Abstract
Background
Longitudinal analyses of comorbid conditions in women with breast cancer are few.
Methods
Using Surveillance, Epidemiology, and End Results–Medicare data, we included 51 950 women aged ≥66 years with in situ and stage I to IV breast cancer diagnosed in 1998–2002. We identified the prevalence and incidence of 34 comorbid conditions in these women, as well as in a matched cohort without cancer whose rates were standardized to the age and race/ethnicity distribution of the cancer patients. We also estimated rates of office encounters and diagnostic or testing procedures during the 12 months before diagnosis.
Results
The prevalence of most conditions at diagnosis was comparable among breast cancer and noncancer patients. New conditions after diagnosis were more common in breast cancer patients, and the incidence rates increased with higher stage at diagnosis. Before diagnosis, women presenting with stage IV disease had 41% [95% confidence interval (CI) 38% to 43%] fewer physician encounters and 34% (95% CI 24% to 31%) fewer unique diagnostic tests than women diagnosed with carcinoma in situ.
Conclusions
Many comorbid conditions are identified as a consequence of the breast cancer diagnosis. There appears to be an important contribution from a lack of interaction with the health care system before diagnosis.
Keywords: adverse events disclosures, breast cancer, comorbidity
introduction
Breast cancer is a common disease in women, and its incidence increases with age [1, 2]. Understanding the interplay between breast cancer and comorbid conditions is important because comorbidities influence decisions about the appropriate course of treatment and are independent risk factors for survival [3–5]. In addition, comorbidities may limit patients' eligibility for clinical trials, and consequently, the generalizability of study results to the overall population [6]. For these reasons, comorbidity burden is a key component of the diagnosis and treatment process as well as postcancer care.
If higher stage at diagnosis were related to greater comorbidity burden, as might be hypothesized, it would suggest that comorbidity considerations become more complicated for women with more advanced-stage breast cancer. Although there is no direct evidence of a significant reservoir of undiagnosed comorbid conditions that increases with stage, there are studies showing that more screening procedures and more ambulatory care visits are each associated with earlier stage at diagnosis [7, 8]. Based on this, it would be reasonable to surmise that those patients who do not seek care for signs and symptoms of cancer, or who delay screening, may ignore other health issues as well [9]. Such undiagnosed conditions would be expected to be picked up in the extensive testing and related evaluations conducted after cancer diagnosis. In the clinical trial setting, any conditions not identified at diagnosis could complicate the analysis and interpretation of the trial data. However, despite its relevance to patient care, the incidence of new comorbid conditions after cancer diagnosis has not, to our knowledge, been compared across stages, or to a control population of individuals without cancer.
Therefore, the purpose of this study was threefold: to quantify the comorbidity burden at the time of diagnosis by comparing the prevalence of a variety of conditions in women with and without breast cancer; to estimate the previously undetected comorbidity burden elicited after cancer diagnosis by estimating incidence rates for a variety of conditions in these women; and to explore whether the identification of comorbid conditions in breast cancer patients is related to the degree of precancer interaction with the health system in these women.
methods
data source
This study used the Surveillance, Epidemiology, and End Results (SEER)–Medicare database, which links SEER cancer registry data with Medicare data. Medicare-eligible persons are primarily individuals aged ≥65 in the United States, although other younger populations are included based on disability or specific medical conditions [10–12]. Our dataset also included a separately created 5% random sample of noncancer patients from the Medicare program in the same catchment areas as those used in the SEER program for use as a reference (i.e. control) population.
study population and observation period
Patients were diagnosed with in situ and stage I to IV breast cancer between 1 January 1998 and 31 December 2002 and had Medicare claims available from 1997 through 2005. Women with previous primary cancer diagnoses in the SEER registry were excluded as were women who died in the month of diagnosis. Follow-up was based on data for covered health care services, including hospital, physician, and outpatient claims. Cancer and noncancer subjects were at least 66 years old to allow at least 12 months of Medicare claims data for identifying prevalent conditions before diagnosis. All patients were required to have both Part A and B Medicare coverage (i.e. fee-for-service) during the observation period, and all patients in managed care plans were excluded because detailed medical claims are not available for these individuals. Follow-up ended at the earliest of the following events: end of the observation period, end of Part A and B coverage, or death.
An identically sized cohort of women without cancer (i.e. noncancer patients) was created by matching on both time of diagnosis and geographic area to the cohort of women with cancer (other variables were controlled using stratification and adjustment as discussed below). For time matching, the SEER month and year of cancer diagnosis for each cancer patient were used to identify potential noncancer matches who had appropriate Medicare coverage on the same date. The first day of this month was assigned as the diagnosis index date for both the breast cancer patient and her randomly selected noncancer control. For geographic area matching, the county of residence was used first and the state of residence was used if no county match was available. Subjects in the noncancer cohort were known to be cancer-free through 2002.
patient characteristics
For women with breast cancer, the SEER data were the source for identifying the date of cancer diagnosis, cancer site, and tumor characteristics. Stage at breast cancer diagnosis was based on the SEER–Modified American Joint Committee on Cancer (AJCC) stage variable [13]. Hormone receptor status included estrogen receptor (ER) and progesterone receptor status (PR). For all patients, age was given in years at the diagnosis index date. Race/ethnicity was based on the following categories: white, black, Hispanic, or other (predominately American Indian, Native Alaskan, Pacific Islander, and Asian).
