Abstract
OBJECTIVE:
The objective of this study was to determine the impact of recombinant human prolactin (r-hPRL) on the nutritional and immunologic composition of breast milk.
METHODS:
We conducted 2 trials of r-hPRL treatment. In the first study, mothers with documented prolactin deficiency were given r-hPRL every 12 hours in a 28-day, open-label trial. In the second study, mothers with lactation insufficiency that developed while they were pumping breast milk for their preterm infants were given r-hPRL daily in a 7-day, double-blind, placebo-controlled trial. Breast milk characteristics were compared before and during 7 days of treatment.
RESULTS:
Among subjects treated with r-hPRL (N = 11), milk volumes (73 ± 36 to 146 ± 54 mL/day; P < .001) and milk lactose levels (155 ± 15 to 184 ± 8 mmol/L; P = .01) increased, whereas milk sodium levels decreased (12.1 ± 2.0 to 8.3 ± 0.5 mmol/L; P = .02). Milk calcium levels increased in subjects treated with r-hPRL twice daily (2.8 ± 0.6 to 5.0 ± 0.9 mmol/L; P = .03). Total neutral (1.5 ± 0.3 to 2.5 ± 0.4 g/L; P = .04) and acidic (33 ± 4 to 60 ± 6 mg/L; P = .02) oligosaccharide levels increased in r-hPRL-treated subjects, whereas total daily milk immunoglobulin A secretionwas unchanged.
CONCLUSIONS:
r-hPRL treatment increased milk volume and induced changes in milk composition similar to those that occur during normal lactogenesis. r-hPRL also increased antimicrobially active oligosaccharide concentrations. These effects were achieved for women with both prolactin deficiency and lactation insufficiency.
Keywords: breastfeeding, prolactin, nutrition, premature, neonate
WHAT'S KNOWN ON THIS SUBJECT:
The direct effects of prolactin on the nutritional and antimicrobial composition of breast milk have not been examined previously in women.
WHAT THIS STUDY ADDS:
The study demonstrates that recombinant human prolactin increases milk volume, induces changes in milk composition consistent with those during normal lactogenesis, and increases antimicrobially active oligosaccharide concentrations. The data suggest that prolactin is an important mediator of normal lactogenesis.
Successful lactation requires a transition from colostrum to copious milk production within the first 2 to 10 days after birth, a process termed lactogenesis. Lactogenesis requires an interplay between hormonal changes, normal breast anatomic features, and sufficient milk removal.1 Prolactin is the critical hormone in the initiation and maintenance of breast milk production, and its absence at any stage results in cessation of lactation.2,3
Prolactin also may mediate changes in breast milk composition during normal lactogenesis.4,5 During lactogenesis, lactose, citrate, and calcium concentrations increase, whereas protein, sodium, and chloride concentrations decrease.4–6 In vitro, prolactin increases the synthesis of α-lactalbumin, a key regulator of lactose synthesis.7 Prolactin also is involved in the closure of epithelial tight junctions between alveolar cells in animals,8 which reduces sodium and chloride levels and increases lactose levels in mature milk.9 Indirect evidence suggests that prolactin also may influence the production of breast milk secretory immunoglobulin A (IgA) and oligosaccharides,10,11 immune factors that inhibit intestinal host cell-enteric pathogen interactions.12 Prolactin increases mammary IgA-secreting plasma cells in mice,13 and the pattern of milk oligosaccharide concentrations (high during early lactation and decreasing over the course of lactation)14 parallels decreases in prolactin levels that occur over time.15–17 These effects suggest that prolactin may contribute to the progressive changes in milk composition.
The coincidence of abnormalities in serum prolactin levels and breast milk composition for mothers of infants born prematurely further suggests a role for prolactin in lactogenesis.18–21 Mothers of preterm infants have lower breast milk lactose levels, smaller milk volumes, and higher sodium concentrations than do mothers of term infants.18,22,23 For preterm mothers using breast pumps to maintain lactation, prolactin concentrations are relatively low.23,24 However, direct effects of prolactin in human mothers had not been studied previously. We conducted 2 clinical trials using recombinant human prolactin (r-hPRL) in cases of lactation insufficiency.25 In these trials, mothers with prolactin deficiency and mothers of preterm infants with lactation insufficiency who were treated with r-hPRL experienced significant increases in milk volume.25 Here we describe the changes in milk chemical and immune composition among mothers treated with r-hPRL.
METHODS
Studies Included
Breast milk composition was measured for mothers participating in 2 clinical trials of r-hPRL, one involving mothers with prolactin deficiency (study 1) and the other involving mothers with lactation insufficiency who were pumping breast milk for their preterm infants (study 2). All studies were approved by the Partners and Boston University Medical Center human research committees, and subjects gave written informed consent for themselves and their infants. These pilot studies are presented together to provide preliminary data on the effects of r-hPRL on breast milk composition. Maternal and neonatal histories, serum prolactin levels, r-hPRL safety and efficacy, details from the subgroups, and follow-up volume data were published separately.25
Subjects
Study 1 was an open-label trial of r-hPRL involving prolactin-deficient mothers (N = 5). Subjects had congenital or acquired prolactin deficiency, with either baseline or peak prolactin levels below the normal range for postpartum date,24 and produced <8 mL of milk per day. All mothers were pumping their breasts (n = 4) or the infant was suckling at the breast by using a Lact-Aid device (Lact-Aid International, Inc, Athens, TN) (n = 1) 8 times per day. Study 2 was a randomized, double blind, placebo-controlled trial of r-hPRL treatment for mothers of premature infants with lactation insufficiency (N = 10). All subjects were pumping breast milk for their preterm infants, reported a decrease in milk production, and produced <750 mL of milk per day.26 No subjects were taking medications known to increase prolactin levels.
