxidant Therapies in Stroke (BEAT-Stroke) study. Stroke. 2008;39:100–104. doi: 10.1161/STROKEAHA.107.488189. [DOI] [PubMed] [Google Scholar]
- 17.Krapfenbauer K. Engidawork E. Cairns N. Fountoulakis M. Lubec G. Aberrant expression of peroxiredoxin subtypes in neurodegenerative disorders. Brain Res. 2003;967:152–160. doi: 10.1016/s0006-8993(02)04243-9. [DOI] [PubMed] [Google Scholar]
- 18.Kuan CY. Whitmarsh AJ. Yang DD. Liao G. Schloemer AJ. Dong C. Bao J. Banasiak KJ. Haddad GG. Flavell RA. Davis RJ. Rakic P. A critical role of neural-specific JNK3 for ischemic apoptosis. Proc Natl Acad Sci U S A. 2003;100:15184–15189. doi: 10.1073/pnas.2336254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee TH. Kim SU. Yu SL. Kim SH. Park DS. Moon HB. Dho SH. Kwon KS. Kwon HJ. Han YH. Jeong S. Kang SW. Shin HS. Lee KK. Rhee SG. Yu DY. Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice. Blood. 2003;101:5033–5038. doi: 10.1182/blood-2002-08-2548. [DOI] [PubMed] [Google Scholar]
- 20.Lee YM. Park SH. Shin DI. Hwang JY. Park B. Park YJ. Lee TH. Chae HZ. Jin BK. Oh TH. Oh YJ. Oxidative modification of peroxiredoxin is associated with drug-induced apoptotic signaling in experimental models of Parkinson disease. J Biol Chem. 2008;283:9986–9998. doi: 10.1074/jbc.M800426200. [DOI] [PubMed] [Google Scholar]
- 21.Lees KR. Zivin JA. Ashwood T. Davalos A. Davis SM. Diener HC. Grotta J. Lyden P. Shuaib A. Hardemark HG. Wasiewski WW. NXY-059 for acute ischemic stroke. N Engl J Med. 2006;354:588–600. doi: 10.1056/NEJMoa052980. [DOI] [PubMed] [Google Scholar]
- 22.Lin HW. Thompson JW. Morris KC. Perez-Pinzon MA. Signal transducers and activators of transcription: STATs-mediated mitochondrial neuroprotection. Antioxid Redox Signal. 2011;14:1853–1861. doi: 10.1089/ars.2010.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu F. Lang J. Li J. Benashski SE. Siegel M. Xu Y. McCullough LD. Sex differences in the response to poly(ADP-ribose) polymerase-1 deletion and caspase inhibition after stroke. Stroke. 2011;42:1090–1096. doi: 10.1161/STROKEAHA.110.594861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Low FM. Hampton MB. Winterbourn CC. Peroxiredoxin 2 and peroxide metabolism in the erythrocyte. Antioxid Redox Signal. 2008;10:1621–1630. doi: 10.1089/ars.2008.2081. [DOI] [PubMed] [Google Scholar]
- 25.Matsuura T. Harrison RA. Westwell AD. Nakamura H. Martynyuk AE. Sumners C. Basal and angiotensin II-inhibited neuronal delayed-rectifier K+ current are regulated by thioredoxin. Am J Physiol Cell Physiol. 2007;293:C211–C217. doi: 10.1152/ajpcell.00615.2006. [DOI] [PubMed] [Google Scholar]
- 26.Mizusawa H. Ishii T. Bannai S. Peroxiredoxin I (macrophage 23 kDa stress protein) is highly and widely expressed in the rat nervous system. Neurosci Lett. 2000;283:57–60. doi: 10.1016/s0304-3940(00)00910-1. [DOI] [PubMed] [Google Scholar]
- 27.Nakaso K. Kitayama M. Mizuta E. Fukuda H. Ishii T. Nakashima K. Yamada K. Co-induction of heme oxygenase-1 and peroxiredoxin I in astrocytes and microglia around hemorrhagic region in the rat brain. Neurosci Lett. 2000;293:49–52. doi: 10.1016/s0304-3940(00)01491-9. [DOI] [PubMed] [Google Scholar]
- 28.Niizuma K. Endo H. Chan PH. Oxidative stress and mitochondrial dysfunction as determinants of ischemic neuronal death and survival. J Neurochem. 2009;109(Suppl 1):133–138. doi: 10.1111/j.1471-4159.2009.05897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okuno S. Saito A. Hayashi T. Chan PH. The c-Jun N-terminal protein kinase signaling pathway mediates Bax activation and subsequent neuronal apoptosis through interaction with Bim after transient focal cerebral ischemia. J Neurosci. 