Abstract
Early life stressors in rodents, including maternal separation and social isolation, have been shown to disrupt brain development and profoundly affect a wide-range of behaviors in adult animals. In this study, we focus on the development of female Sprague-Dawley rats in the presence and absence of conspecifics during the critical period of social play. Similar studies in male rodents have shown that this form of social deprivation results in dysregulated dopaminergic and serotonergic functions in the brain with core features of neuropsychiatric disorders including anxiety disorder and schizophrenia. Here we examined the behavioral and biochemical effects of post-weaning social isolation in female rats. Our findings demonstrated that isolation rearing produced marked deficits in social interaction behaviors and increased anxiety in open-field and novelty-suppressed feeding tests. The expression of synaptic-associated proteins PSD95 and synapsin I as well as glutamate receptors subunits GluR1 and NR1 in the prefrontal cortex (PFC) were significantly reduced in isolation-reared female rats. Current findings provide evidence that in female rats, post-weaning environmental disruption can result in profound dysregulation of synapse-related proteins and behavior.
Keywords: anxiety, isolation rearing, social interaction, prefrontal cortex, female rats, synaptic marker, glutamate receptor
1. Introduction
Numerous studies of social isolation of post-weaning laboratory rodents have shown significant disturbances in a variety of neurochemical systems and behaviors. The behavioral effect of social isolation, rearing rat pups in the absence of conspecifics, has often been referred to as the “isolation syndrome,” a state of hyperactivity, neophobia, perseverative behaviors, and deficits in sensorimotor gating [1]. More recent attempts to summarize the findings demonstrate connections between post-weaning social isolation of rodents and a variety of neuropsychiatric disorders [2]. Dysregulation of dopaminergic and serotonergic function in the nucleus accumbens, prefrontal cortex and hippocampus along with impaired prepulse inhibition, increased startle response, and cognitive rigidity suggest certain symptoms of schizophrenia [2]. Recent work has shown that a variety of typical and atypical antipsychotics alleviate the deleterious effects of social isolation [3]. Other psychopathological disturbances associated with social isolation include hoarding [3] and anxiety [4].
In addition to creating vulnerability to brain and behavioral disturbances, post-weaning social isolation has been a useful developmental model for aspects of psychopathology that relate to early life adversity and stress. In most isolation rearing studies, rodents are singly housed for some portion of the juvenile period. During this period, rats engage in social play, which reaches a plateau of activity between postnatal day (PND) 24 and 52 with a peak at PND 32-40 [5]. Social play consists of sudden episodes of darting movements associated with pouncing on the partner's back, chasing, wrestling and pinning (so-called on-top and on-back postures) [6]. The period of social play is a period of fixed length considered critical to solidifying aspects of lifelong social interactions and reciprocal behavior in the adult rat [7-10]. Rat behaviors that emerge from an adversely manipulated, truncated, or isolated juvenile period result in a phenotype similar to human psychiatric illness with impairments in social skills [11].
In humans, damage to the prefrontal cortex has been shown to impair a variety of social and cognitive behaviors [12, 13] and is strongly implicated in the negative symptoms (asociality and avolition) of psychotic illness. Likewise, volume loss in medial prefrontal cortex has been shown in juvenile rats reared in isolation [14]. Lesions of the neonatal PFC disrupt social play and social behavior in juvenile and adult rats [15]. Social isolation throughout the peri-pubertal period appears to affect synaptic plasticity in the PFC as well. Glutamate is the most abundant excitatory neurotransmitter in the brain and a key mediator of synaptic plasticity. However, to date a limited number of studies have examined the effect of social isolation on glutamatergic neurotransmission. Initial studies have found decreased metabotropic glutamate receptor subtypes 1 and 5 (mGluR1 and mGluR5) protein in the dorsal PFC among juvenile male rats linked to cognitive deficits [16]. In contrast, increased mGluR6 and AMPA glutamate receptor subunits 3 (GluR3) have been identified in the medial PFC of isolation-reared male rats [17].
