Abstract
The aim of the present study was to assess the potential barrier effect of Esoxx®, a new nonprescription medication under development for the relief of gastroesophageal reflux symptoms. Esoxx is based on a mixture of hyaluronic acid and chondroitin sulfate in a bioadhesive suspension of Lutrol® F 127 polymer (poloxamer 407) which facilitates the product adhesion on the esophageal mucosa. The mucosal damage was induced by 15 to 90 minutes of perfusion with an acidic solution (HCl, pH 1.47) with or without pepsin (2000 U/mL, acidified to pH 2; Sigma-Aldrich). Mucosal esophageal specimens were histologically evaluated and Evans blue dye solution was used to assess the permeability of the swine mucosa after the chemical injury. The results show that: (1) esophageal mucosal damage is related to the perfusion time and to the presence of pepsin, (2) mucosal damage is associated with an increased permeability, documented by an evident Evans blue staining, (3) perfusion with Esoxx is able to reduce the permeability of the injured mucosa, even after saline washing of the swine esophagus. These preliminary results support further clinical studies of Esoxx in the topical treatment of gastroesophageal reflux symptoms.
Keywords: bioadhesion, hyaluronic acid, Evans blue dye, animal model, esophagus, reflux esophagitis
Introduction
Esoxx® (Alfa Wassermann, Bologna, Italy) is a new medical device for the topical treatment of the symptoms of gastroesophageal reflux disease (GERD). The product is based on a mixture of hyaluronic acid (HA) and chondroitin sulfate (CS) in a bioadhesive carrier Lutrol® F 127 (poloxamer 407) (BASF, Milan, Italy), which acts as a buffering agent to form a barrier and to prolong the action on esophageal mucosa.1
Hyaluronic acid (hyaluronan; HA) is a glycosaminoglycan made up of glucuronic acid and N-acetylglucosamine disaccharide units. HA is mainly present in the extracellular matrix of soft connective tissues and is involved in several key processes, including cell signaling, wound repair and regeneration, morphogenesis, matrix organization and pathobiology.2 Clinically, it is largely used for the treatment of several diseases. Topical HA preparations are applied in the management of recurrent aphthous ulceration, with immediate reduction of symptoms due to its barrier effect.2–4
Chondroitin sulfate (CS) is a natural glycosaminoglycan present in the extracellular matrix and is formed by the 1–3 linkage of D-glucuronic acid to N-acetylgalactosamine. The mechanism of action of CS explains its beneficial effect on the cartilage, synovial membrane and subchondral bone. CS may be of benefit in diseases where inflammation is an essential marker.5,6
For its component characteristics, Esoxx® is proposed as a protective topical agent towards esophageal and gastric lesions as demonstrated in a preliminary report which shows that its oral administration may significantly improve symptoms in a group of patients affected by GERD or gastritis.7 Given that a direct evaluation of the barrier effect of this product on the esophageal mucosa is lacking, we attempted to prove its efficacy in an ex-vivo swine esophagus with a model similar to that previously used for evaluating the esophageal bioadhesion of alginate suspensions.8 To induce mucosal lesions, we used the perfusion of acid solutions, with and without pepsin. The aim of our study was to assess the barrier effect of Esoxx on swine esophageal mucosa after damage similar to that observed as a consequence of gastroesophageal reflux.
Materials and methods
Experimental animal procedure
Thirty swine esophagi were obtained from commercially available European breed pigs, weighing about 120 kg, slaughtered for food purposes. The pig esophagi arrived at the laboratory within 2 hours from the local slaughter house. Each esophagus was washed with tap water and the mucosa isolated from the muscularis mucosae and kept in vital condition on an oblique polystyrene surface (45°) at 37°C in a thermostated hood.
The tube of esophageal mucosa was cannulated and the top was tied with a surgery suture to a syringe connected to a perfusion pump. The perfusion rate was set to a rate of 1 mL/min. The distal end was closed for 5 minutes to completely fill the tube (Figure 1).
Figure 1.
Experimental model.
Damaging solutions
Esophageal mucosal damage was induced through perfusion with hydrochloric acid solutions in the presence or absence of pepsin as suggested by previous experimental studies.9,10 The acid solution (AS) was prepared with HCl 0.1 N in sterile saline (pH 1.47). The pepsin solution (PS) was prepared by adding porcine pepsin (1%, 2000 U/mL; Sigma-Aldrich, St Louis, MO) to sterile normal saline acidified to pH 2 with HCl 0.1 N.
