Abstract
Background
Many institutions have organized specialized groups of ambulatory surgery anesthesiologists with the aim of improving ambulatory surgery patient care and efficiency. We hypothesized that specialized ambulatory anesthesia teams produce better patient outcomes such as lower postoperative nausea and vomiting (PONV) rates, lower postoperative pain scores, and shorter postanesthesia care unit (PACU) lengths of stay (LOS).
Methods
In this prospective observational study, we collected outcomes data on 1,299 patients including incidence of PONV, PACU LOS, maximum and average pain scores, amount of postoperative opioid use, and rescue antiemetic use.
Results
Ambulatory anesthesiologists had statistically shorter phase 2 PACU LOS times (P < .05) and overall recovery times (P < .01). The PONV incidence odds ratio for ambulatory versus nonambulatory anesthesiologists was 1.31 (95% CI 1.01-1.72). We found no significant difference in the amount of postoperative opioid use, maximum postoperative pain scores, or PACU phase 1 LOS time.
Conclusions
The decreased PACU LOS for the study group's patients occurred despite the increased incidence of PONV. Ambulatory anesthesiologists contributed to decreased PACU LOS while practicing evidence-based anesthesia with regard to PONV and pain control. Ambulatory subspecialization may benefit institutions as a way to increase perioperative efficiency and improve surgeon and patient satisfaction.
Keywords: Ambulatory anesthesia, ambulatory surgery, operating room efficiency
INTRODUCTION
Ambulatory surgery has experienced exponential growth over the past 20 years, and ambulatory surgery unit efficiency has become essential to a successful ambulatory practice. In today's environment of cost cutting, decreasing reimbursements, and competition for patients and surgeons, improved patient outcomes in an ambulatory surgery unit may result in cost savings from decreased unexpected overnight hospital stays and shorter postanesthesia care unit (PACU) recovery times, as well as lead to increased patient convenience and satisfaction.1 Postoperative nausea and vomiting (PONV) and postoperative pain are the most frequent complications in ambulatory surgery patients, affecting patient recovery, discharge, and overall satisfaction.
The incidence of postdischarge symptoms at our institution for gynecologic procedures (predominantly using laparoscopy) has been reported as 17% for nausea and 7% for vomiting, as well as drowsiness in 62%, sore throat in 49%, aches in 47%, headache in 25%, and dizziness in 20%.2 Wu et al3 reviewed the frequency of occurrence of postdischarge symptoms in outpatients in a metaanalysis. The overall incidence of postdischarge symptoms was 45% for pain, 17% for nausea, 8% for vomiting, and 17% for all headaches. They also found a 42% incidence of drowsiness, 18% for dizziness, 21% for fatigue, 31% for myalgia, and 37% for sore throat. Moderate to severe pain occurred in 25%-35% of these outpatients.
Many large tertiary care educational institutions have a specialized group of anesthesiologists dedicated to ambulatory surgery cases. These specialized groups have been developed with the aim of improving patient care through more standardized practices, greater specialized knowledge, and better working relationships with surgical and nursing colleagues. Many large academic anesthesiology practices have accepted the notion that subspecialization provides certain benefits such as more consistent care, a higher degree of state-of-the-art knowledge, better teaching, and better working relationships among the subspecialty anesthesia faculty.4
Our hypothesis was that specialized ambulatory anesthesiologists would demonstrate better patient outcomes than anesthesiologists who rarely perform outpatient surgery cases because of the ambulatory specialists' experience working in a high turnover environment; their greater familiarity with PONV risk stratification, prophylaxis, and treatment guidelines; their use of more effective techniques for treating postoperative pain in a high turnover environment; and their familiarity with the surgeons and specifics of surgical procedures.
No previous studies have specifically compared outcomes for ambulatory versus nonambulatory anesthesia specialists. This prospective observational study evaluated outcomes measures of PONV, pain, and PACU length of stay (LOS) for the 2 anesthesiologist groups.
METHODS
This study was approved by the hospital institutional review board in July 2005. Data were collected for 1,299 ambulatory surgery cases from September 1, 2005, to June 15, 2007, at a major tertiary care academic center in Boston, MA. Patient inclusion criteria were American Society of Anesthesiologists (ASA) class 1 and 2 females undergoing common ambulatory surgery gynecologic procedures under general anesthesia. Patients were excluded from the study if they had a history of chronic pain medication use, significant comorbidities (ie, ASA status ≥ 3), or current pregnancy. The study included cases handled by both ambulatory and nonambulatory anesthesiologists. The study group consisted of day surgery cases performed by an experienced ambulatory anesthesiologist, defined as an established member of the ambulatory team who had performed at least 100 day surgery cases within the previous year that met the inclusion criteria listed above. The control group consisted of ambulatory surgery cases performed by a nonambulatory anesthesiologist: someone who was not a member of the ambulatory anesthesia team and who did not routinely perform ambulatory surgery cases (ie, < 20 total ambulatory cases within the past year). Comparison of patient outcomes for these 2 anesthesiologist groups formed the basis for this study.