Often considered to have greater salience for males of the species, the present study establishes the importance of isolation rearing and the social play period for female rats [18]. Using a series of well-established behavioral tests, we found that isolation reared female rats had impairments in response to novel environments and social interaction behaviors. Furthermore, biochemical studies showed down-regulation of post-synaptic (PSD95) and pre-synaptic (Synapsin 1) markers and glutamate receptor expression, including AMPA receptor subunit GluR1 and NMDA receptor subunit NR1 in the prefrontal cortex.
2.0 Methods
2.1 Subjects
Pregnant Sprague-Dawley dams were purchased from Charles River Laboratories. At PND 19, the beginning of the juvenile play period, female pups were weaned and assigned to group (N=8, two or three animals per cage) versus isolation housing (N=8). Animals were housed in clear plastic bottom cages (25.9 cm × 47.6 cm × 20.9 cm) under a 12h standard light/dark cycle with free access to food and water except during tests of novelty suppressed feeding. Animal use procedures were in accordance with the Yale University Care and Use of Laboratory Animals' Guidelines.
2.2 Experimental Design
The isolated female rats were able to see, smell and hear conspecifics in the shared colony room but were without touch and the affiliative context of group housing. Animals were left in these conditions until PND 70 when the behavioral effects of chronic social isolation were measured using well-established behavioral assessments on consecutive days. The duration of social isolation was based on previous studies of isolation rearing [19, 20]. The behavioral tests included open field test (OFT) on PND 71, novelty suppressed feeding test (NSFT) on PND 72, social interaction test (SIT) on PND 73 (Table 1). Additional measures of physiological function, including weight and phase of estrus were included. At the end of behavioral testing, animals were sacrificed and biochemical measures of synaptic functions in prefrontal cortex (PFC) were obtained.
Table 1. Experimental Design.
| Experimental Design | Post Natal Day |
|---|---|
| Weaning, assignment to group housing vs. isolate housing | 19 |
| Open Field Testing | 71 |
| Novelty Suppressed Feeding | 72 |
| Social Interaction Test | 73 |
| Sacrifice and Tissue Harvest | 73 |
2.3 Physiological Measures: Weight and Estrus
Weight
Animal weights were recorded in grams using a standard laboratory scale. At postnatal day 70, animals weighed between 190 and 290 grams prior to behavioral testing.
Estrus
To avoid potential confounding effects of sex steroids on brain biochemistry assays, measures of ovarian cyclicity were obtained prior to sacrifice using a non-invasive method for determining hormonal status. Vaginal secretion was collected with a plastic pipette filled with normal saline (NaCl 0.9%) by inserting the tip into the rat vagina. Vaginal fluid was placed on glass slides. A different glass slide was used for each animal. One drop was collected with a clean tip from each rat. The slides were subsequently stained with hematoxylin and eosin for cellular categorization using a light microscope with 10 and 40 × objective lenses.
Cellular types from the vaginal smear of each rat were coded per standard protocol [21, 22]. A proestrus smear consisted of a preponderance of nucleated epithelial cells; an estrous smear consisted of a majority of anucleated cornified cells; a metestrus smear consisted of an equal distribution of leukocytes, cornified, and nucleated epithelial cells; and a diestrus smear consisted primarily of leukocytes.
2.4 Open Field Test
The open field test apparatus was a square, polyurethane arena (76.5 cm × 76.5 cm × 40 cm, Plexiglas) divided into sixteen identical size squares by black lines. The animal was placed in the center of the arena, and locomotion (distance traveled calculated as lines crossed), entries into the central zone and time spent in the central zone were recorded for 5 minutes. Behavioral testing took place from 1000 to 1400 h (i.e. in the light phase of the light-dark cycle). The apparatus was cleaned twice with 10% ethanol after each animal exposure.
2.5 Novelty Suppressed Feeding
The test was performed as described previously [23]. The testing apparatus consisted of an open field (76.5 cm × 76.5 cm × 40 cm, Plexiglas). Twenty-four hours before behavioral testing, animals were deprived of food in the home cage. At the time of testing, eight food pellets were placed in the center of the testing apparatus. The test began immediately after the animal was placed in the corner of the field. The latency to approach the pellet and begin feeding was recorded in minutes (maximum time, 8 min). Immediately afterward, the animal was transferred back to its home cage and the amount of food consumed in 5 min was measured [24].
