Abstract
BACKGROUND:
It is hypothesized that a physiological predisposition toward hypertension results from a combination of intrauterine growth restriction or overgrowth and excessive postnatal weight gain. Previous studies were conducted largely in Western countries however the hypothesis may also be relevant in developing countries where metabolic disorders are increasing.
OBJECTIVE:
We investigated the association of birth weight and postnatal weight gain with hypertension among Chinese children.
METHODS:
A population based study was conducted among 15 600 children aged 3 to 6 years from Tianjin, China. Weight was expressed as z scores. Postnatal weight gain was defined as changes in z scores from birth to 3 to younger than 4 years, 4 to younger than 5 years, and 5 to 6 years. Hypertension was defined as greater than the 90th percentile of either systolic or diastolic blood pressure. Logistic regression-derived odds ratios and 95% confidence intervals were generated to estimate the association between birth weight and postnatal weight gain with hypertension risk in childhood.
RESULTS:
Birth weight was positively associated with childhood hypertension in boys and girls (odds ratios [95% confidence interval] comparing extreme quartiles [high versus low] were 5.67 [3.83–8.39] and 2.58 [3.83–8.39], respectively). Postnatal weight gain was positively associated with hypertension and the association did not significantly vary by birth size for gestational age.
CONCLUSIONS:
Greater birth weight or postnatal weight gain was associated with increased childhood hypertension risk, suggesting that intrauterine growth and postnatal weight gain may have implications on health during childhood.
Keywords: birth weight, hypertension, fetal origins
WHAT'S KNOWN ON THIS SUBJECT:
Previous studies have identified an inverse association between birth weight and childhood hypertension. In addition, postnatal weight gain may contribute to this predisposition toward hypertension.
WHAT THIS STUDY ADDS:
Previous studies evaluating the association between birth weight and childhood hypertension were conducted largely in Western countries but may be relevant in developing countries, where the occurrence of metabolic disorders is increasing.
Hypertension is a major modifiable risk factor for cardiovascular disease, a leading cause of death in China.1,2 Given the correlation between childhood and adult hypertension,3 identifying risk factors of childhood hypertension may contribute to the early prevention of hypertension and cardiovascular disorders in adulthood. In addition, childhood hypertension itself recently was identified as a risk factor for premature death.4 Although obesity is a major risk factor for childhood hypertension,5 hypertension is not fully explained by obesity, and additional risk factors should be characterized.
Conventionally, it is hypothesized that a physiological predisposition toward hypertension results from a combination of intrauterine growth restriction and excessive postnatal weight gain.6 The hypothesis proposes that in a nutrient-poor uterine environment, the fetus must make adaptations that promote survival; however, these adaptations are deleterious after birth when nutrients are plentiful. An inverse association has been observed between birth weight and hypertension in childhood through adulthood and also has been observed for cardiovascular disease,7 metabolic syndrome,8 and type 2 diabetes.9 Emerging evidence indicates that the association between birth weight and metabolic disorders may not be linear. A U-shaped association between birth weight and increased risks of developing hypertension and other metabolic disorders later in life was observed,10,11 which leads to the expansion of the developmental origins of health and disease to include the role of excessive fetal growth and early overnutrition. This may be particularly relevant in some developing countries, such as China, where the influx of Western lifestyles are escalating and, as a result, the occurrence of overnutrition, macrosomia, childhood obesity, hypertension, and other metabolic diseases are increasing rapidly.12 Previous studies on the association of birth weight and childhood hypertension, however, were conducted largely in Western countries, and studies among developing countries are sparse. In the present study, we investigated the association between birth weight and hypertension, accounting for variation by adiposity and postnatal weight gain, in a large cohort of 15 600 children aged 3 to 6 years in Tianjin, China.
