Abstract
This study examined adult age differences in reflexive orienting to two types of uninformative spatial cues: central arrows and peripheral onsets. In two experiments using a Posner cuing task, young adults (ages 18 – 28 yrs), young-old adults (ages 60 – 74 yrs), and old-old adults (ages 75 – 92 yrs) responded to targets that were preceded 100–1,000 ms earlier by a central arrow or a peripheral abrupt onset. In Experiment 1, the cue remained present upon target onset. Facilitation effects at early cue-target stimulus onset asynchronies (SOAs) were prolonged in duration for the two older groups relative to the young adults. At later cue-target SOAs, inhibition of return (IOR) that was initiated by peripheral onset cues was observed in the performance of young adults but not in that of the two older groups. In Experiment 2, the cue was presented briefly and removed prior to target onset. The change in cue duration minimized age differences (particularly for young-old adults) in facilitation effects and led to IOR for all three age groups. The findings are consistent with the idea that attentional control settings change with age, with higher settings for older adults leading to delayed disengagement from spatial cues.
Keywords: aging, attention, spatial orienting, young-old and old-old, attentional control settings
Responding quickly to events in a complex visual world often requires rapid shifts of spatial attention. An attentional shift is reflexive when it cannot be prevented, even when the external stimulus that elicits the shift does not provide information regarding the likely location of a desired item. It was originally thought that reflexive orienting occurred only toward stimuli that appeared or changed at a location peripheral to an observer’s direct line of sight. Reflexive orienting is now known to also occur for certain uninformative central stimuli (e.g., Ristic, Friesen, & Kingstone, 2002). In this study, we examined age-related changes in the time course of reflexive spatial orienting to both central arrows and peripheral onset cues. It has been proposed that with age, attentional control settings that regulate reflexive orienting are altered (Klein, 2000). We examined how these changes affect the time course with which older adults reflexively orient to uninformative peripheral and central visual information.
Volitional versus Reflexive Orienting
In the Posner cuing paradigm (Posner & Cohen, 1984), which has been widely used to study spatial orienting, a central fixation point is flanked by visual markers (e.g., two boxes) that indicate potential target locations. One of two types of cues has traditionally been presented: an arrow cue at central fixation that points to one of the potential target locations, or a sudden onset cue at one of the peripheral locations (e.g., a brief highlighting of the outline of one of the boxes). A target is presented shortly after the cue, to which participants make a speeded detection (e.g., press a button as soon as the target is detected) or discrimination (e.g., press a button to indicate the location of the target) response. In early research, central arrows were informative (the target more often appeared at the location indicated by the arrow than at an uncued location) and peripheral cues were uninformative (the target was just as likely to appear at an uncued location as at the cued location). Both types of cues were effective in orienting attention. Observers were faster to respond to targets that were presented at cued locations (validly-cued targets) than to targets presented at uncued locations (invalidly-cued targets). This facilitation effect was attributed to a “spotlight” of attention (Posner & Cohen, 1984). The cue caused the spotlight to be directed toward the cued location, and once attention was engaged there, a target was detected efficiently if it fell under the attentional spotlight. If the target was presented at an uncued location, responses were slowed because attention had to be disengaged from the cued location and the spotlight had to be moved to the target location.
Temporal patterns of orienting were found to vary for the two types of cue (for a review, see Klein, Kingstone, & Pontefract, 1992). With informative central arrow cues, facilitation effects were maximal at approximately 300 ms and were maintained at longer intervals. In contrast, facilitation effects developed rapidly with uninformative peripheral cues (as early as 50 to 100 ms), but were replaced by inhibitory effects at approximately 300 ms, with slower responses to validly-cued targets than to invalidly-cued targets. The contrast in cuing patterns was interpreted to reflect volitional orienting in response to informative central cues and reflexive orienting toward uninformative peripheral cues (Jonides, 1981). With central arrows, observers voluntarily directed their attention to the cued location based on their knowledge that the arrows were predictive of the target’s likely location. Because observers expected the target to appear at the cued location, attention was not quickly withdrawn from cued locations, leading to prolonged facilitation effects. Peripheral cues were thought to summon attention automatically (i.e., attention was reflexively drawn to stimuli moving or appearing in the periphery), leading to facilitation effects at short cue-target intervals. At longer intervals, when the target did not immediately appear at the cued location, attention returned to central fixation because there was no expectation that the target would appear at the cued location. If the target did subsequently appear at the cued location, attention was slower to return because of a bias to explore new locations and to avoid reinspecting previous locations. The resulting slowing in reaction times was termed inhibition of return (IOR; Posner & Cohen, 1984; see Klein, 2000 for a review).
Contrary to early assumptions (e.g., Jonides, 1981), spatial orienting that is guided by central arrow cues can be reflexive as well as voluntary. It has recently been demonstrated that even when an arrow cue is not informative, it can facilitate target detection (e.g., Hommel, Pratt, Colzato, & Godijn, 2001; Kingstone, Smilek, Ristic, Friesen, & Eastwood, 2003; Ristic et al., 2002; Tipples, 2002). Like the orienting pattern identified with peripheral cues, facilitation effects resulting from uninformative central arrows have been observed at short cue-target intervals. In contrast to peripheral cues, inhibitory effects are not observed at longer intervals, consistent with the idea that IOR is linked to the eye movement system, which is activated by a visual transient in the periphery. Because these orienting effects arise early and occur despite the fact that the arrows are not predictive of the likely target location, they can be viewed as reflexive or automatic. Thus, the orienting effects observed with the informative arrows that were used in the past as a measure of volitional orienting likely represented some combination of reflexive and volitional orienting (Ristic & Kingstone, 2006).
Adult Age Differences in Visuospatial Orienting
Arrow cues
Age differences in orienting to arrow cues have been studied with informative arrows only. Older adults’ attention is effectively guided by arrows that predict the likely location of a target. In fact, facilitation effects for older adults are similar in magnitude to those of young adults (Curran, Hills, Patterson, & Strauss, 2001; Tales, Muir, Bayer, & Snowden, 2002; Tellinghuisen, Zimba, & Robin, 1996; Yamaguchi, Tsuchiya, & Kobayashi, 1995) or are even greater in magnitude (Folk & Hoyer, 1992; Hartley, Kieley, & Slabach, 1990; Lincourt, Folk, & Hoyer, 1997; Nissen & Corkin, 1985). Greater facilitation effects have been attributed to either general slowing on the part of older adults (Lincourt et al., 1997) or to age-related difficulties in cue encoding (Folk & Hoyer, 1992). In addition, the time course of facilitation effects tends to be similar for the two age groups (Folk & Hoyer, 1992; Lincourt et al., 1997; Yamaguchi et al., 1995). Typically, facilitation effects for both age groups are minimal at cue-target intervals of 50 ms or less but are clearly evident at stimulus onset asynchronies (SOAs) of 250 ms and longer (up to 3,000 ms), with no indication of IOR at later intervals. In some cases, facilitation effects emerge earlier for older adults relative to young adults (e.g., Hartley et al., 1990) and in other cases, later (e.g., Brodeur & Enns, 1997).
Peripheral cues
The timing of reflexive orienting to uninformative peripheral cues changes with age. Relative to young adults, older adults tend to show extended and enhanced cue facilitation at early cue-target intervals (e.g., 50–200 ms; Brodeur & Enns, 1997; Castel, Chasteen, Scialfa, & Pratt, 2003; Lincourt et al., 1997) and delayed IOR at later intervals (Brodeur & Enns, 1997; Castel et al., 2003), which may be due to older adults having greater difficulty disengaging attention from cues (Castel et al., 1997). When peripheral cues are informative (predictive or counterpredictive), the cuing effects of young and older adults are consistent with rapid and automatic orienting at short cue-target intervals (facilitated responding to cued locations, even when the cue predicts that the target will appear elsewhere; Faust & Balota, 1997; Hartley & Kieley, 1995, Hartley et al., 1990; Yamaguchi et al., 1995).
Visuospatial Orienting for Young-Old and Old-Old Adults
Orienting processes continue to change late in life, at least when the task is sufficiently challenging (Greenwood & Parasuraman, 1994; Greenwood, Parasuraman, & Haxby, 1993). With a simple target detection task, adults across a wide age range (17–85 years) show similar orienting patterns in response to informative arrow cues (Greenwood & Parasuraman, 1994; Greenwood et al., 1993). However, when completing a letter discrimination task, facilitation effects for old-old adults (ages 75–84 years) are greater than those for young-old adults (ages 65–74 years). The age difference appears to be due largely to slower disengagement from an invalid cue rather than faster engagement of a valid cue. Orienting to noninformative cues has not yet been investigated in old-old adults.
Attentional Control Settings
To summarize, both young adults and older adults orient in response to peripheral onset cues and arrow cues.. When orienting to peripheral onset cues, older adults tend to show greater facilitation than young adults do at early cue-target intervals. Furthermore, older adults maintain facilitation longer and demonstrate a delayed onset of IOR relative to young adults. Volitional orienting to informative central arrows is unchanged with age, or if anything, older adults are more sensitive to cue information than young adults (as demonstrated by greater facilitation effects). Further age enhancements in facilitation effects are observed for adults over the age of 75, but only when the task is sufficiently difficult. However, reflexive orienting to noninformative central arrows has yet to be studied in older adults.
