Abstract
Pieces of mitochondrial DNA from a 7000-year-old human brain were amplified by the polymerase chain reaction and sequenced. Albumin and high concentrations of polymerase were required to overcome a factor in the brain extract that inhibits amplification. For this and other sources of ancient DNA, we find an extreme inverse dependence of the amplification efficiency on the length of the sequence to be amplified. This property of ancient DNA distinguishes it from modern DNA and thus provides a new criterion of authenticity for use in research on ancient DNA. The brain is from an individual recently excavated from Little Salt Spring in southwestern Florida and the anthropologically informative sequences it yielded are the first obtained from archaeologically retrieved remains. The sequences show that this ancient individual belonged to a mitochondrial lineage that is rare in the Old World and not previously known to exist among Native Americans. Our finding brings to three the number of maternal lineages known to have been involved in the prehistoric colonization of the New World.
Full text
PDF





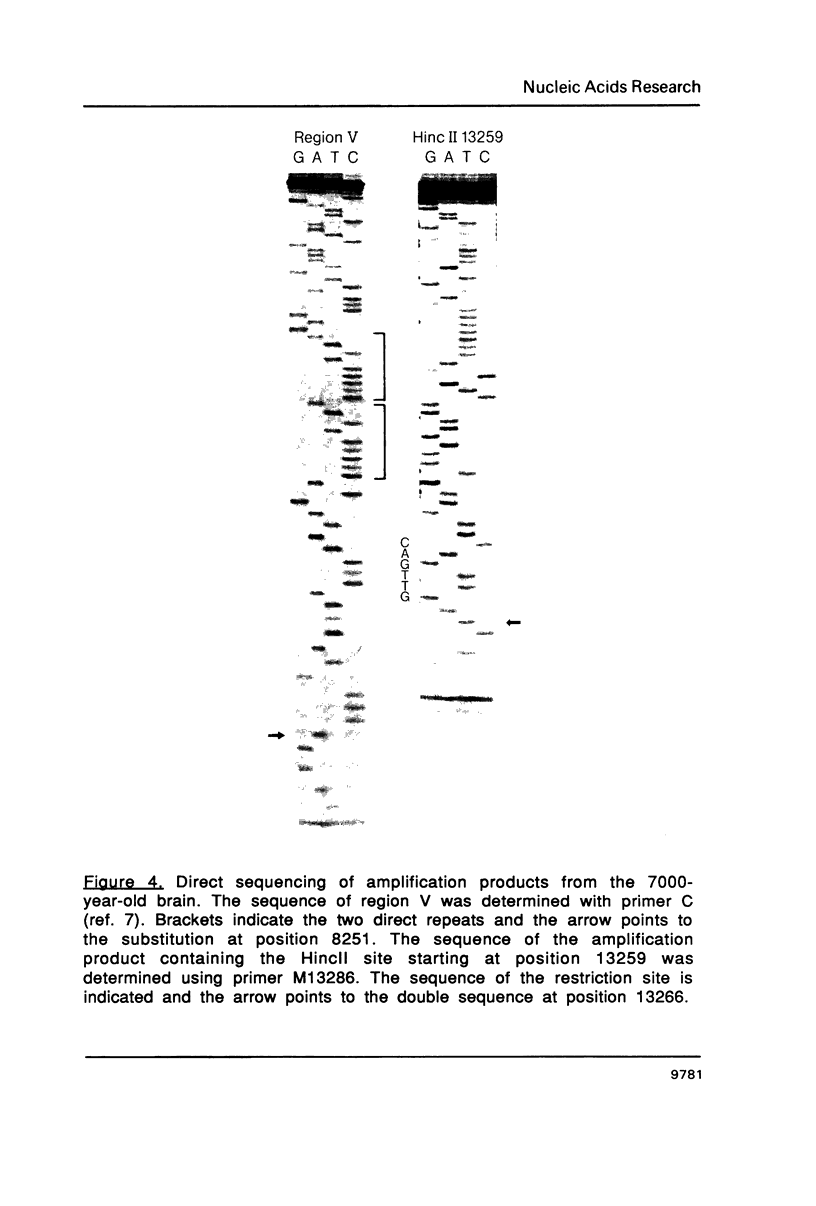






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Blanc H., Chen K. H., D'Amore M. A., Wallace D. C. Amino acid change associated with the major polymorphic Hinc II site of Oriental and Caucasian mitochondrial DNAs. Am J Hum Genet. 1983 Mar;35(2):167–176. [PMC free article] [PubMed] [Google Scholar]
- Cann R. L., Stoneking M., Wilson A. C. Mitochondrial DNA and human evolution. Nature. 1987 Jan 1;325(6099):31–36. doi: 10.1038/325031a0. [DOI] [PubMed] [Google Scholar]
- Clausen C. J., Cohen A. D., Emiliani C., Holman J. A., Stipp J. J. Little salt spring, Florida: a unique underwater site. Science. 1979 Feb 16;203(4381):609–614. doi: 10.1126/science.203.4381.609. [DOI] [PubMed] [Google Scholar]
- Doran G. H., Dickel D. N., Ballinger W. E., Jr, Agee O. F., Laipis P. J., Hauswirth W. W. Anatomical, cellular and molecular analysis of 8,000-yr-old human brain tissue from the Windover archaeological site. 1986 Oct 30-Nov 5Nature. 323(6091):803–806. doi: 10.1038/323803a0. [DOI] [PubMed] [Google Scholar]
