Abstract
Background
Insulin/insulin-like growth factor-I (IGF-I) signaling is a mechanism mediating the promoting effect of type 2 diabetes (DM2) on cancer. Human epidermal growth factor receptor (HER2), insulin receptor and IGF-I receptor involve the same PI3K/AKT/mTOR signaling, and different antidiabetic pharmacotherapy may differentially affect this pathway, leading to different prognoses of HER2+ breast cancer.
Methods
We reviewed 1983 consecutive patients with HER2+ breast cancer treated between 1 January 1998 and 30 September 2010. The overall survival, breast cancer-specific death rate, age, race, nuclear grade, stage, menopausal status, estrogen and progesterone receptor status, body mass index and classes of antidiabetic pharmacotherapy were analyzed.
Results
A Cox regression analysis showed that DM2 [P = 0.026, hazard ratio (HR) = 1.42, 95 % confidence interval (95 % CI) 1.04–1.94] predicted poor survival of stage ≥2 HER2+ breast cancer. In Kaplan–Meier analysis, metformin predicted lengthened survival and so did thiazolidinediones. Analyzing only the diabetics, Cox regression showed that metformin (P = 0.041, HR = 0.52, 95 % CI 0.28–0.97) and thiazolidinediones (P = 0.036; HR = 0.41, 95 % CI 0.18–0.93) predicted lengthened survival, and competing risk analysis showed that metformin and thiazolidinediones were associated with decreased breast cancer-specific mortality (P = 0.023, HR = 0.47, 95 % CI 0.24–0.90 and P = 0.044, HR = 0.42, 95 % CI 0.18–0.98, respectively).
Conclusions
Thiazolidinediones and metformin users are associated with better clinical outcomes than nonusers in diabetics with stage ≥2 HER2+ breast cancer. The choice of antidiabetic pharmacotherapy may influence prognosis of this group.
Keywords: breast cancer-specific mortality, HER2-positive breast cancer, insulin, metformin, secretagogues, thiazolidinediones
introduction
Diabetes mellitus and cancer are major causes of morbidity and mortality worldwide. Extensive epidemiological data suggest important roles of type 2 diabetes mellitus (DM2) in carcinogenesis [1–4]. There is also evidence that DM2 is associated with decreased survival in breast cancer patients. A meta-analysis of association between preexisting diabetes and all-cause mortality in breast cancer patients using data from eight studies [5–12]showed that diabetic breast cancer patients had a 1.49 times higher risk of death, tended to present at later stages, and received less intense treatment regimens than nondiabetic breast cancer patients [13]. A recent retrospective cohort study [14], not included in the above meta-analysis [13], also examined the association of diabetes with the prognosis of early-stage breast cancer and found a close to twofold increase in mortality in diabetics compared with nondiabetics [14]. Similar to DM2, the metabolic syndrome, which is also characterized by insulin resistance and hyperinsulinemia, is a significant prognostic factor in postmenopausal breast cancer patients and is associated with increased risk of recurrence [15, 16].
The current consensus is that activation of insulin receptor and insulin-like growth factor-I (IGF-I) receptor signaling through the PI3K/AKT/mTOR signaling pathway mediates at least in part the promoting effect of DM2 on cancer risk and cancer progression [17]. About 25 % of breast cancers overexpress Human epidermal growth factor receptor 2 (HER2) (ErbB2), which is a growth factor receptor tyrosine kinase that also involves the same downstream signaling via PI3K, AKT and mTOR [18, 19]. Given that AKT/mTOR signaling is already active in HER2-overexpressing (HER2+) cancers [20], an interesting question is whether DM2 can further accelerate their growth. Thus far, most studies have investigated breast cancer inclusive of all subtypes [5–12, 21], and no study has specifically addressed the impact of DM2 on breast cancer-specific survival of patients with this specific subtype of breast cancer.
One major clinical question is whether specific strategies in diabetes care can improve survival of diabetic breast cancer patients. The foundation for this question is based on the fact that insulin and IGFs are key regulators of cell survival, proliferation and energy metabolism [19]; and extensive laboratory, epidermiological and clinical research all support that insulin/IGF signaling pathway underpins the influence of lifestyle and dietary factors on cancer prognosis [22, 23]. Although one may suspect that insulin and insulin analogues may accelerate cancer growth through activation of insulin and IGF-I receptors, evidence is lacking regarding any association of exogenous insulin use with poor breast cancer prognosis. In a prospective cohort study of early-stage breast cancer patients, fasting insulin level was associated with poor breast cancer outcomes in terms of distant recurrence and mortality [24]. In a recent prospective observational study, the association between C-peptide level (a stable surrogate marker of endogenous insulin secretion) and breast cancer-specific death was stronger in women with DM2 than in women without DM2 [25]. These studies suggest that anti-insulin resistance medications such as metformin and thiazolidinediones, which reduce fasting insulin and C-peptide levels in breast cancer patients, may be associated with improved breast cancer-specific survival in diabetic patients [25]. Metformin has also been shown to lower insulin levels in nondiabetic breast cancer patients [26]. Our group has carried out in vitro investigation on the differential impact of antidiabetic medications on cancer cells and found that metformin and thiazolidinediones inhibited cancer cell growth [27], in agreement with results for metformin [28–33] and thiazolidinediones [34–40] by other groups. Our recent retrospective review of diabetic prostate cancer patients revealed that metformin and thiazolidinediones were associated with improved overall survival [41]. Specifically, regarding HER2+ breast cancer, metformin decreases HER2 expression by inhibiting signaling through mTOR in vitro [42], and metformin prolongs survival in a HER2-overexpressing transgenic mouse model of breast cancer [43].