Basic demographic data collected included patient's age, gender, and weight. We noted history of PONV, motion sickness, smoking, and chronic pain, as well the type and duration of surgery. Only female patients undergoing common gynecologic procedures under general anesthesia were included in this study to provide for a more accurate comparison between the study groups. We recorded the times, doses, and routes of administration of analgesics, antiemetics, and local anesthetics administered during the intraoperative course. The type of general anesthesia induction technique and use of nitrous oxide and propofol were recorded. The data collected during the postoperative course included incidence of nausea, vomiting (if any), antiemetic and pain medication use, LOS during PACU phases 1 and 2, and average and maximum pain scores. For each case, we also recorded whether the attending anesthesiologist was a member of the ambulatory or nonambulatory anesthesia group, according to the definitions above.
In this prospective, observational study, care provided by anesthesiologists and nurses was not altered. Each anesthesiologist developed and executed an anesthetic plan—including the administration of any prophylactic antiemetics—according to his or her usual practice. Anesthesia was administered by residents in training, nurse anesthetists supervised by an attending anesthesiologist, or by an attending anesthesiologist working alone. Recovery room nurses followed the standard postoperative protocols at our institution for the administration of antiemetics and analgesics.
The anesthesia providers documented care using paper anesthesia charts in a routine fashion, and a research assistant transferred data from the chart to an electronic database immediately after surgery. The assistant followed each patient through her recovery and recorded data on recovery room parameters, including nausea/vomiting scores, rescue antiemetics received, and recovery room stay times. Missing or unclear data were corrected by consulting the anesthesiologist or nurse responsible for documenting the data. If patient data were incomplete for any reason, the case was excluded from the study.
Times, doses, and routes of administration of induction agents, analgesics, antiemetics, and other drugs used during the perioperative period were recorded until the time of discharge. For analgesics, an equivalent dose was calculated for each drug to permit summing equivalent doses. Celecoxib, rofecoxib, ketorolac, fentanyl, morphine, and hydromorphone were used. The equivalent doses used were obtained from the medical literature.5-7 Numeric pain scores (0 to 10 where 0 = no pain and 10 = worst imaginable pain) were recorded at 30-minute intervals during recovery until discharge. Both average and maximum pain scores were recorded. Other data recorded in the PACU included the time the patient spent in phase 1 and phase 2 recovery, respectively.
We identified the differences between the study and control groups. The primary outcomes of interest were the incidence of PONV and the length of PACU stay for patients whose anesthesia was provided by ambulatory compared to nonambulatory anesthesiologists. We also examined the maximum and average pain scores during recovery, the amount of postoperative narcotic use, and rescue antiemetic use.
We assessed the comparability of patient and procedure characteristics and intraoperative variables between the study group and the control group using the independent samples t test for continuous variables and the chi-square test for dichotomous and categorical variables. For the odds ratios for PONV and PONV requiring treatment, multivariate logistic regression models controlled for history of smoking, history of PONV, history of motion sickness, and type of surgery. Least-squares regression assessed the differences in average and maximum pain score, total postoperative narcotic requirement, and PACU length of stay, controlling for type of surgery. P values ≤ .05 were considered significant. All analyses were performed using SAS 9.1 (SAS Institute Inc, Cary, NC).