The algorithm from the National Cancer Institute (NCI) for constructing a comorbidity index was also used for all patients to estimate a modified Charlson Comorbidity Index incorporating the adaptations suggested by Deyo and Romano (excluding cancer as a condition) [14–16]. Scores were categorized into 0, 1, and ≥2.
definitions of comorbid conditions
Medicare claims data were used for identifying prevalent and incident conditions of interest throughout the observation period. Supplemental Appendix Table S1 (available at Annals of Oncology online) provides the International Classification of Diseases, ninth edition (ICD-9) codes used to identify the 34 conditions in both breast cancer and noncancer women [17]. These conditions were chosen to represent common comorbid conditions in older adults, as well as common consequences of chemotherapy (referred to as ‘adverse events’). Diagnoses recorded on claims for inpatient stays were counted at the time of their first occurrence. Diagnoses in outpatient facility and physician claims were assessed similarly to the NCI comorbidity algorithm, which requires two diagnoses at least 30 days apart to identify a comorbidity (taking the first occurrence as the date of onset). For the subgroup of 10 conditions classified as adverse events, only claims associated with an inpatient or emergency department visit were counted, and, because ‘rule-out’ claims are less of a problem in the hospital setting, only a single diagnosis code was required. In this way, they align with the concept of ‘serious adverse events’ often used in clinical trials, although they may represent chronic conditions and/or be unrelated to cancer-directed therapy.
counts of physician encounters and diagnostic tests
As a simple approach to measuring interactions with the health system, we counted the number of physician encounters and the number of unique diagnostic tests carried out in the breast cancer and noncancer patient cohorts during the 12 months before, and after, the diagnosis index date. Physician encounters were defined as physician office visits using Healthcare Common Procedure Coding System (HCPCS) codes [18]. Unique diagnostic tests included claims with HCPCS codes for the following: radiology, pathology and laboratory, psychiatric diagnostics, gastroenterology diagnostics, echocardiography, intracardiac electrophysiology, cerebrovascular arterial studies, pulmonary testing, glucose monitoring, electroencephalography, and central nervous system assessments (see supplemental Appendix Table S2, available at Annals of Oncology online). One physician encounter per day per patient was counted, and one of each type of diagnostic test (HCPCS code) per patient was counted.
statistical analyses
For each condition, standardized rates were estimated for each of the three observation periods (before, 3 months after, and 12 months after the index date). These rates were estimated overall as well as within age, race/ethnicity, and stage-specific (for cancer patients) strata (data not shown). The cancer rates for each stratum were standardized to the age, race/ethnicity, and stage-specific characteristics of the total breast cancer cohort [19]. Rates in the noncancer patients were standardized to the age and race/ethnicity distribution of the total breast cancer cohort to facilitate comparisons.
Prevalence was defined as the proportion of study subjects with a particular condition as of the index date using claims before the diagnosis index date. Binomial confidence intervals (CIs) were calculated for each prevalence proportion. Incidence rates were defined as new diagnoses after the index date in patients free of the condition at diagnosis. If the comorbid condition was not diagnosed, subjects were censored at death, the end of coverage according to the eligibility criteria, or the end of the observation period. Incidence rates per 1000 person-years were estimated. The 12-month and 3-month rates reflect overlapping time periods.
Zero-inflated negative binomial models were used to estimate the average number of visits or tests during the year before the index date by cancer stage at diagnosis in the breast cancer cohort and to compare the number of visits or tests between cancer and noncancer patients. These models were adjusted for age and race/ethnicity [20, 21].
All analyses were conducted in SAS (version 9.2; SAS Institute Inc., Cary, NC) and Stata (version 10; Stata Corporation, College Station, TX).
results
There were 51 950 women identified in both the breast cancer and the noncancer populations. See Table 1 for details on the cohorts.
Table 1.
Demographic characteristics of the breast cancer and noncancer patient cohorts
| Characteristic | Breast cancer patients | Noncancer patients | |
|---|---|---|---|
| N (%) | N (%) | ||
| Age (years)a | 66–69 | 9690 (18.8) | 9913 (19.2) |
| 70–74 | 13 469 (26.1) | 12 451 (24.1) | |
| 75–79 | 13 039 (25.3) | 11 721 (22.7) | |
| ≥ 80 | 15 392 (29.8) | 17 505 (33.9) | |
| Race/ethnicity | White | 44 712 (86.7) | 43 671 (84.7) |
| Black | 3345 (6.5) | 3781 (7.3) | |
| Asian/Other | 1843 (3.6) | 2999 (5.8) | |
| Hispanic | 1690 (3.3) | 1139 (2.2) | |
| SEER region | Georgia (Atlanta/Rural Georgia) | 2027 (3.9) | 2027 (3.9) |
| Californiab | 15 558 (30.2) | 15 558 (30.2) | |
| Connecticut | 4225 (8.2) | 4225 (8.2) | |
| Hawaii | 876 (1.7) | 876 (1.7) | |
| Iowa | 4544 (8.8) | 4544 (8.8) | |
| Kentucky | 3239 (6.3) | 3239 (6.3) | |
| Louisiana | 2731 (5.3) | 2731 (5.3) | |
| Michigan (Detroit) | 5013 (9.7) | 5013 (9.7) | |
| New Jersey | 6726 (13) | 6726 (13) | |
| New Mexico | 1408 (2.7) | 1408 (2.7) | |
| Utah | 1664 (3.2) | 1664 (3.2) | |
| Washington (Seattle/Puget Sound) | 3579 (6.9) | 3579 (6.9) | |
| NCI comorbidity scorec | 0 | 34 296 (68.6) | 30 721 (63.3) |
| 1 | 10 844 (21.7) | 10 797 (22.2) | |
| ≥2 | 4833 (9.7) | 7033 (14.5) | |
| Stage at diagnosisd | In situ | 7532 (14.6) | N/A |
| I | 22 235 (43.1) | ||
| II | 14 753 (28.6) | ||
| III | 2558 (5) | ||
| IV | 2191 (4.3) | ||
| Unknown | 2282 (4.4) | ||
| Tumor grade at diagnosis | Well differentiated | 9861 (19.1) | N/A |
| Moderately differentiated | 19 057 (26.9) | ||
| Poorly differentiated | 12 564 (24.4) | ||
| Undifferentiated | 1426 (2.8) | ||
| Other and unknown | 8682 (16.8) | ||
| ER/PR status | Positive | 30 613 (59.3) | N/A |
| Negative | 5112 (9.9) | ||
| Unknown | 15 865 (30.8) | ||
| Year of diagnosis | 1998 | 6412 (12.4) | N/A |
| 1999 | 6429 (12.5) | ||
| 2000 | 12 915 (25) | ||
| 2001 | 13 085 (25.4) | ||
| 2002 | 12 749 (24.7) |
aAge at diagnosis index date.
bCalifornia includes Los Angeles, San Francisco/Oakland, San Jose/Monterey, and Greater California.
cComorbidity index based on conditions identified in the 12 months before the diagnosis index date.
dStage based on the American Joint Committee on Cancer's staging system (third edition).