The relevant clinical question in the management of a diabetic patient already diagnosed with cancer is whether the choice of antidiabetic pharmacotherapy can influence the clinical outcome of the malignancy and, ultimately, the breast cancer-specific survival. Because it is very costly and time consuming to examine the impact of antidiabetic drugs on cancer progression by randomized controlled human trials [17], we carried out a retrospective review of HER2+ breast cancer patients evaluated at our institution to examine the impact of different classes of antidiabetic pharmacotherapy on breast cancer-specific survival.
patients and methods
study population
The study was approved by The University of Texas MD Anderson Cancer Center Institutional Review Board in accordance with an assurance filed with and approved by the U.S. Department of Health and Human Services. MD Anderson's Breast Cancer Management System Database was searched, and 2792 consecutive patients with histologically confirmed primary HER2+ breast cancer treated at MD Anderson between 1 January 1998 and 30 September 2010 were identified. Among these breast cancer patients, 238 were diabetic at the time of breast cancer diagnosis. The following exclusion criteria were applied to these 2792 patients: (i) ductal carcinoma in situ or stage 1 disease, (ii) male sex, (iii) unknown estrogen receptor (ER) or progesterone receptor (PR) status, (iv) resolved gestational diabetes, (v) type 1 diabetes mellitus, (vi) diabetic patients on dietary management only and not on any form of antidiabetic pharmacotherapy before and after diagnosis of breast cancer and (vii) incomplete records (including medication records). The final study cohort consisted of 1983 patients: 154 diabetic patients taking antidiabetic medications (diabetic group) and 1829 nondiabetics (nondiabetic group), all of whom had stage ≥2 HER2+ breast cancer.
data collection
Trained personnel reviewed online patient records to collect information on demographics and known or suspected risk factors for breast cancer prognosis. Cancer stage was defined based on the clinical stage at the time of diagnosis using the sixth edition of the American Joint Committee on Cancer's AJCC Cancer Staging Manual. From the pathology reports, we obtained the HER2 status: a tumor was considered HER2+ if it had a staining intensity of 3+ on immunohistochemical analysis or amplification (>2.2 copies) of the HER2 gene as demonstrated by FISH. The nuclear grade, ER and PR expression were also recorded based on the pathology reports. A tumor was considered ER or PR positive if the respective immunostaining showed ≥10 % cells with expression.
Body mass index (BMI) for each patient was calculated using the recorded height and body weights during the first clinic visit at MD Anderson. Based on recent investigations of the relationship between BMI and cause-specific mortality in different ethnic groups [44, 45], we categorized BMI (kg/m2) as follows: <20 (underweight), 20 to <25 (normal), 25 to <30 (overweight), 30 to <35 (obese) and ≥35 (morbidly obese).
DM2 was determined based on the recorded medical history and was confirmed by antidiabetic pharmacotherapy in both the medical and pharmacy records. Overall survival time was defined as the duration between the date of cancer diagnosis and the date of death. For patients who did not die or were not followed closely at our institution near their dates of death, the dates of death were obtained by Customer Service Representatives of the Tumor Registry section of Medical Informatics who follow up by mailed questionnaires to all the patients who had not been seen in our clinics for over 1 year or by search of public databases including Social Security Death Index and other state and local vital records and obituaries. In only ∼2 % of the cases, we had to resort to attributing the survival time as the duration between the date of cancer diagnosis and the date of our last record when the patient was alive. If the patient was not known dead, survival time was censored at the date of last follow-up during the monitoring period. The primary end point addressed in this study was breast cancer-specific death. Death was attributed to HER2+ breast cancer if at least one of the following criteria were met: (i) breast cancer was listed as a cause of death on the death certificate, (ii) there was clinical evidence of breast cancer progression at the time of death despite chemotherapy or palliative radiotherapy or (iii) the patient was receiving hospice care for end-stage breast cancer at the time of death. Patients for whom the cause of death could not be determined were included in the category of ‘other causes’ of death.
statistical analysis
Baseline patient characteristics and risk factors of breast cancer prognosis were compared between groups by the χ2 test, Student's t-test or Mann–Whitney rank sum test where appropriate. The association of HER2+ breast cancer patient survival with each class of antidiabetic pharmacotherapy was evaluated by the Kaplan–Meier method. The relationships of risk factors to overall survival (e.g. age, race, DM2 status, BMI, tumor nuclear grade, hormone receptor status, stage at the time of cancer diagnosis, insulin use, insulin secretagogue use, metformin use, thiazolidinedione use) were analyzed using multivariate Cox regression analysis. Age was categorized as ≤35, >35 to <65 and ≥65 years. BMI was categorized as described above. Antidiabetic pharmacotherapy was classified as (i) insulin or insulin analogues, (ii) biguanides, (iii) thiazolidinediones, (iv) insulin secretagogues (e.g. sulfonylureas and meglitinides) and (v) others (including α-glucosidase inhibitors, dipeptidyl peptidase-4 inhibitors, amylin analogues and glucagon-like peptide 1 analogues). Because many patients used combination therapy, and the drugs or combinations might have changed over time, and the number of patients in each monotherapy group was small, we represented the antidiabetic pharmacotherapy of each patient with four categorical attributes of user versus nonuser of (i) insulin formulations or insulin analogues, (ii) insulin secretagogues, (iii) metformin and (iv) thiazolidinediones. These attributes were classified according to medication use at the time of presentation and subsequent medication records at our institution. ‘Users’ of a class of drug meant that the patients used at presentation or subsequently changed to or added a member of that class of drug. These categorical variables were used in regression models to examine the association with specific types of antidiabetic pharmacotherapy.
Competing risk analysis based on the Fine and Gray model was carried out to analyze HER2+ breast cancer-specific death using the R statistical package [R version 2.13.0 (13 April 2011), The R Foundation for Statistical Computing]. Function cuminc was used to analyze cumulative incidence, and function crr was used to evaluate a regression model for competing risk.