Protocol
All subjects were seen in the General Clinical Research Center at Massachusetts General Hospital or Brigham and Women's Hospital. On day 1, subjects were evaluated and received pumping instructions from the study's lactation consultant. Subjects then pumped both breasts with a hospital-grade breast pump until there was no milk flow for 2 minutes, with the goal of pumping 8 times per day throughout the study. The volume of milk produced at each pumping session was recorded. In both studies, subjects had blood drawn before pumping and at frequent intervals for 180 minutes, to determine the peak prolactin levels.25
In study 1, subjects subsequently self-administered r-hPRL (60 μg/kg) injections subcutaneously every 12 hours for 28 days. On days 7 and 28, blood was sampled for 6 hours with medication. In study 2, subjects returned on day 2 and were assigned randomly to receive subcutaneous injections of r-hPRL (60 μg/kg) every 12 hours (N = 3), alternating doses of r-hPRL and placebo every 12 hours (N = 3), or placebo (normal saline solution) every 12 hours (N = 4) for 7 days. On days 2 and 8, blood was sampled for 6 hours with medication. In both studies, all visits occurred at the same time of day for each subject.
Milk Analyses
Milk samples (1 mL) were collected before the first administration of r-hPRL each day, frozen, and stored at −20°C. Milk components were measured in milk samples pooled from days 1 and 2 (before treatment) and days 7 and 8 (during treatment). Additional analyses were conducted on pooled samples from days 3 and 4 and days 5 and 6 for lactose and oligosaccharide measurements.
Fat concentrations were measured by using the creamatocrit technique.27 Prolactin, protein, α-lactalbumin, IgA, and oligosaccharide assays were performed with the aqueous layer after defatting through centrifugation for 6 minutes at 12 000 rpm. Prolactin levels were measured by using a 2-site, monoclonal antibody, nonisotopic system (Axsym [Abbott Laboratories, Abbott Park, IL]), as described previously.28 Milk protein levels were measured by using the Bio-Rad (Hercules, CA) protein assay, according to the manufacturer's instructions. IgA levels were measured by using a Beckman array nephelometric analyzer (Beckman Instruments, Fullerton, CA).29 Neutral oligosaccharide and α-lactalbumin levels were measured by using high-performance liquid chromatography.17,30,31 Acidic oligosaccharide levels were measured by using capillary electrophoresis.32
Citrate, sodium, calcium, and lactose assays were performed after defatting and deproteination, as described previously.6 Lactose and citrate levels were measured by using a commercial enzymatic assay (R-Biopharm, Darmstadt, Germany) validated for human milk.6 Sodium and calcium were measured by using flame photometry (Hitachi 917 system [F. Hoffmann-LaRoche, Ltd, Basel, Switzerland]).18
Statistical Analyses
Milk samples from mothers who received r-hPRL once or twice daily in studies 1 and 2 were analyzed together and compared with those from mothers who received only placebo. Of note, although the majority of mothers were studied within 12 weeks after birth (n = 13), 2 were studied 39 weeks after birth (1 from each study). Although there are differences in milk composition at 4 to 12 weeks, compared with 39 weeks, during normal lactation,33 the trends of changes in milk components were the same for all subjects; therefore, the data were analyzed together.
Data were logarithmically transformed for analyses. Milk volume and lactose and prolactin concentrations were analyzed by using 2-way, repeated-measures analysis of variance with Holm-Sidak posthoc analysis. The changes in milk components from baseline (days 1–2) to after treatment (days 7–8, or days 3–8 for oligosaccharides) were compared by using paired t tests. Changes in milk components over 28 days were analyzed by using 1-way, repeated-measures analysis of variance. Total secretion of milk components was calculated as concentration × daily milk volume. Correlations among levels of milk components, milk volumes, and serum prolactin concentrations were examined by using Pearson product moment or Spearman rank order tests, as appropriate.