ER, estrogen receptor; NCI, National Cancer Institute; PR, progesterone receptor; SEER, Surveillance, Epidemiology, and End Results.
The standardized prevalence rates for 27 of the 34 conditions were lower in women with cancer compared with women without cancer (Table 2). Certain comorbidities were exceptions to this pattern: atrial fibrillation, hypertension, diabetes, liver disease, osteoarthritis, thromboembolic events, and chronic obstructive pulmonary disease. In terms of incidence rates for all conditions, standardized 3-month and 12-month rates were, with the exception of the 12-month cardiac arrest rate, always higher in the women with breast cancer. See Table 3 for incidence rates.
Table 2.
Prevalence proportions for Comorbid conditions in breast cancer patients and noncancer control patients
| Comorbid condition | Breast cancer patients, % (95% CI) | Noncancer patients, % (95% CI) |
|---|---|---|
| Adverse events | ||
| Anemia | 6.09 (5.89–6.3) | 7.59 (7.36–7.81) |
| Diarrhea | 1.62 (1.51–1.73) | 1.83 (1.71–1.94) |
| Electrolyte disorder | 9.39 (9.15–9.64) | 11.34 (11.07–11.61) |
| Infectious disease | 11.95 (11.68–12.23) | 14.39 (14.09–14.69) |
| Infusion reaction | 0.43 (0.38–0.49) | 0.46 (0.4–0.52) |
| Neutropenia | 0.13 (0.1–0.16) | 0.16 (0.13–0.2) |
| Oral mucositis | 0.03 (0.01–0.04) | 0.03 (0.01–0.04) |
| Skin rash (medication related) | 0.13 (0.1–0.16) | 0.21 (0.17–0.25) |
| Skin rash (other) | 2.02 (1.9–2.14) | 2.36 (2.23–2.49) |
| Thrombocytopenia | 0.55 (0.49–0.62) | 0.68 (0.6–0.75) |
| Cardiac/vascular | ||
| Arrhythmia | 7.24 (7.01–7.46) | 7.41 (7.18–7.63) |
| Arterial thrombosis | 0.29 (0.25–0.34) | 0.47 (0.41–0.53) |
| Atrial fibrillation | 9.19 (8.94–9.43) | 8.61 (8.37–8.85) |
| Coronary artery disease | 18.35 (18.02–18.68) | 19.13 (18.8–19.47) |
| Congestive heart failure | 10.69 (10.43–10.96) | 11.6 (11.33–11.86) |
| Cerebrovascular disease | 8.11 (7.88–8.34) | 9.39 (9.14–9.64) |
| Cardiac arrest | 0.09 (0.06–0.11) | 0.11 (0.08–0.14) |
| Hypertension | 50.74 (50.31–51.16) | 42.93 (42.51–43.35) |
| Myocardial infarction | 4.18 (4.01–4.35) | 4.67 (4.48–4.85) |
| Peripheral vascular disease | 2.86 (2.72–3.01) | 3.47 (3.31–3.63) |
| Thromboembolism | 2.12 (2–2.25) | 2 (1.87–2.12) |
| Gastrointestinal/hepatic | ||
| Cholecystitis | 1.61 (1.5–1.72) | 1.62 (1.51–1.73) |
| Gastric ulcers | 0.74 (0.67–0.81) | 0.94 (0.85–1.02) |
| Liver disease | 0.49 (0.43–0.55) | 0.39 (0.34–0.45) |
| Metabolic | ||
| Diabetes | 14.29 (13.99–14.59) | 12.8 (12.51–13.1) |
| Hyperglycemia | 0.09 (0.07–0.12) | 0.11 (0.08–0.14) |
| Musculoskeletal/rheumatic | ||
| Osteoarthritis | 16.06 (15.74–16.37) | 15.52 (15.2–15.83) |
| Rheumatalogic disease | 2.08 (1.95–2.2) | 2.38 (2.25–2.52) |
| Neurological/psychiatric | ||
| Alzheimer's disease and dementia | 3.79 (3.63–3.95) | 6.67 (6.46–6.87) |
| Depression | 5.56 (5.36–5.76) | 6.27 (6.06–6.48) |
| Hemiplegia | 0.94 (0.86–1.02) | 1.27 (1.18–1.37) |
| Pulmonary | ||
| Chronic obstructive pulmonary disease | 9.83 (9.57–10.08) | 9.77 (9.51–10.03) |
| Renal | ||
| Nephrotic syndrome | 0.06 (0.04–0.09) | 0.08 (0.05–0.1) |
| Renal disease | 1.33 (1.23–1.43) | 1.49 (1.38–1.59) |
Noncancer women are matched to women with breast cancer by time and geographic area. All rates are standardized to the age and race/ethnicity distribution of the cancer population.
CI, confidence inteval.
Table 3.