All other statistical analyses were carried out using SPSS version 18.0 (SPSS, Chicago, IL) and SigmaPlot version 11.0 (Systat, Chicago, IL) software with two-sided tests, with a P-value of ≤0.05 considered statistically significant.
results
patient demographics and clinical characteristics
The median follow-up in the study population was 47.6 months (range 0.3–152.2 months). Demographics and clinical characteristics of the study patients are summarized in Table 1. The mean age of patients in the diabetic group and those in the nondiabetic group were not significantly different, but the distribution among age groups was significantly different (P < 0.001) with no patient in the diabetic group being <35 years old. There was a higher proportion (P < 0.001) of diabetic postmenopausal patients than nondiabetic. The racial distribution is also different between the diabetic and nondiabetic groups (P = 0.004). There were also higher proportions of diabetic patients in the overweight and obese categories than in the nondiabetic group (P < 0.001). The other standard prognostic factors (stage, nuclear grade and ER/PR status) were not significantly different between the diabetic and nondiabetic groups (Table 1). We also evaluated the characteristics of the diabetic patients based on their diabetes medications. There were a higher proportion (P = 0.030) of diabetic patients >65 years in the metformin nonuser group than the metformin user group. There were a higher proportion of stage 2 patients in thiazolidinedione nonusers than users (P = 0.030). These results were consistent with the known facts that DM2 is associated with age and obesity and old women are more likely to be postmenopausal than young women.
Table 1.
Patient demographics and clinical characteristics of the study population
| Characteristic | Metformin user | Metformin nonuser | P-value | Thiazolidinedione user | Thiazolidinedione nonuser | P-value | Insulin user | Insulin nonuser | P-value | Secretagogue user | Secretagogue nonuser | P-value | Diabetic patients | Nondiabetic patients | P-value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at diagnosis (years) | 0.030 | 0.281 | 0.397 | 0.203 | <0.001 | ||||||||||
| Median (range) | 55 (36–77) | 56 (26–83) | 55 (36–81) | 56 (26–83) | 55 (26–82) | 55.5 (36–83) | 55 (36–83) | 55 (26–82) | 55 (26–83) | 49 (21–89) | |||||
| <35 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 218 | |||||
| 35–65 | 75 | 46 | 30 | 91 | 39 | 82 | 57 | 64 | 121 | 1436 | |||||
| >65 | 13 | 19 | 5 | 27 | 8 | 24 | 19 | 13 | 32 | 175 | |||||
| Menopausal status | 0.460 | 0.815 | 0.762 | 0.369 | <0.001 | ||||||||||
| Pre- and perimenopausal | 17 | 16 | 7 | 26 | 11 | 22 | 14 | 19 | 33 | 829 | |||||
| Postmenopausal | 71 | 50 | 28 | 93 | 37 | 84 | 62 | 59 | 121 | 1000 | |||||
| Race | 0.630 | 0.095 | 0.601 | 0.528 | 0.004 | ||||||||||
| White | 50 | 35 | 15 | 70 | 25 | 60 | 40 | 45 | 85 | 1217 | |||||
| Spanish/Hispanic | 19 | 17 | 13 | 23 | 12 | 24 | 17 | 19 | 36 | 270 | |||||
| African-American | 13 | 11 | 6 | 18 | 9 | 15 | 15 | 9 | 24 | 226 | |||||
| Other | 6 | 3 | 1 | 8 | 2 | 7 | 4 | 5 | 9 | 116 | |||||
| BMI (kg/m2) | 0.596 | 0.289 | 0.319 | 0.532 | <0.001 | ||||||||||
| Median (range) | 32.1 (20.6–51.3) | 32.8 (17.7–48.6) | 31.5 (22.1–50.3) | 32.8 (17.7–48.0) | 31.6 (18.7–47.1) | 32.8 (17.7–50.3) | 32.8 (17.7–50.3) | 32.8 (18.7–45.2) | 32.8 (17.7–50.3) | 27.9 (16.5–60.1) | |||||
| Underweight, BMI < 20 | 0 | 3 | 4 | 3 | 2 | 1 | 1 | 2 | 3 | 76 | |||||
| Normal, BMI 20 to <25 | 11 | 3 | 8 | 9 | 2 | 12 | 5 | 9 | 14 | 559 | |||||
| Overweight, BMI 25 to <30 | 21 | 18 | 10 | 32 | 15 | 24 | 22 | 17 | 39 | 638 | |||||
| Obese, BMI 30 to <35 | 25 | 16 | 7 | 31 | 14 | 27 | 18 | 23 | 41 | 344 | |||||
| Morbidly obese, BMI ≥ 35 | 31 | 26 | 6 | 44 | 15 | 42 | 30 | 27 | 57 | 212 | |||||
| Breast cancer stage | 0.770 | 0.030 | 0.215 | 0.143 | 0.978 | ||||||||||
| II | 46 | 36 | 13 | 69 | 22 | 60 | 45 | 37 | 82 | 976 | |||||
| III | 34 | 24 | 19 | 39 | 18 | 40 | 28 | 30 | 58 | 624 | |||||
| IV | 8 | 6 | 3 | 11 | 8 | 6 | 3 | 11 | 14 | 229 | |||||
| Nuclear grade | 0.950 | 0.298 | 0.553 | 0.937 | 0.367 | ||||||||||
| I and II | 17 | 13 | 9 | 21 | 8 | 22 | 15 | 15 | 30 | 414 | |||||
| III | 71 | 53 | 26 | 98 | 40 | 84 | 61 | 63 | 124 | 1415 | |||||
| ER/PR status | 0.070 | 0.506 | 0.249 | 0.627 | 0.355 | ||||||||||
| Both negative | 49 | 27 | 19 | 57 | 27 | 49 | 36 | 40 | 76 | 832 | |||||
| Either one positive | 39 | 39 | 16 | 62 | 21 | 57 | 40 | 38 | 78 | 997 |
BMI, body mass index; ER, estrogen receptor; PR, progesterone receptor.