To interpret the pattern of findings described above, we turn to the attentional control setting (ACS) theory (Folk, Remington, & Johnston, 1992). According to the theory, when a task is more difficult, people allocate more attentional resources for longer periods of time than when the task is simpler. Specifically, the observer sets the level of attention to be allocated to a target based on task difficulty (i.e., low intensity for a simple task; high intensity for a difficult task). The attentional control settings cannot be changed fluidly, so the level put in place to process the target will also apply to the cue. The higher the control setting, the more strongly attention is directed to a cue, leading to extended facilitation and (in the case of peripheral cues) later emergence of IOR. Klein (2000) proposed that the ACS theory could account for task differences and individual differences in the time course of attentional orienting. For example, the ACS theory can account for the later emergence of IOR on discrimination tasks relative to detection tasks (Klein, 2000; Lupiáñez, Milliken, Solano, Weaver, & Tipper, 2001), because greater attention is required for discrimination than for detection. It is also consistent with findings that individuals with poorer attentional control, such as young children (MacPherson, Klein, & Moore, 2003) and individuals with schizophrenia (Huey & Wexler, 1994; Sapir, Henik, Dobrusin, & Hochman, 2001; Spencer et al., 2011), show enhanced facilitation and delayed IOR relative to their respective comparison groups (older children and adult controls).
Older adults, like young children, might apply different attentional control settings to spatial orienting tasks (Klein, 2005). Because the target processing task becomes more difficult with age, older adults may set their control settings at a higher level than young adults do. With greater attention to the cue, older adults would be slower to disengage from the cued location, and thus demonstrate greater and longer-lasting benefits of a valid cue and, with uninformative peripheral cues, later onset of IOR. The age pattern predicted by ACS theory is consistent with older adults’ orienting patterns summarized above.
Does the ACS theory make similar predictions for the orienting patterns resulting from central cues as it does for peripheral cues? It depends on whether the control settings affect engagement and disengagement with the cue itself or with the location indicated by the cue. If a higher setting leads to greater engagement with the cue, then it would seem that engagement of the central arrow would lead to reduced orienting to the cued location. But if the result of a higher control setting is greater attention to the location indicated by the cue (an enhanced orienting effect), then facilitation effects would be enhanced. We propose that attentional control settings impact how strongly observers react to directional information provided by the cue rather than how long attention dwells on the cue, which leads to the prediction that age-related increases in facilitation effects will be observed for both central arrows and peripheral onset cues.
The Present Study
The purpose of the present study was to examine age differences in reflexive orienting to uninformative peripheral onset cues and uninformative central arrow cues. We assessed orienting performance in young adults (ages 18–30 yrs), young-old adults (ages 60–74 yrs), and old-old adults (ages 75+ yrs). If the ACS theory can account for age differences in reflexive orienting, we would expect that both groups of older adults would show facilitation effects of greater magnitude and longer duration compared to younger adults, for both peripheral and central cues. If old-old adults find the target task (location discrimination) particularly difficult, they should implement an even higher control setting than young-old adults, leading this group to have even stronger and longer-lasting facilitation effects. For peripheral cues, the two older groups should demonstrate a later onset of IOR as compared to young adults due to delayed disengagement from the cue. We used a two-location Posner cuing task with a variable cue-target SOA and a location discrimination response. To examine the impact of cue duration on age differences in reflexive orienting, the cue remained on the screen during target presentation in Experiment 1 and was removed prior to target presentation in Experiment 2. If age was associated with difficulty in cue disengagement, age effects were expected to be accentuated for persistent cues.
Experiment 1
The purpose of Experiment 1 was to determine whether reflexive orienting patterns for young adults, young-old adults, and old-old adults would be consistent with age differences predicted by ACS theory. We expected that both peripheral onset cues and central arrow cues would lead to facilitation effects at a cue-target SOA of 100 ms, consistent with reflexive orienting (e.g., Hommel et al., 2001; Klein, 2000; Posner & Cohen, 1984; Ristic et al., 2002; Tipples, 2002). At later intervals (SOAs of 600 ms and 1,000 ms), facilitation effects would resolve for central arrow cues because the arrows would not predict target location (Ristic et al., 2002). Facilitation would turn to IOR for peripheral onset cues (which activate eye movement systems) to bias attention to new locations (Klein, 2000; Posner & Cohen, 1984). Because the location discrimination decision required of the target would be more difficult for older adults than for young adults, we predicted that the orienting performance of the two older groups (young-old and old-old) would reflect greater and longer-lasting facilitation effects (for both peripheral and central cues) and delayed onset of inhibitory effects (for peripheral cues), as the result of a higher attentional control setting that would lead to enhanced processing of cue information.
Method
Participants
Thirty-four young adults (18–28 yrs; 22 women, 12 men), 34 young-old adults (61–73 yrs; 22 women, 12 men), and 34 old-old adults (75–92 yrs; 22 women, 12 men) participated in Experiment 1. Young adults were recruited from psychology courses and received course credit. Older adults were recruited from the Fargo-Moorhead community and received $10/hour for participating. All individuals had at least a high school education and were native English speakers. Participants had corrected near visual acuity of 20/40 or better as assessed by a Snellen eye chart (Precision Vision, La Salle, IL) and were free from serious medical conditions (e.g., cancer, stroke, dementia, or drug and alcohol abuse) according to self-report (Christensen, Moye, Armson, & Kern, 1992). All included participants scored 9 points or lower on the Geriatric Depression Scale (GDS; Yesavage et al., 1982), indicating minimal depressive symptoms, and 26 points or higher on the Mini-Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975), indicating no demonstrable signs of significant cognitive impairment. Demographic and screening data for included participants are provided in Table 1.
Table 1.
Participant Characteristics for Experiments 1 and 2
| Experiment 1
|
Experiment 2
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean
|
SD
|
Mean
|
SD
|
|||||||||
| YA | YO | OO | YA | YO | OO | YA | YO | OO | YA | YO | OO | |
| Age (yrs) | 20.7* | 67.0 | 79.3* | 2.4 | 3.7 | 3.8 | 20.3* | 67.3 | 79.2* | 2.4 | 4.1 | 3.0 |
| Education (yrs) | 14.1 | 15.5 | 15.1 | 1.4 | 3.3 | 2.8 | 14.0 | 14.9 | 14.8 | 1.3 | 2.4 | 2.4 |
| GDS (30 max) | 2.2 | 1.3 | 1.4 | 2.4 | 1.4 | 2.2 | 1.5 | 1.7 | 2.1 | 2.1 | 2.3 | 2.0 |
| WASI Vocab. (80 max) | 58.8* | 68.0 | 66.3 | 6.4 | 7.5 | 9.0 | 58.5* | 65.0 | 64.0 | 5.4 | 8.0 | 6.5 |
| Snellen acuity (20/___) | 16.1* | 22.8 | 24.9 | 4.4 | 6.7 | 6.6 | 15.9* | 24.7 | 25.8 | 4.1 | 6.6 | 7.0 |
| MMSE (30 max) | 29.0 | 29.4 | 29.1 | 1.0 | 0.9 | 0.8 | 29.6 | 29.4 | 29.1 | 0.7 | 1.0 | 1.0 |
Note. SD standard deviation, YA young adults, YO young-old adults, OO old-old adults.
GDS Geriatric Depression Scale. Maximum score is 30, with a higher score indicating more endorsed symptoms of depression.
WASI Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999). Maximum score on the vocabulary subscale is 80 points, with a higher score indicating better performance.
Snellen acuity denominator of the Snellen fraction for corrected near vision. A smaller number indicates better vision.
MMSE Mini Mental State Examination. Maximum score is 30 points, with a higher score indicating better performance.
mean scores differed significantly from the young-old adult group according to Student Newman Keuls t test, p < .05.
Materials and stimuli
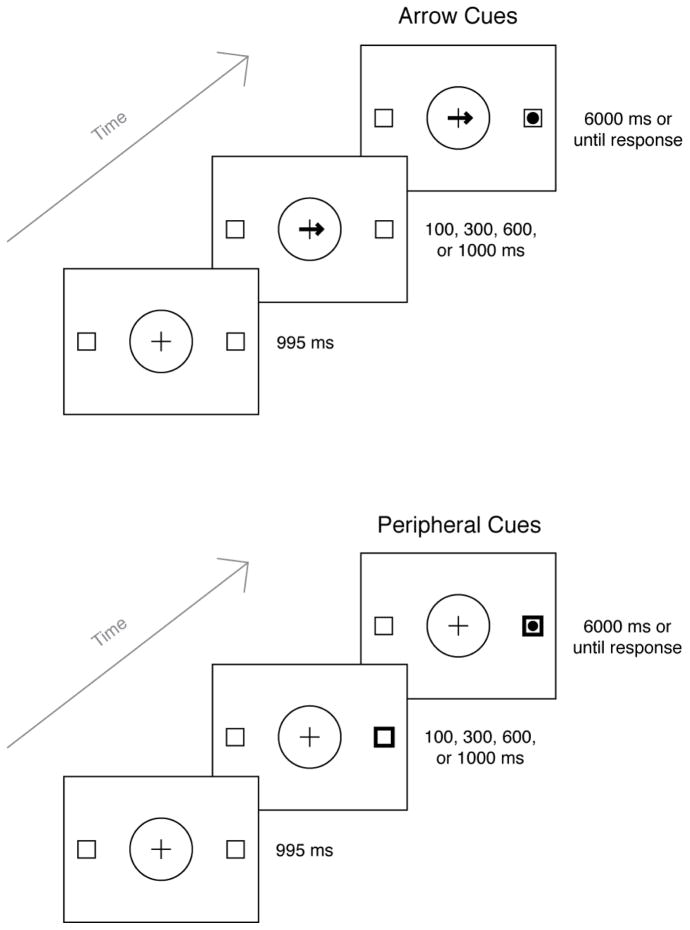
Stimuli were presented on a 17 in. color monitor (refresh rate of 85 Hz) controlled by a PC computer with a Pentium 4 processor. A chin rest maintained participants’ viewing distance at 40 cm. Participants responded to stimuli using a PST Serial Response Box (Psychology Software Tools, Pittsburgh, PA). We used E-Prime 1.1 (Psychology Software Tools) to develop and run the experiment. Stimuli were black line drawings presented against a white background. The initial fixation display consisted of a circle positioned in the middle of the monitor that subtended 14.5° of visual angle. Centered in the circle was a cross with arms that were 4.3° long and 0.1° thick. The circle was flanked to the left and right by two empty 2.9° squares. The outer edges of the squares were located 8.9° from the outer edge of the central circle. Sample stimulus displays are presented in Figure 1. The cue displays (arrow cues and peripheral cues) overlaid the fixation display. The arrow was asymmetrical, with a shaft (4.3° long) and head (1.4° high and 1.0° wide) pointing to the left or right, drawn with lines 0.4° thick. The arrow overlaid the horizontal line of the center fixation cross. The peripheral cue consisted of an empty square which superimposed one of the two outer squares. The outline of the square was thicker than that in the original display (0.6° instead of approximately 0.1°). A black filled circle, 1.7° in diameter, served as the target stimulus for both cue conditions and was presented in the center of one of the two outer squares.