PATIENTS AND METHODS
Study Sample
Between March and September 2005, a retrospective longitudinal cohort study was conducted within 71 kindergartens in Tianjin, China, a city of 11.5 million people.13,14 A multistage cluster sampling was used to obtain a random sample of children in Tianjin, representing both urban and rural districts. Children from 71 of Tianjin's 269 kindergartens, including 29 urban and 42 rural kindergartens, were sampled. All children aged 3 to 6 years in the selected kindergartens were invited to join the survey, and it was completed by a total of 15 928 (95.6%) children. The final sample in the present analyses included 15 600 children after the exclusion of individuals without data on birth weight (n = 76) and gestational age (n = 49) and infants born preterm, defined as less than 37 weeks' gestation (n = 203). The study was approved by the Tianjin Women's and Children's Health Center. The parents gave verbal informed consent.
Assessments of Anthropometric Factors and Covariates
Anthropometric measurements and the survey questionnaire were conducted and verified by intensively trained health workers from the Women's and Children's Health Center of Tianjin. The survey was completed by the parents at the kindergartens and included information on the child's history of illness status (any of the following: seizure; tuberculosis; hepatitis; chronic bronchitis; asthma; nephritis; endocrine disease; or neurologic disease) and parents' (any of the following: diabetes; hypertension; coronary heart disease; or dyslipidemia) current health status, parent's socioeconomic factors (such as occupation, including blue collar, farmer, administrative, uniform service professionals, education/health/technology field, business, or unemployed), as well as medical histories. In addition, the parents abstracted birth data from their child's birth certificate, including birth date, weight, length, and gestational age. Data on birth certificates were added by doctors and nurses at the time of the birth.
Current body weight was measured to the nearest 0.1 kg using a beam-balance scale, with participants wearing light indoor clothing and shoes. Body height was measured to the nearest 0.1 cm using a stadiometer without shoes. BMI was calculated by dividing weight in kilograms by the square of height in meters. Internal standard-derived z scores were calculated for birth weight and childhood weight using population means and SDs. Birth weight z scores were gestational age and sex specific, and childhood weight z scores were age and sex specific.
Blood Pressure Measurements
Blood pressure was measured using a standardized mercury sphygmomanometer, with a cuff-bladder width of 8 cm. The fourth Korotkoff sound was adopted for the diastolic blood pressure recording. The measurement was taken on the right arm of the participant in a comfortable sitting position after at least a 5-minute rest. Mean blood pressure was calculated from 2 readings, unless the difference between these readings was more than 10 mm Hg, in which case a third measurement was taken and the mean of the last 2 measurements was used. Hypertension was defined as greater than the age- and sex-specific 90th percentile in either systolic or diastolic blood pressure, as recommended by the 1996 Task Force Report on High Blood Pressure in Children and Adolescents.15
Statistical Analysis
To evaluate differences in general study characteristics between hypertensive and normotensive children, P values were calculated using a χ2 test for categorical variables and a t test for continuous variables. Logistic regression was used to generate odds ratios (ORs) and 95% confidence intervals (CIs), describing the association between hypertension and multiple independent variables, including birth weight, birth-weight z score, childhood-weight z score, and postnatal weight change, defined as the change in z score from birth to childhood and potential confounding factors. To determine whether the association between birth weight and hypertension was nonlinear, we evaluated fractional polynomials. The fracpoly command in Stata compares the deviance of a linear model to models of various powers (−2, −1, −0.5, 0, 0.5, 1, 2, and 3) and degrees. Because these models can be highly affected by outliers, individuals with birth-weight z scores greater and less than 4 SDs from the mean were dropped for these analyses (n = 30). To visualize the association, we fit restricted cubic splines within logic regression and plotted the ORs stratified by age and gender.16 Additional analyses were conducted for continuous measures of systolic and diastolic blood pressure using linear regression.