Estimates of 3-month and 12-month incidence of comorbid conditions in breast cancer and noncancer patients
| Comorbid condition | Breast cancer patients | Noncancer patients | Breast cancer patients | Noncancer patients |
|---|---|---|---|---|
| 3-month rate/1000 (95% CI) | 3-month rate/1000 (95% CI) | 12-month rate/1000 (95% CI) | 12-month rate/1000 (95% CI) | |
| Adverse events | ||||
| Anemia | 105.65 (99.79–111.52) | 34.66 (31.33–37.99) | 63.63 (61.29–65.97) | 34.51 (32.8–36.21) |
| Diarrhea | 15.61 (13.43–17.8) | 7.09 (5.63–8.55) | 11.01 (10.07–11.95) | 6.88 (6.14–7.62) |
| Electrolyte disorder | 124.04 (117.59–130.49) | 49.17 (45.12–53.21) | 78.69 (76.04–81.34) | 47.13 (45.1–49.16) |
| Infectious disease | 149.82 (142.62–157.02) | 55.02 (50.64–59.39) | 94.64 (91.68–97.6) | 56.78 (54.51–59.06) |
| Infusion reaction | 5.71 (4.4–7.02) | 1.12 (0.53–1.71) | 3.16 (2.66–3.66) | 1.64 (1.28–2.01) |
| Neutropenia | 17.48 (15.19–19.77) | 0.3 (0–0.6) | 15.6 (14.49–16.71) | 0.8 (0.55–1.04) |
| Oral mucositis | 2.02 (1.27–2.77) | 0.07 (0–0.2) | 2.15 (1.73–2.56) | 0.12 (0.02–0.21) |
| Skin rash (medication related) | 2.34 (1.5–3.18) | 0.56 (0.14–0.98) | 1.17 (0.86–1.47) | 0.63 (0.41–0.86) |
| Skin rash (other) | 35.34 (32.04–38.63) | 10.07 (8.32–11.82) | 20.85 (19.55–22.15) | 10.43 (9.53–11.34) |
| Thrombocytopenia | 11.52 (9.66–13.38) | 3.26 (2.26–4.26) | 8.23 (7.43–9.04) | 3.72 (3.18–4.27) |
| Cardiac/vascular | ||||
| Arrhythmia | 52.25 (48.13–56.37) | 15.69 (13.45–17.94) | 25.56 (24.08–27.04) | 16.28 (15.11–17.46) |
| Arterial thrombosis | 2.73 (1.83–3.64) | 1.3 (0.66–1.95) | 1.97 (1.57–2.36) | 1.41 (1.07–1.75) |
| Atrial fibrillation | 58.73 (54.32–63.15) | 21.34 (18.72–23.96) | 30.45 (28.82–32.08) | 21.66 (20.31–23.02) |
| Coronary artery disease | 80.31 (74.85–85.77) | 28.77 (25.48–32.07) | 35.35 (33.49–37.21) | 26.61 (24.99–28.22) |
| Congestive heart failure | 64.58 (59.91–69.25) | 32.49 (29.17–35.8) | 37.71 (35.88–39.54) | 28.61 (27.02–30.19) |
| Cerebrovascular disease | 42.16 (38.45–45.88) | 23.88 (21.08–26.68) | 24.55 (23.09–26) | 22.24 (20.85–23.62) |
| Cardiac arrest | 2.42 (1.57–3.27) | 1.99 (1.22–2.77) | 1.84 (1.46–2.22) | 2.24 (1.82–2.65) |
| Hypertension | 252.56 (239.88–265.24) | 50.75 (45.5–55.99) | 94.51 (90.49–98.53) | 43.54 (41.05–46.02) |
| Myocardial infarction | 45.12 (41.36–48.89) | 15.24 (13.04–17.43) | 21.7 (20.36–23.04) | 16.53 (15.37–17.69) |
| Peripheral vascular disease | 18.15 (15.79–20.52) | 9.38 (7.66–11.1) | 9.83 (8.94–10.72) | 8.68 (7.84–9.51) |
| Thromboembolism | 26.17 (23.34–29) | 5.53 (4.24–6.83) | 20.75 (19.46–22.05) | 6.32 (5.61–7.02) |
| Gastrointestinal/hepatic | ||||
| Cholecystitis | 7.92 (6.37–9.47) | 4.05 (2.9–5.2) | 5.89 (5.2–6.57) | 4.9 (4.26–5.53) |
| Gastric ulcers | 4.63 (3.45–5.81) | 2.93 (2.02–3.85) | 3.29 (2.78–3.8) | 3.42 (2.9–3.93) |
| Liver disease | 3.13 (2.16–4.1) | 1.04 (0.48–1.6) | 1.7 (1.34–2.07) | 1.25 (0.94–1.56) |
| Metabolic | ||||
| Diabetes | 34.96 (31.46–38.46) | 13.48 (11.32–15.64) | 17.17 (15.91–18.43) | 11.32 (10.31–12.33) |
| Hyperglycemia | 1.48 (0.81–2.15) | 0.35 (0.04–0.66) | 0.69 (0.46–0.93) | 0.33 (0.17–0.49) |
| Musculoskeletal/rheumatic | ||||
| Osteoarthritis | 71.85 (66.76–76.93) | 25.85 (22.83–28.87) | 34.1 (32.29–35.9) | 22.35 (20.91–23.79) |
| Rheumatalogic disease | 8.99 (7.34–10.65) | 2.73 (1.8–3.67) | 3.97 (3.4–4.53) | 2.66 (2.19–3.12) |
| Neurological/psychiatric | ||||
| Alzheimer's disease and dementia | 37.9 (34.46–41.34) | 19.02 (16.56–21.48) | 20.78 (19.47–22.08) | 21.3 (19.98–22.62) |
| Depression | 44 (40.26–47.74) | 14.27 (12.14–16.39) | 23.55 (22.14–24.95) | 14.59 (13.49–15.7) |
| Hemiplegia | 7.79 (6.25–9.32) | 4.53 (3.37–5.69) | 4.74 (4.13–5.36) | 4.41 (3.82–4.99) |
| Pulmonary | ||||
| Chronic obstructive pulmonary disease | 78.02 (72.9–83.13) | 19.08 (16.55–21.62) | 33.06 (31.35–34.77) | 18 (16.75–19.24) |
| Renal | ||||
| Nephrotic syndrome | 0.55 (0.14–0.95) | 0.29 (0–0.58) | 0.31 (0.15–0.46) | 0.3 (0.15–0.44) |
| Renal disease | 8.45 (6.85–10.05) | 5.27 (4.01–6.53) | 5.57 (4.91–6.24) | 5.14 (4.51–5.77) |
Noncancer patients are matched to cancer patients on gender, index date, and geographic area. All rates are standardized to the age and race/ethnicity distribution of the cancer population. Rates are expressed per 1000 person-years.
CI, confidence interval.