Figure 1.
Trial sequence for Experiment 1. Stimuli are not scaled to size.
Design and procedure
The testing session (including consent, screening, and computer task) lasted approximately 1 ½ hours. The computer task consisted of two blocks of 80 trials for each cue type (arrow and peripheral), for a total of 320 experimental trials. Blocks alternated between arrow and peripheral cues (ABAB or BABA); whether an arrow or peripheral block was presented first was counterbalanced across participants. Before each block, participants completed eight practice trials which were the same in format as the experimental trials.
As depicted in Figure 1, a trial began with the fixation display presented for 995 ms, followed by a cue display (an arrow pointing left or right, or a thick left or right square outline). After a variable cue-target stimulus onset asynchrony (SOA; specific times are described below), the target (a black circle) appeared in either the left or right outer square. The cue and target remained on the screen for 6,000 ms or until the participant responded. Participants pressed the left or right buttons specified on the response box to indicate the target’s location.
In addition to cue type (arrow or peripheral cue), the variables of interest were cue validity and cue-target SOA. On valid trials, the target appeared at the location indicated by the cue (left or right), whereas on invalid trials the target appeared at the uncued location. The SOAs were four values close to 100, 300, 600, and 1,000 ms (as constrained by the refresh rate of the monitor): 117.6, 317.6, 611.7, and 1011.7 ms. For convenience, we will refer to these SOAs as 100, 300, 600, and 1,000 ms, respectively, throughout the remainder of the paper. All combinations of cue direction/side, target side, and SOA were selected randomly and with equal probability within each block.
The experimenter explained the task to participants using verbal instructions and a drawn representation of stimulus events. Participants were told that the direction or location of the cue (left or right) was random and would not help them predict the target location. They were instructed to rest their index fingers on two buttons of the response box and to indicate the target location with a left or right button press as quickly as possible, but not at the expense of accuracy. Participants took short rests between blocks.
Results
Mean RTs as a function of cue type, age group, cue validity, and cue-target SOA are presented in Table 2. Responses that were less than 150 ms or more than 2,000 ms were considered outliers and removed. For the remaining trials, median response times (RT) were calculated for correct responses. We conducted a 3 × 2 × 4 mixed analysis of variance (ANOVA) for each cue type, with age group (young, young-old, and old-old adults) as the between-subjects factor and cue validity (valid and invalid), and cue-target SOA (100, 300, 600, and 1,000 ms) as the within-subject factors. For all analyses, we used an alpha of .05, and for simple effects analyses of variables with more than two levels, we used Student Newman Keuls (SNK) post hoc tests to reduce experimentwise error rates.
Table 2.
Mean Reaction Times (and Standard Deviations) for Experiment 1
| SOA (ms) | Arrow Cues
|
Peripheral Cues
|
||||||
|---|---|---|---|---|---|---|---|---|
| 100 | 300 | 600 | 1,000 | 100 | 300 | 600 | 1,000 | |
| Young | ||||||||
| Valid | 383 (67) | 342 (59) | 335 (65) | 328 (57) | 395 (68) | 368 (55) | 366 (64) | 352 (65) |
| Invalid | 399 (68) | 348 (63) | 331 (62) | 339 (70) | 415 (66) | 374 (76) | 350 (75) | 343 (60) |
| Young-Old | ||||||||
| Valid | 474 (76) | 437 (65) | 404 (59) | 416 (65) | 486 (68) | 449 (63) | 436 (61) | 439 (69) |
| Invalid | 499 (74) | 464 (67) | 416 (67) | 420 (71) | 531 (71) | 480 (73) | 446 (74) | 438 (78) |
| Old-Old | ||||||||
| Valid | 523 (81) | 483 (85) | 453 (76) | 463 (96) | 544 (80) | 499 (82) | 492 (87) | 500 (94) |
| Invalid | 559 (92) | 521 (87) | 460 (97) | 455 (94) | 597 (95) | 534 (100) | 499 (107) | 496 (108) |
Reaction times are reported in milliseconds. SOA stimulus onset asynchrony.
Arrow cues
For arrow cues, all three main effects were significant: age group, F(2, 99) = 34.48, p < .0001, cue validity, F(1, 99) = 49.60, p < .0001, and cue-target SOA, F(3, 297) = 230.41, p < .0001. Old-old adults were slower to respond than young-old adults, who in turn were slower to respond than young adults (490 ms, 441 ms, and 351 ms, respectively), ps < .05. The main effect for cue validity indicated a significant cue facilitation effect; overall, participants responded more quickly to validly-cued targets (420 ms) than to invalidly-cued targets (434 ms). The main effect of SOA reflected a general decrease in RT as SOA increased (473 ms, 433 ms, 400 ms, and 404 ms for SOAs of 100 ms, 300 ms, 600 ms, and 1,000 ms, respectively), a standard foreperiod effect (see, for example, Friesen & Kingstone, 1998).
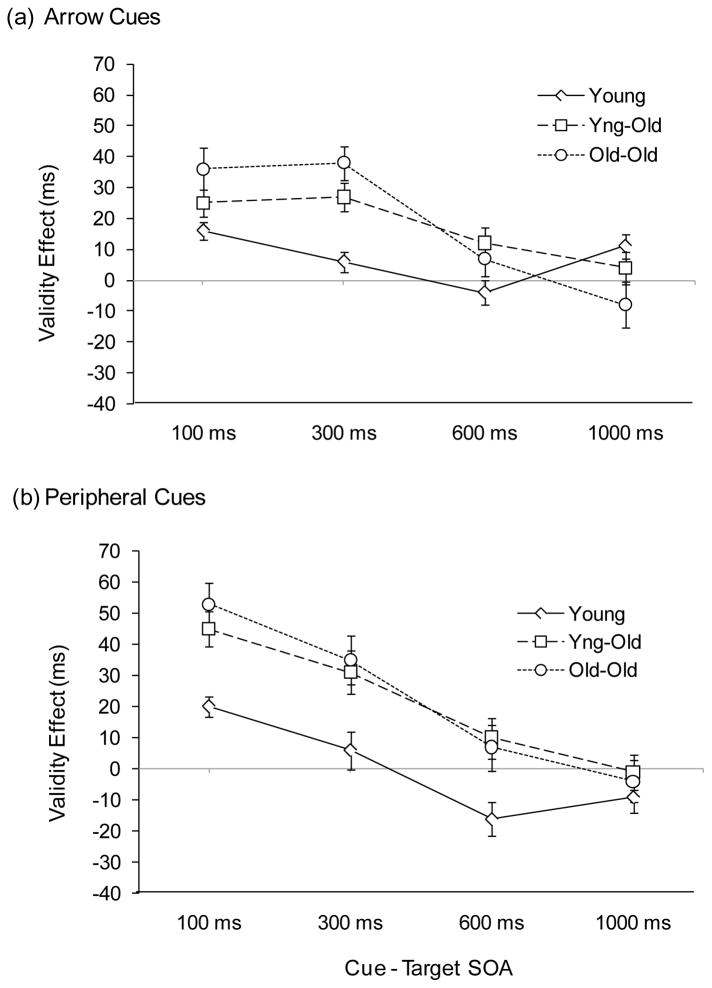
In addition to the main effects, there was a significant two-way interaction between cue validity and SOA (consistent with declining facilitation effects with increasing SOA), F(3, 297) = 16.59, p < .0001, which was qualified by a three-way interaction between age group, cue validity, and SOA, F(6, 297) = 4.59, p < .001. We explored the three-way interaction with simple main effects testing to examine validity effects for each age group at each SOA. The two older groups’ RTs were significantly faster following valid cues than following invalid cues at the 100 ms and 300 ms SOAs, all Fs > 17.0, all ps < .001. In contrast, young adults’ RTs reflected this pattern only at the 100 ms SOA, F(1, 33) = 13.13, p < .001. Consistent with this pattern, when difference scores (invalid RT minus valid RT) were submitted to one-way ANOVAs to assess group differences in validity effects at each of the SOAs, there was a significant age effect at the 300 ms SOA, F(2, 99) = 8.22, p < .001, with the two older groups having significantly greater facilitation effects than the young group, as indicated by SNK post hoc comparisons, ps < .05. Difference scores reflecting facilitation effects for arrow cues are depicted in Figure 2a.
Figure 2.
Validity effects (invalid RT minus valid RT difference scores) as a function of cue-target SOA for young, young-old, and old-old adults in Experiment 1. (a) Uninformative central arrow cues. (b) Uninformative peripheral onset cues. Error bars represent ± 1 SEM.
