Abstract
BACKGROUND:
There is little epidemiologic evidence to assess the maturation of respiratory control in premature infants.
OBJECTIVE:
To measure the success rate or the percentage of infants who have no additional events of various apnea- or bradycardia-free intervals after correcting for gestational age, postmenstrual age of the last apnea or bradycardia event, and the severity of the event.
METHODS:
This was a retrospective cohort study of infants born at 34 weeks' gestational age or earlier at 1 of 5 Kaiser Permanente Medical Care Program hospitals between 1998 and 2001. The success rates of various apnea- or bradycardia-free intervals were calculated after stratifying according to gestational age, postmenstrual age of the last event, or event severity.
RESULTS:
Among the 1403 infants identified in this study, 84.2% did not have an apnea event and 78.5% did not have a bradycardia event after they were otherwise ready for discharge. For the entire cohort, a 95% success rate was statistically reached, with a 7-day apnea- or bradycardia-free interval. Infants with a gestational age of 30 weeks or less had a 5% to 15% lower success rate than infants with a gestational age more than 30 weeks for any given apnea- or bradycardia-free interval. The success rate was reduced by an additional 5% to 10% if the last apnea or bradycardia event occurred at a postmenstrual age of more than 36 weeks. Including only the most severe events slightly improved the success rate of a given interval.
CONCLUSIONS:
The risk of recurrence for apnea or bradycardia differs depending on the gestational age of the infant and the postmenstrual age of the last apnea or bradycardia event.
Keywords: premature infant, apnea of prematurity, bradycardia
WHAT'S KNOWN ON THIS SUBJECT:
The maturation of respiratory control, as measured by the resolution of apnea and bradycardia events, is important for the safe management of prematurely born infants. There is little epidemiologic evidence to assess the maturation of respiratory control in premature infants.
WHAT THIS STUDY ADDS:
Many premature infants do not have an apnea or bradycardia event once they are otherwise ready for discharge. The risk of recurrence for apnea or bradycardia depends on gestational age and the time of the last event.
The discharge of premature infants should be determined by physiologic criteria, such as adequate temperature regulation, oral feeding, and maturation of respiratory control.1 The maturation of respiratory control, with concurrent resolution of apnea and bradycardia events, can vary depending on gestational age and neonatal illnesses, such as bronchopulmonary dysplasia.2 Several studies2,3 suggest that most infants are apnea and bradycardia free by 37 to 40 weeks' postmenstrual age (PMA), although recent studies2,4,5 suggest that extremely premature infants may not achieve this skill until 43 weeks' PMA.
Clinicians determine the maturity of respiratory control through clinical observation. Thus, most NICUs have adopted empiric protocols mandating a period of in-hospital observation time for apnea and bradycardia around the time of hospital discharge, ranging from 1 to 21 days.6 This variation occurs because there is limited information about (1) the recurrence of apnea or bradycardia after a specific apnea- or bradycardia-free interval, (2) the effect of gestational age and the PMA of the last event on this recurrence rate, and (3) the impact of different definitions of apnea or bradycardia on recurrence rates, specifically differentiating episodes that resolved spontaneously from episodes that required bag-mask ventilation or intubation. The objective of this study was to examine these questions in a cohort of 1403 premature infants by calculating the success rate and event identification rate (EIR) of various apnea- and bradycardia-free intervals around the time of discharge, after accounting for differences in gestational age, PMA of the last event, and the severity of the event. The success rate is defined as the percentage of infants who have no additional events after the specified apnea- or bradycardia-free interval, which is more relevant to the clinician seeing an individual child. The EIR is defined as the percentage of recurrences captured by the specific interval and is more generalizable across NICUs because it is not dependent on how often apnea or bradycardia occurs in a given NICU.
METHODS
Study Population
We used data collected within the Kaiser Permanente Medical Care Program (KPMCP) between 1998 and 2001 during the Infant Functional Status Study, as described previously.2,7–9 The goal of the Infant Functional Status Study was to assess discharge decision making for premature infants. Eligible infants were born at 5 KPMCP hospitals between 1998 and 2001 with a gestational age of 34 weeks or less, determined by obstetricians on the basis of the last menstrual period date and available ultrasound data. Exclusion criteria included major congenital anomalies, the need for home mechanical ventilation, ventriculoperitoneal shunt placement, and loss to follow-up within 1 year of discharge. Approximately 20% of eligible infants were excluded. Excluded infants were older and heavier at birth and more likely to be black or Hispanic than the included infants. The included and excluded infant groups were similar in their need for mechanical ventilation and their average length of NICU stay. This study was approved by the institutional review boards of the Children's Hospital of Philadelphia and the KPMCP.