2004;24:7879–7887. doi: 10.1523/JNEUROSCI.1745-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patenaude A. Murthy MR. Mirault ME. Emerging roles of thioredoxin cycle enzymes in the central nervous system. Cell Mol Life Sci. 2005;62:1063–1080. doi: 10.1007/s00018-005-4541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rashidian J. Rousseaux MW. Venderova K. Qu D. Callaghan SM. Phillips M. Bland RJ. During MJ. Mao Z. Slack RS. Park DS. Essential role of cytoplasmic cdk5 and Prx2 in multiple ischemic injury models, in vivo. J Neurosci. 2009;29:12497–12505. doi: 10.1523/JNEUROSCI.3892-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saito A. Maier CM. Narasimhan P. Nishi T. Song YS. Yu F. Liu J. Lee YS. Nito C. Kamada H. Dodd RL. Hsieh LB. Hassid B. Kim EE. Gonzalez M. Chan PH. Oxidative stress and neuronal death/survival signaling in cerebral ischemia. Mol Neurobiol. 2005;31:105–116. doi: 10.1385/MN:31:1-3:105. [DOI] [PubMed] [Google Scholar]
- 33.Saitoh M. Nishitoh H. Fujii M. Takeda K. Tobiume K. Sawada Y. Kawabata M. Miyazono K. Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarafian TA. Verity MA. Vinters HV. Shih CC. Shi L. Ji XD. Dong L. Shau H. Differential expression of peroxiredoxin subtypes in human brain cell types. J Neurosci Res. 1999;56:206–212. [PubMed] [Google Scholar]
- 35.Shuaib A. Lees KR. Lyden P. Grotta J. Davalos A. Davis SM. Diener HC. Ashwood T. Wasiewski WW. Emeribe U. NXY-059 for the treatment of acute ischemic stroke. N Engl J Med. 2007;357:562–571. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- 36.Stankowski JN. Zeiger SL. Cohen EL. DeFranco DB. Cai J. McLaughlin B. C-terminus of heat shock cognate 70 interacting protein increases following stroke and impairs survival against acute oxidative stress. Antioxid Redox Signal. 14:1787–1801. doi: 10.1089/ars.2010.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stetler RA. Cao G. Gao Y. Zhang F. Wang S. Weng Z. Vosler P. Zhang L. Signore A. Graham SH. Chen J. Hsp27 protects against ischemic brain injury via attenuation of a novel stress-response cascade upstream of mitochondrial cell death signaling. J Neurosci. 2008;28:13038–13055. doi: 10.1523/JNEUROSCI.4407-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stetler RA. Gao Y. Zukin RS. Vosler PS. Zhang L. Zhang F. Cao G. Bennett MV. Chen J. Apurinic/apyrimidinic endonuclease APE1 is required for PACAP-induced neuroprotection against global cerebral ischemia. Proc Natl Acad Sci U S A. 2010;107:3204–3209. doi: 10.1073/pnas.1000030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turtzo LC. Siegel C. McCullough LD. X chromosome dosage and the response to cerebral ischemia. J Neurosci. 2011;31:13255–13259. doi: 10.1523/JNEUROSCI.0621-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X. Phelan SA. Forsman-Semb K. Taylor EF. Petros C. Brown A. Lerner CP. Paigen B. Mice with targeted mutation of peroxiredoxin 6 develop normally but are susceptible to oxidative stress. J Biol Chem. 2003;278:25179–25190. doi: 10.1074/jbc.M302706200. [DOI] [PubMed] [Google Scholar]
- 41.Woo HA. Chae HZ. Hwang SC. Yang KS. Kang SW. Kim K. Rhee SG. Reversing the inactivation of peroxiredoxins caused by cysteine sulfinic acid formation. Science. 2003;300:653–656. doi: 10.1126/science.1080273. [DOI] [PubMed] [Google Scholar]
- 42.Woo HA. Kang SW. Kim HK. Yang KS. Chae HZ. Rhee SG. Reversible oxidation of the active site cysteine of peroxiredoxins to cysteine sulfinic acid. Immunoblot detection with antibodies specific for the hyperoxidized cysteine-containing sequence. J Biol Chem. 2003;278:47361–47364. doi: 10.1074/jbc.C300428200. [DOI] [PubMed] [Google Scholar]
- 43.Zeiger SL. Musiek ES. Zanoni G. Vidari G. Morrow JD. Milne GJ. McLaughlin B. Neurotoxic lipid peroxidation species formed by ischemic stroke increase injury. Free Radic Biol Med. 2009;47:1422–1431. doi: 10.1016/j.freeradbiomed.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.