RESULTS
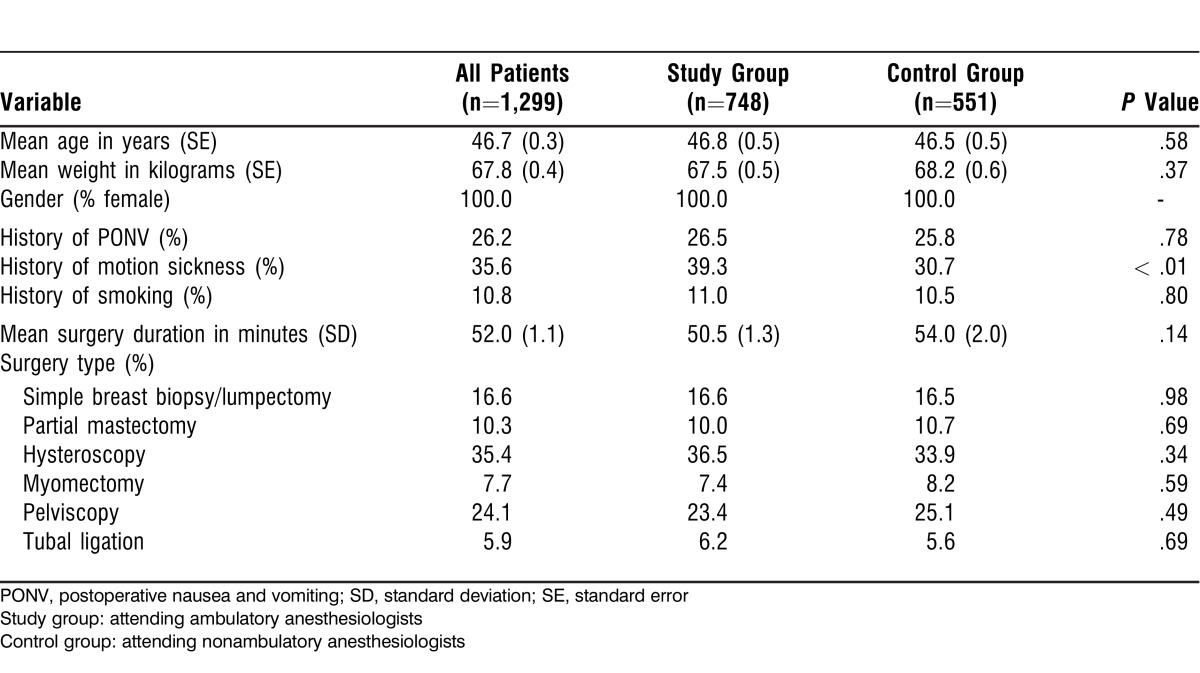
We collected data for 1,299 patients whose surgeries were staffed by either an ambulatory (study group) or a nonambulatory (control group) anesthesiologist. Table 1 shows patient demographic factors and type of surgery for all patients, for the study group, and for the control group. We found no significant difference in patient demographics, surgery characteristics, or PONV risk factors except for a higher incidence of motion sickness history in the study group (39.3% vs 30.7%, P < .01).
Table 1.
Patient Demographics and Surgery Details
Patients underwent 1 of 6 ambulatory surgery gynecologic procedures: breast biopsy, partial mastectomy, hysteroscopy, hysteroscopic myomectomy, pelviscopy, or laparoscopic tubal ligation.
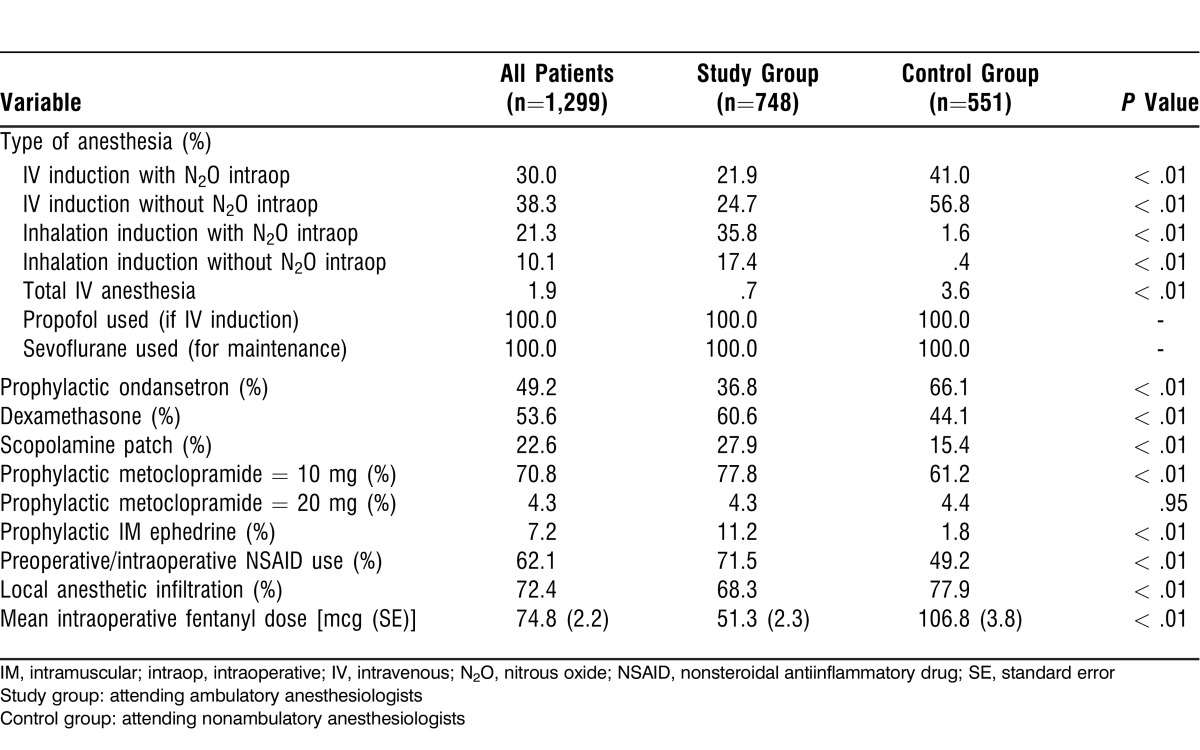
Table 2 shows anesthetic induction and maintenance techniques as well as antiemetics and analgesics administered. The study group had significantly lower use of intravenous induction with and without nitrous oxide compared to the control group (21.9% and 24.7% vs 41.0% and 56.8%, respectively). Inhalation induction use was much higher in the study group (35.8%-17.4% vs 1.6-0.4%, respectively) (all P < .01).