Data Collection During the NICU Stay
Our initial data source was the Kaiser Permanente Neonatal Minimum Data Set, which tracks all NICU admissions in the KPMCP.10 These data were supplemented by daily information on the physiologic maturity of each infant. Chart abstractionists used nursing flow sheets at each KPMCP NICU to collection information on ventilator and incubator settings; body temperature; apnea and bradycardia events from cardiopulmonary monitors, as noted by nurses; use of caffeine or methylxanthines; and feeding method (gavage, bottle, or breast). Abstraction began at either 31 weeks' PMA or at birth if the infant was born after 31 weeks' gestational age and continued to hospital discharge. The decision to discharge an infant was at the discretion of the attending physician, because there were no uniform discharge criteria of premature infants from hospitals within the KPMCP during this study's time frame. An apneic episode was defined as a pause in breathing for more than 20 seconds. A bradycardia episode was defined as any heart rate drift below 80 beats per minute. Severe apnea or bradycardia episodes required bag-mask ventilation, placement on continuous positive airway pressure, or reintubation during the management of the episode. All variables collected in the study had an adequate internal reliability with a Cohen's κ value of at least 0.70 from a previous review of 200 charts by separate abstactors.11
Data Collection After NICU Discharge
For apnea and bradycardia events that occurred after discharge from the NICU, we examined hospital, emergency department, and outpatient visit records for International Statistical Classification of Diseases, Ninth Revision codes for apnea or bradycardia. The dates for each encounter determined the number of days since the last apnea or bradycardia event.
Data Analysis
The primary outcome for this analysis was the success rate and EIR for a specific apnea- or bradycardia-free interval. We first determined the day when a child was classified as being ready for discharge to standardize the point where apnea and bradycardia may influence the decision to discharge a patient. This occurred on the first day when a patient-day met each of the following criteria:
No feeds administered via a naso- or orogastric tube
Temperature maintained off of supplemental heat
Receipt of nasal cannula oxygen or room air
Discontinuation of methylxanthines
Then, we identified the day when the last apnea or bradycardia event occurred before this ready-for-discharge day and included all subsequent days in our analysis. Eligible events for the study are shown in Fig 1. To make this calculation applicable to clinical practice, we only studied those ready-for-discharge days within 21 days from the last apnea or bradycardia event. To calculate the success rate and EIR of each apnea- or bradycardia-free interval, we first determined the number of days that an infant had been apnea or bradycardia free when each recurrence occurred. This interval was defined as the recurrence time. The success rate of a given apnea- or bradycardia-free interval was defined as the the number of patients without an event after this interval divided by the total number of patients. The EIR of a given apnea- or bradycardia-free interval was defined as the number of events in which recurrence time was less than the given interval divided by the total number of recurrences.
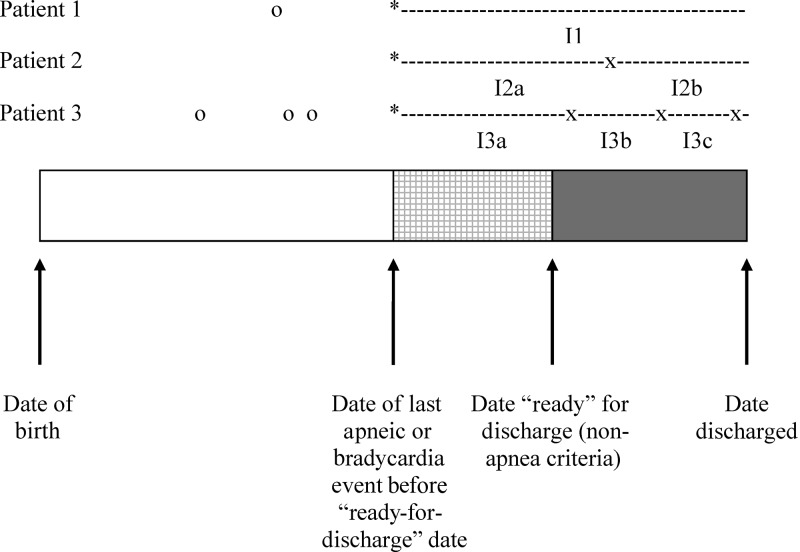
FIGURE 1.
Inclusion criteria for the study project. The bar shows the course of a typical infant from birth (shown on the left-hand edge) through hospital discharge (shown on the right-hand edge). Arrows show important events for this project: the day of the last apnea or bradycardia event before the infant reached the ready-for-discharge date according to other criteria and the day when the infant was first ready for hospital discharge by other criteria. Three theoretical patients are shown above the solid line, and apnea or bradycardia events are shown to the right of the infant. Events shown as circles indicate events that occurred before the infant was ready for discharge according to other criteria and were not included in this study. Events shown as “X” indicate events that were included in the study because they occurred after the infant met other criteria for discharge. Patient 1 had the simplest course: the infant had no events after reaching the ready-for-discharge date. Patients 2 and 3 had events included in the study; the interval times are denoted by the “I2x” and “I3x” periods between events. The observation time began at the day of the last apnea or bradycardia event before the infant reached his or her individual ready-for-discharge date, denoted as the asterisk in the patient time lines.