Given the significant effects of age in the above analysis, we conducted a second set of analyses on transformed reaction times to reduce the influence of general slowing on group differences in facilitation effects (Madden, Pierce, & Allen, 1992; Madden, Whiting, Cabeza, & Huettel, 2004; see also Faust, Balota, Spieler, & Ferraro, 1999 for a similar approach). We used Brinley plot analyses (Cerella, 1994) of the eight condition means to determine the regression equations that best characterized the linear relationship between the condition means of old-old adults with those of the two other groups. We used the resulting equations (see Equations 1 and 2 below) to transform the data of young and young-old participants. The assumption of this approach is that age interactions that remain significant following the transformation can be considered representative of cognitive or perceptual effects that are independent of general slowing. Submitting the transformed data to the 3 × 2 × 4 ANOVA described above, there was no longer a main effect of age, F < 1, as expected. The main effects of cue validity, F(1, 99) = 50.00, p < .0001, and SOA, F(3, 297) = 239.12, p < .0001, remained significant, as did the three-way interaction of age group, cue validity, and SOA, F(6, 297) = 3.70, p < .01. As in the original analysis, cue validity effects of the two older groups were significantly greater than those of young adults at the 300 ms SOA, F(2, 99) = 6.11, p < .01.
| (1) |
| (2) |
Peripheral cues
Replicating the pattern found with arrow cues, the three main effects of the 3 × 2 × 4 ANOVA were significant: age group (old-old adults were significantly slower than young-old adults, who in turn were slower than young adults), F(2, 99) = 37.05, p < .0001, cue validity (participants responded more quickly to validly-cued targets than to invalidly-cued targets), F(1, 99) = 26.16, p < .0001, and cue-target SOA (participants responded more quickly to targets presented at longer SOAs), F(3, 297) = 178.41, p < .0001. In addition, there were two significant two-way interactions: age group × cue validity, F(2, 99) = 6.62, p < .01, and cue validity × SOA, F(3, 297) = 36.32, p < .0001. The three-way interaction was not significant, F(6, 297) = 1.23, p > .20. We explored the age group × cue validity interaction by conducting one-way ANOVAs of validity effects for each age group, collapsed across SOA. Responses were significantly faster to validly-cued targets than to invalidly-cued targets for old-old adults (by 23 ms), F(1, 33) = 13.50, p < .001, and for young-old adults (by 21 ms), F(1, 33) = 18.67, p < .0001, but not for young adults (0 ms), F < 1. We explored the cue validity × SOA interaction by conducting within-subject ANOVAs on validity effects for each SOA. There were significant facilitation effects at the 100 ms SOA (39 ms), F(1, 101) = 74.99, p < .0001, and at the 300 ms SOA (24 ms), F(1, 101) = 35.64, p < .0001. Contrary to predictions, there were not significant IOR effects at the 600 or 1,000 ms SOAs (1 ms and −4 ms, respectively).
To be consistent with the analysis for arrow cues, we examined each age group’s validity effects at each SOA with simple main effects testing. The performance of the two older groups reflected significant facilitation effects at the 100 ms and 300 ms SOAs, all Fs > 27.0, all ps < .0001. Young adults demonstrated significant facilitation at the 100 ms SOA, F(1, 33) = 23.67, p < .0001, and significant IOR effects at the 600 ms and 1,000 ms SOAs, both Fs > 4.2, both ps < .05. Group differences in validity effects, as assessed with difference scores (invalid RT minus valid RT), were significant at SOAs of 100 ms, F(2, 99) = 5.25, p < .01, 300 ms, F(2, 99) = 5.85, p < .01, and 600 ms, F(2, 99) = 3.89, p < .05, with the two older groups demonstrating significantly greater facilitation (or at 600 ms, significantly less inhibition) than the young adult group, p < .05. Validity effect difference scores for peripheral cues are depicted in Figure 2b.
The Brinley analyses on the peripheral cue data produced Equations 3 and 4, which we used to transform the data of young and young-old participants. When submitted to the overall ANOVA, the main effect of age was no longer significant, F < 1, as expected. The main effects of cue validity, F(1, 99) = 23.69, p < .0001, and cue-target SOA, F(3, 297) = 189.49, p < .0001, continued to be significant, as did the interactions between age group and cue validity, F(2, 99) = 5.97, p < .01, and between cue validity and SOA, F(3, 297) = 35.34, p < .0001. All remaining significance patterns remained unchanged from the original analysis, except that the group difference in validity effects at the 100 ms SOA was now marginally significant, F(2, 99) = 2.85, p = .06.
| (3) |
| (4) |
Errors
For both arrow cues and peripheral cues, error rates were low across all conditions for each age group (ranging between 0 and 1.5%), so no further error analyses were conducted.
Discussion
Both central arrows and peripheral onsets were successful in rapidly orienting attention. At the earliest cue-target interval (100 ms SOA), participants were faster to localize targets preceded by valid cues than those preceded by invalid cues. This early facilitation effect for uninformative arrows is consistent with recent proposals (Kingstone et al., 2003; Ristic & Kingstone, 2006) that arrows represent a special form of central symbolic cue that has gained the ability to automatically direct spatial attention. Facilitation effects for arrows were no longer evident by 600 ms, indicating that attention was not maintained at a cued location when the cue was not predictive (Ristic et al., 2002). Peripheral onset cues, in contrast, were associated with IOR (slower responses to validly-cued targets than to invalidly-cued targets) at later SOAs (600 and 1,000 ms), consistent with a slowed return of attention to previously attended locations. This inhibitory effect, which was observed in the responses of young adults, is thought to be linked to oculomotor programming. For cues that trigger the preparation of an eye movement, such as stimuli peripheral to central gaze (Abrams & Dobkin, 1994; Rafal, Calabresi, Brennan, & Sciolto, 1989), attention is slower to return to cued locations. Thus, to summarize, the two types of spatial cues were associated with reflexive orienting patterns that differed in time course.
As was also predicted, orienting patterns differed by age. Facilitation effects were longer in duration for the two older adult groups relative to the young adult group. For arrow cues, young adults’ facilitation effects were observed at 100 ms but had resolved by 300 ms. Older adults’ facilitation effects were still evident at 300 ms, leading to significant age differences (that withstood an analysis accounting for general slowing) in the magnitude of facilitation effects at 300 ms. For peripheral cues, the two older age groups showed greater facilitation than young adults at the 100 and 300 ms SOAs (the age difference was still significant at the 300 ms SOA after accounting for general slowing). Younger adults demonstrated IOR at the later SOAs (600 and 1,000 ms), but the two older groups did not.
The present findings are consistent with the ACS theory (Klein, 2005). Prolonged facilitation effects for older adults suggested that they attended to cued locations with greater intensity than did young adults. According to the theory, this heightened sensitivity resulted because older adults perceived the target localization task to be more difficult, leading them to raise their attentional control setting relative to the setting used by young adults. The higher setting was applied to cues as well as to targets, which delayed the disengagement of attention from cued locations (leading to prolonged facilitation effects and delayed IOR). Old-old adults and young-old adults had similar orienting patterns, indicating that old-old adults did not need to further raise their ACS level to successfully perform the task.
Given the relatively long cue-target intervals (600 and 1,000 ms SOAs), we were surprised to find that IOR did not develop for the two older groups. Using a single-cue task similar to that used in the present study, Castel et al. (2003) found that IOR was delayed with age but was still observed by approximately 600 ms. However, an important difference between the two study designs is that the cue remained on the screen during target presentation in the current study. If older adults were slow to disengage from the location indicated by the cue due to a high control setting, the continued presence of the cue may have exacerbated this delay in the onset of IOR. Indeed, leaving the cue on the screen throughout the experimental trial may have (a) enhanced age differences in facilitation effects at early intervals for both types of cues, and (b) delayed the onset of older adults’ IOR at later intervals for peripheral cues. To assess this possibility, we removed the cue prior to target presentation in Experiment 2 to see whether age differences in initial orienting and IOR would diminish.
Experiment 2
In Experiment 2 we examined the impact of cue duration on age differences in the time course of orienting. Experiment 2 was identical to Experiment 1 except that the cue was presented for a fixed duration and was removed prior to target presentation. As observed in Experiment 1, we expected to find age differences in the magnitude and duration of cue validity effects. However, we predicted that removing the cue would facilitate endogenous disengagement from the cued location, particularly for older adults, and thus reduce age differences in facilitation effects. Furthermore, with earlier disengagement, IOR to peripherally-cued locations would be more likely to be observed at the sampled SOAs, although IOR onset might still be delayed for older adults due to continued group differences in control settings.
Method
Participants
Forty young adults (18–28 yrs; 27 women, 13 men), 40 young-old adults (60–74 yrs; 27 women, 13 men), and 40 old-old adults (75–86 yrs; 28 women, 12 men) participated in Experiment 2 (see Table 1 for participants’ screening and psychometric data). Participants were recruited and screened in the same manner as described in Experiment 1, but there were no participants in common across the two experiments.