Esophagi were perfused at 37°C for 15, 30, 60, and 90 minutes with AS; and for 15, 30, and 60 minutes with PS. Each procedure was repeated on three different esophagi.
Assessment of mucosal injury
At the end of each time point of perfusion, three specimens of mucosal rings were cut and fixed. Whole-wall mucosa samples were fixed in cold neutral formaldehyde 4% for 1 hour and then placed in 200 mL Dulbecco’s phosphate-buffered saline 25% sucrose at 4°C for cryoprotection, and finally embedded in optimal cutting temperature tissue freezing medium. Five-micron-thick sections of mucosa were cut, serially mounted on glass, and processed for routine hematoxylin–eosin staining.
Preparations, mounted with Mowiol® 4–88 reagent (Calbiochem, Giessen, Germany), were examined by light microscopy; representative photomicrographs were taken using a DS-5M digital camera (Nikon Instruments, Florence, Italy).
The mucosal damage severity was classified according to the following scores: Grade 0, no damage; Grade 1, (mild damage) erosion involving less than 10% of mucosal area, localized on superficial epithelial layers; Grade 2, (moderate damage) erosion involving 10% to 50% of mucosal area, localized on superficial layers, with dropout of keratinic cells; Grade 3, (severe damage) erosion involving more than 50% of mucosal area and extending through >50% of epithelial stratified layer.
Assessment of mucosal permeability and evaluation of the barrier effect of Esoxx
In order to assess the permeability of esophageal mucosa, the Evans blue (EB) dye solution was used. EB is known to bind to albumin in vivo and in vitro and is used as an indicator of vascular11 and intestinal permeability.12 In our experiment, we perfused with EB (10 mg/mL in sterile saline) both undamaged esophagi (negative control) and esophagi with severe mucosal lesions (see above) to allow EB penetration within the mucosal layer.
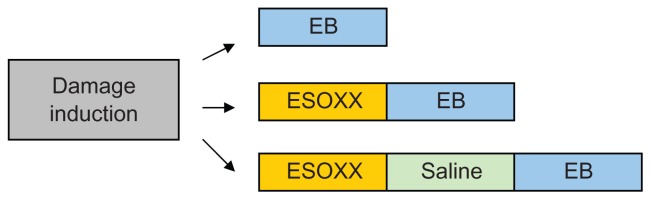
The damaged mucosa was washed with saline for 5 minutes and divided into three portions. The first (used as positive control) was perfused with EB (10 minutes at 1 mL/minute); the second, with Esoxx (10 minutes, 1 mL/minute) followed by EB (10 minutes at 1 mL/minute). The third segment was perfused with Esoxx (10 minutes at 1 mL/minute), then washed with saline for 30 seconds, and finally perfused with EB (10 minutes at 1 mL/minute). This last experiment aimed to assess the adhesive property of Esoxx (Figure 2).
Figure 2.
Perfusion sequences used for the evaluation of mucosal permeability and Esoxx effect.
Abbreviation: EB, Evans blue dye.
The presence of EB staining was evaluated by light microscopy and scored with a 3-point scale: 0 (no stain); 1 (weak stain); 2 (strong stain). We used two undamaged esophagi for the negative control and six esophagi with severe mucosal lesions.
Results
Assessment of mucosal injuries
The severity of the lesions induced by damaging solutions on the swine esophageal mucosa is shown in Table 1. The perfusions were performed on three different esophagi and three specimens were obtained from each of them. Nine mucosal specimens were examined for each experimental group.
Table 1.
Severity of histological damage induced with acidic and pepsin solutions
| Time (min) | Grade 0 | Grade 1 | Grade 2 | Grade 3 | |
|---|---|---|---|---|---|
| Acidic solution | 15 | 9 (100%) | – | – | – |
| 30 | 1 (11%) | 7 (78%) | 1 (11%) | – | |
| 60 | – | 1 (11%) | 7 (78%) | 1 (11%) | |
| 90 | – | – | – | 9 (100%) | |
| Pepsin solution | 15 | – | 7 (78%) | 2 (22%) | – |
| 30 | – | – | 7 (78%) | 2 (22%) | |
| 60 | – | – | – | 9 (100%) |
Notes: Data expressed as absolute frequency (%) of score attributed (grade 0 = no damage; grade 1 = mild damage; grade 2 = moderate damage; grade 3 = severe damage).