RESULTS
Postpartum dates, baseline milk volumes, and prolactin levels in studies 1 and 2 are shown in Table 1. All mothers had small milk volumes and/or decreases from baseline values. Prolactin-deficient mothers (study 1) by definition had low baseline or peak prolactin levels, or both, despite continued pumping or breast stimulation in an attempt to lactate. Baseline (15.2 ± 3.5 vs 101.8 ± 24.1 μg/L; P < .01) and peak (75.7 ± 28.9 vs 211.8 ± 19.3 μg/L; P < .001) prolactin levels increased into the high-normal postpartum range for subjects treated with r-hPRL.24,25 Prolactin levels did not increase for subjects treated with placebo (baseline: 56.5 ± 40.9 vs 98.8 ± 82.9 μg/L; peak: 111.7 ± 68.1 vs 129.9 ± 57.9 μg/L; both P = .2). Milk volumes and compositions at baseline and after treatment are reported in Table 2, which combines data for all women receiving r-hPRL. Milk volumes increased in the group treated with r-hPRL but not in the placebo group (Table 2). Two subjects in the placebo group produced 0 to 1 mL of milk per day, which excluded them from milk composition analyses. The other 2 subjects produced the greatest milk volumes, which resulted in a wide variation in milk volumes in the placebo group.
TABLE 1.
Baseline Characteristics of Subjects in r-hPRL Trials
| Study Group | Time After Birth, Mean (Range), wk | Milk Production, Mean (Range), mL/da | Serum Prolactin Level, Mean (Range), μg/Lb |
|
|---|---|---|---|---|
| Baseline | Peak | |||
| Study 1: prolactin-deficient mothers (N = 5) | 12.4 (5–39) | 3.4 (0–8) | 11.1 (0–34.5) | 27.9 (0–93.1) |
| Study 2: lactation-insufficient mothers of premature infants (N = 10) | 11.3 (3.5–39) | 190 (0–726) | 33.2 (5.7–178) | 107 (8.4–308) |
TABLE 2.
Milk Volumes and Composition in r-hPRL and Placebo Groups Before and After Treatment
| r-hPRL (N = 11) |
Placebo (N = 2–4)a |
|||||
|---|---|---|---|---|---|---|
| Baseline | Treatment | Pb | Baseline | Treatment | Normalc | |
| Milk volume, mL/d | 73 ± 36 | 146 ± 54 | <.001 | 319 ± 188 | 348 ± 202 | 749–1181 |
| Lactose level, mmol/L | 155 ± 15.2 | 184 ± 7.6 | .01 | 207; 236 | 207; 274 | 159–214 |
| Sodium level, mmol/L | 12.1 ± 2.0 | 8.3 ± 0.5 | .02 | 8; 8 | 7; 7 | 4–16 |
| Calcium level, mmol/L | 2.8 ± 1.2 | 3.9 ± 1.6 | .23 | 1.1; 1.5 | 2.0; 2.3 | 4.3–9.0 |
| Calcium level with twice-daily r-hPRL, mmol/L | 2.8 ± 0.6 | 5.0 ± 0.9 | .03 | NA | NA | 4.3–9.0 |
| Protein level, g/L | 13.2 ± 2.6 | 11.2 ± 2.1 | .33 | 7.2; 6.8 | 8.2; 2.9 | 7–11 |
| Fat proportion, % | 7.2 ± 1.0 | 7.8 ± 0.8 | .65 | 9.1; 8.8 | 9.3; 10.7 | 2–8 |
| Citrate level, mmol/L | 3.9 ± 2.1 | 3.0 ± 1.6 | .79 | 8.3; 0.5 | 8.3; 0.5 | 2.3–4.4 |
| α-Lactalbumin level, g/L | 3.6 ± 0.3 | 3.6 ± 0.6 | .9 | 3.6; 3.5 | 3.4; 3.5 | 3.2 ± 1.0 |
| Neutral oligosaccharide level, g/L | 1.5 ± 0.3 | 2.5 ± 0.4 | .04 | 0.5; 1.3 | 1.4; 1.5 | 0.1–1.9 |
| Acidic oligosaccharide level, mg/L | 33.2 ± 4.5 | 60.3 ± 6.3 | .02 | 52.8; 12.8 | 87.2; 16.7 | NA |
| IgA level | ||||||
| g/L | 2.5 ± 0.8 | 0.8 ± 0.2 | .03 | 0.2; 0.2 | 0.2; 0.2 | 0.5–1.5 |
| mg/d | 73 ± 27 | 76 ± 31 | .92 | 73; 167 | 92; 134 | NA |
| Milk prolactin level, μg/L | 47.4 ± 7.7 | 118 ± 25 | .03 | 21.5; 1.8 | 59.9; 12.6 | 4–254 |
Baseline indicates the average for days 1 to 2, and treatment indicates the average for days 7 to 8. NA indicates not available.
N = 4 for milk volume; N = 2 for all other milk parameters (milk volume was 0–2 mL for 2 subjects). Where N = 2, raw data are presented; otherwise, data are expressed as mean ± SEM.
P values are for paired t tests or repeated-measures analysis of variance (see text).
Values indicate normal milk volumes after 1 to 6 months of exclusive breastfeeding,57 normal values for lactose, sodium, calcium, protein, fat, citrate, and IgA levels in mature milk during 3 weeks to 6 months of successful lactation,31,33 normal oligosaccharide concentrations during 0 to 10 weeks of lactation,42 normal α-lactalbumin levels in mature milk from US mothers,30 and normal milk prolactin levels for breast milk at any time after birth.7,48
Lactose concentrations increased (Table 2) and sodium concentrations decreased within 7 days for subjects treated with r-hPRL. Of note, abnormally high sodium and low lactose levels for 3 subjects reached the normal range. The lactose and sodium concentrations remained stable after day 7 for prolactin-deficient mothers who were treated for 28 days (n = 5; data not shown).