Materials and procedure
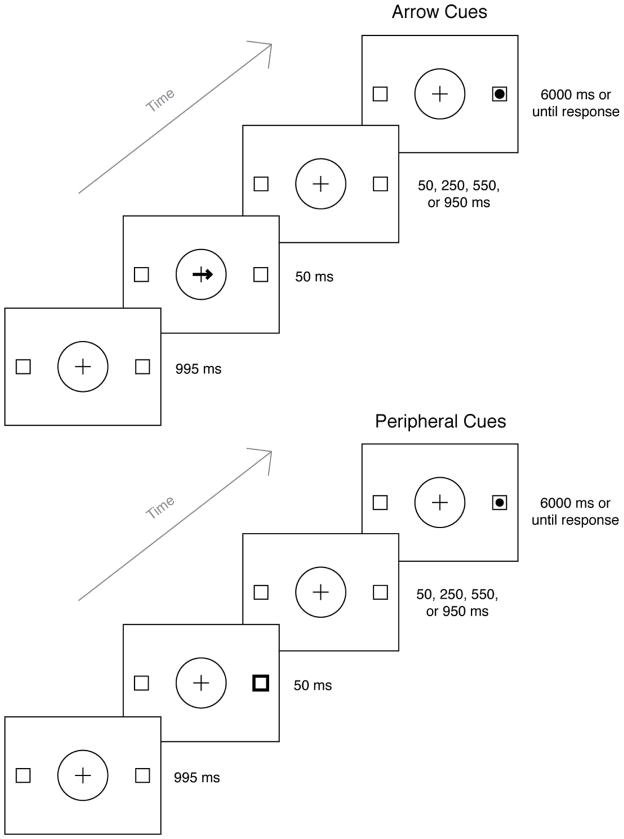
The stimuli, design, and procedure were the same as those described in Experiment 1, except that instead of overlapping temporally with the target, the cue was presented for 50 ms and was then removed. After an inter-stimulus interval of 50, 250, 550, or 950 ms (corresponding to cue-target SOAs of 100, 300, 600, and 1,000 ms), the target was presented until the participant responded or 6,000 ms had elapsed. Sample trial sequences are presented in Figure 3. As in Experiment 1, participants were told that the direction or location of the cue was random and would not assist them in predicting the target location. Participants completed two blocks of 80 trials for each cue type (arrow and peripheral), for a total of 320 experimental trials. Each block began with eight practice trials.
Figure 3.
Trial sequence for Experiment 2. Stimuli are not scaled to size.
Results
Table 3 displays the mean RTs and error rates for Experiment 2 as a function of cue type, age group, cue validity, and cue-target SOA. After removing outliers and error trials, median RTs were submitted to separate 3 × 4 × 2 mixed ANOVAs for the two types of cues. We again used an alpha level of .05 and SNK post-hoc tests on variables involving more than two levels.
Table 3.
Mean Reaction Times (and Standard Deviations) for Experiment 2
| SOA (ms) | Arrow Cues
|
Peripheral Cues
|
||||||
|---|---|---|---|---|---|---|---|---|
| 100 | 300 | 600 | 1,000 | 100 | 300 | 600 | 1,000 | |
| Young | ||||||||
| Valid | 364 (56) | 326 (60) | 321 (53) | 311 (52) | 380 (52) | 357 (60) | 359 (62) | 341 (57) |
| Invalid | 387 (58) | 333 (65) | 325 (60) | 316 (60) | 400 (59) | 357 (61) | 334 (59) | 320 (58) |
| Young-Old | ||||||||
| Valid | 471 (87) | 440 (83) | 412 (76) | 414 (75) | 497 (72) | 451 (67) | 457 (81) | 444 (74) |
| Invalid | 504 (87) | 463 (81) | 407 (72) | 405 (73) | 521 (82) | 460 (76) | 428 (82) | 414 (77) |
| Old-Old | ||||||||
| Valid | 528 (105) | 493 (90) | 470 (90) | 462 (107) | 542 (104) | 500 (91) | 499 (90) | 505 (100) |
| Invalid | 556 (96) | 516 (87) | 474 (99) | 454 (102) | 594 (113) | 530 (101) | 494 (102) | 481 (94) |
Reaction times are reported in milliseconds. SOA stimulus onset asynchrony.
Arrow cues
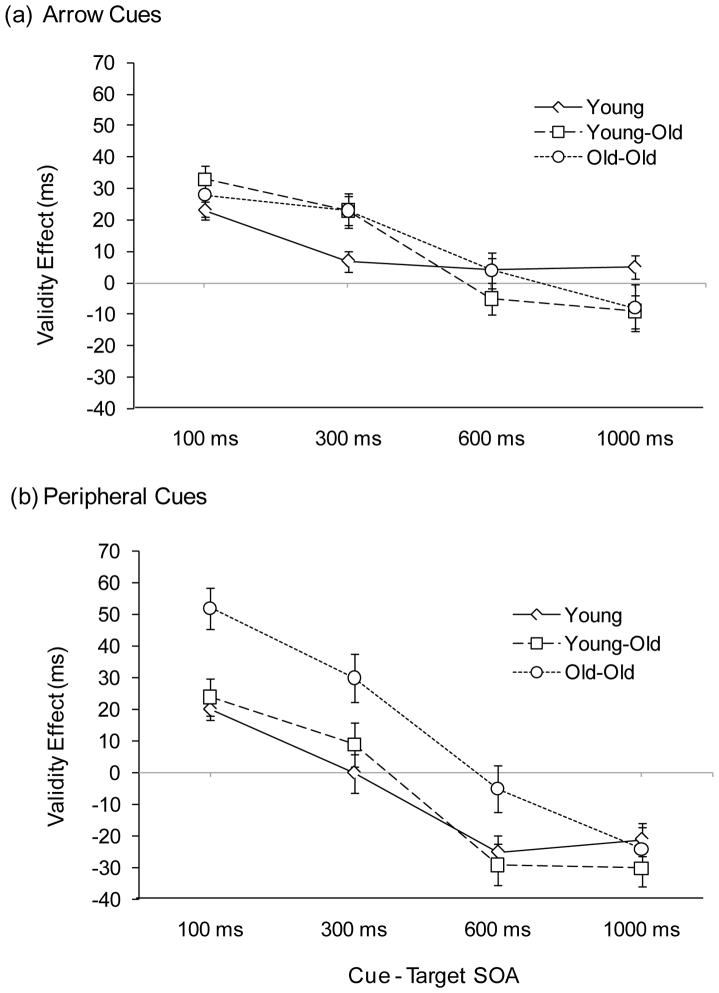
As in Experiment 1, the ANOVA for arrow cues revealed the three main effects to be significant: age group, F(2, 117) = 46.05, p < .0001, cue validity, F(1, 117) = 35.43, p < .0001, and cue-target SOA, F(3, 351) = 200.05, p < .0001. In addition, there was a significant two-way interaction between cue validity and SOA (reflecting declining facilitation effects with increasing SOA), F(3, 351) = 31.19, p < .0001, which was qualified by a three-way interaction between age group, cue validity, and SOA, F(6, 351) = 2.97, p < .01. Validity effects, when examined at each SOA with simple effects testing, reflected significant facilitation for all three age groups at the 100 ms and 300 ms SOAs, all Fs > 5.0, all ps < .05. Group differences in the magnitude of facilitation at these SOAs (Figure 4a), as assessed with difference scores (invalid RT minus valid RT), were significant at the 300 ms SOA, F(2, 117) = 4.02, p < .05. Young-old adults and old-old adults had significantly greater facilitation effects than young adults, ps < .05.
Figure 4.
Validity effects (invalid RT minus valid RT difference scores) as a function of cue-target SOA for young, young-old, and old-old adults in Experiment 2. (a) Uninformative central arrow cues. (b) Uninformative peripheral onset cues. Error bars represent ± 1 SEM.
Following the Brinley transformation to the data for young and young-old participants using Equations 5 and 6, the main effect of age from the overall ANOVA was no longer significant, F < 1, as expected. The other effects from the original analysis remained significant, including the three-way interaction of age group, cue validity, and SOA, F(6, 351) = 2.27, p < .05. However, when age differences in facilitation effects were explored at each SOA, the age difference at the 300 ms SOA was now marginally significant, F(2, 117) = 2.64, p = .08.
| (5) |
| (6) |
Peripheral cues
For peripheral cues, two main effects were significant: age group, F(2, 117) = 48.22, p < .0001, and SOA, F(3, 351) = 205.05, p < .0001. There were two significant two-way interactions: age group × cue validity, F(2, 117) = 8.16, p < .001, and cue validity × SOA, F(3, 351) = 75.84, p < .0001, which were qualified by a three-way interaction between age group, cue validity, and SOA, F(6, 351) = 2.46, p < .05. Simple main effects testing assessed validity effects for each age group at each SOA. Facilitation effects were significant for all three age groups at the 100 ms SOA, all Fs > 16.5, all ps < .001. Only the old-old group continued to show facilitation at the 300 ms SOA, F(1, 39) = 15.12, p < .001. At 600 ms and 1,000 ms, IOR effects were significant for young adults and young-old adults, all Fs > 17.0, all ps < .001. IOR effects for old-old adults were significant at 1,000 ms, F(1, 39) = 13.12, p < .001, but not at 600 ms, F < 1. Group differences in validity effects, as assessed with difference scores (invalid RT minus valid RT), were significant at SOAs of 100 ms, F(2, 117) = 10.39, p < .0001, 300 ms, F(2,117) = 5.10, p < .01, and 600 ms, F(2, 117) = 4.30, p < .05. Old-old adults had significantly greater facilitation (or at 600 ms, significantly less inhibition) than young-old and young adults, ps < .05. Difference scores reflecting validity effects are presented in Figure 4b.
Following the Brinley transform using Equations 7 and 8, the main effect of group from the overall ANOVA was no longer significant, F < 1. The two-way interactions between age group and cue validity, F(2, 117) = 7.95, p < .001, and between cue validity and SOA, F(3, 351) = 75.99, p < .0001, continued to be significant, but the three-way interaction was no longer significant, F(6, 351) = 1.47, p > .15. All other significance patterns remained unchanged from the original analysis, including significant group differences in validity effects at SOAs of 100, 300, and 600 ms, all Fs > 4.4, all ps < .05, with old-old adults having greater facilitation effects (or at 600 ms, smaller inhibition effects) than the other two age groups.
| (7) |
| (8) |
Errors
For both arrow and peripheral cues, error rates were low across all conditions for each age group (ranging between 0.1 and 2.4%), so no further error analyses were conducted.