association of DM2 with overall survival of patients with HER2+ breast cancer after controlling for age, ER/PR status, nuclear grade, BMI and stage
A multivariate Cox proportional hazards regression model examined DM2 along with race, age, ER/PR status, nuclear grade, BMI and stage as predictive factors for overall survival of these patients with HER2+ breast cancer. As listed in Table 2, DM2 was a significant independent predictor [hazard ratio (HR) = 1.42, 95 % confidence interval (CI): 1.04–1.94, P = 0.026] of decreased overall survival of patients with stage ≥2 HER2+ breast cancer after adjustment for age, ER/PR status, nuclear grade, BMI and stage. Other significant factors were age (<35 years compared with 35–65 years: HR = 1.51, 95 % CI 1.15–1.97, P = 0.003), age (>65 years compared with 35–65 years: HR = 1.39, 95 % CI 1.08–1.79, P = 0.010), stage (stage 4 compared with stage 2: HR = 4.74, 95 % CI 3.83–5.88, P < 0.001; stage 3 compared with stage 2: HR = 1.68, 95 % CI 1.39–2.03, P < 0.001), both ER and PR negative (HR = 1.54, 95 % CI 1.30–1.82, P < 0.001) and BMI (30 to <35 versus 20 to <25 kg/m2: HR = 0.74, 95 % CI 0.57–0.95, P = 0.017; ≥35 versus 20 to <25 kg/m2: HR = 0.73, 95 % CI 0.55–0.97, P = 0.030). Therefore, DM2, age at diagnosis, stage at diagnosis, ER/PR status and BMI are significant independent predictors of overall survival of patients with stage ≥2 HER2+ breast cancer.
Table 2.
Multivariate Cox proportional hazards regression model for overall survival of patients with stage ≥2 HER2+ breast cancer
| Variables | P-value | Hazard ratio | 95 % CI for hazard ratio |
|
|---|---|---|---|---|
| Lower | Upper | |||
| White (yes versus no) | 0.471 | 1.07 | 0.89 | 1.27 |
| DM2 (yes versus no) | 0.026 | 1.42 | 1.04 | 1.94 |
| Stage (3 versus 2) | <0.001 | 1.68 | 1.39 | 2.03 |
| Stage (4 versus 2) | <0.001 | 4.74 | 3.83 | 5.88 |
| ER and PR negative (yes versus no) | <0.001 | 1.54 | 1.30 | 1.82 |
| High nuclear grade (yes versus no) | 0.490 | 0.93 | 0.76 | 1.14 |
| Age (<35 versus 35 to 65 years) | 0.003 | 1.51 | 1.15 | 1.97 |
| Age (>65 versus 35 to 65 years) | 0.010 | 1.39 | 1.08 | 1.79 |
| BMI (<20 versus 20 to <25 kg/m2) | 0.545 | 1.14 | 0.75 | 1.72 |
| BMI (25–<30 versus 20 to <25 kg/m2) | 0.487 | 0.93 | 0.76 | 1.14 |
| BMI (30–<35 versus 20 to <25 kg/m2) | 0.017 | 0.74 | 0.57 | 0.95 |
| BMI (≥35 versus 20 to <25 kg/m2) | 0.030 | 0.73 | 0.55 | 0.97 |
BMI, body mass index; CI, confidence interval; DM2, type 2 diabetes mellitus; ER, estrogen receptor; PR, progesterone receptor.
association of antidiabetic pharmacotherapies with overall survival
In Kaplan–Meier analysis, diabetics who used insulin had a significantly decreased survival duration compared with diabetic nonusers (log-rank test, P = 0.029; Figure 1A) and compared with nondiabetics (log-rank test, P = 0.005; Figure 1A). The median survival of diabetic insulin nonusers was 42.2 months compared with 37.8 months in insulin users. However, insulin secretagogue usage was not a significant predictor of survival (Figure 1B).
Figure 1.
Insulin use is associated with decreased overall survival while metformin use and thiazolidinedione use are associated with improved survival of diabetic patients with stage ≥2 HER2+ breast cancer. Kaplan–Meier survival curves comparing users (red curves), diabetic nonusers (blue curves) and nondiabetic patients (black curves) are shown for insulins (A), insulin secretagogues (B), insulins and/or insulin secretagogues (C), metformin (D), thiazolidinediones (E), and metformin and/or thiazolidinediones (F).
In stark, contrast diabetic patients who received metformin therapy had a significantly longer survival duration compared with diabetics who received no metformin therapy (log-rank test, P = 0.045; Figure 1D) and nondiabetics (log-rank test, P = 0.007; Figure 1D). The median survival of diabetic metformin nonusers was 37.4 months compared with 42.4 months in metformin users. Diabetics who received thiazolidinedione therapy also had a significantly longer survival duration compared with diabetics who did not receive thiazolidinedione therapy (log-rank test, P = 0.034; Figure 1E) and nondiabetics (log-rank test, P = 0.014; Figure 1E). The median survival of diabetic thiazolidinedione nonusers was 37.3 months compared with 49.8 months in thiazolidinedione users. Likewise, patients who received metformin and/or thiazolidinediones had a significantly longer survival duration compared with diabetic nonusers (log-rank test, P = 0.002; Figure 1F) and nondiabetics (log-rank test, P = 0.001; Figure 1F). The median survival of diabetic metformin ± thiazolidinedione nonusers was 36.9 months compared with 42.4 months in metformin ± thiazolidinedione users. Life table analysis of these data was shown in Table S1 (available as supplementary data in Annals of Oncology online). Our results suggested that insulin therapy was associated with a prognosis worse than that of nondiabetics, while metformin and thiazolidinedione therapies were associated with a prognosis indistinguishable from that of nondiabetics.
association of antidiabetic pharmacotherapies with overall survival of diabetic patients with stage ≥2 HER2+ breast cancer in multivariate analysis
Multivariate Cox proportional hazards regression analysis of overall survival of diabetic patients with stage ≥2 HER2+ breast cancer was carried out using a model consisting of the categorical covariates: age at diagnosis, BMI, stage at diagnosis, ER/PR both negative, insulin usage, insulin secretagogue usage, thiazolidinedione usage and metformin usage (Table 3). Race and nuclear grade were not included in the model because they were not significant predictors of overall survival (Table 2). As expected, stage at diagnosis (stage 3 & 4 versus stage 2) was a significant (P = 0.005) predictor of overall survival (HR = 2.45, 95 % CI 1.31–4.59) (Table 3). Thiazolidinedione usage was a significant (P = 0.033) predictor of favorable survival (HR = 0.41, 95 % CI 0.18–0.93) and so was metformin usage (P = 0.041, HR = 0.52, 95 % CI 0.28–0.97). ER and PR negative, age at diagnosis over 65 years and BMI ≥30 kg/m2 were near significant factors with the respective P-values of 0.069, 0.072 and 0.093, respectively. Therefore, stage at diagnosis, metformin therapy and thiazolidinedione therapy are significant predictors of overall survival of diabetic patients with stage ≥2 HER2+ breast cancer after adjustment for age, BMI, ER/PR status, insulin therapy and insulin secretagogue therapy.