There was no change in calcium concentrations among all subjects treated with r-hPRL; however, calcium concentrations increased among subjects treated twice daily (n = 8). All changes resulted in values that fell within normal limits. There were no changes in protein, fat, citrate, or α-lactalbumin concentrations among mothers treated with r-hPRL; there also were no changes in these parameters for the subset of prolactin-deficient mothers who were treated for 28 days (data not shown). There were no differences in any of the parameters between the r-hPRL and placebo groups.
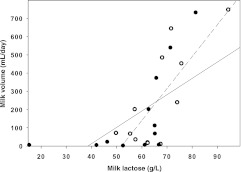
Milk volumes were correlated with milk lactose levels at baseline (r = 0.7; P = .01) and after treatment (r = 0.6; P < .05) (Fig 1). There was an inverse correlation between milk sodium levels and volumes after treatment (r = −0.7; P = .02). There was no relationship between levels of the other milk components and volumes.
FIGURE 1.
Correlation between milk lactose levels and milk volume before (n = 11) (closed circles and solid line) and after (n = 11) (open circles and dashed line) treatment with r-hPRL.
Neutral and acidic oligosaccharide contents increased for subjects treated with r-hPRL (Table 2). Although milk IgA concentrations decreased, the total IgA secretion over 24 hours did not change, because of increases in milk volumes for treated subjects. At baseline (r = −0.8; P = .004) and after treatment (r = −0.7; P = .03), IgA concentrations were correlated inversely with milk volumes.
The average prolactin concentration for mothers treated with r-hPRL was higher than the values for the 2 mothers treated with placebo (90.0 ± 15.3 vs 10 and 55 μg/L). For treated mothers, milk prolactin levels were higher on days 5 to 7 than at baseline (P < .01) (Fig 2). Among subjects treated for 28 days, milk prolactin levels decreased to baseline by day 14 (P < .001) (Fig 2). Milk prolactin levels were not correlated with serum prolactin levels before or after treatment. Milk prolactin levels were inversely correlated with milk volumes (r = −0.6; P < .001) and lactose levels after treatment (r = −0.6; P < .04). Milk prolactin levels were correlated with neutral oligosaccharide levels at baseline (r = 0.6; P < .05) and acidic oligosaccharide levels after treatment (r = 0.6; P < .05).
FIGURE 2.
Mean ± SE milk prolactin concentrations for prolactin-deficient mothers treated with r-hPRL (n = 5) (closed circles) and mothers of preterm infants treated with r-hPRL (n = 6) (closed triangles) and individual prolactin concentrations for mothers of preterm infants treated with placebo (n = 2) (open squares). Prolactin concentrations were higher on days 5 to 7, compared with baseline, for mothers treated with r-hPRL (*P < .01). Prolactin concentrations returned to baseline on day 14 for prolactin-deficient mothers treated with r-hPRL for 28 days (n = 5; **P < .001).
DISCUSSION
This study provides the first direct evidence for a role of prolactin in human lactogenesis, through direct administration of prolactin to mothers with prolactin deficiency and lactation insufficiency. Among subjects treated with r-hPRL, baseline and peak serum prolactin levels increased to normal postpartum levels. The increased prolactin levels resulted in increased milk volumes, increased lactose and calcium concentrations, and decreased sodium concentrations, with all falling within normal ranges.24 Therefore, r-hPRL administration is associated with compositional changes that mirror those in women undergoing normal lactogenesis in the first 2 to 10 days after birth.7,18,30–33 Furthermore, r-hPRL administration increased oligosaccharide levels, whereas total daily IgA levels did not change, which potentially improved the antimicrobial properties of breast milk. These findings suggest that r-hPRL improves breast milk quantity, maturity, and immunity for mothers with lactation insufficiency.
The increases in milk volumes and lactose concentrations with r-hPRL treatment and the correlation between milk volumes and lactose concentrations suggest that r-hPRL increases milk volumes through lactose production. Synthesis and retention of lactose, the major milk osmolyte, allow fluid to be drawn into milk secretory vesicles, increasing milk volume.7 The slopes of the lactose concentration-volume relationships before and after r-hPRL treatment are consistent with prolactin increasing the osmotic effect of lactose. It is possible that prolactin increases lactose concentrations by increasing synthesis of α-lactalbumin, a protein that regulates the rate of lactose synthesis by lactose synthase.34 r-hPRL treatment prevented the normal decrease in milk α-lactalbumin levels that occurs as lactose levels increase during lactogenesis.35
Along with the increase in lactose concentrations, the decrease in milk sodium concentrations with r-hPRL treatment is consistent with closure of tight junctions between mammary epithelial cells, which prohibits sodium entry from the interstitium into milk and traps lactose in the ducts. This hypothesis is supported by animal studies demonstrating that prolactin establishes and maintains impermeability of the mammary epithelium barrier.8,9 When tight junctions open, milk production decreases,4 because osmotically active milk components cannot be retained in the duct. Studies with women have shown consistently that decreases in milk sodium concentrations are associated with successful lactation, whereas high milk sodium levels are associated with breastfeeding failure, which suggests that closure of tight junctions also is necessary for the establishment of successful lactation in humans.4,5,19
Milk calcium concentrations increased in the smaller subset of subjects treated twice daily with r-hPRL (n = 8). Prolactin may increase breast milk calcium levels through increased synthesis of parathyroid hormone-related protein, which is not demonstrable with once-daily dosing, or increased calcium transport into mammary epithelial cells.28,36 Milk calcium levels were lower than reported previously,33 but they were measured in defatted, deproteinated milk, which excluded calcium associated with casein micelles, α-lactalbumin, and milk fat.7 Together, these results provide preliminary evidence that twice-daily dosing is necessary to affect calcium handling in both lactating and nonlactating women.