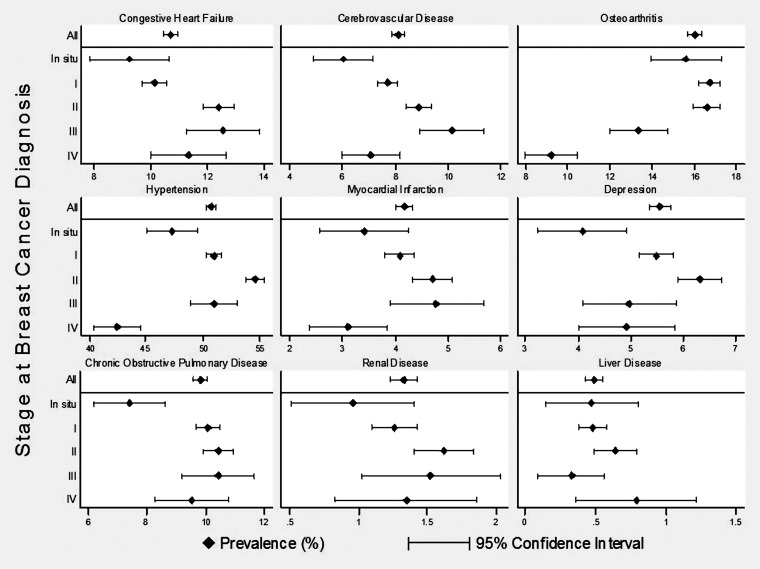
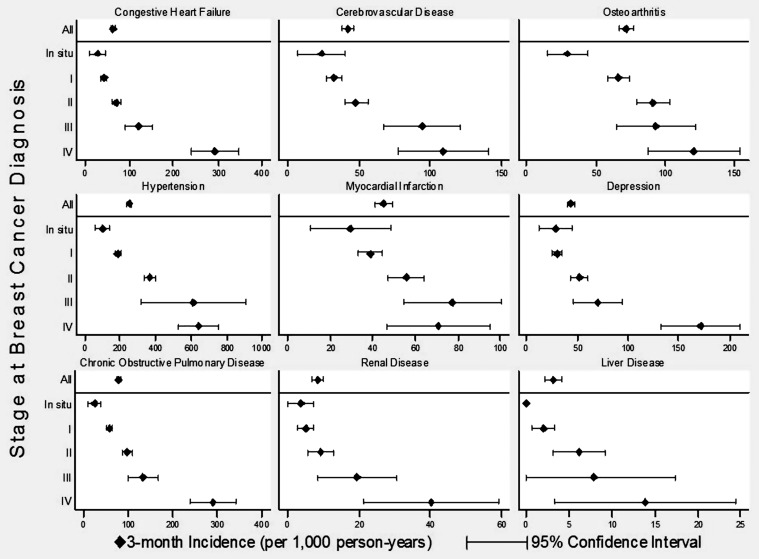
When stratified by stage at breast cancer diagnosis, for many comorbid conditions, the prevalence rates were quite variable (Figure 1 and supplemental Appendix Table S3, available at Annals of Oncology online). In contrast, the incidence rates for most conditions increased with higher stage at breast cancer diagnosis (Figure 2 and supplemental Appendix Table S4, available at Annals of Oncology online).
Figure 1.
The prevalence of selected comorbid conditions in breast cancer patients by diagnosis stage. Comorbid conditions were selected to include a variety of systems. See supplemental Appendix materials (available at Annals of Oncology online) for data on other conditions.
Figure 2.
The 3-month incidence of selected comorbid conditions in breast cancer patients by diagnosis stage. Comorbid conditions were selected to include a variety of systems. See supplemental Appendix materials (available at Annals of Oncology online) for data on other conditions.
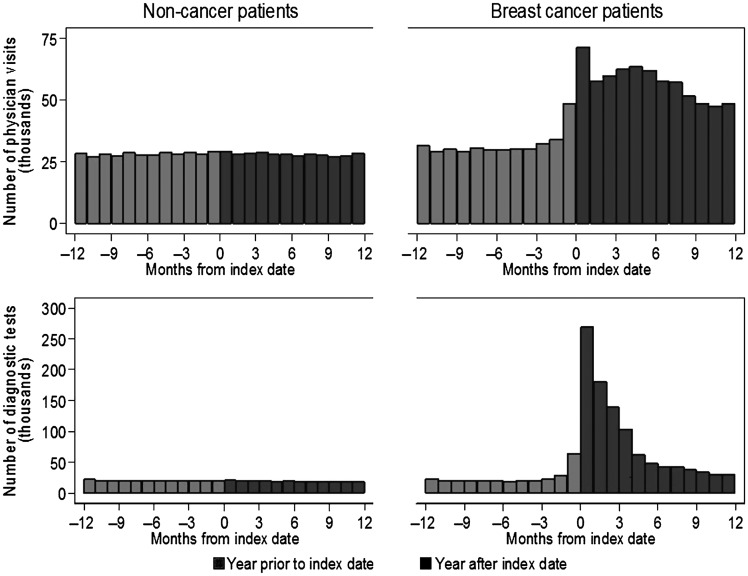
Women with and without breast cancer had comparable numbers of physician encounters in the window of time beginning 12 months before the cancer diagnosis date and ending 4 months prior, as seen in the unadjusted counts in Figure 3. For women with breast cancer, the counts of both physician encounters and new diagnostic tests during the 12-month precancer diagnosis period appeared to increase beginning 3 months before the cancer diagnosis date. Statistical models to estimate counts and rates in the precancer diagnosis period were consistent with these figures after adjusting for age and race/ethnicity, as well as accounting for censoring (i.e. losses to follow-up and death). When we excluded the 3-month pre-diagnosis period from the statistical models comparing utilization between women with and without breast cancer, most of the difference in physician encounters and virtually all of the difference in diagnostic testing were removed (Table 4).
Figure 3.
The unadjusted number of physician office visits and unique diagnostic tests before and after the diagnosis index date. Counts in the above figures do not show losses to follow-up after diagnosis. At 3 months the breast cancer and noncancer populations were 96% and 98% of the baseline total, respectively (51 590); at 6 months they were 94% and 96% of baseline, respectively; and at 12 months they were 90% and 93% of baseline, respectively.
Table 4.