Table 3.
Multivariate Cox proportional hazards regression model for overall survival of diabetic patients with stage ≥2 HER2+ breast cancer
| Variables | P-value | Hazard ratio | 95 % CI for hazard ratio |
|
|---|---|---|---|---|
| Lower | Upper | |||
| Stage (3 & 4 versus 2) | 0.005 | 2.45 | 1.31 | 4.59 |
| ER and PR negative (yes versus no) | 0.069 | 0.56 | 0.30 | 1.05 |
| Insulin (users versus nonusers) | 0.336 | 1.38 | 0.72 | 2.67 |
| Insulin secretagogues (users versus nonusers) | 0.573 | 1.19 | 0.64 | 2.21 |
| Thiazolidinediones (users versus nonusers) | 0.033 | 0.41 | 0.18 | 0.93 |
| Metformin (users versus nonusers) | 0.041 | 0.52 | 0.28 | 0.97 |
| Age (>65 versus ≤65 years) | 0.072 | 1.80 | 0.95 | 3.42 |
| BMI (<20 versus 20 to <30 kg/m2) | 0.234 | 0.28 | 0.04 | 2.25 |
| BMI (≥30 versus 20 to <30 kg/m2) | 0.093 | 0.59 | 0.32 | 1.09 |
BMI, body mass index; CI, confidence interval; ER, estrogen receptor; PR, progesterone receptor.
association of antidiabetic pharmacotherapies with breast cancer-specific death
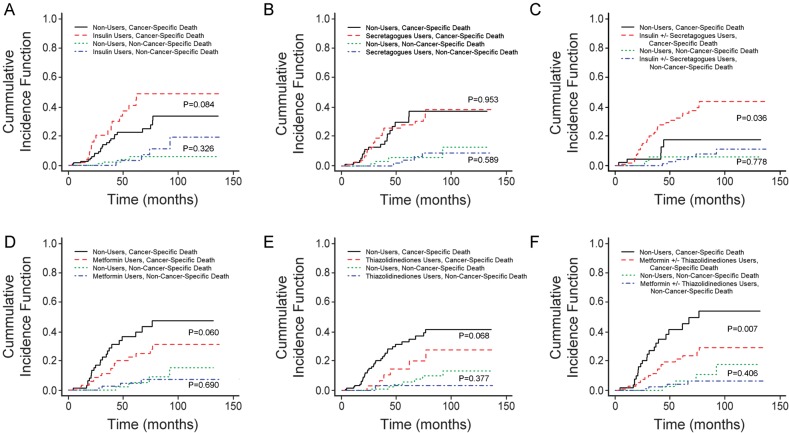
Competing risk analysis was carried out using the R statistics package [46] to analyze breast cancer-specific mortality in diabetic patients with stage ≥2 HER2+ breast cancer. Cumulative incidence estimate plots for breast cancer-specific death among patients grouped by antidiabetic medications are presented in Figure 2. When comparing insulin users versus insulin nonusers, there was no significant increase in breast cancer-specific mortality and no significant difference in mortality from other causes (Figure 2A). When comparing insulin secretagogue users versus nonusers, there was no significant difference in breast cancer-specific mortality and no significant difference in mortality from other causes (Figure 2B). When comparing insulin ± secretagogue users versus nonusers, there was a significant (P = 0.036) increase in breast cancer-specific mortality and no significant difference in mortality of other causes (Figure 2C). When comparing metformin users versus nonusers, there was no significant increase in breast cancer-specific mortality and no significant difference in mortality from other causes (Figure 2D). When comparing thiazolidinedione users versus nonusers, there was no significant difference in breast cancer-specific mortality and no significant difference in mortality of other causes (Figure 2E). When comparing metformin ± thiazolidinedione users versus nonusers, there was a significant (P = 0.007) increase in breast cancer-specific mortality and no significant difference in mortality from other causes (Figure 2F). These results suggested that insulin ± secretagogue therapy was associated with increased, while metformin ± thiazolidinedione therapy was associated with decreased cumulative incidence of breast cancer-specific death in diabetic patients with stage ≥2 HER2+ breast cancer.
Figure 2.
Metformin and thiazolidinediones are associated with decreased cumulative incidence of breast cancer-specific mortality of diabetic patients with HER2+ breast cancer. Cumulative incidence curves of breast cancer-specific deaths comparing users (red curves) and diabetic nonusers (black curves) and those of non-breast cancer-specific deaths comparing users (blue curves) and diabetic nonusers (green curves) are shown for insulin (A), insulin secretagogues (B), insulin and/or insulin secretatgogues (C), metformin (D), thiazolidinediones (E) and metformin and/or thiazolidinediones (F). The P-values are as labeled close to the compared curves.
association of antidiabetic pharmacotherapies with breast cancer-specific mortality in multivariate analysis
In multivariate regression analysis of risk of competing events, we examined factors for breast cancer-specific mortality in diabetic patients with stage ≥2 HER2+ breast cancer using a regression model consisting of the same factors as the model reported in Table 3 (Table 4). The presence of advanced-stage (stage 3 & 4) breast cancer was associated with significantly (P = 0.004) increased breast cancer-specific mortality (HR = 2.70, 95 % CI 1.38–5.27). Both metformin usage and thiazolidinedione usage were associated with significantly decreased HRs for breast cancer-specific mortality (P = 0.023, HR = 0.47, 95 % CI 0.24–0.90 and P = 0.044, HR = 0.42, 95 % CI 0.18–0.98, respectively). Therefore, stage at diagnosis, metformin therapy and thiazolidinedione therapy are significant predictors of breast cancer-specific mortality in diabetic patients with stage ≥2 HER2+ breast cancer.