2.6 Social Interaction Test
Social encounters lasted 5 min, and took place in a Plexiglas box (76.5 cm × 76.5 cm × 40 cm, Plexiglas) located in the colony room. Behavioral testing took place from 1000 to 1400 h (i.e. in the light phase of the light-dark cycle). An experimental animal belonging to one of the two groups was placed in the apparatus together with an animal from the same housing condition. Each animal was tested once. Group housed animals were tested with animals from different group cages; isolated animals were tested with other isolated animals. Social interaction initiated by either member of the pair was scored, per established protocol, resulting in a single score for each pair of rats [25]. Therefore, it was important to test two animals with identical social backgrounds so that the initiation of a social encounter was not biased by one of the pair having had a more socially enriched developmental experience. Weights of each animal were matched within 3-20% of the weight of the animal in the shared social encounter. The behavior of each pair was video-recorded using a camcorder. Social interaction was scored for 5 minutes
2.8 Western Blot Analyses
On PND 73, rats were sacrificed by rapid decapitation; brains were immediately removed and frozen on dry ice. The prefrontal cortex was dissected per established laboratory protocol [26]. Tissue samples were subsequently sonicated in protein lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 2 mM EDTA, 1 mM NaVO3, 5 mM NaF and 1× protease inhibitor cocktail). Protein concentration was determined by BCA protein assay. For Western Blotting, equal amount of proteins (10-20 μg) for each sample were loaded into 10% SDS PAGE gel for electrophoresis. Polyvinylidene difluoride (PVDF) membranes with transferred proteins were blocked with 2% BSA in PBST (PBS + 0.1% Tween-20) for 1 h and kept with antibodies for Synapsin I (Abcam), PSD95 (Millipore), GluR1 (Abcam) and NR1 (Cell Signaling) overnight at 4 °C. All antibodies were made in rabbits. The next day, blots were washed three times in PBST and incubated with horseradish peroxidase conjugated anti-rabbit secondary antibody (Vector Labs, Burlingame, CA) for 1 h. After three final washes with PBST, bands were detected using enhanced chemiluminescence (ECL). The blots were then incubated in stripping buffer (2% SDS, 100mM beta-mercaptoethanol, 50mM Tris pH 6.8) for 30 min at 50-55 °C followed by three washes with PBST. The stripped blots were kept in blocking solution for 1 h and incubated with the primary antibody directed against GAPDH for loading control. Densitometric analysis of immunoreactivity was conducted using NIH Image J software and normalized to group housed animals.
2.8 Statistical Analysis
Data were analyzed using StatView 5.0 software (SAS Institute, Cary, NC). For all experiments except the novelty-suppressed feeding test, student t-tests were performed. The significance level was set at p < 0.05 with two-tailed tests. In the novelty-suppressed feeding test, collected data was in a non-normal distribution and was therefore analyzed with a non-parametric test, the Mann–Whitney U test.
3. Results
3.1 Social Housing and Body Weight
On PND 70, despite having had a greater per animal area for locomotion, isolated female rats weighed more than group housed females, isolated female rats weighed 10% more than age matched group housed counterparts. Mean weights ± S.E.M, isolates 244.25 ± 7.28 g; group 221.13 ± 6.31 g, n = 8 per condition, t(14) = 2.40, p < 0.05. (Data not shown.)
3.2 Effects of Social Isolation on Responses to Novelty
Across three tests of unconditioned responses to potential threat, isolated rats displayed higher degrees of maladaptive inhibition including significantly more thigmotaxis, hyponeophagia, and social avoidance (Figs 1 and 2).
Figure 1. Behavioral measures of anxiety in open field and novelty suppressed feeding.

Results showing behavioral dysregulation among socially isolated animals in the open-field (A, B) and novelty suppressed feeding (C) tests. In the open field test, time in the center (A) and visits to the center were determined, and in novelty suppressed feeding, latency to feed was measured (C). Results for open field testing, are the mean ± S.E.M., n = 7 per condition, * p < 0.05 compared to control (t-test). Results for novelty suppressed feeding are the mean ± S.E.M., n=8 per condition, ** p < 0.001 compared to control (Mann Whitney U).
Figure 2. Social Interaction Test.