Table 2.
Anesthetic Information
The ambulatory (study) group had significantly higher use of intraoperative dexamethasone, scopolamine patch, intraoperative metoclopramide 10 mg, intramuscular ephedrine, and nonsteroidal antiinflammatory drugs (NSAIDs) but significantly less use of the intraoperative opioid fentanyl. Intraoperative ondansetron was administered significantly more frequently by the control group, and surgeon infiltration of the wound with local anesthetic occurred more frequently in the control group as well.
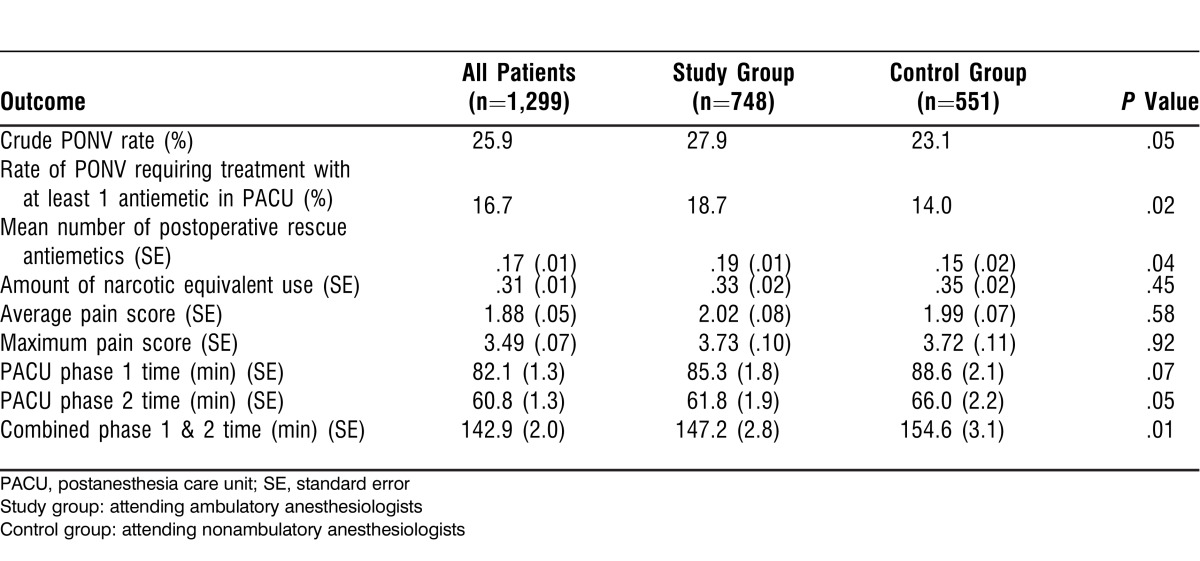
Table 3 presents PONV outcomes measures. Even after controlling for various patient-, anesthetic-, and surgery-related factors (gender, history of smoking, history of PONV or motion sickness, narcotic use, and surgery type and duration) in a multivariate analysis, the ambulatory anesthesiologists had higher PONV rates. The PONV incidence odds ratio for ambulatory compared to nonambulatory anesthesiologists was 1.31 (95% CI 1.01-1.72), and the odds ratio for the rate of PONV requiring treatment with at least 1 antiemetic was 1.44 (95% CI 1.05-1.98).
Table 3.