Histologic damage was found to be directly related to perfusion time. The acidic solution alone, perfused for 15 minutes, did not give rise to evident histological damage, while more prolonged perfusion caused a progressive involvement of the inner mucosal layers. Adding pepsin to the acid solution during the damage induction phase produced an appearance of histological damage at earlier time points. Severe histological lesions were always observed after AS and PS perfusion for 90 and 60 minutes, respectively. Figure 3 shows some examples of the different degrees of damaged mucosa.
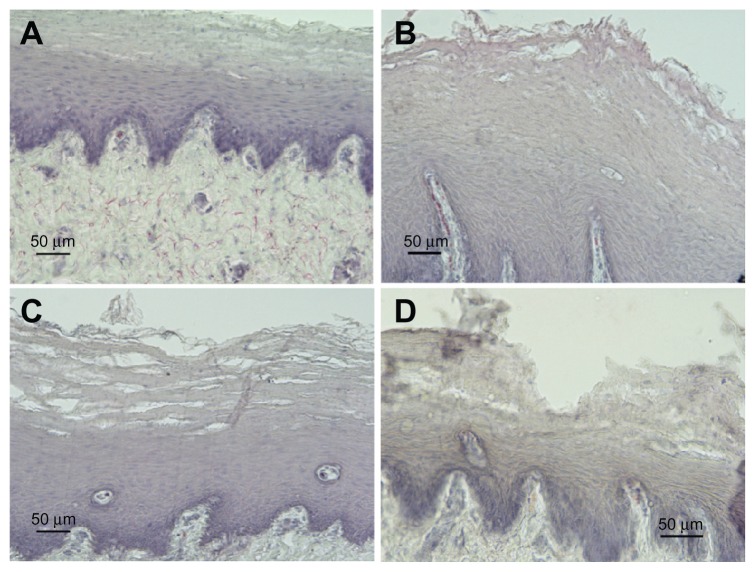
Figure 3.
Representative examples of cross-sections of esophageal mucosa (H&E) treated with saline (A) or different damaging solutions (B–D). (A) No damage (grade 0). (B) Acid solution (60 minutes): mild damage (grade 1) extended throughout one or two epithelial layers. (C) Pepsin solution (30 minutes); moderate damage (grade 2) mainly localized on superficial layers. A disorganization of epithelial layers was observed along the tissue, with some intact areas and areas in which erosion interested from 30% to 50% of mucosal thickness. (D) Acid solution (90 minutes); severe damage (grade 3) and complete erosion of keratinic epithelial layers, with injury extending through more than 50% of epithelial stratified layer.
Assessment of mucosal permeability and evaluation of the barrier effect of Esoxx
Results of EB staining on different mucosal samples are presented in Table 2. No EB staining was detected on the epithelial layer of control mucosa, while EB staining was evident in all the samples of damaged mucosa with 67% of them showing a strong stain. Perfusion of Esoxx after damaging acid solution for 90 minutes and pepsin solution for 60 minutes was able to completely prevent the EB staining in all the mucosal samples examined (Figure 4). This effect of Esoxx was not reversed by a short period of saline perfusion.
Table 2.
Evans blue (EB) staining on control tissue and damaged mucosa with or without Esoxx application
| 0 (no stain) | 1 (weak stain) | 2 (strong stain) | |
|---|---|---|---|
| No damaged mucosa + EB | 2 (100%) | – | – |
| Damaged mucosa + EB | – | 2 (33%) | 4 (67%) |
| Damaged mucosa + Esoxx + EB | 6 (100%) | – | – |
| Damaged mucosa + Esoxx + saline + EB | 6 (100%) | – | – |
Note: Data expressed as frequency of staining score observed in each experimental group (six mucosal samples).