r-hPRL did not affect milk fat, protein, or citrate levels, which suggests that prolactin is not involved in regulating these nutrients in breast milk. Our findings are consistent with those observed in a recent study in which domperidone increased maternal prolactin levels and milk calcium concentrations with no significant changes in milk protein or fat contents.37
Typical IgA concentrations in mature milk range from 0.5 to 1.5 g/L, but levels can be much higher in colostrum produced during pregnancy and 2 to 5 days after birth, before the completion of lactogenesis.31,38,39 On average, mothers in the current study were assessed at 12 weeks after birth but had milk IgA levels of 2.53 g/L. The high IgA levels may be partially explained by preterm delivery and smaller milk volumes. IgA levels are greater in mothers of preterm infants for ≥12 weeks after birth21,40 and milk volumes are smaller, which results in similar levels of total IgA secretion per day, compared with mothers of term infants.21 Consistent with these observations, treatment with r-hPRL increased milk volume but did not decrease total IgA secretion into milk.
Oligosaccharide concentrations increased with r-hPRL treatment. Oligosaccharides are antimicrobial complex carbohydrate structures and the third largest component in human milk.41,42 Oligosaccharides containing sialic acid (acidic oligosaccharides) stimulate growth of intestinal commensal bacteria, such as Lactobacillus bifidus,43 which acidify the gut, bind to potential colonization sites, and prevent colonization by harmful pathogens.12 The glycan component of oligosaccharides resembles the cell surface glycans of the intestine and competitively binds enteric pathogens.12,32 Oligosaccharides are associated with lower levels of diarrhea in breastfed infants.14
Oligosaccharide concentrations are highest immediately after birth and decrease exponentially in the first 2 months of breastfeeding.42 In contrast to the decrease during lactogenesis, levels of both neutral and acidic oligosaccharides increased with r-hPRL treatment, and milk prolactin levels were correlated with oligosaccharide concentrations, which suggests that prolactin plays a role in the regulation of neutral and acidic oligosaccharides. Therefore, the study provides the first evidence that prolactin increases oligosaccharide levels in human milk, which potentially increases its immune benefits.
In the current study, r-hPRL treatment increased milk prolactin levels. Although r-hPRL cannot be distinguished from endogenous prolactin in milk, it is likely that r-hPRL transfer into the milk was responsible. In addition to local mammary prolactin synthesis, circulating prolactin binds to prolactin receptors on alveolar cells and is secreted into the ducts,44,45 in direct relation to the plasma concentration.46 During normal lactogenesis, milk prolactin levels decrease sharply with overall protein levels after postpartum day 335 and continue to decrease over the course of lactation,7,47,48 which suggests that the decrease is an effect of dilution.35 Consistently, milk prolactin levels decreased with increased milk volume in the current study, as expected with dilution of a fixed dose of r-hPRL. Importantly, prolactin levels never exceeded those noted during normal lactogenesis.7,35,47,49
The study is limited by the small sample size. Nevertheless, it provides the first pilot data demonstrating the direct effect of prolactin on milk composition. There was heterogeneity in postpartum dates, gestational ages, and underlying maternal diagnoses associated with lactation insufficiency. It is noteworthy that all subjects treated with r-hPRL exhibited increases in breast milk volume25 and qualitatively similar changes in levels of individual milk components, despite the heterogeneity. The effects of r-hPRL were studied only to 28 days; longer-term effects of r-hPRL use and possible additive effects of nonhormonal galactogogues will require additional study. Finally, additional components of milk important for its immune properties, such as white blood cells and lactoferrin, were not assessed. Studies are needed to examine these components and the long-term effects of r-hPRL with larger numbers of subjects.