Postoperative Nausea and Vomiting (PONV) Outcomes
PACU pain outcomes and treatments were not different between the 2 groups (Table 3). The difference in PACU phase 1 stay time was not statistically significant. However, the study group had a statistically shorter phase 2 PACU stay time and, consequently, a statistically shorter total PACU stay time than the control group.
DISCUSSION
Formal grouping of anesthesiologists practicing in a specific subspecialty area is common in pain management, cardiac surgery, intensive care, and pediatrics. Many anesthesiologists have received additional training in their area of clinical interest. Theoretically, subspecialization in anesthesia can lead to better evidence-based care, better adherence to institutional care guidelines, better knowledge of surgical procedures, and better rapport with surgical and nursing colleagues.1 On the other hand, much of the published literature does not consistently provide conclusive data on the relative benefits or costs of subspecialization in anesthesiology.8 No study to date has specifically examined the effect of ambulatory anesthesia subspecialization on patient outcomes such as PONV, pain, and PACU LOS.
The present study examined whether anesthesiologists subspecializing in ambulatory anesthesia demonstrated better patient outcomes than nonambulatory anesthesiologists. The outcomes examined included PONV, PACU LOS, antiemetic use, maximum pain scores, and total postoperative narcotic requirements. Comparison of patient outcomes showed conflicting results. The most significant finding was that patients who received anesthesia by ambulatory anesthesiologists had significantly lower PACU phase 2 and total PACU LOS times (147.2 minutes vs 154.6 minutes, P = .01) compared to nonambulatory anesthesiologists after controlling for patient factors and surgery length. Motion sickness was more common in patients in the ambulatory group. However, even after controlling for high PONV risk patients and surgery length, the ambulatory group had higher incidence of PONV (27.9% vs 23.1%, P = .05) and of PONV requiring at least 1 treatment in the PACU (18.7% vs 14.0%, P = .02). No significant difference in postoperative pain scores between the two groups was found.
The decreased PACU total LOS time for the ambulatory group is consistent with our hypothesis but is rare in the existing literature. Röhm et al9 showed no difference in PACU LOS and recovery of cognitive function regardless of whether a total intravenous anesthesia or a desflurane/fentanyl general anesthetic technique was used. Similarly, Montes et al10 compared recovery characteristics of remifentanil/propofol and sevoflurane/fentanyl techniques, showing that variability in anesthetic technique helped to achieve home readiness criteria in a significantly shorter time but did not shorten PACU LOS. The authors attributed this result to multiple nonmedical and administrative factors. Chung and Mezei11 identified predictors for prolonged postoperative stay after ambulatory surgery, concluding that the PACU LOS is mostly determined by the type and length of surgery; by adverse events such as excessive pain, PONV, dizziness, and drowsiness; and by patient comorbidities such as congestive heart failure. Seago et al12 found that postoperative pulmonary and cardiovascular status, overall pain, and length of surgery contributed significantly to PACU LOS. However, the authors admitted that intraoperative factors and individual physician practice styles may have contributed to the variability in LOS, in addition to nursing staffing and other organizational factors. In our study, the shorter LOS may be related to the ambulatory specialists' practice patterns, such as the goal to titrate minimal necessary anesthetic depth, as well as the experience of the ambulatory team.
The ambulatory anesthesiologist group had a significantly higher percentage of patients with PONV (Table 3). The ambulatory group followed the then-current PONV consensus guidelines (2003)13 that recommended the use of multimodal therapy for high-risk patients14,15 but did not preferentially recommend the use of a serotonin antagonist. Also, at that time, our institution recommended minimizing the use of ondansetron in prophylaxis as a cost-conservation measure; the ambulatory group largely followed the institution's recommendation, using ondansetron primarily for treatment (1 mg). The use of ondansetron for treatment rather than prophylaxis followed the then-available data from Scuderi et al16 that showed no difference in time to discharge, rate of unanticipated admission, time to return to normal activity, and patient satisfaction scores when patients were given PONV treatment compared to prophylaxis.
Generic ondansetron became available in our institution in March 2007, and only a small number of study patients would have been affected by this change. Our supplementary statistical analysis that took this change into consideration did not show any statistically significant effect on PONV rates, PACU LOS, or pain scores for patients after March, probably because the institution's recommendation had not yet been modified. The 2007 PONV guidelines17 developed by the Society for Ambulatory Anesthesia appeared after the enrollment of patients in this study. A follow-up study at our institution could be performed to ascertain whether the 2007 published guidelines affected practice patterns.