We further evaluated whether the association of postnatal weight gain with hypertension was modified by birth size for gestational age (small for gestational age, appropriate for gestational age, and large for gestational age) using stratified analysis and by evaluating interaction terms. Interaction was assessed via a multiplicative interaction term of birth size for gestational age and postnatal weight gain. Small for gestational age was defined as age- and gender-specific birth weight less than the 10th percentile of this population, large for gestational age was defined as gender and sex-specific birth weight more than the 90th percentile, and all other infants were appropriate for gestational age. All models evaluating the association of birth weight and postnatal weight gain with hypertension risk were stratified on the children's age at the time that blood pressure was measured. Finally, we evaluated the joint effects of birth weight and childhood weight by using the “centile crossing” methods, as previously described.17 We created 3 centiles of childhood weight to correspond with clinically relevant birth weights, including less than 2500 g, 2500 to 4000 g, and 4,000 g or more. For example, 2500 g represents just under the first percentile of birth weight. The corresponding percentile of childhood weight is 12.7 kg. Therefore, a child who is underweight at birth and underweight in childhood will have a birth weight less than 2500 g and a weight in childhood of less than 12.7 kg. A weight of 4000 g represents the 88th percentile of birth weight. We initially created 9 categories (data not shown) to evaluate in logistic regression models with normal birth weight and normal childhood weight as the reference, but because of missing cells, we combined categories of birth and childhood weight.
All statistical analyses were conducted using Stata 9.0 (Stata Corp, College Station, TX), except for the spline regression and plotting, which was conducted using SAS software (version 9.1; SAS Institute, Cary, NC).
RESULTS
Characteristics of the study population are presented in Table 1. Overall, ∼1% of the population was born with low birth weight. Compared with children with normal blood pressure, hypertensive children were significantly shorter at birth, were heavier, more frequently resided in urban areas, had parents who achieved a higher education level, had differing parental occupations, had a greater history of disease in both the father and mother, and had a higher family income, which was significantly different for the highest level of income.
TABLE 1.
Characteristics of Study Participants by Hypertensive Status Among 15 600 Children Aged 3 to 6 Years, Tianjin, China
| Hypertension, n = 2552a | Normotensive, n = 13 048 | P | |
|---|---|---|---|
| Age, y | |||
| 3 to younger than 4 | 644 (24.78) | 3384 (25.53) | .42 |
| 4 to younger than 5 | 841 (32.36) | 4117 (43.40) | .19 |
| 5–6.11 | 1114 (42.86) | 3752 (43.40) | .61 |
| Male gender (%) | 1309 (51.29) | 1243 (48.71) | .22 |
| Gestational age, wk | 39.48 (4.25) | 39.47 (3.38) | .54 |
| Birth weight, g | 3450.19 (452.46) | 3424.02 (445.09) | .99 |
| Birth recumbent length, cm | 53.95 (12.26) | 55.06 (14.22) | .0002 |
| Body weight at age 3–6 years, kg | 20.37 (5.10) | 18.41 (3.48) | <.0001 |
| Body height at age 3–6 years, kg | 110.67 (8.40) | 108.51 (7.93) | <.0001 |
| BMI at age 3–6 years, kg/m2 | 16.42 (2.36) | 15.52 (1.48) | <.0001 |
| Body weight for age z score | 0.76 (1.27) | 0.18 (0.96) | <.0001 |
| Body height for age z score | 0.55 (1.00) | 0.17 (0.96) | <.0001 |
| BMI for age z score | 0.62 (1.38) | 0.09 (1.00) | <.0001 |
| Birth-weight categories, g | |||
| <2500 | 28 (1.08) | 139 (1.05) | .90 |
| 2500–2999 | 255 (9.81) | 1350 (10.19) | .56 |
| 3000–3499 | 997 (38.36) | 5437 (41.02) | .01 |
| 3500–3999 | 978 (37.63) | 4691 (35.40) | .03 |
| ≥4000 | 341 (13.12) | 1636 (12.34) | .27 |
| BMI category, % | |||
| Normal | 1798 (69.18) | 11.106 (83.80) | <.0001 |
| Overweight, >85% | 801 (30.82) | 2147 (16.20) | <.0001 |
| Obesity I, >95% | 468 (18.01) | 795 (6.00) | <.0001 |
| Obesity II, >97.7% | 353 (13.58) | 482 (3.64) | <.0001 |
| Weight gain, change in z score | −0.44 (.47) | 0.09 (1.18) | <.0001 |