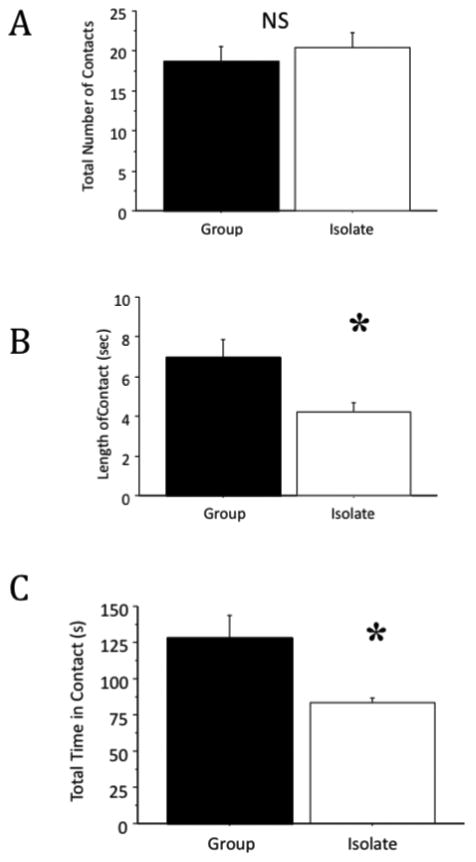
Social interaction initiated by either member of the pair was scored resulting in a single score for each pair of rats. Measures included total number of social contacts (A), average length of time/contact (B), and total time in contact (C) across the testing period. Isolated rats spent 40% less time in social behaviors than group housed animals. Results are the mean ± S.E.M., n = 8 per condition, * p < 0.05 compared to control (t-test).
Open Field Testing
In this measure of exploration stress, socially isolated animals made fifty percent fewer trips to the center of the arena than group housed animals, t(14) = 2.71, p < 0.05. As compared to group housed animals, isolates also spent less than half the time in the center of the arena, t(14) = 2.74, p < 0.05 (Fig. 1 A-B). Independent t-test demonstrated that isolation-reared rats had a significantly higher number of crossings, (isolate crossings, 104.86 ± 5.93; group crossings, 87.86 ± 5.3, t(14) = 2.14, p < 0.05), showing the typical hyperactivity to novelty environment observed in isolation-reared rats.
Novelty Suppressed Feeding
Socially isolated rats had a two-fold increase over group housed rats in latency to feed in NSFT. Median latencies of group housed and isolated rats were 174 (s) and 417 (s); the distributions in the latencies of the two groups differed as well. A non-parametric statistical test was used to determine statistical significance in the latencies across housing, (Mann-Whitney U prime = 64, n=8 in each condition, p < 0.001) (Fig. 1 C). In the home cage feeding control test, group housed animals consumed more food than socially isolated (isolate consumption, 1.25 g ± 0.21; group consumption 2.25 ± 0.05, t(16)=5.27, p < 0.005). This measure was confounded by hoarding behaviors among socially isolated animals on return to the home cage. Hoarding behavior/caching of food in socially isolated rats is an established finding [3].
Social Interaction
Measures included total number of contacts, average length of time/contact, and total time in contact. We did not observe significant differences across housing condition in total number of social contacts (t(6) = 0.68, p = 0.53). However, in measures of discrete social contact and overall time spent in contact during the five minute testing phase, significant differences across housing were observed (length of discrete social contacts; t(6) = 2.64, p < 0.05), and overall time spent in contact during the five minute testing phase; t(6) = 2.71, p < 0.05) (Fig. 2 A-C).
3.3 Measures of Synaptic Proteins in Pre-Frontal Cortex
At the conclusion of behavioral testing, animals were sacrificed and biochemical measures of prefrontal cortex (PFC) synaptic function were obtained. These measures included western blot analysis of synaptic-associated proteins; including synaptic markers PSD95 and Synapsin I, as well as glutamate receptor subtypes NR1 and GluR1. The results revealed lower levels of all measured synaptic-associated proteins in the PFC of isolated animals: Synapsin 1 (t(6) = 4.42, p < 0.01); PSD95, (t(6) = 4.13, p < 0.01); GluR1, t(6) = 7.01, p < 0.001); NR1, (t(6) = 6.59, p < 0.01) (Fig. 3 A-B).