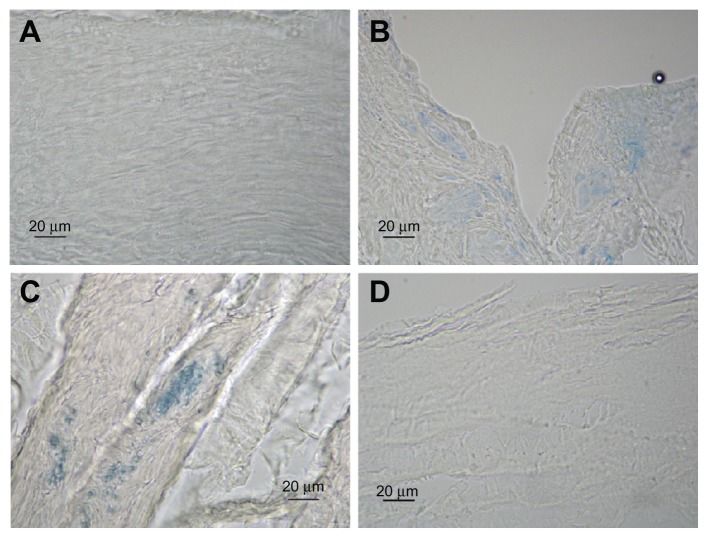
Figure 4.
Representative examples of cross-sections of esophageal mucosa after EB perfusion. (A) No stain; undamaged mucosa + EB. (B) Weak stain; damaged mucosa (acid solution 90 minutes ) + EB. (C) Strong stain; damaged mucosa (pepsin solution 60 minutes) + EB. (D) No stain; damaged mucosa (acid solution 90 minutes) + Esoxx + EB.
Note: No stain also in the damaged mucosa (pepsin solution 60 minutes) + Esoxx + EB.
Abbreviation: EB, Evans blue dye.
Discussion
Previous experimental studies carried out both in vitro13 and in vivo in animal models9,10 have clearly demonstrated that perfusion with solutions containing HCl and pepsin may induce esophageal mucosal damage that mimics the lesions induced by gastroesophageal reflux. Porcine esophagus is considered an appropriate model for ex-vivo studies and has been used in several experimental pharmacology studies to assess the adhesive property of drugs.14–16 As suggested in previous studies,8,17 it can be mounted as a mucosal tube in order to reproduce a kind of flow along the esophageal lumen. In our study we used acidic solutions, with or without pepsin, perfused for different time periods in an ex-vivo swine model to induce esophageal mucosal damage. Mucosal lesions were assessed by applying a histologic score that took into account both the superficial extent and the depth of the mucosal damage. In our model, the severity of the lesions was directly influenced by the duration of the perfusion and by the presence of pepsin. This is in agreement with the results obtained with the perfusion of acidified pepsin of an in vivo rabbit esophagus.10
We found that the most severe lesions, characterized by erosions involving more than 50% of mucosal area and extending through more than 50% of the epithelial stratified layer, were observed in all the specimens perfused with acid solution for 90 minutes or with acid plus pepsin for 60 minutes. We considered this a suitable and reproducible model to induce a chemical lesion of esophageal mucosa.
In normal conditions, the esophageal mucosa is protected against injurious agents by its stratified, multilayered squamous epithelium which represents a true mucosal barrier. All the damaging substances, such as hydrochloric acid and pepsin contained in the gastric refluxate, may impair this barrier and as a consequence may increase the mucosal permeability.18 In our experimental model we evaluated the esophageal mucosal permeability with a high-molecular-weight dye, EB, which has been extensively used to study microvascular permeability.11–19 This dye has also been used as an endoluminal marker of mucosal permeability of the jejunum in rat12 and of the colon in mouse.20 These authors perfused EB for long periods of time (30 to 120 minutes) in in-vivo models and estimated the uptake of the dye into the gut wall, both qualitatively and quantitatively. Our study was carried out in different experimental conditions (ex-vivo porcine esophagus) and with a short time of EB perfusion (10 minutes) of both a segment of undamaged esophagus (negative control) and segments of mucosa with severe lesions. Our results show that EB does not penetrate undamaged esophageal mucosa, while it is clearly detectable, even with light microscopy, in the specimens of mucosa damaged by acid and peptic solutions. For this reason, we tested the hypothesis that prevention of the increased permeability due to the presence of mucosal breaks could be accomplished with Esoxx, which can coat the damaged mucosa with its components, HA and CS. HA is an extraordinarily versatile glycosaminoglycan currently receiving attention across a wide range of research areas. It has a very high molar mass, usually in the order of millions of Daltons, and possesses interesting viscoelastic properties based on its polymeric and polyelectrolyte characteristics. Its length, coupled with its high hydrating property, allows many HA polymers to organize in a reticular structure, which in turn produces a molecular framework. Such scaffolding, besides supporting the tone and shape of tissues, acts as a filter to prevent the diffusion of high-molecular-weight substances and dissemination of infectious agents.2,21–24 CS may be of benefit in diseases where inflammation is an essential marker.6
The results showed that Esoxx perfused at 1 mL/minute for 10 minutes, after mucosal damage induction, preventing the penetration of the EB dye in the mucosa and acting as a topical mucosal barrier.