| Preterm birth, % | 35 (1.35) | 168 (1.27) | .74 |
| Urban | 1425 (54.83) | 6810 (51.38) | .001 |
| History of any disease, % | 30 (1.15) | 179 (1.35) | .42 |
| Having any current disease, % | 26 (1.00) | 133 (1.00) | .99 |
| Father's education, % | |||
| Junior middle school or lower | 729 (28.05) | 4475 (33.77) | <.0001 |
| Senior middle school | 771 (29.67) | 3413 (25.75) | <.0001 |
| University or higher | 1099 (42.29) | 5365 (40.48) | .09 |
| Mother's education, % | |||
| Junior middle school or lower | 757 (29.13) | 4621 (34.87) | <.0001 |
| Senior middle school | 796 (30.63) | 3603 (27.19) | <.0001 |
| University or higher | 1046 (40.25) | 5029 (37.95) | .027 |
| Father's occupation, % | |||
| Blue collar | 659 (25.36) | 3035 (22.90) | .007 |
| Farmer | 374 (14.39) | 2846 (21.47) | <.0001 |
| Office worker | 808 (31.09) | 4007 (30.23) | .39 |
| Service professional worker | 411 (15.81) | 1920 (14.49) | .08 |
| Unemployed worker | 96 (3.69) | 391 (2.95) | .045 |
| Other | 251 (9.66) | 1054 (7.95) | .004 |
| Mother's occupation, % | |||
| Blue collar | 528 (20.32) | 2346 (17.70) | .002 |
| Farmer | 407 (15.66) | 3064 (23.12) | <.0001 |
| Office worker | 794 (30.55) | 4008 (30.24) | .76 |
| Service professional worker | 300 (11.54) | 1336 (10.08) | .03 |
| Unemployed worker | 286 (11.00) | 1238 (9.34) | .009 |
| Other | 284 (10.93) | 1261 (9.51) | .026 |
| Family income, % | |||
| <1000 yuan per mo | 545 (20.97) | 2984 (22.52) | .08 |
| 1000–3000 yuan per mo | 1404 (54.02) | 7198 (54.31) | .79 |
| >3000 yuan per mo | 650 (25.01) | 3071 (23.17) | .04 |
| Father with history of disease, % | |||
| Yes | 76 (2.92) | 293 (2.21) | .03 |
| Mother with history of disease, % | |||
| Yes | 33 (1.27) | 95 (0.72) | .004 |
Hypertension was defined as greater than the 90th percentile in either systolic or diastolic blood pressure, as recommended by the 1996 Task Force Report on High Blood Pressure in Children and Adolescents.15
Birth-weight z score was significantly and positively associated with the risk of hypertension among both girls and boys across different age groups. For instance, after the adjustment of postnatal weight change, height, and father's occupation, ORs (95% CIs) for the association were 5.67 (3.83–8.39) for boys aged 5 years and older and 2.58 (1.79–3.73) for girls of the same age group, comparing the highest to lowest quartile of birth-weight z score percentile (Table 2). The regression splines demonstrated linear associations between birth weight and hypertension (P values for curvature all > 0.05) (Fig 1). The association was slightly stronger among boys than girls. When blood pressure was analyzed as a continuous variable, there also was a positive association between birth-weight z score and systolic blood pressure for both boys and girls (parameter estimate: 0.004 [95% CI: 0.003–0.005] for boys and parameter estimate: 0.003 [95% CI: 0.002–0.003] for girls) after the adjustment for postnatal weight change and other factors.
TABLE 2.
Association of Birth-Weight z Score (Quartiles) and Postnatal Weight Gain With Hypertension Stratified by Child's Age When Blood Pressure Was Measured Among Boys and Girls
| OR (95% CI)a |
OR (95% CI)a |
|||||
|---|---|---|---|---|---|---|
| Boys |
Girls |
|||||
| Age 3–4 years, n = 2040 | Age 4–5 years, n = 2581 | Age ≥5 years, n = 3555 | Age 3–4 years, n = 1939 | Age 4–5 years, n = 2285 | Age ≥5 years, n = 3200 | |
| Birth-weight z score percentile | ||||||
| <25th | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 25th–50th | 1.20 (0.83–1.72) | 1.46 (1.05–2.03) | 1.96 (1.45–2.63) | 1.00 (0.68–1.45) | 1.01 (0.71–1.44) | 1.50 (1.12–2.02) |
| 50th–75th | 1.95 (1.29–2.93) | 1.58 (1.09–2.29) | 3.21 (2.32–4.45) | 1.07 (0.72–1.58) | 1.31 (0.93–1.85) | 1.97 (1.47–2.65) |
| >75th | 2.33 (1.43–3.81) | 2.31 (1.50–3.56) | 5.67 (3.83–8.39) | 1.36 (0.84–2.19) | 1.78 (1.17–2.71) | 2.58 (1.79–3.73) |
| Weight gain since birthb | 1.66 (1.42–1.93) | 1.26 (1.11–1.44) | 2.06 (1.83–2.31) | 1.24 (1.06–1.45) | 1.36 (1.19–1.55) | 1.56 (1.39–1.75) |
Adjusted for height, father's occupation, and the other weight variables in the table (ie, birth weight and postnatal weight gain were mutually adjusted).