Figure 3. Biochemical measures of synaptic proteins in the prefrontal cortex.
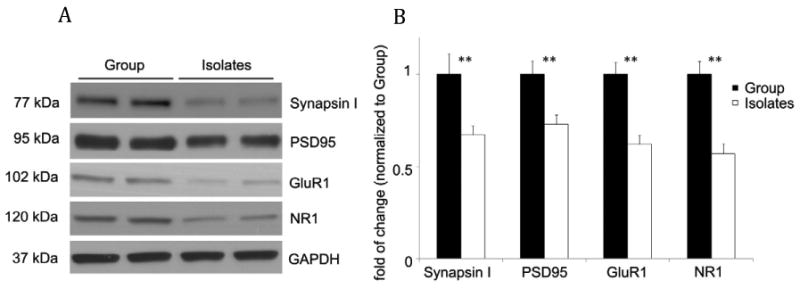
Protein blotting revealed significantly lower levels of all measured glutamate receptor subtypes (NR1 and GluR1) synaptic-associated (synapsin I and PSD95) proteins in the PFC of isolated females. Levels of GAPDH were determined as a control for Western Blot analysis. Results are the mean ± S.E.M, n = 6, 3 per housing condition and counterbalanced hormonal condition (estrus and proestrus) * p < 0.05 compared to control (t-test).
3.4 Ovarian Steroids
Estradiol has been shown to have potent effects on synaptogenesis in the hippocampus and other brain regions [27-29]. The effect of ovarian steroids on synaptic proteins in the prefrontal cortex is not known. In order to control for potential confounding effects, categorization of each vaginal smear from animals in this study revealed a subset of animals in estrus or proestrus. Animals in proestrus or estrus were included in the brain based biochemical studies and were counterbalanced in equal numbers across housing conditions.
4.0 Discussion
4.1 Social Isolation and Maladaptive Responses to Diverse Stimuli
The present work demonstrates the importance of the juvenile period for neural development, adaptive responses to environmental stimuli and sociality. While previous research has shown that brief periods of social isolation can be used to enhance social play and social interaction in rats [5], results of this study show that protracted social isolation from the juvenile period through young adulthood results in a profound disruption of natural forms of social behaviors. Isolated female rats had social encounters, which were limited in length and therefore attenuated in the kind of social complexity observed in rats with normal juvenile development. This kind of asociality is reminiscent of the social disconnectedness observed in psychotic illness in humans.
In at least one respect, current findings represent a significant departure from established findings among isolated male rats, which have shown increased sociality in the social interaction test. These findings appear to be secondary to increased aggression in male rats [30]. Several reports on play and sociality among rats suggest that aggressive behavior along with quantity of social contacts is hormonally driven. Indeed, female rats that have been androgenized are more socially aggressive [31-33]. Conversely, male rats exposed to androgen antagonists during the social play period are less likely to engage in social interactions [34, 35]. In our hands, no aggressive behavior among socially isolated or group housed females was observed during the social interaction test; the absence of aggression no doubt contributes to these findings.
Whether social isolation exerts anxiogenic effects in isolated rodents has also been a source of controversy. A comprehensive review of work in the area shows inconsistent results across research groups even using the same tests of anxiety such as elevated plus maze, novelty testing, and cage escape latency [2]. In the current study, behavioral measures including OFT and NSFT, suggested that chronic social isolation was anxiogenic for female rats. In particular, NSFT, a highly validated test of anxiety, one that is sensitive to anxiolytic treatment [36] showed significantly increased latencies among isolated animals with concomitant hoarding (food caching) tendencies.
4.2 Psychosocial Neglect and Physiological Regulation
Housing in this study allowed for greater cage area per rat among isolates than group housed animals. The absence of conspecifics appears to be a necessary condition for the evolution of social play but also for the vigorous, energy intensive activity needed for weight regulation during the juvenile period. Our report of weight differences across housing is consistent with an early report on hyperphagy in isolated rodents [37]. The significant difference in weight across housing conditions raises a question as to whether the critical period for social play overlaps with a newly defined sensitive period for eating behavior and leptin sensitivity in rodents [38], and if so whether the presence of conspecifics facilitates or inhibits feeding during this sensitive period. Of additional and related importance is the potential effect of these weight differences on neural development. Recent studies in humans have shown an association between aerobic fitness, hippocampal volume, and memory performance in pre-adolescent children [39]. The effect of excess weight on the prefrontal cortex of rodents is not known but cannot be dismissed as a potential contributing factor to the neurochemical differences observed in the present study.