Table 4.
Competing risks regression model for breast cancer-specific deaths in diabetic patients with stage ≥2 HER2+ breast cancer
| Variables | P-value | Hazard ratio | 95 % CI for hazard ratio |
|
|---|---|---|---|---|
| Lower | Upper | |||
| Stage (3 & 4 versus 2) | 0.004 | 2.70 | 1.38 | 5.27 |
| ER and PR negative (yes versus no) | 0.118 | 1.73 | 0.87 | 3.42 |
| Insulin (users versus nonusers) | 0.466 | 1.31 | 0.64 | 2.69 |
| Insulin secretagogues (users versus nonusers) | 0.562 | 1.21 | 0.64 | 2.28 |
| Thiazolidinediones (users versus nonusers) | 0.044 | 0.42 | 0.18 | 0.98 |
| Metformin (users versus nonusers) | 0.023 | 0.47 | 0.24 | 0.90 |
| Age (>65 versus ≤65) | 0.262 | 1.51 | 0.74 | 3.11 |
| BMI (<20 versus 20 to <30) | <0.001 | <0.001 | <0.001 | <0.001 |
| BMI (≥30 versus 20 to <30) | 0.178 | 0.64 | 0.33 | 1.23 |
BMI, body mass index; CI, confidence interval; ER, estrogen receptor; PR, progesterone receptor.
discussion
After reviewing close to 2000 medical records in detail, our analysis of the association of DM2 and antidiabetic pharmacotherapy with overall survival in stage ≥2 HER2+ breast cancer found that DM2, age at diagnosis, stage at diagnosis and ER/PR status are significant predictors of overall survival in multivariate analysis (Table 2). This is the first report of the association of DM2 with poor prognosis in this particular subtype of breast cancer. Since HER2, insulin receptor and IGF-I receptor all involve the same downstream signaling via PI3K, AKT and mTOR [47], our finding suggests that DM2 can further accelerate growth of HER2+ breast cancer given that AKT/mTOR signaling is already active [48]. This conclusion is also in agreement with our published in vitro data that increased glucose and insulin concentrations in culture media, mimicking hyperglycemia and hyperinsulinemia, respectively, in DM2, promote growth of HER18 cells (an MCF7 subline stably transfected to overexpress HER2) [27].
Our Kaplan–Meier analyses of nondiabetics and diabetics categorized based on use of a particular class of antidiabetic pharmacotherapy are revealing. Bowker et al. [49] found that DM2 patients treated with sulfonylureas and insulin had a significantly higher risk of cancer-related mortality compared with patients treated with metformin. The denominator for cancer-related mortality rate in this study included DM2 patients without cancer, and this observed difference between factors was the combined effect on both carcinogenesis (cancer risk) and cancer progression (prognosis after diagnosis). Three possible explanations for the observed difference were offered: (i) a ‘deleterious effect of sulfonylurea and insulin’, (ii) a ‘protective effect of metformin’ or (iii) ‘some unmeasured effect related to both choice of therapy and cancer risk’ [49]. With the nondiabetic group included in our analyses (Figure 1), it appears that insulin therapy may be associated with a prognosis worse than that of nondiabetics, while insulin secretagogue treatments may be neutral. However, our retrospective study cannot rule out unrecognized confounding factors related to choice of pharmacotherapy for DM2 and cancer progression.
Different classes of antidiabetic pharmacotherapy have a differential impact on the progression of cancer cells [27] and on the survival of pancreatic cancer patients [50]. Similar to the results of our analysis in diabetic prostate cancer patients [41], we found that metformin therapy and thiazolidinedione therapy were significant predictors of increased overall survival of diabetic patients with stage ≥2 HER2+ breast cancer (Table 3). Insulin ± secretagogue therapy was associated with increased cumulative incidence, while metformin ± thiazolidinedione therapy was associated with decreased cumulative incidence of breast cancer-specific death in this patient population (Figure 2). In multivariate regression, stage at diagnosis, metformin therapy and thiazolidinedione therapy were significant predictors of breast cancer-specific mortality (Table 4).
Hyperinsulinemia, hyperglycemia, adiposity, inflammation and diabetes treatment are all potential factors in diabetic patients that may mediate the impact of DM2 on breast cancer prognosis [51]. It is challenging to evaluate the impact of pharmacotherapy for DM2 for several reasons: (i) there is often limited statistical power to detect modest differences, (iii) patients often receive combination treatment with more than one antidiabetic medication, and (iii) patients often have changes in their pharmacotherapy over time [17]. Evidence is accumulating to suggest that both metformin [28–32] and thiazolidinediones [34–40] inhibit cancer proliferation through a variety of mechanisms beyond their impact on systemic insulin sensitivity.
The current consensus regarding the choice of antidiabetic pharmacotherapy in diabetic cancer patients is that the currently available evidence is not conclusive and the choice of pharmacotherapy for DM2 ought not to be based on the potential impact on cancer progression [51, 52]. However, our study provides the first clinical evidence for the potential benefits of metformin and thiazolidinediones for a defined patient population (i.e. diabetic patients with stage ≥2 HER2+ breast cancer). More research (including randomized prospective clinical trials to evaluate the survival benefits of metformin and thiazolidinediones, alone or in combination, for well-defined cancer patient groups) will be necessary to confirm our findings and may lead to changes in the standard of clinical management of DM2 in breast cancer patients to maximize survival in the future.
funding
This work was supported by an R01 grant from the U. S. National Institutes of Health (NIHRO1CA 089266 to M-HL); a Department of Defense, Breast Cancer Research Program (BCRP) of Congressionally Directed Medical Research Programs (CDMRP) Synergistic Idea Development Award (BC062166 to S-CJY and M-HL); Susan G Komen For the Cure, PROMISE grant (KG081048 to S-CJY, M-HL and FJE); and a National Institutes of Health Cancer Center Support Grant (CA16672) to The University of Texas MD Anderson Cancer Center.
disclosure
The authors declare no conflicts of interest.