Weight gain from birth through the time of the blood pressure measurement was expressed as a change in z score.
FIGURE 1.
Estimated spline transformation to describe birth weight in relation to hypertension adjusted for covariates (weight gain, height and SES) and stratified by both age and gender. Solid lines represent odds ratios adjusted for weight gain, height and SES. Dashed lines represent 95% confidence intervals.
Similarly, postnatal change in weight z score was significantly and positively associated with hypertension risk among both boys and girls across different age groups. Among boys, each unit augment in weight z score from birth to 3 to younger than 4 years was associated with a more than 1.6-fold-increased risk (OR: 1.66 [95% CI: 1.42–1.93]). The corresponding ORs (95% CIs) for ages 4 to younger than 5 years and 5 years or older were 1.26 (1.11–1.44) and 2.06 (1.83–2.31), respectively. The corresponding effect measures for girls were OR: 1.24 (95% CI: 1.06–1.45), OR: 1.36 (95% CI: 1.19–1.55), and OR: 1.56 (95% CI: 1.39–1.75). In addition, the above observed associations did not differ significantly by birth size for gestational age (P values for interaction were all > 0.1), although the magnitude of the associations varied slightly across stratum by birth size for gestational age (Table 3).
TABLE 3.
Association of Birth Weight (g) and Postnatal Weight Gaina With Hypertension Stratified by the Child's Age When Blood Pressure Was Measured and Birth Size for Gestational Age Among Boys and Girls
| Adjusted OR (95% CI)b |
|||
|---|---|---|---|
| Age 3 to Younger Than 4 Years | Age 4 to Younger Than 5 Years | Age >5 Years | |
| Boys | |||
| Small for gestational age, n = 589 | n = 154 | n = 177 | n = 258 |
| Birth weight, g | 1.001 (0.998–1.003) | 1.001 (0.998–1.004) | 1.001 (0.999–1.003) |
| Weight gain from birth, change in z score | 2.12 (1.78–3.83) | 0.69 (0.30–1.61) | 2.09 (1.12–3.91) |
| Appropriate for gestation age, n = 6405 | n = 1624 | n = 2018 | n = 2763 |
| Birth weight, g | 1.001 (1.0007–1.002) | 1.001 (1.00–1.0012) | 1.0015 (1.001–1.002) |
| Weight gain from birth, change in z score | 1.80 (1.48–2.18) | 1.37 (1.17–1.61) | 2.17 (1.88–2.51) |
| Large for gestational age, n = 1182 | n = 262 | n = 386 | n = 534 |
| Birth weight, g | 1.001(0.999–1.0020) | 1.001 (1.00–1.002) | 1.002 (1.001–1.003) |
| Weight gain from birth, change in z score | 1.52 (1.06–2.17) | 1.36 (1.00–1.86) | 2.76 (2.04–3.72) |
| Girls | |||
| Small for gestational age, n = 684 | n = 184 | n = 217 | n = 293 |
| Birth weight, g | 1.00 (0.997–1.002) | 1.00 (0.998–1003) | 1.004 (1.001–1.006) |
| Weight gain from birth, change in z score | 1.04 (0.54–2.01) | 1.69 (1.00–2.86) | 2.91 (1.66–5.10) |
| Appropriate for gestational age, n = 5857 | n = 1540 | n = 1800 | n = 2517 |
| Birth weight, g | 1.00 (0.9997–1.0001) | 1.001 (1.00–1.0012) | 1.001 (1.0006–1.002) |
| Weight gain from birth, change in z score | 1.28 (1.05–1.56) | 1.42 (1.20–1.69) | 1.59 (1.39–1.82) |
| Large for gestational age, n = 873 | n = 215 | n = 268 | n = 390 |
| Birth weight, g | 1.002 (1.0002–1.003) | 1.0003 (0.999–1.002) | 1.002 (1.001–1.003) |
| Weight gain from birth, change in z score | 1.40 (0.92–2.15) | 1.40 (0.98–2.00) | 1.75 (1.22–2.52) |
Adjusted for height, father's occupation, and the other weight variables in the table (ie, birth weight and postnatal weight gain were mutually adjusted).