The differences in PONV rates may also have been the result of variations in intraoperative anesthetic practices such as prophylactic antiemetic use, stricter adherence to antiemetic protocols, and better adherence to evidence-based practice in the ambulatory anesthesiologist group. In addition, general anesthetic induction and maintenance techniques were different between the 2 groups. Intravenous induction with or without maintenance with nitrous oxide was performed more frequently by nonspecialists in the control group, whereas inhalation induction with or without nitrous oxide was more common in the ambulatory specialist group. Reasons for this difference might include the ambulatory specialists' desire to teach residents inhalation induction and airway management techniques, or the nonspecialists' lack of experience with this technique. Philip et al18 compared vital capacity inhaled induction with sevoflurane and nitrous oxide to intravenous induction with propofol for adult ambulatory surgery in a protocol using no intraoperative opioid. The inhalation induction produced faster loss of consciousness and had side effects, recovery times, and patient satisfaction similar to those of propofol. On the other hand, a later metaanalysis comparing sevoflurane with propofol for induction (without opioid restriction) found that the two are comparable in terms of induction time and complications, yet sevoflurane was associated with significantly more PONV.19
Confounding variables may also include other patient factors not captured in the models. Neostigmine and fluid administration histories are incomplete. In addition, differences between the study groups may have been minimized by the transmission of ambulatory principles to nonambulatory staff, via formal and informal teaching. Also, residents practiced with both ambulatory and nonambulatory anesthesiologists during the study period and may have transferred practices that they learned.
Pain is an important patient outcome of interest in ambulatory surgery. Poorly controlled pain can delay discharge.20 Excessive use of opioids for perioperative analgesia can lead to acute opioid tolerance and hyperalgesia, sedation, PONV, urinary retention, and/or hypoventilation, thus delaying hospital discharge and adding to the cost of care.21,22 The concept of multimodal analgesia has gained acceptance as a way to adequately treat pain by using a combination of opioids and opioid-sparing drugs such as NSAIDs, cyclooxygenase-2 inhibitors, acetaminophen, gabapentin, beta-blockers, and alpha-2 adrenergic agonists. The multimodal approach may minimize the side effects that can arise from the use of larger doses of any of these drugs alone23 and can improve overall pain control. Postoperative pain is also related to PONV. Pain may directly worsen PONV,24 increased opioid use may induce PONV,25 and PONV may in turn exacerbate the level of pain.26 These issues make effective pain management using multimodal techniques critical.27 Pavlin et al20 found that the maximum pain score was predictive of total PACU recovery time in ambulatory surgery. Several metaanalyses have confirmed the opioid dose-sparing effects of NSAIDs and the decrease in opioid-related side effects of PONV and sedation.28-30
In our study, the ambulatory specialists were significantly more likely to use NSAIDs (71.5% vs 49.2%, P < .01) and to use less fentanyl (51.3 mcg vs 106.8 mcg, P < .01) than the control group. Pain scores were similar between the 2 groups and low despite the significantly lower use of fentanyl by the ambulatory specialists. These results may be caused by the group's higher use of NSAIDs. Additionally, the control group's use of higher doses of opioids may have produced acute opioid tolerance and hyperalgesia. A confounding factor is the surgeons' less frequent use of local anesthetic wound infiltration in the ambulatory specialist group. This result may be related to 1 high-volume surgeon who declines to use infiltration and is more likely to work with the ambulatory anesthesiologists.
This study raises the need for practitioner-specific outcomes data. If the PONV data had been given to practitioners during the study period, practice could have been changed. Neither the anesthesiology department nor the institution provides outcomes data to practitioners.
One limitation of this study was that providers' practices were not controlled. However, use of multivariate analyses minimized bias and allowed comparison of patient outcomes between the 2 anesthesiologist groups. We did not obtain any long-term follow-up outcomes data, including postdischarge nausea and vomiting. This information may have been helpful in further delineating outcomes between the 2 groups, but the goal of this study was to determine immediate postoperative outcomes. Another limitation was a narrow patient selection that only included females undergoing gynecologic procedures. Hence, applying the results and conclusions of this study to a wider patient population undergoing other procedure types must be done with caution.
CONCLUSION
This study shows that ambulatory anesthesia specialists contributed to decreased PACU LOS. Thus, ambulatory subspecialization may benefit institutions as a way to increase perioperative efficiency while practicing evidence-based anesthesia. The ambulatory specialists obtained similar postoperative analgesia with lower intraoperative opioid use and more NSAID use. However, PONV was higher because of separate practice decisions. Providing practitioner-specific outcomes data and comparing anesthetic practices between ambulatory specialist groups may help institutions establish specific anesthetic guidelines that lead to better patient outcomes.
Footnotes
This research was supported in part by grant T15-LM-07092 from the National Library of Medicine of the National Institutes of Health and by funding from the Brigham and Women's Hospital Department of Anesthesiology, Perioperative and Pain Medicine.