- Higuchi R. G., Wrischnik L. A., Oakes E., George M., Tong B., Wilson A. C. Mitochondrial DNA of the extinct quagga: relatedness and extent of postmortem change. J Mol Evol. 1987;25(4):283–287. doi: 10.1007/BF02603111. [DOI] [PubMed] [Google Scholar]
- Higuchi R., Bowman B., Freiberger M., Ryder O. A., Wilson A. C. DNA sequences from the quagga, an extinct member of the horse family. Nature. 1984 Nov 15;312(5991):282–284. doi: 10.1038/312282a0. [DOI] [PubMed] [Google Scholar]
- Higuchi R., von Beroldingen C. H., Sensabaugh G. F., Erlich H. A. DNA typing from single hairs. Nature. 1988 Apr 7;332(6164):543–546. doi: 10.1038/332543a0. [DOI] [PubMed] [Google Scholar]
- Horai S., Matsunaga E. Mitochondrial DNA polymorphism in Japanese. II. Analysis with restriction enzymes of four or five base pair recognition. Hum Genet. 1986 Feb;72(2):105–117. doi: 10.1007/BF00283927. [DOI] [PubMed] [Google Scholar]
- Päbo S. Molecular cloning of Ancient Egyptian mummy DNA. Nature. 1985 Apr 18;314(6012):644–645. doi: 10.1038/314644a0. [DOI] [PubMed] [Google Scholar]
- Päbo S. Molecular genetic investigations of ancient human remains. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):441–446. [PubMed] [Google Scholar]
- Päbo S., Wilson A. C. Polymerase chain reaction reveals cloning artefacts. Nature. 1988 Aug 4;334(6181):387–388. doi: 10.1038/334387b0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace D. C., Garrison K., Knowler W. C. Dramatic founder effects in Amerindian mitochondrial DNAs. Am J Phys Anthropol. 1985 Oct;68(2):149–155. doi: 10.1002/ajpa.1330680202. [DOI] [PubMed] [Google Scholar]
- Wrischnik L. A., Higuchi R. G., Stoneking M., Erlich H. A., Arnheim N., Wilson A. C. Length mutations in human mitochondrial DNA: direct sequencing of enzymatically amplified DNA. Nucleic Acids Res. 1987 Jan 26;15(2):529–542. doi: 10.1093/nar/15.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]