Weight gain from birth through the time of the blood pressure measurement.
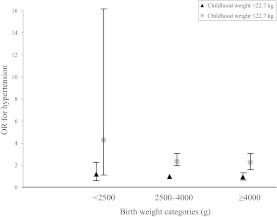
Lastly, we evaluated the joint effect of birth weight and childhood weight on the risk of childhood hypertension (Fig 2). Children who were born small but grew to be relatively large during childhood were at the highest risk. For instance, children who were of low birth weight (<2500 g) but of childhood weight equal or greater than 22.7 kg had more than fourfold-increased risk of hypertension (OR: 4.30 [95% CI: 1.15–16.08]) compared with children with birth weight in the normal range (2500–4000g) and with childhood weight less than 22.7 kg. The extremely small number of children of low birth weight limited our capacity to further examine its joint effect with greater childhood weight.
FIGURE 2.
Multivariate adjusted OR and 95% CI of the joint effects of birth weight and childhood weight for boys and girls compared to children who were born of normal weight and were a normal weight at the time of the blood pressure measurement.
DISCUSSION
In this population-based study of 15 600 children aged 3 to 6 years from Tianjin, China, children of higher weight percentiles at birth had a significantly increased risk of hypertension in both boys and girls. Moreover, children whose postnatal weight percentiles moved upward from birth to childhood had a significantly increased risk for hypertension. The associations of birth weight and postnatal weight change with childhood hypertension were not significantly modified by birth size for gestational age.
Previous studies on the association of birth weight and childhood hypertension were conducted largely in Western countries. Studies on birth weight in Chinese populations and other developing countries are sparse. Caution should be taken when generalizing findings from Western countries to other populations because of differences in genetic predisposition, the distribution of anthropomentrics, behaviors, and other factors that contribute to hypertension across populations. Findings from the present study do correspond with those of a study conducted within a US population.18 This study identified an increased risk for high systolic and diastolic blood pressure with increasing birth weights among participants in the US Collaborative Perinatal Project. In addition, children who traversed weight percentiles upward were at increased risk of high blood pressure in early childhood.18 However, in contrast, a majority of published studies reported an inverse association between birth weight and hypertension in childhood.19 Such inconsistency in findings may be attributed, at least in part, to differences in study population and analytical approaches across studies. Different from the majority of previous studies, we did not control for children's current weight in the analyses of the association between birth weight and hypertension because of the concern of over adjustment.
Current weight may mediate the association between birth weight and hypertension.20 In addition, the observed significant association may be attributed to a common cause of both birth weight and hypertension. For example, genetic factors may predispose an individual to a particular birth weight and hypertension.21 Adjustment for current weight may artificially attenuate or reverse the association between birth weight and hypertension. Nevertheless, our findings of a positive association between birth weight and risk for childhood hypertension were comparable to findings from the Collaborative Perinatal Project,18 adapting the similar analytical approach of not adjusting for current childhood weight.