Discussion
The time course of spatial orienting for young adults was relatively constant across the two experiments. Orienting was facilitated by valid arrow cues at the shortest cue-target interval (100 ms SOA), and this facilitation effect diminished at longer intervals. With peripheral cues, young adults’ responses reflected facilitation at the earliest interval (100 ms SOA) and inhibition at the later intervals (600 and 1,000 ms SOAs). Thus, overall, the observed orienting pattern for young adults was relatively unaffected by cue duration, although casual comparison of Figures 2 and 3 suggests that young adults’ IOR effects were greater in magnitude following brief peripheral cues (Experiment 2) than following persistent cues (Experiment 1).
Removing the cue prior to target presentation was effective in modifying age-related patterns of orienting, but more markedly so for peripheral cues. For arrow cues, as in Experiment 1, facilitation effects were greater in magnitude for the two older groups than for young adults at the 300 ms SOA, although the age difference did not survive the Brinley transform. Thus, from a conservative standpoint, a brief arrow cue led to less pronounced age differences in facilitation effects than a persistent cue. Both older groups demonstrated IOR when the peripheral cue was removed prior to target presentation (Experiment 2), but not when the cue remained present (Experiment 1), With a brief cue, the young-old group’s validity effects were now virtually identical to those of young adults (facilitation at the 100 ms SOA and IOR at the 600 and 1,000 ms SOAs, with no differences between the two groups in the magnitude of the effects). The old-old groups’ performance did not benefit as much from a shortened cue. They continued to show greater facilitation than the other two age groups at the early SOAs (100 and 300 ms) and a delayed development of IOR at the later SOAs (with IOR at 1,000 ms but not at 600 ms).
In accord with predictions of ACS theory, age differences in orienting performance were still evident in Experiment 2. With a short-duration arrow cue, both older groups showed greater facilitation effects than young adults, suggesting slower-resolving orienting effects, although general slowing likely contributed to this age difference. For peripheral cues, enhanced facilitation effects were limited to the old-old group. Both older groups showed IOR, although the onset of IOR was delayed for old-old adults. Although older adults may have set attentional controls to a higher setting due to greater perceived task difficulty, these settings appeared to have had less of an impact on orienting to peripheral cues when the cue was removed prior to target presentation. Removing the cue likely facilitated endogenous disengagement of attention from the cued location (and thus facilitated IOR), and perhaps more so for young-old adults (whose performance became similar to that of young adults) than for old-old adults.
General Discussion
Reflexive Orienting Initiated by Central Arrow Cues and Peripheral Onset Cues
We examined the time course of reflexive orienting to two types of visuospatial cues, central arrow cues and peripheral onset cues. The cues did not reliably predict the location of an upcoming target, yet young adults and older adults reliably shifted attention based on location information provided by the cues. The early time point at which cues influenced orienting (100 ms between the onsets of the cues and targets) indicated that attentional orienting was reflexively guided. Facilitation effects (faster responses to validly-cued targets than to invalidly-cued targets) at early cue-target intervals were replaced by IOR (slower responses to validly-cued targets than to invalidly-cued targets) at later cue-target intervals, but only following peripheral onset cues, consistent with the idea that peripheral cues uniquely activate components of the eye movement system (e.g., frontal eye fields, superior colliculus) that are associated with attentional inhibition (Lepsien & Pollman, 2002; Posner, Rafal, Choate, & Vaughan, 1985; Sapir, Soroker, Berger, & Henik, 1999). Facilitation effects resulting from arrow cues were not followed by inhibition effects, and these facilitation effects were largely resolved by 600 ms.
How do central symbolic cues assume reflexive orienting properties? In the case of the arrow, it is a symbol that is pervasively used in our daily lives to indicate the locations of objects or destinations (e.g., traffic exits, turn lanes, airport gates, stores in the mall). Arrows in these contexts are highly predictive of desired locations. Thus, with accumulating experience, observers may come to automatically orient in the direction indicated by an arrow. Another possibility is that orienting in response to the directional information provided by arrows is not learned but occurs naturally because arrows are inherently directional (Freyd & Pantzer, 1995). This notion is consistent with the results of a study by Ristic and colleagues (2002), in which four- and five-year-old children oriented in response to uninformative arrow cues despite their limited experience with the predictive properties of arrows in everyday life. In either case, findings from the present study suggest that automatic orienting in response to arrows does not diminish with age, even in advanced old age. In fact, it appears that the effect of arrow cues on attentional orienting is actually enhanced later in life.
Age Differences in Reflexive Orienting to Peripheral Onset Cues
We found that age and cue duration interacted to influence the temporal patterns of orienting to peripheral onset cues. With a persistent cue (Experiment 1), both young-old and old-old adults showed enhanced facilitation effects relative to young adults. With a brief cue (Experiment 2), only old-old adults continued to show enhanced facilitation effects. Cue duration impacted IOR effects as well. Young adults demonstrated IOR toward peripheral cues that remained on the screen (Experiment 1), but older adults did not. In contrast, when peripheral cues were removed prior to target onset (Experiment 2), all three age groups demonstrated IOR, although IOR onset was delayed for old-old adults. Thus, the facilitation and IOR patterns suggest that reducing cue duration was successful in prompting cue disengagement for older adults, particularly for young-old adults.
The above cuing patterns indicate that peripheral cues were effective in capturing older adults’ attention. In fact, older adults may have been particularly prone to automatic capture. The age-related increase in the magnitude and duration of facilitation effects, consistent with findings from other studies (Brodeur & Enns, 1997; Castel et al., 2003; Faust & Balota, 1997; Lincourt et al., 1997), likely reflected older adults’ stronger engagement with and delayed disengagement from peripheral cues. In addition, the extended duration of cue engagement may have interfered with the expression of IOR (Klein, 2000; Klein, 2005). When a peripheral cue remained visible over the cue-target interval, thus promoting continued engagement at the cued location, older adults failed to demonstrate IOR (Experiment 1). In contrast, when the cue was removed prior to the target’s appearance, older adults’ endogenous disengagement from the cue was facilitated and consequently IOR to the cued location was revealed (Experiment 2), although IOR onset was still delayed for old-old adults. In studies using a cue-back IOR task (in which a second central cue is used to draw attention back to fixation), older adults show no delay in the development of IOR (Faust & Balota, 1997; Langley, Fuentes, Vivas, & Saville, 2007), reinforcing the idea that if conditions are conducive to older adults removing attention from a cued location, IOR will emerge for this group. Young adults, on the other hand, appear to be able to efficiently remove attention from the cue even when the cue remains physically present (thus leading to IOR in Experiment 1), suggesting greater flexibility in attentional shifting in this age group, and the least flexibility in shifting performance of old-old adults.
The IOR pattern of the present study differs from that identified by Castel et al. (2003). Using a brief-duration cue (50 ms, like Experiment 2 of the present study), Castel and colleagues found that IOR onset was observed in young adults at a cue-target SOA of 250 ms but was delayed for older adults until an SOA of 750 ms. With a brief cue (Experiment 2), we found a delay in IOR onset only for old-old adults. One difference between studies is the time point at which IOR was first observed in young adults (IOR emerged at a 600 ms SOA in the present study and at a 250 ms SOA in the Castel et al. study). Differences in the target task may account for between-study differences in IOR onset; we used a discrimination task (indicate the left or right location of the target), whereas Castel et al. used a detection task (press a button as soon as the target is detected). Onset of IOR is known to lag in discrimination tasks relative to detection tasks (Lupiáñez, Milán, Tornay, Madrid, & Tudela, 1997). However, task differences cannot explain the difference between studies in age patterns of IOR. Castel et al. found age differences at 250 ms (young adults showed IOR, whereas older adults showed facilitation) and at 500 ms (older adults showed less IOR than younger adults). In contrast, we found no age differences in IOR between the young-old and the young group when IOR was first observed at 600 ms. Consistent with what we found (at SOAs of 600 and 1,000 ms), both age groups in Castel et al. showed IOR at 750 and 1,000 ms. It is possible that we would have observed age differences in the onset of IOR (with young adults developing IOR sooner than young-old adults) in Experiment 2 if we had sampled SOAs between 300 and 600 ms. Nevertheless, it is still the case that we found an impact of cue duration on observed age differences in IOR, with age differences greatly reduced (older adults now showed IOR, although still delayed in old-old adults) when the cue was removed prior to target presentation.
The pattern of results with peripheral cues is largely consistent with Klein’s (2005) ACS theory of attentional orienting. It is important to note that, based on the findings from the Brinley plot transforms, the observed age differences in facilitation effects and IOR effects were not completely accounted for by general slowing on the part of older adults (Faust et al., 1999). This leaves open the possibility that attention-specific accounts, such as the ACS theory, could explain the observed age patterns. In accord with the ACS theory, we interpreted the results to indicate that older adults used a higher control setting than younger adults because they found the location discrimination task more difficult. The higher setting heightened the intensity of attention to the cue as well as to the target, leading cue-induced orienting to be associated with enhanced facilitation and delayed inhibition. The control setting’s influence on older adults’ reflexive orienting was most evident for persistent cues, which, at the cued location, would have been particularly difficult to disengage from.
We found evidence that old-old adults used a higher attentional control setting than young-old adults, but only with brief cues. With persistent cues, the two older groups oriented to peripheral cues in a similar manner (with little difference in the magnitude and time course of validity effects). With brief peripheral cues (Experiment 2), old-old adults showed greater-magnitude facilitation at early cue-target intervals than did young-old adults, and later-developing IOR. This pattern suggested that cue offset was less effective in encouraging old-old adults’ early endogenous disengagement from the cued location. However, old-old adults did demonstrate IOR in Experiment 2, indicating improved cue disengagement relative to Experiment 1. The enhanced early facilitation effect for old-old adults is consistent with Greenwood and Parasuraman’s (1994) findings that old-old adults had greater cuing effects than young-old adults when orienting to informative peripheral arrows. Because we used uninformative peripheral onset cues, we can be more certain that our results reflect changes in reflexive orienting (as opposed to some combination of reflexive and volitional orienting). Together, the findings suggest that the control setting used by old-old adults was at the same level or perhaps slightly higher than the setting used by young-old adults. In addition, cue offset was not as effective in this group at discouraging prolonged attentional engagement. It is worth investigating age differences for an orienting task requiring a detection response; it is possible that had we used a simpler task (thus lowering the control setting), age differences in orienting would have diminished.