references
- 1.Nilsen TI, Vatten LJ. Prospective study of colorectal cancer risk and physical activity, diabetes, blood glucose and BMI: exploring the hyperinsulinaemia hypothesis. Br J Cancer. 2001;84:417–422. doi: 10.1054/bjoc.2000.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verlato G, Zoppini G, Bonora E, et al. Mortality from site-specific malignancies in type 2 diabetic patients from verona. Diabetes Care. 2003;26:1047–1051. doi: 10.2337/diacare.26.4.1047. [DOI] [PubMed] [Google Scholar]
- 3.Richardson LC, Pollack LA. Therapy insight: influence of type 2 diabetes on the development, treatment and outcomes of cancer. Nat Clin Pract Oncol. 2005;2:48–53. doi: 10.1038/ncponc0062. [DOI] [PubMed] [Google Scholar]
- 4.Coughlin SS, Calle EE, Teras LR, et al. Diabetes mellitus as a predictor of cancer mortality in a large cohort of us adults. Am J Epidemiol. 2004;159:1160–1167. doi: 10.1093/aje/kwh161. [DOI] [PubMed] [Google Scholar]
- 5.Lipscombe LL, Goodwin PJ, Zinman B, et al. The impact of diabetes on survival following breast cancer. Breast Cancer Res Treat. 2008;109:389–395. doi: 10.1007/s10549-007-9654-0. [DOI] [PubMed] [Google Scholar]
- 6.Yancik R, Wesley MN, Ries LA, et al. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285:885–892. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 7.van de Poll-Franse LV, Houterman S, Janssen-Heijnen ML, et al. Less aggressive treatment and worse overall survival in cancer patients with diabetes: a large population based analysis. Int J Cancer. 2007;120:1986–1992. doi: 10.1002/ijc.22532. [DOI] [PubMed] [Google Scholar]
- 8.Srokowski TP, Fang S, Hortobagyi GN, et al. Impact of diabetes mellitus on complications and outcomes of adjuvant chemotherapy in older patients with breast cancer. J Clin Oncol. 2009;27:2170–2176. doi: 10.1200/JCO.2008.17.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tammemagi CM, Nerenz D, Neslund-Dudas C, et al. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA. 2005;294:1765–1772. doi: 10.1001/jama.294.14.1765. [DOI] [PubMed] [Google Scholar]
- 10.Du W, Simon MS. Racial disparities in treatment and survival of women with stage i-iii breast cancer at a large academic medical center in metropolitan Detroit. Breast Cancer Res Treat. 2005;91:243–248. doi: 10.1007/s10549-005-0324-9. [DOI] [PubMed] [Google Scholar]
- 11.Fleming ST, Pursley HG, Newman B, et al. Comorbidity as a predictor of stage of illness for patients with breast cancer. Med Care. 2005;43:132–140. doi: 10.1097/00005650-200502000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Fleming ST, Rastogi A, Dmitrienko A, et al. A comprehensive prognostic index to predict survival based on multiple comorbidities: a focus on breast cancer. Med Care. 1999;37:601–614. doi: 10.1097/00005650-199906000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Peairs KS, Barone BB, Snyder CF, et al. Diabetes mellitus and breast cancer outcomes: a systematic review and meta-analysis. J Clin Oncol. 2011;29:40–46. doi: 10.1200/JCO.2009.27.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schrauder MG, Fasching PA, Haberle L, et al. Diabetes and prognosis in a breast cancer cohort. J Cancer Res Clin Oncol. 2011;137:975–983. doi: 10.1007/s00432-010-0960-2. [DOI] [PubMed] [Google Scholar]
- 15.Pasanisi P, Berrino F, De Petris M, et al. Metabolic syndrome as a prognostic factor for breast cancer recurrences. Int J Cancer. 2006;119:236–238. doi: 10.1002/ijc.21812. [DOI] [PubMed] [Google Scholar]
- 16.Benson JR, Jatoi I, Keisch M, et al. Early breast cancer. Lancet. 2009;373:1463–1479. doi: 10.1016/S0140-6736(09)60316-0. [DOI] [PubMed] [Google Scholar]
- 17.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esteva FJ, Hortobagyi GN, Sahin AA, et al. Expression of erbB/HER receptors, heregulin and p38 in primary breast cancer using quantitative immunohistochemistry. Pathol Oncol Res. 2001;7:171–177. doi: 10.1007/BF03032345. [DOI] [PubMed] [Google Scholar]
- 19.Jin Q, Esteva FJ. Cross-talk between the ErbB/HER family and the type I insulin-like growth factor receptor signaling pathway in breast cancer. J Mammary Gland Biol Neoplasia. 2008;13:485–498. doi: 10.1007/s10911-008-9107-3. [DOI] [PubMed] [Google Scholar]
- 20.Esteva FJ, Sahin AA, Cristofanilli M, et al. Molecular prognostic factors for breast cancer metastasis and survival. Semin Radiat Oncol. 2002;12:319–328. doi: 10.1053/srao.2002.35251. [DOI] [PubMed] [Google Scholar]
- 21.Brewster AM, Hortobagyi GN, Broglio KR, et al. Residual risk of breast cancer recurrence 5 years after adjuvant therapy. J Natl Cancer Inst. 2008;100:1179–1183. doi: 10.1093/jnci/djn233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 23.Esteva FJ, Sahin AA, Smith TL, et al. Prognostic significance of phosphorylated p38 mitogen-activated protein kinase and HER-2 expression in lymph node-positive breast carcinoma. Cancer. 2004;100:499–506. doi: 10.1002/cncr.11940. [DOI] [PubMed] [Google Scholar]
- 24.Goodwin PJ, Ennis M, Pritchard KI, et al. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol. 2002;20:42–51. doi: 10.1200/JCO.2002.20.1.42. [DOI] [PubMed] [Google Scholar]
- 25.Irwin ML, Duggan C, Wang CY, et al. Fasting C-peptide levels and death resulting from all causes and breast cancer: the health, eating, activity, and lifestyle study. J Clin Oncol. 2011;29:47–53. doi: 10.1200/JCO.2010.28.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodwin PJ, Pritchard KI, Ennis M, et al. Insulin-lowering effects of metformin in women with early breast cancer. Clin Breast Cancer. 2008;8:501–505. doi: 10.3816/CBC.2008.n.060. [DOI] [PubMed] [Google Scholar]