Studies associating birth weight with childhood- and adult-onset diseases are motivated by the fetal origins or developmental programming hypotheses, which describe how exposures at critical periods of development can affect long-term developmental changes.22 Birth weight, as a proxy for the nutritional status of the intrauterine environment, in a majority of studies has been found to be inversely associated with hypertension as a result of impaired renal development.23 However, this association between low birth weight and future hypertension may only describe 1 tail of the distribution. Indeed, the association between birth weight and metabolic disorders may not be linear; rather, it may be described by a J- or U-shaped association.22 Studies have identified an increased risk for metabolic disorders at both ends of the birth-weight distribution, suggesting a role for early overnutrition in the development of hypertension.10,11 A U-shaped association also has been identified between birth weight and cardiovascular risk factors,24 type 2 diabetes,25 and all-cause mortality,26 such that both the lowest and the highest birth weights are associated with increased risk. This observation leads to the expansion of the developmental origins of health and disease theory to include the role of excessive fetal growth and early overnutrition. This may particularly be relevant in Chinese populations where the influx of the Western lifestyle is escalating, and, as a result, the occurrence of macrosomia, childhood obesity, hypertension, and other metabolic diseases are increasing rapidly.12 Moreover, in China, conventionally, greater birth weight is an indicator of healthy infants. Because of the 1-child policy, pregnant women are highly encouraged to consume more food and nutrients during pregnancy to ensure a healthy infant and an infant of higher birth weight, as evidenced by the very low prevalence of low birth weight in the present study.
The prevalence of low birth weight in this population was very low (∼1%), which limited the statistical power to detect a significant association between low birth weight and hypertension. It is the higher end of the birth weight distribution that is captured to a greater degree in the present analyses. We did identify a moderate but nonsignificant increase in the risk for hypertension among male infants of low birth weight (<2500 g) compared with those with normal weight (2500–3000 g) (OR: 1.71 [95% CI: 0.94–3.11]).
These analyses were conducted in a large, representative cohort of children from Tianjin, China. Additional study strengths include the validity of measurements, including birth weights and lengths abstracted from birth certificates and accurate current hypertension and weight measurements, which were administered by intensively trained health workers. Analytical strengths include the assessment of confounding factors, such as socioeconomic factors and evaluation of the full range of potential association between birth weight and hypertension via splines and fractional polynomials.
There are several limitations. The childhood weight measurements were taken at a single time point. Although cross-sectional analyses evaluating weight gain from birth to different age points suggest that the age of weight gain is important, a longitudinal assessment would be necessary to determine whether cumulative weight gain versus weight gain at a specific age contribute to hypertension.
CONCLUSIONS
Findings from the present study suggest potential adverse health consequences of maternal overnutrition and excessive postnatal weight gain. This is especially true in developing countries, where the number of infants born with high birth weights and childhood obesity is increasing. Given the large influx of the Western lifestyle, metabolic disorders, including hypertension, in subsequent generations may be a growing public health concern. Preventing infants from being born with a high birth weight or from being large for gestational age, and preventing excessive weight gain during childhood, could provide strategies for cardiovascular disease prevention in childhood and beyond.
ACKNOWLEDGMENTS
Katherine Bowers, Mark Klebanoff, Edwina Yeung, and Cuilin Zhang were supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
All of the authors are responsible for the reported research; all have participated in the concept and design of the study; the analysis and interpretation of the data; and the drafting or revising of the manuscript. All have approved the article as submitted.
FINANCIAL DISCLOSURE: The authors have indicated that they have no personal financial relationships relevant to this article to disclose.