Despite the limitations, our findings have several important implications for feeding of preterm, very low birth weight (VLBW) infants. Although the beneficial effects of breast milk feeding are well established,50 increasing evidence supports a critical role for breast milk feeding for VLBW infants. Breast milk rather than formula feeding of VLBW infants is associated with decreased risks of late-onset sepsis and necrotizing enterocolitis,51–53 complications of prematurity that cause significant short-term morbidity and death and are associated with poor long-term neurologic outcomes.54 Breast milk feeding also influences intestinal bacterial colonization and may have global effects on health.55 Lactation insufficiency is a primary reason for formula-feeding of VLBW infants. Administration of r-hPRL to increase maternal milk production may be one approach to achieving exclusive breast milk feeding of VLBW infants, one that may be complementary to donor milk feeding. Our findings suggest that the changes in milk composition induced by r-hPRL may increase the nutritional value of breast milk for VLBW infants, especially by increasing calcium content, and may decrease the need to supplement breast milk with human milk fortifiers. The increase in potentially protective oligosaccharides with maintenance of white blood cells, IgA, lysozyme, and lactoferrin in mother's milk, which are lost during pasteurization of donor milk,56 also may be critical to decreasing the risk of infection and necrotizing enterocolitis for VLBW infants.
CONCLUSIONS
For mothers with prolactin deficiency and lactation insufficiency, r-hPRL treatment resulted in increased milk volume and maturation of milk composition. Milk prolactin levels increased with r-hPRL treatment but only to levels seen during normal lactogenesis. These findings suggest that prolactin induces changes in milk composition during normal lactogenesis. Our data also suggest that prolactin plays a role in the synthesis of immunologically important oligosaccharides. Additional study of the use of r-hPRL in lactation insufficiency is warranted.
ACKNOWLEDGMENTS
This work was supported by the Food and Drug Administration (grant FD-R-003014), the March of Dimes Birth Defects Foundation (research grant 6-FY04-76), and the National Center for Research Resources General Clinical Research Centers Program (grant M01-RR-01066).
This trial has been registered at www.clinicaltrials.gov (identifiers NCT00181623 and NCT00181610).
FINANCIAL DISCLOSURE: The recombinant human prolactin was provided by Genzyme. Dr Welt received an unrestricted grant from Genzyme. Genzyme was not involved in study design, subject recruitment, data analysis, or manuscript preparation.
- r-hPRL
- recombinant human prolactin
- IgA
- immunoglobulin A
- VLBW
- very low birth weight
REFERENCES
- 1. Neifert MR. Prevention of breastfeeding tragedies. Pediatr Clin North Am. 2001;48(2):273–294 [DOI] [PubMed] [Google Scholar]
- 2. Brun del Re R, del Pozo E, de Grandi P, Friesen H, Hinselmann M, Wyss H. Prolactin inhibition and suppression of puerperal lactation by a Br-ergocryptine (CB 154). A comparison with estrogen. Obstet Gynecol. 1973;41(6):884–890 [PubMed] [Google Scholar]
- 3. Kauppila A, Chatelain P, Kirkinen P, Kivinen S, Ruokonen A. Isolated prolactin deficiency in a woman with puerperal alactogenesis. J Clin Endocrinol Metab. 1987;64(2):309–312 [DOI] [PubMed] [Google Scholar]
- 4. Neville MC, Allen JC, Archer PC, et al. Studies in human lactation: milk volume and nutrient composition during weaning and lactogenesis. Am J Clin Nutr. 1991;54(1):81–92 [DOI] [PubMed] [Google Scholar]
- 5. Kulski JK, Hartmann PE. Changes in human milk composition during the initiation of lactation. Aust J Exp Biol Med Sci. 1981;59(1):101–114 [DOI] [PubMed] [Google Scholar]
- 6. Arthur PG, Smith M, Hartmann PE. Milk lactose, citrate, and glucose as markers of lactogenesis in normal and diabetic women. J Pediatr Gastroenterol Nutr. 1989;9(4):488–496 [DOI] [PubMed] [Google Scholar]
- 7. Ostrom KM. A review of the hormone prolactin during lactation. Prog Food Nutr Sci. 1990;14(1):1–43 [PubMed] [Google Scholar]
- 8. Linzell JL, Peaker M, Taylor JC. The effects of prolactin and oxytocin on milk secretion and on the permeability of the mammary epithelium in the rabbit. J Physiol. 1975;253(2):547–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nguyen DA, Neville MC. Tight junction regulation in the mammary gland. J Mammary Gland Biol Neoplasia. 1998;3(3):233–246 [DOI] [PubMed] [Google Scholar]
- 10. Richards SM, Murphy WJ. Use of human prolactin as a therapeutic protein to potentiate immunohematopoietic function. J Neuroimmunol. 2000;109(1):56–62 [DOI] [PubMed] [Google Scholar]