Outpatient visits and unique diagnostic tests in the year before the diagnosis index date for breast cancer and noncancer patients (both overall and by stage)
| Population | Physician
encounters |
Unique diagnostic
tests |
|||
|---|---|---|---|---|---|
| Mean | Rate ratio (95% CI) | Mean | Rate ratio (95% CI) | ||
| Months −12 to −1 before diagnosis index date | |||||
| Noncancer (overall) | 4.99 | 1.00 (ref) | 4.83 | 1.00 (ref) | |
| Breast cancer (overall) | 7.43 | 1.49 (1.47–1.51) | 5.73 | 1.17 (1.16–1.19) | |
| By stage | In situ | 8.26 | 1.00 (ref) | 6.41 | 1.00 (ref) |
| I | 7.86 | 0.95 (0.93–0.97) | 6.01 | 0.94 (0.91–0.97) | |
| II | 7.14 | 0.86 (0.84–0.88) | 5.39 | 0.84 (0.82–0.87) | |
| III | 5.52 | 0.66 (0.63–0.68) | 4.15 | 0.65 (0.62–0.68) | |
| IV | 5.24 | 0.63 (0.60–0.65) | 4.64 | 0.72 (0.69–0.76) | |
| Unknown | 6.68 | 0.79 (0.76–0.82) | 5.63 | 0.89 (0.84–0.93) | |
| Months −12 to −4 before diagnosis index date | |||||
| Noncancer (overall) | 4.87 | 1.00 (ref) | 3.62 | 1.00 (ref) | |
| Breast cancer (overall) | 5.22 | 1.08 (1.06–1.09) | 3.58 | 0.98 (0.96–1.00) | |
| By stage | In situ | 5.77 | 1.00 (ref) | 3.96 | 1.00 (ref) |
| I | 5.54 | 0.95 (0.93–0.98) | 3.76 | 0.95 (0.92–0.99) | |
| II | 5.04 | 0.86 (0.84–0.88) | 3.41 | 0.86 (0.83–0.89) | |
| III | 3.92 | 0.66 (0.64–0.69) | 2.70 | 0.68 (0.64–0.73) | |
| IV | 3.48 | 0.59 (0.57–0.62) | 2.62 | 0.66 (0.62–0.71) | |
| Unknown | 4.72 | 0.80 (0.76–0.83) | 3.57 | 0.91 (0.85–0.97) | |
Estimated by negative binomial regression and adjusted for age at diagnosis and race/ethnicity.
CI, confidence interval.
Analyses of the numbers of physician encounters and unique diagnostic tests by stage showed that each measure was inversely related to the stage of breast cancer at diagnosis. This trend was consistent for both the entire 12-month period before diagnosis, as well as the period excluding the 3-month pre-diagnosis period. In particular, patients presenting with stage IV disease had 41% (95% CI 38% to 43%) fewer physician encounters and 34% (95% CI 24% to 31%) fewer unique diagnostic tests than women diagnosed with carcinoma in situ when ignoring the 3-month pre-diagnosis period (Table 4).
discussion
This study shows that older women, at the time of breast cancer diagnosis, have a comparable prevalence of comorbid conditions to women who do not have breast cancer. In addition, stage at diagnosis is associated with variability in the prevalence of many conditions, but the pattern of the association is quite heterogeneous. In contrast, the incidence rates of comorbid conditions, stratified by stage at diagnosis, show that more advanced cancer stage is associated with a greater likelihood of identifying new comorbid conditions. Most importantly from a public health perspective, more advanced stage at diagnosis is also associated with the degree of precancer health system interaction, as measured by office visits and unique diagnostic tests. Hence, there is evidence for a health care seeking behavioral component to the undiagnosed comorbidity burden in breast cancer patients.
Looking more closely, older women with breast cancer tended to have slightly lower prevalence rates for most comorbid conditions compared with women without cancer, even after accounting for age, race, time, and geographic area. Some of this is likely to be related to an under-diagnosis of conditions in women with later-stage disease, a gap that shrinks after the cancer diagnosis. However, the prevalence of several conditions was higher in women with breast cancer than in those without, in contrast to the overall pattern. For hypertension, diabetes, thromboembolic events, and osteoarthritis, there is an established association with higher body mass index (BMI), a confounder that could not be controlled through standardization in these data [22–28]. That is, because higher BMI is a risk factor for breast cancer, our breast cancer population may have been heavier, which may have increased the prevalence of conditions associated with higher BMI [29, 30]. Similar reasoning may apply to alcohol consumption and liver disease [31, 32].
There are a variety of conflicting studies evaluating the cross-sectional association between comorbidity burden and breast cancer stage at diagnosis. Yancik et al. [4] found no association between comorbidity and breast cancer stage. Fleming et al. [33] found that the association between comorbid conditions and advanced-stage diagnosis depended on the comorbid condition. Vaeth et al. [34] found that women with conditions causing functional limitations were half as likely to be diagnosed with advanced-stage breast cancer. Gonzalez et al. [35] found that a higher comorbidity index was associated with higher odds of advanced-stage breast cancer. In addition, similarly conflicting results exist for other tumors as well [36, 37]. The presence of undiagnosed conditions, many of which are found after cancer diagnosis, appears to confound associations at the time of diagnosis.
In addition to our study, others have suggested that interaction with the health system is a key factor in stage at diagnosis. Gornick et al. [7] showed that the use of preventive services was associated with a lower likelihood of late-stage diagnosis for breast cancer, colorectal cancer, and prostate cancer. Keating et al. [8] showed that women who saw a medical provider in the 2 years before diagnosis were significantly less likely to be diagnosed with advanced-stage disease. Furthermore, several studies have evaluated the use of mammography and have shown that its use is associated with a less-advanced stage at diagnosis [38, 39]. Our findings add additional evidence to support the idea that cancer can be identified early if women interact sufficiently, and appropriately, with the system.
Our findings extend this previous work in several ways. First, in the year before diagnosis (particularly when ignoring the 3-month pre-diagnosis period), the overall patterns of medical resource use for women with and without breast cancer were remarkably similar. Based on this, there does not appear to be any excess utilization in the year immediately preceding diagnosis. Second, the increase in resource use that occurs around diagnosis begins as early as 3 months before the month of diagnosis. Hence, the time it takes to diagnose a woman with breast cancer is variable, occurring over several months. Third, the rate of newly diagnosed conditions is very high in the 3-month period after the breast cancer diagnosis. These findings have implications for researchers as well as clinicians, particularly for researchers studying, or adjusting for, the effect of comorbidity on outcomes.