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care, Systems-Based Practice, and Practice-Based Learning and Improvement.
REFERENCES
- 1.Overdyk FJ, Harvey SC, Fishman RL, Shippey F. Successful strategies for improving operating room efficiency at academic institutions. Anesth Analg. 1998 Apr;86(4):896–906. doi: 10.1097/00000539-199804000-00039. [DOI] [PubMed] [Google Scholar]
- 2.Philip BK. Patients' assessment of ambulatory anesthesia and surgery. J Clin Anesth. 1992 Sep-Oct;4(5):355–358. doi: 10.1016/0952-8180(92)90155-t. [DOI] [PubMed] [Google Scholar]
- 3.Wu CL, Berenholtz SM, Pronovost PJ, Fleisher LA. Systematic review and analysis of postdischarge symptoms after outpatient surgery. Anesthesiology. 2002 Apr;96(4):994–1003. doi: 10.1097/00000542-200204000-00030. [DOI] [PubMed] [Google Scholar]
- 4.Lubarsky DA, Reves JG. Effect of subspecialty organization of an academic department of anesthesiology on faculty perceptions of the workplace. J Am Coll Surg. 2005 Sep;201(3):434–437. doi: 10.1016/j.jamcollsurg.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Beaver WT. Aspirin and acetaminophen as constituents of analgesic combinations. Arch Intern Med. 1981;141:293–300. doi: 10.1001/archinte.141.3.293. Feb 23. (3 Spec No) [DOI] [PubMed] [Google Scholar]
- 6.Habib S, Matthews RW, Scully C, Levers BG, Shepherd JP. A study of the comparative efficacy of four common analgesics in the control of postsurgical dental pain. Oral Surg Oral Med Oral Pathol. 1990 Nov;70(5):559–563. doi: 10.1016/0030-4220(90)90396-a. [DOI] [PubMed] [Google Scholar]
- 7.Drugs for pain. Med Lett Drugs Ther. 2000 Aug 21;42(1085):73–78. [PubMed] [Google Scholar]
- 8.Desjardins G, Cahalan MK. Subspecialty accreditation: is being special good? Curr Opin Anaesthesiol. 2007 Dec;20(6):572–575. doi: 10.1097/ACO.0b013e3282f18bd8. [DOI] [PubMed] [Google Scholar]
- 9.Röhm KD, Piper SN, Suttner S, Schuler S, Boldt J. Early recovery, cognitive function and costs of a desflurane inhalational vs. a total intravenous anaesthesia regimen in long-term surgery. Acta Anaesthesiol Scand. Acta Anaesthesiol Scand. 2006 2011 Jan;5055(1)(7):14–18. 903. doi: 10.1111/j.1399-6576.2006.00905.x. Retraction in. Aug. [DOI] [PubMed] [Google Scholar]
- 10.Montes FR, Trillos JE, Rincón IE, et al. Comparison of total intravenous anesthesia and sevoflurane-fentanyl anesthesia for outpatient otorhinolaryngeal surgery. J Clin Anesth. 2002 Aug;14(5):324–328. doi: 10.1016/s0952-8180(02)00367-7. [DOI] [PubMed] [Google Scholar]
- 11.Chung F, Mezei G. Factors contributing to a prolonged stay after ambulatory surgery. Anesth Analg. 1999 Dec;89(6):1352–1359. doi: 10.1097/00000539-199912000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Seago JA, Weitz S, Walczak S. Factors influencing stay in the postanesthesia care unit: a prospective analysis. J Clin Anesth. 1998 Nov;10(7):579–587. doi: 10.1016/s0952-8180(98)00084-1. [DOI] [PubMed] [Google Scholar]
- 13.Gan TJ, Meyer T, Apfel CC, et al. Department of Anesthesiology, Duke University Medical Center. Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg. 2003 Jul;97(1):62–71. doi: 10.1213/01.ane.0000068580.00245.95. table of contents. [DOI] [PubMed] [Google Scholar]
- 14.Apfel CC, Greim CA, Haubitz I, et al. A risk score to predict the probability of postoperative vomiting in adults. Acta Anaesthesiol Scand. 1998 May;42(5):495–501. doi: 10.1111/j.1399-6576.1998.tb05157.x. [DOI] [PubMed] [Google Scholar]
- 15.Apfel CC, Kranke P, Greim CA, Roewer N. What can be expected from risk scores for predicting postoperative nausea and vomiting? Br J Anaesth. 2001 Jun;86(6):822–827. doi: 10.1093/bja/86.6.822. [DOI] [PubMed] [Google Scholar]