- OR
- odds ratio
- CI
- confidence interval
REFERENCES
- 1. Wang X, Jiang G, Wang D, Pan Y, Boulton M. All-cause mortality in Tianjin, China, 1999–2004. Prev Chronic Dis. 2009;6(4):A132. [PMC free article] [PubMed] [Google Scholar]
- 2. He J, Gu D, Wu X, et al. Major causes of death among men and women in China. N Engl J Med. 2005;353(11):1124–1134 [DOI] [PubMed] [Google Scholar]
- 3. Chen X. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117(25):3171–3180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Franks P, Hanson R, Knowler W, Sievers M, Bennett P, Looker H. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med. 2010;362(6):485–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiolero A, Cachat F, Burnier M, Paccaud F, Bovet P. Prevalence of hypertension in schoolchildren based on repeated measurements and association with overweight. J Hypertens. 2007;25(11):2209–2217 [DOI] [PubMed] [Google Scholar]
- 6. Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298(6673):564–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barker DJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2(8663):577–580 [DOI] [PubMed] [Google Scholar]
- 8. Ramadhani M, Grobbee D, Bots M, et al. Lower birth weight predicts metabolic syndrome in young adults: the Atherosclerosis Risk in Young Adults (ARYA) Study. Atherosclerosis. 2006;184(1):21–27 [DOI] [PubMed] [Google Scholar]
- 9. Whincup P, Kaye S, Owen C, et al. Birth weight and risk of type 2 diabetes: a systematic review. JAMA. 2008;300(24):2886–2897 [DOI] [PubMed] [Google Scholar]
- 10. Curhan GC, Chertow GM, Willett WC, et al. Birth weight and adult hypertension and obesity in women. Circulation. 1996;94(6):1310–1315 [DOI] [PubMed] [Google Scholar]
- 11. Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ. Birth weight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation. 1996;94(12):3246–3250 [DOI] [PubMed] [Google Scholar]
- 12. Ma R, Chan J. Pregnancy and diabetes scenario around the world: China. Int J Genaecol Obstet. 2009;104(Suppl 1):S42–S45 [DOI] [PubMed] [Google Scholar]
- 13. Tian Z, Ye T, Zhang X, et al. Sleep duration and hyperglycemia among obese and nonobese children aged 3 to 6 years. Arch Pediatr Adolesc Med. 2010;164(1):46–52 [DOI] [PubMed] [Google Scholar]
- 14. Zhang X, Liu E, Tian Z, et al. High birth weight and overweight or obesity among Chinese children 3–6 years old. Prev Med. 2009;49(2–3):172–178 [DOI] [PubMed] [Google Scholar]
- 15. National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents: a working group report from the National High Blood Pressure Education Program. Pediatrics. 1996;98(4 pt 1):649–658 [PubMed] [Google Scholar]
- 16. Harrell FE., Jr Regression Modeling Strategies. New York, NY: Springer-Verlag; 2001 [Google Scholar]
- 17. Rich-Edwards JW, Kleinman K, Michels KB, et al. Longitudinal study of birth weight and adult body mass index in predicting risk of coronary heart disease and stroke in women. BMJ. 2005;330(7500):1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hemachandra AH, Howards PP, Furth SL, Klebanoff MA. Birth weight, postnatal growth, and risk for high blood pressure at 7 years of age: results from the Collaborative Perinatal Project. Pediatrics. 2007;119(6). Available at: www.pediatrics.org/cgi/content/full/119/6/e1264 [DOI] [PubMed] [Google Scholar]
- 19. Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens. 2000;18(7):815–831 [DOI] [PubMed] [Google Scholar]
- 20. Kramer MS. Invited commentary: association between restricted fetal growth and adult chronic disease: is it causal? Is it important? Am J Epidemiol. 2000;152(7):605–608 [DOI] [PubMed] [Google Scholar]
- 21. Saad MJA. Birth weight and type 2 diabetes in adults. JAMA. 2009;301(15):1539–1541 [DOI] [PubMed] [Google Scholar]
- 22. Warner MJ, Ozanne SE. Mechanisms involved in the developmental programming of adulthood disease. Biochem J. 2010;427(3):333–347 [DOI] [PubMed] [Google Scholar]
- 23. Brenner BM. Congenital oligonephropathy and the etiology of adult hypertension and progressive renal injury. Am J Kidney Dis. 1994;23(2):171–175 [PubMed] [Google Scholar]
- 24. Huang RC. Perinatal and childhood origins of cardiovascular disease. Int J Obes. 2007;31(2):236–244 [DOI] [PubMed] [Google Scholar]
- 25. Harder T, Rodekamp E, Schellong K, Dudenhausen J, Plagemann A. Birth weight and subsequent risk of type 2 diabetes: a meta-analysis. Am J Epidemiol. 2007;165(8):849–857 [DOI] [PubMed] [Google Scholar]
- 26. Baker J, Olsen L, Sorensen T. Weight at birth and all-cause mortality in adulthood. Epidemiology. 2008;19(2):197–203 [DOI] [PubMed] [Google Scholar]