Each patient contributed at least 1 observation to the analysis. However, some patients could contribute more than 1 observation, depending on the number of apnea or bradycardia recurrences they had. To control for this fact, we averaged the success rate and EIR of all observation intervals for an individual child, so that in the final analysis, each child contributed only 1 set of values to the analysis. We performed nonparametric bootstrap analysis to calculate 95% confidence intervals (CIs) around each estimate.
For the second aim of the study, we calculated the success rate and EIR of each apnea- or bradycardia-free interval after stratifying by the gestational age of the infant or the PMA of the last apnea or bradycardia event. We chose 2-week groups for gestational age or the PMA of the last event to reduce variation between consecutive groups. For the third aim of the study, we calculated the success rate and EIR of each apnea- or bradycardia-free interval using only severe apnea or bradycardia events. As a sensitivity analysis, we reran all analyses after requiring that methylxanthines be discontinued for 3 and 5 days, on the basis of the half-life of these medications. We used coefficients of variation, defined as the highest success rate divided by the lowest success rate, to determine the variation in the success rate of various observation periods between NICUs.
RESULTS
We identified 1403 eligible infants, with a total of 22 122 patient-days within 21 days of the last apnea or bradycardia event. The average gestational age was 31.1 ± 2.6 weeks, and the average PMA at discharge was 35.6 ± 1.8 weeks. Using standard definitions, 10.0% of the infants had a diagnosis of bronchopulmonary dysplasia, 1.0% had necrotizing enterocolitis, 5.8% had stage II or greater retinopathy of prematurity, and 1.4% had grade III or IV intraventricular hemorrhage.12–15 For the patient-days examined in this study, there was a 7.2% recurrence rate for any apnea episode, a 8.3% recurrence rate for any bradycardia episode, a 3.8% recurrence rate for severe apnea, and 4.3% recurrence rate for severe bradycardia. After discharge from the NICU, 2 infants had an apnea or bradycardia event unrelated to a viral infection within 21 days of the last apnea or bradycardia event, and 7 infants had apnea related to viral symptoms.
Once the infants were otherwise ready for discharge, 84.2% did not have an apnea event and 78.5% did not have a bradycardia event (Table 1). These infants tended to be larger and less likely to have complications of premature birth compared with infants with a future event (Table 2). A 5-day or 7-day apnea- or bradycardia-free period had a success rate between 94% and 96%. The success rate of both apnea- and bradycardia-free intervals was statistically more than 95% at 7 days for both types of events (Table 3). On the other hand, the EIRs for 7-day apnea- and bradycardia-free intervals were between 50% and 60%.
TABLE 1.
Percentage of Infants Without Future Apnea or Bradycardia Events Near Discharge (N = 1403)
| Infants With Future Event, n (%) | Infants Without Future Event, n (%) | |
|---|---|---|
| Apnea | 222 (15.8) | 1181 (84.2) |
| Bradycardia | 302 (21.5) | 1101 (78.5) |
| Severe apnea | 81 (5.8) | 1322 (94.2) |
| Severe bradycardia | 101 (7.2) | 1302 (92.8) |
TABLE 2.
Differences Between Infants With Future Apnea or Bradycardia Events and Infants Without Future Events
| Apnea |
Bradycardia |
|||
|---|---|---|---|---|
| No Future Events, n (%) | Future Events, n (%) | No Future Events, n (%) | Future Events, n (%) | |
| Male gendera | 605 (51.1) | 118 (53.9) | 566 (51.3) | 157 (52.5) |
| Gestational age | ||||
| <26 wk | 61 (65.6) | 32 (34.4) | 54 (58.1) | 39 (41.9) |
| 27–28 wk | 113 (73.4) | 41 (26.6) | 102 (66.2) | 52 (33.8) |
| 29–30 wk | 196 (77.2) | 58 (22.8) | 172 (67.7) | 82 (32.3) |
| 31–32 wk | 316 (86.8) | 48 (13.2) | 295 (81.0) | 69 (19.0) |
| 33–34 wk | 498 (92.6) | 40 (7.4) | 481 (89.4) | 57 (10.6) |
| Discharged on oxygen | 54 (4.6) | 25 (11.4) | 51 (4.6) | 28 (9.4) |
| Bronchopulmonary dysplasia | 94 (7.9) | 47 (21.5) | 84 (7.6) | 57 (19.1) |
| Necrotizing enterocolitisa | 13 (1.1) | 2 (0.9) | 12 (1.1) | 3 (1.0) |
| Intraventricular hemorrhage stages III–IV | 11 (0.9) | 7 (3.2) | 10 (0.9) | 8 (2.7) |
Infants with future events not statistically significant from infants without future events for both apnea and bradycardia.