- 16.Scuderi PE, James RL, Harris L, Mims GR., 3rd Antiemetic prophylaxis does not improve outcomes after outpatient surgery when compared to symptomatic treatment. Anesthesiology. 1999 Feb;90(2):360–371. doi: 10.1097/00000542-199902000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Gan TJ, Meyer TA, Apfel CC, et al. Society for Ambulatory Anesthesia. Society for Ambulatory Anesthesia guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2007 Dec;105(6):1615–1628. doi: 10.1213/01.ane.0000295230.55439.f4. table of contents. [DOI] [PubMed] [Google Scholar]
- 18.Philip BK, Lombard LL, Roaf ER, Drager LR, Calalang I, Philip JH. Comparison of vital capacity induction with sevoflurane to intravenous induction with propofol for adult ambulatory anesthesia. Anesth Analg. 1999 Sep;89(3):623–627. doi: 10.1097/00000539-199909000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Joo HS, Perks WJ. Sevoflurane versus propofol for anesthetic induction: a meta-analysis. Anesth Analg. 2000 Jul;91(1):213–219. doi: 10.1097/00000539-200007000-00040. [DOI] [PubMed] [Google Scholar]
- 20.Pavlin DJ, Chen C, Penaloza DA, Polissar NL, Buckley FP. Pain as a factor complicating recovery and discharge after ambulatory surgery. Anesth Analg. 2002 Sep;95(3):627–634. doi: 10.1097/00000539-200209000-00025. table of contents. [DOI] [PubMed] [Google Scholar]
- 21.Guignard B, Bossard AE, Coste C, et al. Acute opioid tolerance: intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiology. 2000 Aug;93(2):409–417. doi: 10.1097/00000542-200008000-00019. [DOI] [PubMed] [Google Scholar]
- 22.Oderda GM, Evans RS, Lloyd J, et al. Cost of opioid-related adverse drug events in surgical patients. J Pain Symptom Manage. 2003 Mar;25(3):276–283. doi: 10.1016/s0885-3924(02)00691-7. [DOI] [PubMed] [Google Scholar]
- 23.Coloma M, Zhou T, White PF, Markowitz SD, Forestner JE. Fast-tracking after outpatient laparoscopy: reasons for failure after propofol, sevoflurane, and desflurane anesthesia. Anesth Analg. 2001 Jul;93(1):112–115. doi: 10.1097/00000539-200107000-00024. [DOI] [PubMed] [Google Scholar]
- 24.Chia YY, Kuo MC, Liu K, Sun GC, Hsieh SW, Chow LH. Does postoperative pain induce emesis? Clin J Pain. 2002 Sep-Oct;18(5):317–323. doi: 10.1097/00002508-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Stadler M, Bardiau F, Seidel L, Albert A, Boogaerts JG. Difference in risk factors for postoperative nausea and vomiting. Anesthesiology. 2003 Jan;98(1):46–52. doi: 10.1097/00000542-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Reurer M, Hueppe M, Klotz KF, et al. Detection of causal relationships between factors influencing adverse side-effects from anaesthesia and convalescence following surgery: a path analytical approach. Eur J Anaesthesiol. 2004 Jun;21(6):434–442. doi: 10.1017/s0265021504006040. [DOI] [PubMed] [Google Scholar]
- 27.Jensen K, Kehlet H, Lund CM. Post-operative recovery profile after laparoscopic cholecystectomy: a prospective, observational study of a multimodal anaesthetic regime. Acta Anaesthesiol Scand. 2007 Apr;51(4):464–471. doi: 10.1111/j.1399-6576.2006.01251.x. [DOI] [PubMed] [Google Scholar]
- 28.Cepeda MS, Carr DB, Miranda N, Diaz A, Silva C, Morales O. Comparison of morphine, ketorolac, and their combination for postoperative pain: results from a large, randomized, double-blind trial. Anesthesiology. 2005 Dec;103(6):1225–1232. doi: 10.1097/00000542-200512000-00018. [DOI] [PubMed] [Google Scholar]
- 29.Marret E, Kurdi O, Zufferey P, Bonnet F. Effects of nonsteroidal antiinflammatory drugs on patient-controlled analgesia morphine side effects: meta-analysis of randomized controlled trials. Anesthesiology. 2005 Jun;102(6):1249–1260. doi: 10.1097/00000542-200506000-00027. [DOI] [PubMed] [Google Scholar]
- 30.Elia N, Lysakowski C, Tramèr MR. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology. 2005 Dec;103(6):1296–1304. doi: 10.1097/00000542-200512000-00025. [DOI] [PubMed] [Google Scholar]