4.3 Effects of Social-Rearing on Synaptic Function in the PFC
Earlier work examining the effects of social-rearing on glutamatergic function in has shown that isolation rearing decreases glutamate NMDA receptor subunit 1A (NR1A) mRNA expression in the striatum and prefrontal cortex [40]. More recently, Zhao and colleagues found that NR2A mRNA was down-regulated in the prefrontal cortex as well [19]. Consistent with those studies, we have shown that social isolation remarkably decreased both NMDA receptor subunit NR1and AMPA receptor subunit GluR1 in the PFC in female rats.
Glutamate is an important mediator of synaptic plasticity. Synaptic-associate proteins, which serve a scaffolding function for AMPA and NMDA receptors at the synapse, play a critical role in signal transduction during brain maturation [41]. Previous studies showed that isolation rearing could affect synaptic plasticity in adult animals. Synaptophysin, a pre-synaptic marker, is significantly down-regulated in hippocampus in response to social isolation [42]. The current results further corroborate and extend the findings to both pre- and post-synaptic markers (Synapsin I and PSD95, respectively). Isolation rearing induced impairment of synaptogenesis in PFC and other key brain regions that control emotion and/or cognition such as hippocampus, could potentially account for behavioral dysregulations observed in adult animals. In support of this hypothesis, recent studies from Bianchi and colleagues shows that social isolation decreases the expression of a neuronal dendritic marker microtubule associated protein-2 (MAP-2) in hippocampus, which strongly correlate with deficits in memory performance [43].
4.4 The Importance of Female Models of Developmental Psychopathology
There is a dearth of literature on female rodents in neurobehavioral modeling of developmental psychopathology in the context of early life stress. In the absence of a body of work on female rodents, researchers are left to generalize across sex in rodents (what applies to male rats must apply to females) and to the extent that preclinical studies apply to humans, generalize from male preclinical models to human males and females. The importance of doing female work is to establish whether what has been found in male rats applies to female rats and whether there are important differences across sexes that have relevance for clinical settings and ultimately intervention and treatment.
5.0 Summary
Results of this study contribute additional insights into the neurodevelopmental consequences of early life stress and chronic adversity. Specifically, sustained early life exposure to social isolation for female rats had significant impact on behavior and brain, including the ability to respond to novelty, patterns of sociality, and synaptic plasticity. Ongoing studies are being undertaken to further confirm the present results and to provide additional insights into the underlying neurobiological mechanism of this isolation syndrome. The behavioral dysregulation and impairments in PFC neurochemistry seen in this study have translational value for a host of neuropsychiatric disorders and could ultimately help in screening novel medications for those diseases.