Studies of the comorbid conditions identified after breast cancer diagnosis are few. However, our results are comparable to those from a recent study of comorbid conditions in 1183 breast cancer patients in the Health, Eating, Activity, and Lifestyle Study (HEALS) [40]. In both HEALS and our study, hypertension was the most common comorbid condition at the time of diagnosis and also the most commonly identified new condition after cancer diagnosis. In contrast, our sample had higher rates of cardiovascular disease, which is not surprising given that our SEER–Medicare population was notably older. In addition, our study shows that many of these newly identified conditions appear shortly after diagnosis (within 3 months), and in a period of time associated with a substantial increase in the use of diagnostic tests and the initiation of interventions.
There are several key strengths to our analytic approach. The use of a noncancer control group has not been used in other related studies. Its inclusion is important because while claims data are limited in their ability to identify all clinically relevant disease, the control group facilitates internally consistent comparisons. Also, the calculation of both incidence and prevalence allows us to understand the complete picture of comorbidity around the time of diagnosis. The large sample size allows for more accurate rate estimation, particularly for less common conditions. Finally, matching by time and geographic area allows us to control for temporal trends and geographic variation (as well as socioeconomic factors to a limited degree), which can be difficult to adjust for, while allowing for analyses by race, stage, and age (not all of which are shown).
However, the limitations of these analyses also deserve discussion. The SEER–Medicare merged data lack certain variables (e.g. BMI) that would be useful for comparing women with and without breast cancer more precisely. In addition, we did not have complete medical histories for patients, particularly from their pre-Medicare coverage. Also, we were limited to diagnoses that are included in claims data. While studies have generally confirmed that claims data are reasonably sensitive and specific, there are limits to the reliability of claims data for identifying comorbid conditions [41, 42]. It is possible that some of the newly identified conditions are the result of cancer-directed therapy initiated shortly after diagnosis and are not previously undiagnosed conditions. On the other hand, the strong and consistent relationship across conditions between incidence and stage suggests otherwise, particularly in conditions that should not be related to breast cancer interventions (e.g. osteoarthritis). Finally, our measures of physician encounters and unique diagnostic testing are intentionally simplistic, and more sophisticated measures focusing on specific diagnostic tools and their utilization (as used by others) might provide improved insights into the nature of the interactions between providers and patients with respect to cancer diagnosis.
Even with these limitations, this study demonstrates that older women with breast cancer suffer from a myriad of comorbid conditions that may affect treatment and outcomes. Many of these are identified as a consequence of the cancer diagnosis. While some may result from common biological pathways, there is also an important contribution from health-seeking behavior before the cancer diagnosis. To the extent that this behavior is modifiable, particularly with screening-friendly reimbursement policies, it may be possible to find both cancer and comorbid conditions earlier and improve survival.
funding
This research was funded by Amgen, Inc. through a contract with Outcomes Insights, Inc. This contract specifies that the authors are free to publish findings based on this research without restriction. Outcomes Insights, Inc. has provided outcomes research and consulting services related to breast cancer to Amgen Inc., Celgene Inc., and Genentech Inc.
disclosures
This research was funded by Amgen. All authors work for Outcomes Insights, Inc., except Dr. O'Malley who is an employee of Amgen, Inc.
Supplementary Material
acknowledgements
We would like to thank Robert Herbert for his programming assistance with this project.
This study used the linked SEER–Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS) Inc.; and the SEER Program tumor registries in the creation of the SEER–Medicare database.
Institutional Review Board (IRB) approval: At the time of study approval in July 2008, IRB approval was not required. Analyses of the SEER–Medicare data were considered to be exempt from the need for IRB review according to the National Institutes of Health's Office of Human Subjects Research.
references
- 1.American Cancer Society. Breast Cancer Facts & Figures 2009-2010 [Internet] Atlanta, GA: American Cancer Society, Inc; 2009. http://www.cancer.org/downloads/STT/F861009_final%209-08-09.pdf. (21 May 2010, date last accessed) [Google Scholar]
- 2.National Cancer Institute. SEER Stat Fact Sheets: Breast [Internet] Bethesda, MD: National Cancer Institute 2010; http://seer.cancer.gov/statfacts/html/breast.html. (21 May 2010, date last accessed) [Google Scholar]
- 3.Satariano WA, Ragland DR. The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med. 1994;120(2):104–110. doi: 10.7326/0003-4819-120-2-199401150-00002. [DOI] [PubMed] [Google Scholar]
- 4.Yancik R, Wesley MN, Ries LA, et al. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285(7):885–892. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 5.Klabunde CN, Legler JM, Warren JL, et al. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17(8):584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21(7):1383–1389. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Gornick ME, Eggers PW, Riley GF. Associations of race, education, and patterns of preventive service use with stage of cancer at time of diagnosis. Health Serv Res. 2004;39(5):1403–1427. doi: 10.1111/j.1475-6773.2004.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keating NL, Landrum MB, Ayanian JZ, et al. The association of ambulatory care with breast cancer stage at diagnosis among Medicare beneficiaries. J Gen Intern Med. 2005;20(1):38–44. doi: 10.1111/j.1525-1497.2004.40079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tromp DM, Brouha XD, Hordijk GJ, et al. Medical care-seeking and health-risk behavior in patients with head and neck cancer: the role of health value, control beliefs and psychological distress. Health Educ Res. 2005;20(6):665–675. doi: 10.1093/her/cyh031. [DOI] [PubMed] [Google Scholar]