- 27.Feng YH, Velazquez-Torres G, Gully C, et al. The impact of type 2 diabetes and antidiabetic drugs on cancer cell growth. J Cell Mol Med. 2011;15:825–836. doi: 10.1111/j.1582-4934.2010.01083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alimova IN, Liu B, Fan Z, et al. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle. 2009;8:909–915. doi: 10.4161/cc.8.6.7933. [DOI] [PubMed] [Google Scholar]
- 29.Hirsch HA, Iliopoulos D, Tsichlis PN, et al. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69:7507–7511. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zakikhani M, Dowling R, Fantus IG, et al. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269–10273. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 31.Zhuang Y, Miskimins WK. Cell cycle arrest in metformin treated breast cancer cells involves activation of AMPK, downregulation of cyclin D1, and requires p27Kip1 or p21Cip1. J Mol Signal. 2008;3:18. doi: 10.1186/1750-2187-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhuang Y, Miskimins WK. Metformin induces both caspase-dependent and poly(ADP-ribose) polymerase-dependent cell death in breast cancer cells. Mol Cancer Res. 2011;9:603–615. doi: 10.1158/1541-7786.MCR-10-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dowling RJ, Zakikhani M, Fantus IG, et al. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67:10804–10812. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 34.Elstner E, Muller C, Koshizuka K, et al. Ligands for peroxisome proliferator-activated receptorgamma and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proc Natl Acad Sci U S A. 1998;95:8806–8811. doi: 10.1073/pnas.95.15.8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin F, Wakino S, Liu Z, et al. Troglitazone inhibits growth of MCF-7 breast carcinoma cells by targeting G1 cell cycle regulators. Biochem Biophys Res Commun. 2001;286:916–922. doi: 10.1006/bbrc.2001.5491. [DOI] [PubMed] [Google Scholar]
- 36.Yang Z, Bagheri-Yarmand R, Balasenthil S, et al. HER2 regulation of peroxisome proliferator-activated receptor gamma (PPARgamma) expression and sensitivity of breast cancer cells to PPARgamma ligand therapy. Clin Cancer Res. 2003;9:3198–3203. [PubMed] [Google Scholar]
- 37.Tikoo K, Kumar P, Gupta J. Rosiglitazone synergizes anticancer activity of cisplatin and reduces its nephrotoxicity in 7, 12-dimethyl benz{a}anthracene (DMBA) induced breast cancer rats. BMC Cancer. 2009;9:107. doi: 10.1186/1471-2407-9-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang PS, Chou FS, Bloomston M, et al. Thiazolidinediones downregulate Wnt/beta-catenin signaling via multiple mechanisms in breast cancer cells. J Surg Res. 2009;153:210–216. doi: 10.1016/j.jss.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 39.Rashid-Kolvear F, Taboski MA, Nguyen J, et al. Troglitazone suppresses telomerase activity independently of PPARgamma in estrogen-receptor negative breast cancer cells. BMC Cancer. 2010;10:390. doi: 10.1186/1471-2407-10-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friday E, Oliver R, III, Welbourne T, et al. Glutaminolysis and glycolysis regulation by troglitazone in breast cancer cells: relationship to mitochondrial membrane potential. J Cell Physiol. 2011;226:511–519. doi: 10.1002/jcp.22360. [DOI] [PubMed] [Google Scholar]
- 41.He XX, Tu SM, Lee MH, et al. Thiazolidinediones and metformin associated with improved survival of diabetic prostate cancer patients. Ann Oncol. 2011;22:2640–2645. doi: 10.1093/annonc/mdr020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. The antidiabetic drug metformin suppresses HER2 (erbB-2) oncoprotein overexpression via inhibition of the mTOR effector p70S6K1 in human breast carcinoma cells. Cell Cycle. 2009;8:88–96. doi: 10.4161/cc.8.1.7499. [DOI] [PubMed] [Google Scholar]
- 43.Anisimov VN, Egormin PA, Piskunova TS, et al. Metformin extends life span of HER-2/neu transgenic mice and in combination with melatonin inhibits growth of transplantable tumors in vivo. Cell Cycle. 2010;9:188–197. doi: 10.4161/cc.9.1.10407. [DOI] [PubMed] [Google Scholar]
- 44.Zheng W, McLerran DF, Rolland B, et al. Association between body-mass index and risk of death in more than 1 million Asians. N Engl J Med. 2011;364:719–729. doi: 10.1056/NEJMoa1010679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40:381–387. doi: 10.1038/sj.bmt.1705727. [DOI] [PubMed] [Google Scholar]
- 47.Dean-Colomb W, Esteva FJ. HER2-positive breast cancer: herceptin and beyond. Eur J Cancer. 2008;44:2806–2812. doi: 10.1016/j.ejca.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 48.Esteva FJ, Yu D, Hung MC, et al. Molecular predictors of response to trastuzumab and lapatinib in breast cancer. Nat Rev Clin Oncol. 2010;7:98–107. doi: 10.1038/nrclinonc.2009.216. [DOI] [PubMed] [Google Scholar]
- 49.Bowker SL, Majumdar SR, Veugelers P, et al. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29:254–258. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 50.Li D, Yeung SC, Hassan MM, et al. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137:482–488. doi: 10.1053/j.gastro.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Renehan A, Smith U, Kirkman MS. Linking diabetes and cancer: a consensus on complexity. Lancet. 2010;375:2201–2202. doi: 10.1016/S0140-6736(10)60706-4. [DOI] [PubMed] [Google Scholar]
- 52.Gouveri E, Papanas N, Maltezos E. The female breast and diabetes. Breast. 2011;20:205–211. doi: 10.1016/j.breast.2011.02.019. [DOI] [PubMed] [Google Scholar]