Acknowledgments
Research supported by NIMH 5T32 MH-19961, Clinical Neuroscience Research Training in Psychiatry to Yale University, The Hope for Depression Research Foundation, New York, NY, and the Department of Psychiatry and the American Psychiatric Institute for Research and Education/Janssen Resident Psychiatric Research Scholars Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Einon DF, Morgan MJ. A critical period for social isolation in the rat. Dev Psychobiol. 1977;10(2):123–32. doi: 10.1002/dev.420100205. [DOI] [PubMed] [Google Scholar]
- 2.Fone KC, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev. 2008;32(6):1087–102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Heidbreder CA, et al. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience. 2000;100(4):749–68. doi: 10.1016/s0306-4522(00)00336-5. [DOI] [PubMed] [Google Scholar]
- 4.Weiss IC, et al. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res. 2004;152(2):279–95. doi: 10.1016/j.bbr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Panksepp J. The ontogeny of play in rats. Dev Psychobiol. 1981;14(4):327–32. doi: 10.1002/dev.420140405. [DOI] [PubMed] [Google Scholar]
- 6.Pellis SM, Pellis VC. The prejuvenile onset of play fighting in laboratory rats (Rattus norvegicus) Dev Psychobiol. 1997;31(3):193–205. [PubMed] [Google Scholar]
- 7.Hol T, et al. Isolation during the play period in infancy decreases adult social interactions in rats. Behav Brain Res. 1999;100(1-2):91–7. doi: 10.1016/s0166-4328(98)00116-8. [DOI] [PubMed] [Google Scholar]
- 8.van den Berg CL, et al. Play is indispensable for an adequate development of coping with social challenges in the rat. Dev Psychobiol. 1999;34(2):129–38. [PubMed] [Google Scholar]
- 9.Vanderschuren LJ, Niesink RJ, Van Ree JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21(3):309–26. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- 10.Klein ZA, Padow VA, Romeo RD. The effects of stress on play and home cage behaviors in adolescent male rats. Dev Psychobiol. 2010;52(1):62–70. doi: 10.1002/dev.20413. [DOI] [PubMed] [Google Scholar]
- 11.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 12.Eslinger PJ, Flaherty-Craig CV, Benton AL. Developmental outcomes after early prefrontal cortex damage. Brain Cogn. 2004;55(1):84–103. doi: 10.1016/S0278-2626(03)00281-1. [DOI] [PubMed] [Google Scholar]
- 13.Anderson SW, et al. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat Neurosci. 1999;2(11):1032–7. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- 14.Day-Wilson KM, et al. Medial prefrontal cortex volume loss in rats with isolation rearing-induced deficits in prepulse inhibition of acoustic startle. Neuroscience. 2006;141(3):1113–21. doi: 10.1016/j.neuroscience.2006.04.048. [DOI] [PubMed] [Google Scholar]
- 15.Schneider M, Koch M. Deficient social and play behavior in juvenile and adult rats after neonatal cortical lesion: effects of chronic pubertal cannabinoid treatment. Neuropsychopharmacology. 2005;30(5):944–57. doi: 10.1038/sj.npp.1300634. [DOI] [PubMed] [Google Scholar]
- 16.Melendez RI, et al. Impoverished rearing environment alters metabotropic glutamate receptor expression and function in the prefrontal cortex. Neuropsychopharmacology. 2004;29(11):1980–7. doi: 10.1038/sj.npp.1300507. [DOI] [PubMed] [Google Scholar]
- 17.Levine JB, et al. Isolation rearing and hyperlocomotion are associated with reduced immediate early gene expression levels in the medial prefrontal cortex. Neuroscience. 2007;145(1):42–55. doi: 10.1016/j.neuroscience.2006.11.063. [DOI] [PubMed] [Google Scholar]
- 18.Auger AP, Olesen KM. Brain sex differences and the organisation of juvenile social play behaviour. J Neuroendocrinol. 2009;21(6):519–25. doi: 10.1111/j.1365-2826.2009.01871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao X, et al. Isolation rearing induces social and emotional function abnormalities and alters glutamate and neurodevelopment-related gene expression in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(7):1173–7. doi: 10.1016/j.pnpbp.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Li N, et al. Auditory fear conditioning modulates prepulse inhibition in socially reared rats and isolation-reared rats. Behav Neurosci. 2008;122(1):107–18. doi: 10.1037/0735-7044.122.1.107. [DOI] [PubMed] [Google Scholar]
- 21.Long JA, Evans HM. (Mem Univ Calif).The oestrous cycle in the rat and its associated phenomena. 1922;6:1–148. [Google Scholar]
- 22.Mandl AM. The phases of the estrous cycle in the adult white rat. Journal Experimental Biology. 1951;28:576–584. [Google Scholar]