TABLE 3.
Success Rate and EIR of Various Apnea- or Bradycardia-Free Intervals, All Patients
| Interval Length, d | Apnea |
Bradycardia |
Severe Apnea |
Severe Bradycardia |
||||
|---|---|---|---|---|---|---|---|---|
| Success Rate (N = 1403), % | EIR (N = 219), % (95% CI) | Success Rate (N = 1403), % (95% CI) | EIR (N = 299), % (95% CI) | Success Rate (N = 1403), % (95% CI) | EIR (N = 81), % (95% CI) | Success Rate (N = 1403), % (95% CI) | EIR (N = 101), % (95% CI) | |
| 1 | 90.2 (89.0–91.4) | 0.0 | 86.5 (85.1–87.8) | 0.0 | 96.5 (95.8–97.3) | 0.0 | 95.6 (94.8–96.5) | 0.0 |
| 2 | 93.1 (92.2–94.0) | 25.0 (20.0–29.2) | 90.8 (89.8–91.8) | 27.7 (23.9–31.4) | 97.4 (96.8–98.0) | 21.5 (14.7–28.4) | 96.8 (96.1–97.4) | 22.9 (16.5–29.3) |
| 3 | 94.4 (93.6–95.2) | 38.0 (33.0–43.1) | 92.7 (91.8–93.5) | 40.9 (36.5–45.3) | 97.7 (97.1–98.2) | 30.5 (22.7–38.9) | 97.1 (96.5–97.7) | 30.1 (22.9–37.5) |
| 4 | 95.2 (94.4–95.9) | 45.4 (40.0–50.8) | 93.6 (92.8–94.4) | 47.5 (42.9–52.2) | 98.0 (97.4–98.5) | 37.2 (28.4–45.9) | 97.4 (96.8–98.0) | 35.7 (27.8–43.4) |
| 5 | 95.6 (94.9–96.3) | 49.9 (44.4–55.4) | 94.7 (94.0–95.4) | 55.7 (51.0–60.4) | 98.2 (97.7–98.6) | 42.9 (34.0–52.3) | 97.7 (97.2–98.2) | 43.5 (35.5–51.4) |
| 6 | 96.1 (95.4–96.7) | 54.8 (49.2–60.5) | 95.5 (94.7–96.1) | 61.7 (57.0–66.6) | 98.3 (97.9–98.8) | 48.3 (38.5–58.3) | 97.9 (97.4–98.4) | 48.5 (40.2–56.9) |
| 7 | 96.5 (95.9–97.1) | 59.3 (53.4–65.0) | 95.9 (95.2–96.5) | 64.8 (60.1–69.5) | 98.5 (98.1–98.9) | 52.7 (42.8–62.5) | 98.2 (97.7–98.7) | 54.8 (46.5–63.2) |
| 8 | 96.8 (96.2–97.3) | 62.2 (56.6–67.8) | 96.3 (95.6–96.9) | 68.5 (63.9–73.0) | 98.5 (98.1–98.9) | 52.9 (42.9–62.7) | 98.3 (97.8–98.8) | 57.8 (49.0–66.2) |
| 9 | 97.3 (96.7–97.8) | 67.9 (62.3–73.4) | 96.7 (96.1–97.3) | 72.1 (67.5–76.4) | 98.6 (98.2–99.0) | 55.8 (46.0–65.6) | 98.5 (98.1–98.9) | 62.6 (53.5–71.0) |
| 10 | 97.4 (96.8–97.9) | 69.5 (64.1–75.0) | 96.9 (96.3–97.4) | 73.9 (69.2–78.1) | 98.6 (98.2–99.0) | 56.2 (46.4–65.9) | 98.5 (98.1–99.0) | 63.0 (53.9–71.4) |
| 11 | 97.7 (97.1–98.2) | 73.1 (67.7–78.4) | 97.2 (96.7–97.8) | 76.8 (72.3–80.8) | 98.7 (98.2–99.0) | 56.6 (46.7–66.5) | 98.6 (98.2–99.0) | 64.1 (55.0–72.6) |
| 12 | 97.8 (97.3–98.3) | 74.3 (69.0–79.4) | 97.8 (96.8–97.9) | 77.8 (73.4–81.7) | 98.8 (98.3–99.1) | 59.3 (49.3–69.4) | 98.7 (98.3–99.1) | 65.8 (56.8–74.4) |
| 13 | 97.9 (97.4–98.4) | 75.9 (70.7–80.9) | 97.5 (96.9–98.0) | 78.4 (74.0–82.3) | 98.9 (98.5–99.3) | 63.8 (53.8–73.6) | 98.8 (98.4–99.2) | 69.3 (60.6–77.3) |
| 14 | 98.2 (97.7–98.6) | 78.3 (73.8–83.5) | 97.7 (97.2–98.2) | 80.4 (76.2–84.1) | 99.0 (98.6–99.3) | 66.7 (56.7–76.3) | 99.0 (98.6–99.3) | 73.3 (65.4-80.8) |
95% CIs were determined by the bootstrap methodology.