- 11. Boyle-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev. 1998;19(3):225–268 [DOI] [PubMed] [Google Scholar]
- 12. Newburg DS, Ruiz-Palacios GM, Morrow AL. Human milk glycans protect infants against enteric pathogens. Annu Rev Nutr. 2005;25:37–58 [DOI] [PubMed] [Google Scholar]
- 13. Weisz-Carrington P, Roux ME, McWilliams M, Phillips-Quagliata JM, Lamm ME. Hormonal induction of the secretory immune system in the mammary gland. Proc Natl Acad Sci U S A. 1978;75(6):2928–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morrow AL, Ruiz-Palacios GM, Altaye M, et al. Human milk oligosaccharides are associated with protection against diarrhea in breast-fed infants. J Pediatr. 2004;145(3):297–303 [DOI] [PubMed] [Google Scholar]
- 15. Battin DA, Marrs RP, Fleiss PM, Mishell DR. Effect of suckling on serum prolactin, luteinizing hormone, follicle-stimulating hormone, and estradiol during prolonged lactation. Obstet Gynecol. 1985;65(6):785–788 [PubMed] [Google Scholar]
- 16. Hennart P, Delogne-Desnoeck J, Vis H, Robyn C. Serum levels of prolactin and milk production in women during a lactation period of thirty months. Clin Endocrinol (Oxf). 1981;14(4):349–353 [DOI] [PubMed] [Google Scholar]
- 17. Chaturvedi P, Warren CD, Altaye M, et al. Fucosylated human milk oligosaccharides vary between individuals and over the course of lactation. Glycobiology. 2001;11(5):365–372 [DOI] [PubMed] [Google Scholar]
- 18. Cregan MD, De Mello TR, Kershaw D, McDougall K, Hartmann PE. Initiation of lactation in women after preterm delivery. Acta Obstet Gynecol Scand. 2002;81(9):870–877 [DOI] [PubMed] [Google Scholar]
- 19. Morton JA. The clinical usefulness of breast milk sodium in the assessment of lactogenesis. Pediatrics. 1994;93(5):802–806 [PubMed] [Google Scholar]
- 20. Henderson JJ, Hartmann PE, Newnham JP, Simmer K. Effect of preterm birth and antenatal corticosteroid treatment on lactogenesis II in women. Pediatrics. 2008;121(1). Available at: www.pediatrics.org/cgi/content/full/121/1/e92 [DOI] [PubMed] [Google Scholar]
- 21. Gross SJ, Buckley RH, Wakil SS, McAllister DC, David RJ, Faix RG. Elevated IgA concentration in milk produced by mothers delivered of preterm infants. J Pediatr. 1981;99(3):389–393 [DOI] [PubMed] [Google Scholar]
- 22. Anderson GH, Atkinson SA, Bryan MH. Energy and macronutrient content of human milk during early lactation from mothers giving birth prematurely and at term. Am J Clin Nutr. 1981;34(2):258–265 [DOI] [PubMed] [Google Scholar]
- 23. Hill PD, Aldag JC, Demirtas H, et al. Association of serum prolactin and oxytocin with milk production in mothers of preterm and term infants. Biol Res Nurs. 2009;10(4):340–349 [DOI] [PubMed] [Google Scholar]
- 24. Noel GL, Suh HK, Frantz AG. Prolactin release during nursing and breast stimulation in postpartum and nonpostpartum subjects. J Clin Endocrinol Metab. 1974;38(3):413–423 [DOI] [PubMed] [Google Scholar]
- 25. Powe C, Allen M, Puopolo KM, et al. Recombinant human prolactin for the treatment of lactation insufficiency. Clin Endocrinology (Oxf). 2010;73(5):645–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hill PD, Brown LP, Harker TL. Initiation and frequency of breast expression in breastfeeding mothers of LBW and VLBW infants. Nurs Res. 1995;44(6):352–355 [PubMed] [Google Scholar]
- 27. Lucas A, Gibbs JAH, Lyster RLJ, Baum JD. Creamatocrit: simple clinical technique for estimating fat concentration and energy value of human milk. Br Med J. 1978;1(6119):1018–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Page-Wilson G, Smith PC, Welt CK. Short-term prolactin administration causes expressible galactorrhea but does not affect bone turnover: pilot data for a new lactation agent. Int Breastfeed J. 2007;2(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matera L. Endocrine, paracrine and autocrine actions of prolactin on immune cells. Life Sci. 1996;59(8):599–614 [DOI] [PubMed] [Google Scholar]
- 30. Jackson JG, Janszen DB, Lonnerdal B, Lien EL, Pramuk KP, Kuhlman CF. A multinational study of α-lactalbumin concentrations in human milk. J Nutr Biochem. 2004;15(9):517–521 [DOI] [PubMed] [Google Scholar]
- 31. Lönnerdal B. Nutritional and physiologic significance of human milk proteins. Am J Clin Nutr. 2003;77(6):1537S–1543S [DOI] [PubMed] [Google Scholar]
- 32. Bao Y, Newburg DS. Capillary electrophoresis of acidic oligosaccharides from human milk. Electrophoresis. 2008;29(12):2508–2515 [DOI] [PubMed] [Google Scholar]
- 33. Allen JC, Keller RP, Archer P, Neville MC. Studies in human lactation: milk composition and daily secretion rates of macronutrients in the first year of lactation. Am J Clin Nutr. 1991;54(1):69–80 [DOI] [PubMed] [Google Scholar]