- 23.Warner-Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci U S A. 2007;104(11):4647–52. doi: 10.1073/pnas.0610282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–9. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 25.File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463(1-3):35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- 26.Li N, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–64. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McEwen BS, et al. Ovarian steroids and the brain: implications for cognition and aging. Neurology. 1997;48(5 Suppl 7):S8–15. doi: 10.1212/wnl.48.5_suppl_7.8s. [DOI] [PubMed] [Google Scholar]
- 28.McEwen B, A K, Alves S, Brake WG, Bulloch K, Lee S, Li C, Yuen G, Milner TA. Tracking the estrogen receptor in neurons: implications for estrogen-induced synapse formation. Proc Natl Acad Sci. 2001;98(13):7093–100. doi: 10.1073/pnas.121146898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jelks KB, W R, Floyd CL, McAllister AK, Wise P. Estradiol targets synaptic proteins to induce glutamatergic synapse formation in cultured hippocampal neurons: critical role of estrogen receptor-alpha. J Neurosci. 2007;27(26):6903–13. doi: 10.1523/JNEUROSCI.0909-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng Q, et al. Peri-adolescence isolation rearing alters social behavior and nociception in rats. Neurosci Lett. 2010;480(1):25–9. doi: 10.1016/j.neulet.2010.05.067. [DOI] [PubMed] [Google Scholar]
- 31.Thor DH, Holloway WR., Jr Social play soliciting by male and female juvenile rats: effects of neonatal androgenization and sex of cagemates. Behav Neurosci. 1986;100(2):275–9. doi: 10.1037//0735-7044.100.2.275. [DOI] [PubMed] [Google Scholar]
- 32.Meaney MJ, McEwen BS. Testosterone implants into the amygdala during the neonatal period masculinize the social play of juvenile female rats. Brain Res. 1986;398(2):324–8. doi: 10.1016/0006-8993(86)91492-7. [DOI] [PubMed] [Google Scholar]
- 33.Tonjes R, Docke F, Dorner G. Effects of neonatal intracerebral implantation of sex steroids on sexual behaviour, social play behaviour and gonadotrophin secretion. Exp Clin Endocrinol. 1987;90(3):257–63. doi: 10.1055/s-0029-1210699. [DOI] [PubMed] [Google Scholar]
- 34.Casto JM, Ward OB, Bartke A. Play, copulation, anatomy, and testosterone in gonadally intact male rats prenatally exposed to flutamide. Physiol Behav. 2003;79(4-5):633–41. doi: 10.1016/s0031-9384(03)00120-3. [DOI] [PubMed] [Google Scholar]
- 35.Hotchkiss AK, et al. An environmental antiandrogen, vinclozolin, alters the organization of play behavior. Physiol Behav. 2003;79(2):151–6. doi: 10.1016/s0031-9384(03)00093-3. [DOI] [PubMed] [Google Scholar]
- 36.Bodnoff SR, et al. A comparison of the effects of diazepam versus several typical and atypical anti-depressant drugs in an animal model of anxiety. Psychopharmacology (Berl) 1989;97(2):277–9. doi: 10.1007/BF00442264. [DOI] [PubMed] [Google Scholar]
- 37.Shelley HP. Eating behavior: social facilitation or social inhibition? Psychonomic Sci. 1965;3:521–522. [Google Scholar]
- 38.Mainardi M, et al. A sensitive period for environmental regulation of eating behavior and leptin sensitivity. Proc Natl Acad Sci U S A. 2010;107(38):16673–8. doi: 10.1073/pnas.0911832107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaddock L, et al. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res. 2010;1358:172–83. doi: 10.1016/j.brainres.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hall FS, et al. The effects of isolation rearing on glutamate receptor NMDAR1A mRNA expression determined by in situ hybridization in Fawn hooded and Wistar rats. Pharmacol Biochem Behav. 2002;73(1):185–91. doi: 10.1016/s0091-3057(02)00796-7. [DOI] [PubMed] [Google Scholar]
- 41.Sans N, et al. A developmental change in NMDA receptor-associated proteins at hippocampal synapses. J Neurosci. 2000;20(3):1260–71. doi: 10.1523/JNEUROSCI.20-03-01260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varty GB, Marsden CA, Higgins GA. Reduced synaptophysin immunoreactivity in the dentate gyrus of prepulse inhibition-impaired isolation-reared rats. Brain Res. 1999;824(2):197–203. doi: 10.1016/s0006-8993(99)01173-7. [DOI] [PubMed] [Google Scholar]
- 43.Bianchi M, et al. Chronic fluoxetine differentially modulates the hippocampal microtubular and serotonergic system in grouped and isolation reared rats. Eur Neuropsychopharmacol. 2009;19(11):778–90. doi: 10.1016/j.euroneuro.2009.06.005. [DOI] [PubMed] [Google Scholar]