Faust and Balota (1997) found that older adults showed greater facilitation effects than young adults (tested at a cue-target SOA of 200 ms) and proposed that it was due to a breakdown in the posterior attention system, which led to a reduced ability to localize objects in the visual field. As a result, older adults benefited more so than young adults from an onset cue that drew attention to a location. A reduced ability to localize objects could account for the present age differences in the benefit of a valid location cue, but it could not explain why there would be age differences in the time course of cuing effects (reflecting delayed disengagement from cues, particularly when cues remained physically present). The ACS theory provides an alternative explanation for age-related increases in facilitation effects that also addresses changes in the time course of cue validity effects. According to the theory, age-related increases in task difficulty influenced attentional control settings as applied to cues as well as to targets. As a result of higher control settings, older adults produced enhanced and prolonged facilitation effects and delayed inhibition effects.
What the ACS theory does not specify is what makes the target processing task more difficult for older adults than for young adults, thus leading to higher control settings. The present study did not address the source of task difficulty, but there are at least three possibilities. First, age-related changes in central or peripheral acuity may have influenced how quickly targets in the periphery were detected and processed (Schneider & Pichora-Fuller, 2000). Although we limited the sample to individuals with visual acuities of 20/40 or better, age differences in central acuity were observed and may have influenced target processing, and changes in peripheral acuity (not assessed) may have influenced initial detection. Second, age-related general slowing (evidenced in the present study in terms of age-related increases in overall RT) may have delayed or hampered target processing for older adults (Kramer & Madden, 2008). Higher control settings associated with orienting to cues and targets may have compensated for this slowing in stimulus processing. Finally, age-related changes specific to response selection in a forced-choice task may have influenced task difficulty, above and beyond general slowing. That said, similar age patterns in orienting effects (enhanced and prolonged facilitation effects for older adults) have been found with single-choice detection tasks, suggesting that age differences in task difficulty are still found on tasks that are less demanding of response selection processes (e.g., Castel et al., 2003). Future studies will need to delineate the age-related factors that influence task difficulty in orienting tasks.
Age Differences in Reflexive Orienting to Central Arrow Cues
In the present study, young adults and older adults showed similar orienting patterns to uninformative central arrow cues. Responses were facilitated by valid cues relative to invalid cues at the earliest cue-target interval (100 ms SOA), indicating reflexive and rapid orienting of attention. Facilitation effects were resolved by 300 ms for young adults and by 600 ms for young-old and old-old adults. This relatively brief influence on orienting is in contrast to sustained facilitation effects typically found with informative central arrows (Olk, Cameron, & Kingstone, 2008; Ristic & Kingstone, 2006) and suggests that our participants did not have the same incentive (in terms of likely target appearance) to maintain attention at the cued location. In Experiments 1 and 2, facilitation effects for older adults were maintained at 300 ms, whereas these effects for young adults had diminished by the same time point. In contrast to the pattern observed with peripheral cues, cue duration had less of an impact on age differences in orienting to central arrows. When cue duration was shortened, both older groups continued to have greater facilitation effects than young adults (although not so after controlling for general slowing).
Applying ACS theory to central arrow cues is not as straightforward as applying it to peripheral onset cues. Do the control settings affect engagement and disengagement with the arrow cue itself or with the location indicated by the cue? Because a peripheral cue occurs at the cued location, engagement and disengagement from a cue also reflects engaged and disengaged attention from the cued location. However, in the case of a central arrow, greater engagement with the cue itself would mean greater attention at the center location. However, if the result of a higher setting is greater attention to the location indicated by the cue (left location for a left arrow, right location for a right arrow), then facilitation effects would be enhanced. The finding that central arrow cues led to enhanced cuing effects for older adults relative to young adults suggests that older adults had greater engagement of the cued location rather than of the cue directly. Thus, if one accepts that attention control settings impacted how strongly observers reacted to the directional information provided by the cue rather than how strongly attention was directed to the location of the cue, then ACS theory can explain orienting in response to both arrow and peripheral cues. This interpretation may explain why cue duration had relatively little effect on orienting to arrow cues. Once attention had been shifted to the cued location based on the arrow information, there was no need to disengage from the arrow cue itself, even when it remained present. Together, there is mounting evidence that the ACS theory can account for age patterns in reflexive orienting to both central arrows and peripheral onset cues.
Central arrow cues in past aging studies have consistently been informative (e.g., Folk & Hoyer, 1992; Lincourt et al., 1997; Tellinghuisen et al., 1996; Yamaguchi et al., 1995), and orienting to such cues was thought to reflect volitional shifts of attention. In these past studies, both young adults and older adults demonstrated reliable facilitation effects, and in some cases, facilitation effects were greater in magnitude for older adults (Curran et al., 2001; Hartley et al., 1990; Lincourt et al., 1997; Nissen & Corkin, 1985). The pattern was interpreted to indicate that volitional orienting remained intact with age. Future studies will need to disentangle the contributions of volitional and reflexive orienting to older adults’ responses to predictive central arrow cues. Recent work indicates that predictive arrows trigger an interaction between volitional and reflexive orienting that produces orienting effects that exceed the predicted additive combination of the individual orienting processes (Olk et al., 2008; Ristic & Kingstone, 2006). Age differences in either reflexive or volitional orienting alone or age differences in both processes may change how these processes interact to produce orienting to predictive central arrows.
Implications
The question remains whether age differences in orienting represent an adaptive or maladaptive change for older adults. The answer depends in part on how we use peripheral onset and arrow cues in real world contexts (Kingstone et al., 2003). It is easy to imagine contexts in which we encounter orienting cues – driving, navigating unfamiliar environments (e.g., the airport, the mall, an amusement park), or searching for information on internet sites. For example, internet advertisers have discovered that advertisements that involve dynamic visual displays (that function as peripheral cues) are particularly difficult to ignore (due to reflexive shifts of attention). In addition, arrows presented on internet sites direct us to further information and links (and these arrows are likely to be both reflexively and volitionally attended). In real life situations, how will age-related changes in the magnitude and timing of orienting influence the success of such cues in guiding attention? In the lab, enhanced orienting toward valid cues can help older adults more rapidly localize sought-after information, which certainly could be adaptive in real life situations, particularly given age-related slowing in cognitive processing. However, more intense processing of invalid cues hinders attentional shifts to desired target locations, and slower cue disengagement delays IOR to previously-searched locations. These alterations have implications for spatial orienting in the real world, particularly in situations in which misleading visual cues automatically capture older adults’ attention but do not predict the location of desired information. Delayed disengagement may slow older adults’ visual search in real world contexts and cause them to prematurely return attention to searched locations. Thus, the adaptiveness of these age-related changes in orienting may depend on the visual context and the predictability of visual cues.
Acknowledgments
This work was supported by Centers of Biomedical Research Excellence (COBRE) Grant P20 RR020151 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). The contents of this report are solely the responsibility of the authors and do not necessarily reflect the official views of the NIH or NCRR. We are grateful to Ganesh Padmanabhan for programming the experiments and to Shanna Morlock, Jaryn Allen, Laura Klubben, Savannah Kraft, Melissa Tarasenko, Lindsay Anderson, Heather Wadeson, Heather Joyce, Tanya Peterson, RaeAnn Levang, Veselin Marinov, Joseph Gabel, and Nicole Kiewel for collecting the data.