- 34. Kleinberg DL, Todd J, Niemann W. Prolactin stimulation of α-lactalbumin in normal primate mammary gland. J Clin Endocrinol Metab. 1978;47(2):435–441 [DOI] [PubMed] [Google Scholar]
- 35. Healy DL, Rattigan S, Hartmann PE, Herington AC, Burger HG. Prolactin in human milk: correlation with lactose, total protein, and α-lactalbumin levels. Am J Physiol. 1980;238(1):E83–E86 [DOI] [PubMed] [Google Scholar]
- 36. VanHouten JN, Wysolmerski JJ. Transcellular calcium transport in mammary epithelial cells. J Mammary Gland Biol Neoplasia. 2007;12(4):223–235 [DOI] [PubMed] [Google Scholar]
- 37. Campbell-Yeo ML, Allen AC, Joseph KS, et al. Effect of domperidone on the composition of preterm human breast milk. Pediatrics. 2010;125(1). Available at: www.pediatrics.org/cgi/content/full/125/1/e107 [DOI] [PubMed] [Google Scholar]
- 38. Picciano MF. Representative values for constituents of human milk. Pediatr Clin North Am. 2001;48(1):263–264 [DOI] [PubMed] [Google Scholar]
- 39. Goldman AS, Garza C, Nichols BL, Goldblum RM. Immunologic factors in human milk during the first year of lactation. J Pediatr. 1982;100(4):563–567 [DOI] [PubMed] [Google Scholar]
- 40. Goldman AS, Garza C, Nichols B, Johnson CA, Smith EO, Goldblum RM. Effects of prematurity on the immunologic system in human milk. J Pediatr. 1982;101(6):901–905 [DOI] [PubMed] [Google Scholar]
- 41. Wang B, Brand-Miller J, McVeagh P, Petocz P. Concentration and distribution of sialic acid in human milk and infant formulas. Am J Clin Nutr. 2001;74(4):510–515 [DOI] [PubMed] [Google Scholar]
- 42. Carlson SE. N-Acetylneuraminic acid concentrations in human milk oligosaccharides and glycoproteins during lactation. Am J Clin Nutr. 1985;41(4):720–726 [DOI] [PubMed] [Google Scholar]
- 43. György P, Jeanloz RW, von Nicolai H, Zilliken F. Undialyzable growth factors for Lactobacillus bifidus var. pennsylvanicus: protective effect of sialic acid bound to glycoproteins and oligosaccharides against bacterial degradation. Eur J Biochem. 1974;43(1):29–33 [DOI] [PubMed] [Google Scholar]
- 44. Nolin JM. The prolactin incorporation cycle of the milk secretory cell: an integral component of the prolactin response cycle. J Histochem Cytochem. 1979;27(8):1203–1204 [DOI] [PubMed] [Google Scholar]
- 45. Ollivier-Bousquet M, Kann G, Durand G. Prolactin transit through mammary epithelial cells and appearance in milk. Endocr Regul. 1993;27(3):115–124 [PubMed] [Google Scholar]
- 46. Grosvenor CE, Whitworth NS. Incorporation of rat prolactin into rat milk in vivo and in vitro. J Endocrinol. 1976;70(1):1–9 [PubMed] [Google Scholar]
- 47. Yuen BH. Prolactin in human milk: the influence of nursing and the duration of postpartum lactation. Am J Obstet Gynecol. 1988;158(3):583–586 [DOI] [PubMed] [Google Scholar]
- 48. Cregan MD, Mitoulas LR, Hartmann PE. Milk prolactin, feed volume and duration between feeds in women breastfeeding their full-term infants over a 24 h period. Exp Physiol. 2002;87(2):207–214 [DOI] [PubMed] [Google Scholar]
- 49. Gala RR, Singhakaowinta A, Brennan MJ. Studies on prolactin in human serum, urine and milk. Horm Res. 1975;6(5–6):310–320 [DOI] [PubMed] [Google Scholar]
- 50. Gartner LM, Morton J, Lawrence RA, et al. Breastfeeding and the use of human milk. Pediatrics. 2005;115(2):496–506 [DOI] [PubMed] [Google Scholar]
- 51. Meinzen-Derr J, Poindexter B, Wrage L, Morrow AL, Stoll B, Donovan EF. Role of human milk in extremely low birth weight infants' risk of necrotizing enterocolitis or death. J Perinatol. 2009;29(1):57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sullivan S, Schanler RJ, Kim JH, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr. 2010;156(4):562–567 [DOI] [PubMed] [Google Scholar]
- 53. Manzoni P, Rinaldi M, Cattani S, et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. JAMA. 2009;302(13):1421–1428 [DOI] [PubMed] [Google Scholar]
- 54. Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292(19):2357–2365 [DOI] [PubMed] [Google Scholar]
- 55. Mshvildadze M, Neu J. The infant intestinal microbiome: friend or foe? Early Hum Dev. 2010;86(suppl 1):67–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Czank C, Prime DK, Hartmann B, Simmer K, Hartmann PE. Retention of the immunological proteins of pasteurized human milk in relation to pasteurizer design and practice. Pediatr Res. 2009;66(4):374–379 [DOI] [PubMed] [Google Scholar]
- 57. Kent JC, Mitoulas L, Cox DB, Owens RA, Hartmann PE. Breast volume and milk production during extended lactation in women. Exp Physiol. 1999;84(2):435–447 [PubMed] [Google Scholar]