In addition, the ability of the bioadhesive polymer to produce a persistent mucosal barrier effect was also demonstrated after a brief washing of the esophageal mucosa with saline after perfusion with Esoxx.
The esophagus is an organ of transport with a very short transit time that does not favor drug contact or delivery. Several pharmaceutical studies have been carried out to investigate bioadhesive dosage forms that adhere to esophageal mucosa and prolong contact. The retention of sucralfate or alginate formulations were studied both with in-vitro models16,25 and with ex-vivo porcine esophageal tube.8 To the best of our knowledge, there are no studies that have investigated the direct effect of topically acting drugs on esophageal mucosal lesions. In our experimental model perfusion with Esoxx for a relatively short time period was able to exert a barrier effect on the damaged esophageal mucosa by reducing its permeability. This effect should be looked at with interest given that the increased permeability due to chemical damage such as that induced by pathological gastroesophageal reflux, represents the main mechanism for the development not only of the mucosal breaks but also of symptoms (ie, heartburn, pain) even in the absence of detectable lesions.18
Further studies are needed to better evaluate the effect of this compound on the esophageal mucosal barrier and particularly its bioadhesive properties and the duration of the effect. If these preliminary results are clinically confirmed, the possibility of a new topical therapy to be included among the treatment options for the management of reflux disease should be evaluated.
Footnotes
Disclosure
This study was sponsored by Alfa Wassermann SpA and Prof. Fabio Baldi who is on the Alfa Wassermann’s Steering Committee (Esoxx® Product).
References
- 1.Batchelor HK. Novel bioadhesive formulations in drug delivery. Drug Delivery Companies Report Autumn/Winter. 2004:16–19. [Google Scholar]
- 2.Volpi N, Schiller J, Stern R, Soltés L. Role, metabolism, chemical modifications and applications of hyaluronan. Curr Med Chem. 2009;16(14):1718–1745. doi: 10.2174/092986709788186138. [DOI] [PubMed] [Google Scholar]
- 3.Nolan A, Baillie C, Badminton J, Rudralingham M, Seymour RA. The efficacy of topical hyaluronic acid in the management of recurrent aphthous ulceration. J Oral Pathol Med. 2006;35(8):461–465. doi: 10.1111/j.1600-0714.2006.00433.x. [DOI] [PubMed] [Google Scholar]
- 4.Kapoor P, Sachdeva S, Sachdeva S. Topical hyaluronic Acid in the management of oral ulcers. Indian J Dermatol. 2011;56(3):300–302. doi: 10.4103/0019-5154.82485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lauder RM. Chondroitin sulphate: a complex molecule with potential impacts on a wide range of biological systems. Complement Ther Med. 2009;17(1):56–62. doi: 10.1016/j.ctim.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Du Souich P, García AG, Vergés J, Montell E. Immunomodulatory and anti-inflammatory effects of chondroitin sulphate. J Cell Mol Med. 2009;13(8A):1451–1463. doi: 10.1111/j.1582-4934.2009.00826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmieri B, Corbascio D, Capone S, Lodi D. Preliminary clinical experience with a new natural compound in the treatment of oesophagitis and gastritis: symptomatic effect. Trends Med. 2009;9(4):219–225. [Google Scholar]
- 8.Richardson JC, Dettmar PW, Hampson FC, Melia CD. Esophageal bioadhesion of sodium alginate suspensions. Suspension behaviour on esophageal mucosa. Eur J Pharm Sci. 2005;24:107–114. doi: 10.1016/j.ejps.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Pursnani KG, Mohiuddin MA, Geisinger KR, Weinbaum G, Katzka DA, Castell DO. Experimental study of acid burden and acute oesophagitis. Br J Surg. 1998;85(5):677–680. doi: 10.1046/j.1365-2168.1998.00687.x. [DOI] [PubMed] [Google Scholar]