- 10.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(Suppl 8):IV3–IV18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 11.National Cancer Institute. Overview of the SEER Program [Internet] Bethesda, MD: National Cancer Institute; 2010. http://seer.cancer.gov/about/ (21 May 2010, date last accessed) [Google Scholar]
- 12.National Cancer Institute. SEER-Medicare: How the SEER & Medicare Data are Linked [Internet] Bethesda, MD: National Cancer Institute; 2010. http://healthservices.cancer.gov/seermedicare/overview/linked.html. (21 May 2010, date last accessed) [Google Scholar]
- 13.Fritz A, Ries L, editors. SEER Program Code Manual. 3rd edition. Bethesda: National Cancer Institute; 1998. [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 16.Romano PS, Roos LL, Luft HS, et al. A comparison of administrative versus clinical data: coronary artery bypass surgery as an example. Ischemic Heart Disease Patient Outcomes Research Team. J Clin Epidemiol. 1994;47(3):249–260. doi: 10.1016/0895-4356(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 17.Practice Management Information Corporation. ICD-9-CM. 6th edition. Los Angeles, CA: Practice Management Information Corporation; 2005. [Google Scholar]
- 18.Practice Management Information Corporation. HCPCS. Los Angeles, CA: Practice Management Information Corporation; 2005. [Google Scholar]
- 19.Rothman KJ, Greenland S. Modern Epidemiology. 2nd edition. Pliladelphia, PA: Lippincott Williams & Wilkins; 1998. [Google Scholar]
- 20.Hilbe JM. Negative Binomial Regression. New York: Cambridge University Press 2007; 2007. [Google Scholar]
- 21.McCullagh P, Nelder JA. Generalized Linear Models. 2nd edition. London: Chapman and Hall; 1998. [Google Scholar]
- 22.Rywik SL, Williams OD, Pajak A, et al. Incidence and correlates of hypertension in the Atherosclerosis Risk in Communities (ARIC) study and the Monitoring Trends and Determinants of Cardiovascular Disease (POL-MONICA) project. J Hypertens. 2000;18(8):999–1006. doi: 10.1097/00004872-200018080-00002. [DOI] [PubMed] [Google Scholar]
- 23.Kannel WB. Risk stratification in hypertension: new insights from the Framingham Study. Am J Hypertens. 2000;13(1 Pt 2):3S–10S. doi: 10.1016/s0895-7061(99)00252-6. [DOI] [PubMed] [Google Scholar]
- 24.de MP, Wutschert R, Heinzmann M, et al. Superficial vein thrombosis of lower limbs: influence of factor V Leiden, factor II G20210A and overweight. Thromb Haemost. 1998;80(2):239–241. [PubMed] [Google Scholar]
- 25.Heit JA, Silverstein MD, Mohr DN, et al. The epidemiology of venous thromboembolism in the community. Thromb Haemost. 2001;86(1):452–463. [PubMed] [Google Scholar]
- 26.Narayan KM, Boyle JP, Thompson TJ, et al. Effect of BMI on lifetime risk for diabetes in the U.S. Diabetes Care. 2007;30(6):1562–1566. doi: 10.2337/dc06-2544. [DOI] [PubMed] [Google Scholar]
- 27.Reijman M, Pols HA, Bergink AP, et al. Body mass index associated with onset and progression of osteoarthritis of the knee but not of the hip: the Rotterdam Study. Ann Rheum Dis. 2007;66(2):158–162. doi: 10.1136/ard.2006.053538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grotle M, Hagen KB, Natvig B, et al. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet Disord. 2008;9:132. doi: 10.1186/1471-2474-9-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ballard-Barbash R, Swanson CA. Body weight: estimation of risk for breast and endometrial cancers. Am J Clin Nutr. 1996;63(Suppl 3):437S–441S. doi: 10.1093/ajcn/63.3.437. [DOI] [PubMed] [Google Scholar]
- 30.McTiernan A. Associations between energy balance and body mass index and risk of breast carcinoma in women from diverse racial and ethnic backgrounds in the U.S. Cancer. 2000;88(Suppl 5):1248–1255. doi: 10.1002/(sici)1097-0142(20000301)88:5+<1248::aid-cncr12>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 31.Allen NE, Beral V, Casabonne D, et al. Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst. 2009;101(5):296–305. doi: 10.1093/jnci/djn514. [DOI] [PubMed] [Google Scholar]
- 32.Lew JQ, Freedman ND, Leitzmann MF, et al. Alcohol and risk of breast cancer by histologic type and hormone receptor status in postmenopausal women: the NIH-AARP Diet and Health Study. Am J Epidemiol. 2009;170(3):308–317. doi: 10.1093/aje/kwp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fleming ST, Pursley HG, Newman B, et al. Comorbidity as a predictor of stage of illness for patients with breast cancer. Med Care. 2005;43(2):132–140. doi: 10.1097/00005650-200502000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Vaeth PA, Satariano WA, Ragland DR. Limiting comorbid conditions and breast cancer stage at diagnosis. J Gerontol A Biol Sci Med Sci. 2000;55(10):M593–M600. doi: 10.1093/gerona/55.10.m593. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez EC, Ferrante JM, Van Durme DJ, et al. Comorbid illness and the early detection of cancer. South Med J. 2001;94(9):913–920. [PubMed] [Google Scholar]
- 36.Tetsche MS, Dethlefsen C, Pedersen L, et al. The impact of comorbidity and stage on ovarian cancer mortality: a nationwide Danish cohort study. BMC Cancer. 2008;8:31. doi: 10.1186/1471-2407-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zafar SY, Abernethy AP, Abbott DH, et al. Comorbidity, age, race and stage at diagnosis in colorectal cancer: a retrospective, parallel analysis of two health systems. BMC Cancer. 2008;8:345. doi: 10.1186/1471-2407-8-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mouchawar J, Taplin S, Ichikawa L, et al. Late-stage breast cancer among women with recent negative screening mammography: do clinical encounters offer opportunity for earlier detection? J Natl Cancer Inst Monogr. 2005;(35)):39–46. doi: 10.1093/jncimonographs/lgi036. [DOI] [PubMed] [Google Scholar]
- 39.Badgwell BD, Giordano SH, Duan ZZ, et al. Mammography before diagnosis among women age 80 years and older with breast cancer. J Clin Oncol. 2008;26(15):2482–2488. doi: 10.1200/JCO.2007.12.8058. [DOI] [PubMed] [Google Scholar]
- 40.Harlan LC, Klabunde CN, Ambs AH, et al. Comorbidities, therapy, and newly diagnosed conditions for women with early stage breast cancer. J Cancer Surviv. 2009;3(2):89–98. doi: 10.1007/s11764-009-0084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fowles JB, Lawthers AG, Weiner JP, et al. Agreement between physicians' office records and Medicare Part B claims data. Health Care Financ Rev. 1995;16:189–199. [PMC free article] [PubMed] [Google Scholar]
- 42.Warren JL, Klabunde CN, Mariotto AB, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med. 2009;150(12):849–857. doi: 10.7326/0003-4819-150-12-200906160-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.