References
- Abrams RA, Dobkin RS. Inhibition of return: Effects of attentional cuing on eye movement latencies. Journal of Experimental Psychology: Human Perception and Performance. 1994;20:467–477. doi: 10.1037/0096-1523.20.3.467. [DOI] [PubMed] [Google Scholar]
- Brodeur DA, Enns JT. Covert visual orienting across the lifespan. Canadian Journal of Experimental Psychology. 1997;51:20–35. doi: 10.1037/1196-1961.51.1.20. [DOI] [PubMed] [Google Scholar]
- Castel AD, Chasteen AL, Scialfa CT, Pratt J. Adult age differences in the time course of inhibition of return. Journal of Gerontology: Psychological Sciences. 2003;58B:256–259. doi: 10.1093/geronb/58.5.p256. [DOI] [PubMed] [Google Scholar]
- Cerella J. Generalized slowing in Brinley plots. Journal of Gerontology: Psychological Sciences. 1994;49B:65–71. doi: 10.1093/geronj/49.2.p65. [DOI] [PubMed] [Google Scholar]
- Christensen KJ, Moye J, Armson RR, Kern TM. Health screening and random recruitment for cognitive aging research. Psychology and Aging. 1992;7:204–208. doi: 10.1037//0882-7974.7.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T, Hills A, Patterson MB, Strauss ME. Effects of aging on visuospatial attention: An ERP study. Neuropsychologia. 2001;39:288–301. doi: 10.1016/S0028-3932(00)00112-3. [DOI] [PubMed] [Google Scholar]
- Faust ME, Balota DA. Inhibition of return and visuospatial attention in healthy older adults and individual with dementia of the Alzheimer type. Neuropsychology. 1997;11:13–29. doi: 10.1037//0894-4105.11.1.13. [DOI] [PubMed] [Google Scholar]
- Faust ME, Balota DA, Spieler DH, Ferraro FR. Individual differences in information-processing rate and amount: Implications for group differences in response latency. Psychological Bulletin. 1999;125:777–799. doi: 10.1037//0033-2909.125.6.777. [DOI] [PubMed] [Google Scholar]
- Folk CL, Hoyer WJ. Aging and shifts of visual spatial attention. Psychology and Aging. 1992;7:453–465. doi: 10.1037//0882-7974.7.3.453. [DOI] [PubMed] [Google Scholar]
- Folk CL, Remington R, Johnston JC. Involuntary covert orienting is contingent on attentional control settings. Journal of Experimental Psychology: Human Perception and Performance. 1992;18:1030–1044. doi: 10.1037//0096-1523.18.4.1030. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A practical method for grading the cognitive state of the patient for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Freyd JJ, Pantzer TM. Static patterns moving in the mind. In: Smith SM, Ward TB, Finke RA, editors. The creative cognition approach. Cambridge MA: MIT Press; 1995. pp. 181–204. [Google Scholar]
- Friesen CK, Kingstone A. The eyes have it! Reflexive orienting is triggered by nonpredictive gaze. Psychonomic Bulletin and Review. 1998;5:490–495. [Google Scholar]
- Greenwood PM, Parasuraman R. Attentional disengagement deficit in nondemented elderly over 75 years of age. Aging and Cognition. 1994;1:188–202. doi: 10.1080/13825589408256576. [DOI] [Google Scholar]
- Greenwood PM, Parasuraman R, Haxby JV. Changes in visuospatial attention over the adult lifespan. Neuropsychologia. 1993;31:471–485. doi: 10.1016/0028-3932(93)90061-4. [DOI] [PubMed] [Google Scholar]
- Hartley AA, Kieley JM. Adult age differences in the inhibition of return of visual attention. Psychology and Aging. 1995;10:670–683. doi: 10.1037//0882-7974.10.4.670. [DOI] [PubMed] [Google Scholar]
- Hartley AA, Kieley JM, Slabach EH. Age differences and similarities in the effects of cues and prompts. Journal of Experimental Psychology: Human Perception and Performance. 1990;16:523–537. doi: 10.1037//0096-1523.16.3.523. [DOI] [PubMed] [Google Scholar]
- Hommel B, Pratt J, Colzato L, Godijn R. Symbolic control of visual attention. Psychological Science. 2001;12:360–365. doi: 10.1111/1467-9280.00367. [DOI] [PubMed] [Google Scholar]
- Huey ED, Wexler BE. Abnormalities in rapid, automatic aspects of attention in schizophrenia: Blunted inhibition of return. Schizophrenia Research. 1994;14:57–63. doi: 10.1016/0920-9964(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Jonides J. Voluntary versus automatic control over the mind’s eye’s movement. In: Long J, Baddeley A, editors. Attention and performance IX. Hillsdale NJ: Erlbaum; 1981. pp. 187–203. [Google Scholar]
- Kingstone A, Smilek D, Ristic J, Friesen CK, Eastwood JD. Attention, researchers! It is time to take a look at the real world. Current Directions in Psychological Science. 2003;12:176–180. doi: 10.1111/1467-8721.01255. [DOI] [Google Scholar]
- Klein RM. Inhibition of return. Trends in Cognitive Sciences. 2000;4:138–147. doi: 10.1016/B978-012375731-9/50020-3. [DOI] [PubMed] [Google Scholar]
- Klein RM. On the role of endogenous orienting in the inhibitory aftermath of exogenous orienting. In: Mayr U, Awh E, Keele SW, editors. Developing individuality in the human brain: A tribute to Michael I Posner. Washington DC: APA; 2005. pp. 45–64. [DOI] [Google Scholar]
- Klein RM, Kingstone A, Pontefract A. Orienting of visual attention. In: Rayner K, editor. Eye movements and visual cognition: Scene perception and reading. Amsterdam: Elsevier Science; 1992. pp. 46–63. [Google Scholar]
- Kramer AF, Madden DJ. Attention. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 3. New York: Psychology Press; 2008. pp. 189–249. [Google Scholar]
- Langley LK, Fuentes LJ, Vivas AB, Saville AL. Aging and temporal patterns of inhibition of return. Journal of Gerontology: Psychological Sciences. 2007;62B:71–77. doi: 10.1093/geronb/62.2.p71. [DOI] [PubMed] [Google Scholar]
- Lepsien J, Pollman S. Covert reorienting and inhibition of return: An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14:127–144. doi: 10.1162/089892902317236795. [DOI] [PubMed] [Google Scholar]
- Lincourt AE, Folk CL, Hoyer WJ. Effects of aging on voluntary and involuntary shifts of attention. Aging, Neuropsychology, and Cognition. 1997;4:290–303. doi: 10.1080/13825589708256654. [DOI] [PubMed] [Google Scholar]
- Lupiáñez J, Milán EG, Tornay FJ, Madrid E, Tudela P. Does IOR occur in discrimination tasks? Yes, it does, but later. Perception and Psychophysics. 1997;59:1241–1254. doi: 10.3758/bf03214211. [DOI] [PubMed] [Google Scholar]
- Lupiáñez J, Milliken B, Solano C, Weaver B, Tipper SP. On the strategic modulation of the time course of facilitation and inhibition of return. The Quarterly Journal of Experimental Psychology. 2001;54A:753–773. doi: 10.1080/02724980042000453. [DOI] [PubMed] [Google Scholar]
- MacPherson AC, Klein RM, Moore CM. Inhibition of return in children and adolescents. Journal of Experimental Child Psychology. 2003;85:337–351. doi: 10.1016/S0022-0965(03)00104-8. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Pierce TW, Allen PA. Adult age differences in attentional allocation during memory search. Psychology and Aging. 1992;7:594–601. doi: 10.1037//0882-7974.7.4.594. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Cabeza R, Huettel SA. Age-related preservation of top-down attentional guidance during visual search. Psychology and Aging. 2004;19:304–309. doi: 10.1037/0882-7974.19.2.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen MJ, Corkin S. Effectiveness of attentional cueing in older and younger adults. Journal of Gerontology. 1985;40:185–191. doi: 10.1093/geronj/40.2.185. [DOI] [PubMed] [Google Scholar]
- Olk B, Cameron B, Kingstone A. Enhanced orienting effects: Evidence for an interaction principle. Visual Cognition. 2008;16:979–1000. doi: 10.1080/13506280701848921. [DOI] [Google Scholar]
- Posner MI, Cohen YA. Components of visual orienting. In: Bouma H, Bouwhuis DG, editors. Attention and performance X. Hillsdale NJ: Erlbaum; 1984. pp. 531–554. [Google Scholar]
- Posner MI, Rafal RD, Choate LS, Vaughan J. Inhibition of return: Neural basis and function. Journal of Cognitive Neuropsychology. 1985;2:211–228. doi: 10.1080/02643298508252866. [DOI] [Google Scholar]
- Rafal RD, Calabresi PA, Brennan CW, Sciolto TK. Saccade preparation inhibits reorienting to recently attended locations. Journal of Experimental Psychology: Human Perception and Performance. 1989;15:673–685. doi: 10.1037//0096-1523.15.4.673. [DOI] [PubMed] [Google Scholar]
- Ristic J, Friesen CK, Kingstone A. Are eyes special? It depends on how you look at it. Psychonomic Bulletin and Review. 2002;9:507–513. doi: 10.3758/bf03196306. [DOI] [PubMed] [Google Scholar]
- Ristic J, Kingstone A. Attention to arrows: Pointing to a new direction. Quarterly Journal of Experimental Psychology. 2006;59:1921–1930. doi: 10.1080/17470210500416367. [DOI] [PubMed] [Google Scholar]
- Sapir A, Henik A, Dobrusin M, Hochman EY. Attentional asymmetry in schizophrenia: Disengagement and inhibition of return deficits. Neuropsychology. 2001;5:361–370. doi: 10.1037//0894-4105.15.3.361. [DOI] [PubMed] [Google Scholar]
- Sapir A, Soroker N, Berger A, Henik A. Inhibition of return in spatial attention: Direct evidence for collicular generation. Nature Neuroscience. 1999;2:1053–1054. doi: 10.1038/15977. [DOI] [PubMed] [Google Scholar]
- Schneider BA, Pichora-Fuller MK. Implications of perceptual deterioration for cognitive aging research. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 2. Mahwah, NJ: Lawrence Erlbaum; 2000. pp. 155–219. [Google Scholar]
- Spencer KM, Nestor PG, Valdman O, Niznikiewicz MA, Shenton ME, McCarley RW. Enhanced facilitation of spatial attention in schizophrenia. Neuropsychology. 2011;25:76–85. doi: 10.1037/a0020779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tales A, Muir JL, Bayer A, Snowden RJ. Spatial shifts in visual attention in normal ageing and dementia of the Alzheimer type. Neuropsychologia. 2002;40:2000–2012. doi: 10.1016/S0028-3932(02)00057-X. [DOI] [PubMed] [Google Scholar]
- Tellinghuisen DJ, Zimba LD, Robin DA. Endogenous visuospatial precuing effects as a function of age and task demands. Perception and Psychophysics. 1996;58:947–958. doi: 10.3758/bf03205496. [DOI] [PubMed] [Google Scholar]
- Tipples J. Eye gaze is not unique: Automatic orienting in response to uninformative arrows. Psychonomic Bulletin & Review. 2002;9:314–318. doi: 10.3758/BF03196287. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Yamaguchi S, Tsuchiya H, Kobayashi S. Electrophysiologic correlates of age effects on visuospatial attention shift. Cognitive Brain Research. 1995;3:41–49. doi: 10.1016/0926-6410(95)00017-8. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VD. Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]