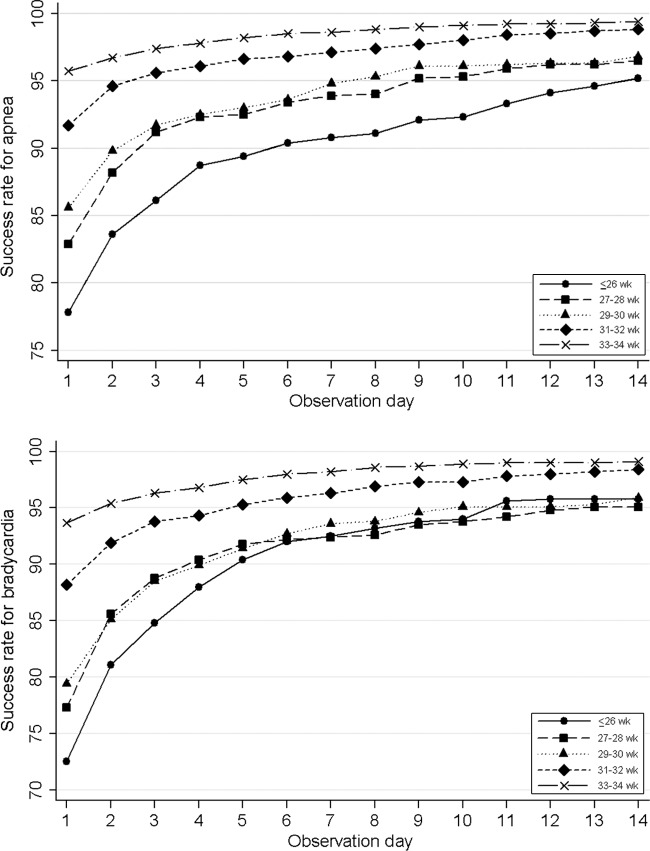
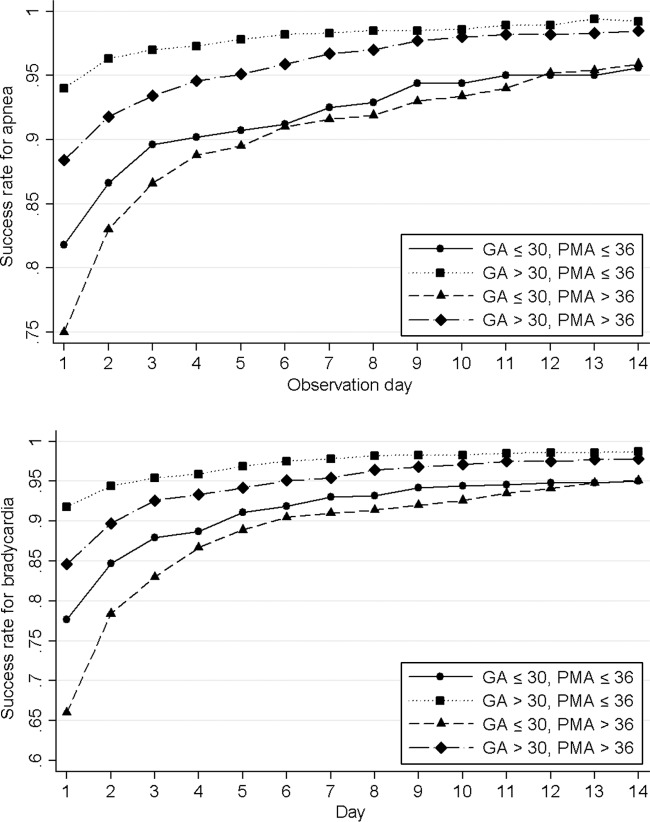
Infants of younger gestational age had lower success rates for the same apnea- or bradycardia-free interval (Fig 2). A 95% success rate apnea threshold was not reached until 13 days for infants with a gestational age of less than 26 weeks and 9 days for infants with a gestational age of 27 to 28 weeks. In contrast, infants of a gestational age of 30 weeks or more reached this threshold between 1 and 3 days after the last apnea event. Bradycardia showed similar trends. Beginning the observation time after a PMA of 36 weeks reduced the success rate by 5% to 10% regardless of the gestational age of the infant, compared with a PMA of 36 weeks or less (Fig 3). Infants of younger gestational age had, on average, an older PMA for their last event than older–gestational age infants. Daily success rates and EIRs are shown in the Supplemental Appendix 1.