- 10.Lanas A, Royo Y, Ortego J, Molina M, Sáinz R. Experimental esophagitis induced by acid and pepsin in rabbits mimicking human reflux esophagitis. Gastroenterology. 1999;116(1):97–107. doi: 10.1016/s0016-5085(99)70233-7. [DOI] [PubMed] [Google Scholar]
- 11.Kaya M, Ahishali B. Assessment of permeability in barrier type of endothelium in brain using tracers: Evans blue, sodium fluorescein, and horseradish peroxidase. Methods Mol Biol. 2011;763:369–382. doi: 10.1007/978-1-61779-191-8_25. [DOI] [PubMed] [Google Scholar]
- 12.Lange S, Delbro DS, Jennische E. Evans blue permeation of intestinal mucosa in the rat. Scand J Gastroenterol. 1994;29:38–46. doi: 10.3109/00365529409090435. [DOI] [PubMed] [Google Scholar]
- 13.Cheng L, Cao W, Fiocchi C, Behar J, Biancani P, Harnett KM. In vitro model of acute esophagitis in the cat. Am J Physiol Gastrointest Liver Physiol. 2005;289:G860–G869. doi: 10.1152/ajpgi.00260.2005. [DOI] [PubMed] [Google Scholar]
- 14.Al-Dujaili H, Florence AT, Salole EG. The adhesiveness of proprietary tables and capsules to porcine oesophageal tissue. Int J Pharm. 1986;34:75–79. [Google Scholar]
- 15.Honkanen O, Pia L, Marvola J, Sari E, Raimo T, Marvola M. Bioavailability and in vitro oesophageal sticking tendency of hydroxypropyl methylcellulose capsule formulations and corresponding gelatine capsule formulations. Eur J Pharm Sci. 2002;15:479–488. doi: 10.1016/s0928-0987(02)00032-5. [DOI] [PubMed] [Google Scholar]
- 16.Batchelor HK, Banning D, Dettmar PW, Hampson FC, Jolliffe IG, Craig DQ. An in vitro mucosal model for prediction of the bioadhesion of alginate solutions to the oesophagus. Int J Pharm. 2002;238:123–132. doi: 10.1016/s0378-5173(02)00062-5. [DOI] [PubMed] [Google Scholar]
- 17.Marvola M, Vahervuo K, Sothmann A, Marttila E, Rajaniemi M. Development of a method for study the tendency of drug products to adhere to the esophagus. J Pharm Sci. 1982;71:975–977. doi: 10.1002/jps.2600710904. [DOI] [PubMed] [Google Scholar]
- 18.Orlando RC. The integrity of the esophageal mucosa. Balance between offensive and defensive mechanisms. Best Pract Res Clin Gastroenterol. 2010;24:873–882. doi: 10.1016/j.bpg.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saria A, Lundberg JM. Evans blue fluorescence: quantitative and morphological evaluation of vascular permeability in animal tissues. J Neurosci Methods. 1983;8(1):41–49. doi: 10.1016/0165-0270(83)90050-x. [DOI] [PubMed] [Google Scholar]
- 20.Kitajima S, Takuma S, Morimoto M. Changes in colonic mucosal permeability in mouse colitis induced with dextran sulfate sodium. Exp Anim. 1999;48:137–143. doi: 10.1538/expanim.48.137. [DOI] [PubMed] [Google Scholar]
- 21.Gaffney J, Matou-Nasri S, Grau-Olivares M, Slevin M. Therapeutic applications of hyaluronan. Mol Biosyst. 2010;6(3):437–443. doi: 10.1039/b910552m. [DOI] [PubMed] [Google Scholar]
- 22.Burdick JA, Prestwich GD. Hyaluronic acid hydrogels for biomedical applications. Adv Mater. 2011;23(12):H41–H56. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ardizzoni A, Neglia RG, Baschieri MC, et al. Influence of hyaluronic acid on bacterial and fungal species, including clinically relevant opportunistic pathogens. J Mater Sci Mater Med. 2011;22(10):2329–2338. doi: 10.1007/s10856-011-4408-2. [DOI] [PubMed] [Google Scholar]
- 24.Kogan G, Soltés L, Stern R, Gemeiner P. Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol Lett. 2007;29(1):17–25. doi: 10.1007/s10529-006-9219-z. [DOI] [PubMed] [Google Scholar]
- 25.Dobrozsi DJ, Smith RL, Sakr AA. Comparative mucoretention of sucralfate suspensions in an everted rat esophagus model. Int J Pharm. 1999;189:81–89. doi: 10.1016/s0378-5173(99)00244-6. [DOI] [PubMed] [Google Scholar]