FIGURE 2.
Success rate of various observation times, stratified according to gestational age.
FIGURE 3.
Success rate of various observation times, stratified according to the PMA of the last event and gestational age (GA).
When we examined these results according to NICU, we found differences in the success rate of various apnea- or bradycardia-free intervals between the centers, especially for periods less than 3 days (Table 4). Coefficients of variations for these rates varied from 2% to 8% for apnea and 2% to 16% for bradycardia. After accounting for differences in the percentage of infants whose observation times began after 38 weeks' PMA and the percentage of infants born with a gestational age less than 30 weeks, the success rates of individual apnea- or bradycardia-free intervals were relatively similar, with a reduction in the coefficient of variation between NICUs to 0% to 4% for apnea and 0% to 8% for bradycardia.
TABLE 4.
Differences in the Success Rate of Various Apnea- or Bradycardia-Free Intervals, According to NICU
| No. of Observation Days | NICU A, % | NICU B, % | NICU C, % | NICU D, % | NICU E, % | Coefficient of Variation, Unadjusted, %a | Coefficient of Variation, Adjusted, %b |
|---|---|---|---|---|---|---|---|
| Apnea | |||||||
| 1 | 92.0 | 89.5 | 92.8 | 90.4 | 85.0 | 9.2 | 4.4 |
| 3 | 95.0 | 95.1 | 95.3 | 93.6 | 92.6 | 2.9 | 0.8 |
| 5 | 95.7 | 96.8 | 96.0 | 95.3 | 94.1 | 2.9 | 0.3 |
| 7 | 96.0 | 97.8 | 96.5 | 97.0 | 95.5 | 2.4 | 1.1 |
| 10 | 96.7 | 98.4 | 97.6 | 97.5 | 96.9 | 1.8 | 0.4 |
| Bradycardia | |||||||
| 1 | 87.3 | 86.3 | 91.6 | 85.3 | 78.9 | 16.1 | 7.9 |
| 3 | 93.8 | 92.4 | 94.9 | 91.6 | 89.1 | 6.5 | 3.3 |
| 5 | 95.4 | 95.3 | 95.9 | 93.7 | 92.5 | 3.7 | 1.1 |
| 7 | 96.1 | 96.7 | 96.4 | 94.9 | 94.7 | 2.1 | 0.9 |
| 10 | 96.9 | 96.9 | 97.7 | 96.4 | 96.1 | 1.5 | 1.0 |
| Infants born at ≤30 wk gestational age, % | 34.0 | 40.9 | 38.5 | 34.8 | 29.3 | ||
| Observations at PMA > 38 wk % | 5.4 | 6.4 | 7.0 | 9.0 | 11.9 |
Coefficient of variation is equal to the highest success rate divided by the lowest success rate.
Coefficient of variation after adjusting for the percentage of infants with a gestational age of less than 30 weeks and the percentage of observations at a PMA of 38 weeks or more at individual NICUs.
For severe apnea or bradycardia events, we found similar results to our primary analysis for all events (Table 3). There were few infants who had a severe apnea or bradycardia event after they reached the ready-for-discharge day. A 5-day observation time had a 97% to 98% success rate for all infants, except for those with a gestational age of 26 weeks or less, whereas a 7-day observation time had success rate of 98%. A 1- to 2-day interval was sufficient for the success rate to be statistically higher than 95%, whereas a 7-day apnea-free interval and a 9-day bradycardia-free interval was needed for the success rate to be statistically higher than 98%.
In our sensitivity analysis, we included a requirement for methylxanthines to be discontinued for 3 or 5 days. For a 3-day discontinuation requirement, infants reached the 95% success rate threshold with a 3-day apnea observation period and a 4-day bradycardia observation period. For a 5-day discontinuation requirement, infants reached the 95% success rate threshold with a 2-day apnea observation period and a 3-day bradycardia observation period.
DISCUSSION
The maturation of respiratory control is an important part of the discharge-planning pathway. Clinicians typically assess this maturation through clinical observation of infants in the hospital, the times for which can range from 1 to 21 days.6 However, many of these observation times are the same regardless of the characteristics of the infant or when this observation period occurs. Our study of more than 1400 premature infants suggests that a large number of infants do not have future apnea or bradycardia events once they are otherwise ready for discharge on the basis of other physiologic characteristics. Younger gestational age, older chronologic age at the time of observation, and site of care were associated with lower success rates. We found higher success rates when we restricted the analysis to infants who were off methylxanthines for 3 or 5 days. These data suggest that guidelines on the basis of patient characteristics, such as gestational age, chronologic age, and days off of methylxanthines, would optimize the discharge planning of premature infants and discontinuation of home monitoring by primary care physicians.
Previous work5 suggests that apnea and bradycardia are more common in premature infants than in healthy term infants or infants with idiopathic apnea and that this risk remains elevated until 43 weeks' PMA. Much of the earlier work on the epidemiology of apnea and bradycardia within premature infants centers on the effect of gestational age on the timing of the last apnea or bradycardia event. Darnall et al6 examined 91 infants with a gestational age of less than 32 weeks who were born between 1992 and 1993. These infants had their last apnea event at an average PMA of 37.7 ± 0.3 weeks. Infants with a gestational age of 28 weeks or less had their last event ∼2 weeks later than older infants. Similar results were found in a single-center study4 of 226 infants with a gestational age between 24 and 28 weeks. In this study, ∼20% of infants with a gestational age of 26 weeks or less had an apnea event at 40 weeks' PMA or later. Severe events resolved at an earlier PMA than self-resolved events, similar to our study results. Our data also suggest that gestational age may influence the resolution of apnea or bradycardia events once the infants are ready for discharge because younger infants, especially those with a gestational age of less than 26 weeks, were more likely to have a future event than infants of older gestational ages.
The PMA of the last event also may be an important factor in the epidemiology of apnea and bradycardia. Apnea and bradycardia events were common several days or weeks after the last event if the last event occurred at a PMA of 36 weeks or older, regardless of the gestational age of the infant. Compared with term infants, prematurely born infants have a blunted response to a hypercapnea challenge,16 a diminished glossopharyngeal muscular contraction in response to periodic breathing,17 and a higher percentage of swallowing events that result in apnea when attempting to oral feed.18 These physiologic responses mirror infants born at term as the premature infant matures.18,19 Infants with persistent apnea or bradycardia may have delayed maturation of these physiologic skills or, as suggested by Table 2, increased severity of common complications of prematurity that may influence the resolution of apnea or bradycardia events.
Our study found inter-NICU differences in the success rates of various observation time periods, similar to previous work20 that found wide inter-NICU variations in the time to the last apnea or bradycardia events. Our results suggest that the case mix of the NICU may influence the success rates of various observation time periods because NICUs with a larger percentage of infants observed after 38 weeks' PMA had lower overall success rates. Accounting for process differences between NICUs reduced inter-NICU variation in success rates by 50% to 90%. These results emphasize the importance of processes of care in understanding differences in inter-NICU outcomes.
Finally, these data show the difficulty in applying statistics for diagnostic tests to the care of an individual patient. For each observation time, the EIR of the observation interval was much lower than the success rate of the test. This fact occurred because more than 75% of the infants did not have a future apnea or bradycardia event once they met other criteria for discharge. For a clinician observing a specific newborn, the more relevant statistic is the success rate if the apnea or bradycardia recurrence in their NICU was similar to those units included in this study. However, the EIR can be used to generate NICU-specific success rates when the overall recurrence risk of apnea or bradycardia differs from those units included in this study.
There are several limitations to this study. First, all events were captured through a nursing report in the medical chart, which were standardized between the multiple sites in the KPMCP health care system. Short, spontaneously resolving events may have been missed if the nurse was distracted when the event occurred.21 Previous studies2,4,6,20 have used a similar data-collection methodology. Nursing records should, however, capture all events that required respiratory intervention by a health care provider. When we only examined these events, we found similar results to the data that used all events. Also, we only were able to use events after discharge that resulted in a visit to a health care provider. Short, self-limited events or obstructive events without apnea may have been missed.5 As a result, when a health care provider decided to discharge a patient may marginally affect these results for those infants discharged relatively soon after their last apnea or bradycardia event. This study also used information from a limited number of NICUs because of the difficulty in collecting detailed data on respiratory maturity from a large number of premature infants with complete postdischarge data. The statistical power provided by this cohort is important to understanding the relationship between gestational age, PMA of the last event, and the future risk of apnea or bradycardia. Finally, the clinical relevance of a 95% or 98% success rate threshold is not certain in the context of apnea or bradycardia.
CONCLUSIONS
Our data suggests that the risk of recurrence for an infant depends on the recurrence rate accepted by practitioners, the gestational age of the infant, and the PMA of the last apnea or bradycardia event. Practitioners should use these data to personalize the discharge planning of premature infants.
Supplementary Material
ACKNOWLEDGMENTS
This study was funded through Maternal and Child Health Bureau grant R40 MC00236 and a grant from the McCabe Foundation of the University of Pennsylvania School of Medicine, the Permanente Medical Group, and Kaiser Foundation Hospitals.
FINANCIAL DISCLOSURE: The authors have indicated they have no personal financial relationships relevant to this article to disclose.
Abbreviations:
- PMA
- postmenstrual age
- EIR
- event identification rate
- KPMCP
- Kaiser Permanente Medical Care Program
- CI
- confidence interval
REFERENCES
- 1. American Academy of Pediatrics, Committee on Fetus and Newborn Hospital discharge of the high-risk neonate. Pediatrics. 2008;122(5):1119–1126 [DOI] [PubMed] [Google Scholar]
- 2. Bakewell-Sachs S, Medoff-Cooper B, Escobar GJ, et al. Infant functional status: the timing of physiologic maturation of premature infants. Pediatrics. 2009;123(5). Available at: www.pediatrics.org/cgi/content/full/123/5/e878 [DOI] [PubMed] [Google Scholar]
- 3. Henderson-Smart DJ. The effect of gestational age on the incidence and duration of recurrent apnoea in newborn babies. Aust Paediatr J. 1981;17(4):273–276 [DOI] [PubMed] [Google Scholar]
- 4. Eichenwald EC, Aina A, Stark AR. Apnea frequently persists beyond term gestation in infants delivered at 24 to 28 weeks. Pediatrics. 1997;100(3 pt 1):354–359 [DOI] [PubMed] [Google Scholar]
- 5. Ramanathan R, Corwin MJ, Hunt CE, et al. Cardiorespiratory events recorded on home monitors: comparison of healthy infants with those at increased risk for SIDS. JAMA. 2001;285(17):2199–2207 [DOI] [PubMed] [Google Scholar]
- 6. Darnall RA, Kattwinkel J, Nattie C, et al. Margin of safety for discharge after apnea in preterm infants. Pediatrics. 1997;100(5):795–801 [DOI] [PubMed] [Google Scholar]
- 7. Lorch SA, Wade KC, Bakewell-Sachs S, et al. Racial differences in the use of respiratory medications in premature infants after discharge from the neonatal intensive care unit. J Pediatr. 2007;151(6):604–610 [DOI] [PubMed] [Google Scholar]
- 8. Lorch SA, Wade KC, Bakewell-Sachs S, et al. Antibiotic use in premature infants after discharge from the neonatal intensive care unit. Clin Pediatr (Phila). 2010;49(3):249–257 [DOI] [PubMed] [Google Scholar]
- 9. Silber JH, Lorch SA, Rosenbaum PR, et al. Time to send the preemie home? Additional maturity at discharge and subsequent healthcare costs and outcomes. Health Serv Res. 2009;44(2 pt 1):444–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Escobar GJ, Fischer A, Kremers R, et al. Rapid retrieval of neonatal outcomes data: the Kaiser Permanente Neonatal Minimum Data Set. Qual Manag Health Care. 1997;5(4):19–33 [PubMed] [Google Scholar]
- 11. Cohen JA. A coefficient of agreement for normal scales. Educ Psychol Meas. 1960;20(1):37–46 [Google Scholar]
- 12. Shennan AT, Dunn MS, Ohlsson A, et al. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics. 1988;82(4):527–532 [PubMed] [Google Scholar]
- 13. Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33(1):179–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Papile LA, Burstein J, Burstein R, et al. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1 500 gm J Pediatr. 1978;92(4):529–534 [DOI] [PubMed] [Google Scholar]
- 15. An international classification of retinopathy of prematurity. Pediatrics. 1984;74(1):127–133 [PubMed] [Google Scholar]
- 16. Carlo WA, Martin RJ, Difiore JM. Differences in CO2 threshold of respiratory muscles in preterm infants. J Appl Physiol. 1988;65(6):2434–2439 [DOI] [PubMed] [Google Scholar]
- 17. Gauda EB, Miller MJ, Carlo WA, et al. Genioglossus response to airway occlusion in apneic versus nonapneic infants. Pediatr Res. 1987;22(6):683–687 [DOI] [PubMed] [Google Scholar]
- 18. Gewolb IH, Vice FL. Maturational changes in the rhythms, patterning, and coordination of respiration and swallow during feeding in preterm and term infants. Dev Med Child Neurol. 2006;48(7):589–594 [DOI] [PubMed] [Google Scholar]
- 19. Hofstetter AO, Legnevall L, Herlenius E, et al. Cardiorespiratory development in extremely preterm infants: vulnerability to infection and persistence of events beyond term-equivalent age. Acta Paediatr. 2008;97(3):285–292 [DOI] [PubMed] [Google Scholar]
- 20. Eichenwald EC, Blackwell M, Lloyd JS, et al. Inter-neonatal intensive care unit variation in discharge timing: influence of apnea and feeding management. Pediatrics. 2001;108(4):928–933 [DOI] [PubMed] [Google Scholar]
- 21. Muttitt SC, Finer NN, Tierney AJ, et al. Neonatal apnea: diagnosis by nurse versus computer. Pediatrics. 1988;82(5):713–720 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.